Abstract
This chapter discusses students’ intuitive energy conceptions in socio-ecological systems (i.e., coupled human and natural systems). We begin with a historical and linguistic examination of how the scientific view of energy is different from a variety of informal views of energy that appeared earlier in the history of science and are embedded in colloquial English. Our analyses indicate that the scientific view of energy is different from the informal views of energy in two important ways: association and tracing. First, in the scientific view, energy is associated with its indicators in specialized ways, whereas in the informal views, energy is often associated with life, feeling, perceptions, or emotions. Second, the scientific view highlights energy as a constraint, whereas the informal views treat energy as a cause. We then describe how these ideas were used to develop a learning progression for energy. The learning progression depicts two trends of student development. Regarding association, there is a trend from broad to restricted association. Regarding tracing, the trend is from tracing the cause-and-effect chain to the specialized scientific way of tracing energy—tracing energy separately from matter and with heat dissipation. Finally, we discuss the implications of the learning progression for teaching energy at the K-12 level.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Energy is identified as a crosscutting concept in A Framework for K-12 Science Education because it can serve as a unifying and organizational framework for students to connect knowledge from the various disciplines into a coherent and scientifically-based view of the world (National Research Council [NRC] 2011). A thorough understanding of energy is fundamental to all science disciplines and therefore essential to scientific literacy. As anthropogenic carbon emission is becoming the major contributor to the global climate change (Intergovernmental Panel on Climate Change [IPCC] 2007), using a scientific view of energy to understand human’s impact on climate has also become a critical component of environmental literacy (NGSS Consortium of Lead States 2013).
Energy, as a concept central to scientific literacy and environmental literacy, has been emphasized in science standards across all grade levels for many years (e.g., NRC 1996). However, current teaching in schools tends to focus on quantitative calculation and does not prepare students to apply knowledge of energy in real-life situations (Nordine et al. 2011). Empirical studies have documented many intuitive energy conceptions students hold. For example, both students and their teachers tend to confuse energy with force and power (Trumper 1998; Watts 1983) as well as effort (Driver and Warrington 1985). In addition, they often think that energy only exists in the bodies of living things (Gilbert and Watts 1983; Watts 1983) or when motion is involved (Trumper 1998). As students are learning about different forms of energy, they tend to think that different kinds of energy exist (Gilbert and Watts 1983; Kaper and Goedhart 2002; Schmid 1982). Among the different forms of energy, heat and chemical energy are the most difficult ones. Students usually see temperature as the measure of heat (Baierlein 1990; Laburu and Niaz 2002; Lewis and Linn 1994; Loverude et al. 2002). They often equate chemical energy with organic molecules (Jin and Anderson 2012a; Mohan et al. 2009). In biological contexts, students often think that energy is the vital power of living organisms (Barak et al. 1997).
Regarding energy principles, researchers found that students seldom use the energy conservation principle to solve problems about mechanical systems (Driver 1994; Duit 1984) or to explain biological and chemical events (Barak et al. 1997; Boo 1998). They usually do not recognize the connections between energy conservation and energy degradation; therefore, students see these two principles as contradictory (Duit 1984; Pinto et al. 2005). They seldom recognize heat dissipation in food chains and often use matter-energy conversion to reason about biological processes (Jin and Anderson 2012a; Lin and Hu 2003; Mohan et al. 2009).
As elaborated above, students encounter tremendous difficulties in learning about energy. Why is the concept of energy so difficult for students? How do students use ideas of energy to understand real-world phenomena? How can instructional approaches help students develop a coherent and sophisticated understanding of energy? This chapter explores these questions as they relate to socio-ecological systems—coupled human and natural systems (Liu et al. 2007; Long Term Ecological Research Network [LTER] 2007). In particular, we focus on environmental events that affect global climate. These events include an oak tree growing, a baby girl growing, people losing weight, a dead tree decaying, a flame burning, and a car running.
In this chapter, we first conduct a historical analysis to better understand how the scientific view of energy differs from the various views of energy that appeared earlier in the history of science. There is a parallel between conceptual change in the history of science and students’ development of scientific concepts (Carey 1985). Therefore, an examination of how the concept of energy was constructed in the history of science will provide significant implications for our understanding of students’ intuitive ideas of energy. Second, we conduct a linguistic analysis to examine how the scientific view of energy differs from the informal views of energy that are embedded in colloquial English. Energy is not just a scientific term. It is also a common word used in everyday language. An examination of colloquial meanings of energy will enable us to better understand common intuitive ideas that hinder student learning of the scientific view of energy. Finally, we describe how we used the ideas from the historical analysis and linguistic analysis to develop a learning progression for energy in socio-ecological systems. This chapter provides an example of how ideas about the history and nature of science and ideas from linguistics can inform learning progression research. The empirical study on how we developed the learning progression for energy in socio-ecological systems is reported in another paper of the project (Jin and Anderson 2012a).
2 Historical Analysis
We study how students use the energy concept to understand environmental events such as an oak tree growing, a baby girl growing, a flame burning, a car running, etc. These environmental events are about fire and life. Therefore, in the historical analysis, we specifically explore how scientists constructed the concept of energy in their inquiries into fire and life. For centuries, scientists have wrestled with essential questions about fire and life. What is it? What is the cause? Why does it happen this way? Is there anything that is conserved? Scientists’ inquiry into these questions gradually differentiated the energy concept from matter and life, and eventually established energy as a universally conserved quantity.
2.1 Inquiry into Fire: How Energy Was Differentiated from Matter
Since ancient time, humans have been asking questions about fire: Why do some materials burn while others do not? What is fire? Why is it hot? Aristotle believed that flammable materials contain a “fire element”. Based on this idea, the alchemists of the 1600s developed the phlogiston theory (Cobb and Goldwhite 1995). According to this theory, all flammable materials contain phlogiston, a substance that is given off in burning; the ash of the burnt material always weighs less due to the emission of phlogiston. In retrospect, it is apparent that alchemists conflated matter and energy into an undifferentiated concept of phlogiston.
About a century later, the French chemist Lavoisier challenged the phlogiston theory through experiment (Cobb and Goldwhite 1995). He demonstrated the important role of oxygen in combustion and formulated the law of mass conservation. His work laid the foundation for modern chemistry. However, one question remained unanswered: Why do some materials burn while others do not? To answer this question, Lavoisier proposed the caloric theory: Flammable materials contain caloric, a special form of matter; caloric can pass freely through the pores of dense materials and becomes manifest in explosions; because caloric is imponderable, no change in weight is observed in the reaction (Morris 1972). The caloric theory indicates the beginning of matter-energy differentiation. Unlike phlogiston, caloric has almost no weight. However, matter and energy are not completely differentiated, because caloric is a fluid or semi-matter that flows from one place to another. This view is different from the modern energy view, from which heat is an abstract quantity associated with the kinetic motion of atoms and molecules. After Mayer and Joule discovered the mechanical equivalent of heat, the caloric theory was superseded by the motion theory of heat (Coopersmith 2010). This indicates a complete differentiation between energy and matter.
2.2 Inquiry into Life: How Energy Was Differentiated from Life
The study of life began with several essential questions: What is life? How are living things different from non-living things? Why can living things grow and reproduce, but non-living things cannot? Again, the first theory of life is generally credited to Aristotle. According to him, all living things have “soul”, and soul is the cause of life. Plants have “vegetative souls” that cause growth and reproduction. Animals have “sensitive souls” that cause not only growth and reproduction, but also motion and sensation. People have “rational souls” that enable us to do all the above and reasoning (Shanks 2001). Soul is a concept that does not differentiate biological entities from psychological ones.
In his book, The web of life: A New Scientific Understanding of Living Systems, Capra (1997) traces the establishment of biology as a distinct discipline and details the paradigm shift in biology. The following description is drawn from Capra’s book. In the nineteenth century, the study of life bifurcated into two disciplines. Mechanistic biology studied life in terms of mechanical and chemical structures and processes, whereas psychology investigated thinking and reasoning. Modern biology was thus established and separated from psychology but at the expense of reducing life into mechanical processes. Many biologists believed that physics and chemistry were insufficient to understand life phenomena. Among them, vitalists proposed that vital power was the cause of life events such as cell reproduction. Vital power was once considered as the “energy” in biology. Organismic biologists proposed a rival theory that viewed life as an emergent property of autopoietic systems. The atoms and molecules that compose the cells do not have life. However, when they form a living network (cell), life is emerged out of the special organization of atoms, molecules, and organelles. It is also important to note that organic molecules in the cell provide energy, but they do not have “life”. As emphasized by Capra, life is a pattern. In this sense, the organismic theory differentiates energy completely from life, and it differentiates both energy and life from psychological entities such as soul.
As discussed above, it took scientists hundreds of years to understand fire and life. Why are these two everyday phenomena so difficult to understand? The reason is because there always seemed to be a quantity that determined what was possible and what was impossible; this quantity was always present but it was never visible. Now we know this quantity is energy. Energy is a powerful concept to understand various environmental events. In particular, our historical analysis indicates two important aspects of the contemporary scientific view of energy. First, energy is an abstract quantity; it should be differentiated from matter, life, and psychological entities. Second, energy is about constraint rather than cause; energy is always conserved and yet degrades. This is a law that constrains our explanations of any environmental events. Similar ideas are also discussed in several other chapters in this book from different angles (See Chaps. 5, 7, and 8).
3 Linguistic Analysis
Many scientific terms have been adopted from colloquial languages. The scientific meanings of these terms could be very different from their vernacular senses. Ordinary words with scientific meanings are a major source of students’ confusions and learning difficulties (Fang 2006). Energy is one of those tricky words. It has a specialized meaning in science, but it is used in non-scientific ways in everyday language. Therefore, understanding the colloquial meanings of the word energy will enable us to better understand students’ naïve conceptions and learning difficulties.
3.1 Definitions of Energy in English Dictionaries
We therefore conducted an analysis of the various meanings of energy in English. Reliable sources for colloquial meanings of words are dictionaries because they contain precise, intelligible, and complete definitions of words. When creating definitions for a word, lexicographers use a variety of strategies to capture the essence of the word’s meanings as well as the word’s unique roles in language (McKeown 1991). These strategies include describing semantic relations among words using synonyms and antonyms, using a strictly controlled vocabulary to define all entries in the dictionary, and using example sentences and collocations (the company in which words customarily appear) to depict word meanings in linguistic contexts. Different dictionaries may use one or more of these strategies. For example, the Longman Dictionary of Contemporary English uses a strictly controlled vocabulary of 2,000 words to define all words, which leads to a greater clarity compared to other dictionaries. The Merriam-Webster Thesaurus uses synonyms and antonyms to depict the semantic boundaries precisely. Therefore, including entries from different dictionaries will allow us to achieve a valid and comprehensive interpretation of the meanings of the word energy. We chose four dictionaries as our data sources: New Oxford Dictionary (3rd edition), Merriam-Webster Thesaurus (online), Dictionary.com, and Longman Dictionary of Contemporary English (online). These dictionaries are widely used today. They also cover the major strategies that lexicographers adopt to depict word meanings.
In the analysis, we first found entries of energy in all four dictionaries. We used the thematic analysis technique (Boyatzis 1998) to analyze the data. The coding units were the definitions of the word energy in the selected dictionaries. We first read and familiarized ourselves with the definitions and generated a set of initial codes to identify important features of the definitions. Then, we used an iterative process to code the data and revise the codes. We found that the dictionaries depict the meanings of energy in terms of three categories: sources of energy, nature of energy, and causal reasoning. The coding scheme was developed based on this finding. It is presented in Table 9.1.
The results of the data analysis are presented in Table 9.2. Our linguistic analysis indicates five definitions of energy: (1) a person’s physical or mental strength or power, (2) life energy of living things, (3) vital power of places, (4) energy sources utilized by people, and (5) the ability to do work.
3.2 Informal Views of Energy
In the paragraphs that follow, we describe the informal views of energy embedded in the above definitions in terms of three categories: sources of energy, nature of energy, and causal reasoning.
3.2.1 Sources of Energy
Four definitions of energy explicitly state the sources of energy. Definitions One and Two describe energy as a type of vital power possessed by living things. Definition Three describes energy as a type of vital power existing in certain places. As shown in many of the example sentences (e.g., There was a lot of energy in the room this morning. Did you feel it?), these definitions associate energy with living things or places based on feelings. This is very different from the scientific view, in which energy is associated with its indicators (e.g., light, special chemical structure, movement, etc.) rather than feelings. Definition Four describes multiple sources of energy in ways very close to the scientific view of energy. However, it does not explicitly distinguish between energy and its sources, which could cause confusion especially in situations involving foods and fuels. Foods and fuels are organic matter that provides energy in carbon-transforming processes; they are not energy. Definition Five does not explicitly state where energy comes from, but it defines energy as an “ability”, which could lead students to think that only living things possess energy. This is because the word ability in colloquial English is often associated with living things. For example, we often say that living animals have the ability to grow and to move, whereas dead animals do not have this ability.
3.2.2 Nature of Energy
Definitions One, Two, and Three define energy as a psychological entity; they also associate energy with life, feelings, or emotions (e.g., being energetic and excited). This is very different from the scientific view of energy, in which energy is not differentiated from life, feelings, or emotions. For example, in everyday situations, we may say: I have a lot of “energy” to begin the day, because I had a good night’s sleep. However, from the scientific view, the body has less energy due to heat dissipation in cellular respiration over the night. In Definition Four and Definition Five, energy is defined as a physical entity—the power provided by certain sources or the ability to do work. Although these two definitions differentiate energy from psychological entities, they do not explicitly define energy as an abstract quantity. In particular, by defining energy using another abstract term (work), Definition Five does not provide any useful information to students.
3.2.3 Causal Reasoning
As elaborated in the historical analysis, the scientific view of energy emphasizes energy as a constraint. However, an informal view embedded in the five definitions is that energy is a cause. Definitions One, Two, Three, and Four all describe energy as the cause of a variety of effects such as life, certain feelings, movement, machines working, etc. In Definition Five, energy is described as both cause and constraint. One example sentence used in Definition Five is about energy being transferred, which indicates a sense of “constraint”. That is, energy must go somewhere. However, this definition also describes energy as the cause of motion or interactions of molecules.
In summary, the linguistic analysis indicates two patterns. First, the scientific view differentiates energy from matter, life, and psychological entities, whereas the informal views do not. This is reflected in the different ways of association. In the scientific view, energy is associated with its indicators in specialized ways, whereas in the informal views, energy is often associated with life, feeling, perceptions, or emotions. Second, the scientific view highlights energy as a constraint, whereas the informal views treat energy as a cause. When we treat energy as constraint, we use energy conservation and degradation to constrain our accounts about events. That is, we trace energy in a specialized way, namely separately from matter and including heat dissipation. When we treat energy as cause, we do not trace energy consistently. Instead, we trace a cause-and-effect chain; energy is often treated as the cause in this chain. This relation can be presented as: energy → effects such as machines moving, people running, etc.
4 The Learning Progression for Energy in Socio-ecological Systems
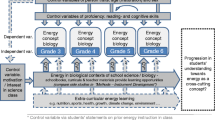
Our historical and linguistic analyses indicate that the scientific view of energy differs from everyday views of energy in two important ways. First, energy is an abstract quantity from the scientific view, whereas it is often equated with matter, life, and psychological entities in colloquial English. Second, energy is about constraint from the scientific view, whereas it is often treated as cause in everyday situations. Within the scope of the Environmental Literacy Project (Michigan State University), we have conducted several learning progression studies to examine how students use energy to explain environmental events in socio-ecological systems (Jin and Anderson 2012a, b; Jin et al. 2013). Our data indicate that students tend to use the informal views of energy to explain environmental events. Based on the historical and linguistic analyses, we identified two progress variables to assess and measure student performance. The first progress variable is association. Scientists use specialized ways to associate energy with its indicators (see more explanation about energy indicator in Nordine et al. 2011) whereas students often associate energy with perceptions, feelings, and life. The second progress variable is tracing. Scientists trace energy in a specialized way, whereas students often lack the ability to trace energy consistently and successfully. This specialized way of tracing emphasizes that energy must come from somewhere and go somewhere, and that heat is always released, so it is aligned with the idea of “energy as constraint”. The scientific ways of association and tracing are listed below:
-
Association. Energy is associated with a set of indicators: kinetic energy → motion; light energy → light; heat energy → differences in temperature; electrical energy → electricity; chemical energy → organic molecules.
-
Tracing. There are specialized ways of tracing energy in carbon-transforming processes: tracing energy separately from matter and with the recognition of conservation and heat dissipation.
4.1 The Learning Progression for Energy
We have used these two progress variables to develop a learning progression for energy (Table 9.3). In the learning progression, each progress variable contains four achievement levels. Each level is about a specific way of association and a specific way of tracing. Levels one, two, and three are about students’ informal views of energy; they describe idiosyncratic ways of association and tracing. Level 4 represents the specialized ways of association and tracing emphasized in the scientific view of energy.
4.2 Trends of Development
The learning progression framework suggests two trends of development. In the paragraphs that follow, we use examples in Table 9.3 to describe these trends.
4.2.1 From a Broad Association to a Restricted Association
Regarding the association progress variable, there is a trend from broad to restricted association. At level one, students tell stories about the actor (i.e., a living organism, flame, or a car) and its needs. Energy usually does not play a role in their stories. However, when being asked to define what energy was and whether energy was involved in the events, all of our participant students were able to incorporate energy into their explanations. They tended to associate energy with conditions, feelings, and emotions. These characteristics are illustrated in the interview episode at level one. The student associated energy with feelings and conditions such as being healthy not sick, being hyper, and being active.
At level two, energy is treated as a physical necessity, an “essence” required to power hidden processes. Energy is no longer associated with psychological characteristics such as feelings and emotions; it is only associated with physical and mechanical characteristics. However, energy is not differentiated from matter in general. As illustrated in the interview episode at level two, the student stated that gasoline was a form of energy.
At level three, the association of energy is even more restricted. Students are able to associate energy with its common indicators such as light and motion. They are also able to relate energy to organic molecules, but they often state that organic molecules such as glucose and ATP are energy.
At level four, students understand energy as an abstract quantity that is associated with its indicators in specialized ways. They are able to recognize that organic molecules provide chemical energy, but they are not energy. This restricted reasoning is illustrated in the interview episode at level four. The student explained that sand and limestone were not fuels because they did not contain high-energy bonds and therefore did not provide energy.
4.2.2 From Tracing the Cause-and-Effect Chain to Tracing Energy Separately from Matter and with Heat Dissipation
Regarding the tracing progress variable, the trend is from tracing the cause-and-effect chain to the specialized way of tracing energy—tracing energy separately from matter and with heat dissipation. At level one, students trace the cause-and-effect chain: When the actor has its needs, it grows and moves. This characteristic is illustrated in the interview episode at level one. The student explained that the causes of the growth of the baby’s body are the enablers/needs such as foods, water, and enough sleep.
At level two, students trace a cause-and-effect chain that involves energy. Students begin to develop the idea that a physical necessity such as energy is required to power hidden processes, but they usually do not spontaneously think about where energy goes when the event is over. When being asked to explain where energy goes, they often come up with some plausible explanations. In the interview episode at level two, the student explained that energy/gasoline was burned to power the car movement. When being asked to explain where the energy went after that, he first admitted that he couldn’t figure out where energy went, and then he guessed that energy could be released from the exhaust pipe as a pollutant.
At level three, students begin to trace matter and energy, but without making necessary distinctions between them. They also do not trace energy with recognition of heat dissipation. In the written example at level three, the student stated that it is impossible for one glucose molecule to provide energy for finger movement and heat at the same time. The student did not recognize that when chemical energy transforms into kinetic energy in cellular respiration, heat is always released as a by-product.
Level four represents the scientific view. Students are able to trace energy in a specialized way—tracing energy separately from matter and with recognition of conservation and heat dissipation. In the interview episode at level four, the student traced matter and energy separately. He was able to explain that the energy of the match was transformed into light energy and heat.
5 Implication for Teaching Energy
The learning progression presented above provides rich information about student intuitive ideas about energy in socio-ecological systems. Based on the learning progression, we propose that an effective teaching approach could focus on association and tracing of energy. A detailed report on this teaching approach is described in another chapter of this book (see Chap. 4).
We suggest that an effective instructional approach to energy in socio-ecological systems should contain two components. The first component is using “forms of energy” to teach the specialized ways of association. Several researchers point out that the term “forms of energy” is problematic, because it implies the existence of many different kinds of energy (Gilbert and Watts 1983; Kaper and Goedhart 2002; Schmid 1982). This point is also articulated in the NRC framework (p. 122). We argue, however, in order for students to learn the abstract energy concept, a bridge between the abstract meaning of energy and daily experience is indispensible; this bridge is “forms of energy”. We do agree that traditional ways of teaching “forms of energy” are problematic. In science classrooms, many forms of energy are taught, but the overlaps among some forms of energy (e.g., solar energy and light energy, kinetic energy, wind energy, and sound energy, etc.) and the distinction between energy and its indicators/manifestations are seldom explicitly addressed. As the result, students often hold very vague ideas about forms of energy. We suggest teaching “forms of energy” with the focus on the specialized ways of association. That is, students understand that energy is an abstract quantity and that quantity is associated with a limited number of indicators (Nordine et al. 2011) in specialized ways. In the socio-ecological systems, the following forms of energy and specialized ways of association are critical: kinetic energy (associated with motion), light energy (associated with light), heat energy (associated with temperature change), and chemical energy (associated with C–C and C–H bonds of organic molecules). Learning these specialized ways of association is very important, because the lower anchor and intermediate levels of the learning progression indicate that students tend to associate energy with a broad range of phenomena including feelings and perceptions, and that they cannot successfully differentiate foods and fuels from materials that do not provide energy. By teaching the specialized ways of association, the teacher will be able to present the concept of “forms of energy” in depth and clarity and therefore help students identify energy in environmental events.
In another chapter of this book, Millar proposes to use forms of energy ONLY for the different ways in which energy can be “stored”. According to Millar, light energy should not be used, because it is often about pathways not stores. In another article, Millar (2005) provides more detailed description of the problem with light energy. He discusses two types of situations. In most situations, it is important to know the rate at which energy is being transferred from one place to another by light (i.e., pathways). In other situations, which are rare, it is important to calculate the amount of energy provided by photons. In socio-ecological systems, light energy is an important form of energy involved in photosynthesis. Specifically, the first stage of photosynthesis is light absorption, in which a photon strikes a pigment molecule and passes on part of its energy to the electrons of that pigment molecule. In this sense, light energy in socio-ecological systems is about stores rather than pathways. Therefore, our approach is not contradictory to Millar’s approach.
The second component is teaching the specialized ways of tracing matter and energy. We suggest teaching the three fundamental principles of matter and energy—matter conservation, energy conservation and energy degradation at the same time rather than in any particular sequence. As presented in Fig. 9.1, the three principles can be integrated into a framework that emphasizes two specialized ways of tracing: tracing energy separately from matter and with conservation and degradation, tracing matter with conservation. The learning progression indicates that students usually cannot successfully trace matter and energy. They use many informal ways of tracing when explaining environmental events. They often trace the cause-and-effect relations rather than matter and energy. They usually cannot differentiate between matter transformation and energy transformation, and therefore use matter-and-energy conversion to reason about phenomena. When tracing energy, they often do not recognize heat dissipation. By introducing the specialized ways of tracing matter and energy, the teacher will be able to help students better understand the connections among the three fundamental principles and use energy as a conceptual tool to analyze environmental events.
References
Baierlein, R. (1990). The meaning of temperature. The Physics Teacher, 28(2), 94–96.
Barak, J., Gorodetsky, M., & Chipman, D. (1997). Understanding of energy in biology and vitalistic conceptions. International Journal of Science Education, 19(1), 21–30.
Boo, H. K. (1998). Students’ understanding of chemical bonds and the energetics of chemical reactions. Journal of Research in Science Teaching, 35(5), 569–581.
Boyatzis, R. E. (1998). Transforming qualitative information: Thematic analysis and code development. Thousand Oaks: Sage Publications.
Capra, F. (1997). The web of life: A new scientific understanding of living systems. New York: Anchor Books.
Carey, S. (1985). Conceptual change in childhood. Cambridge, MA: MIT Press.
Cobb, C., & Goldwhite, H. (1995). Creation of fire: Chemistry’s lively history from alchemy to the atomic age. New York: Plenum Press.
Coopersmith, J. (2010). Energy, the subtle concept: The discovery of Feynman’s blocks from Leibniz to Einstein. Oxford: Oxford University Press.
Driver, R. (1994). Making sense of secondary science: Research into children’s ideas. London/New York: Routledge.
Driver, R., & Warrington, L. (1985). Students’ use of the principle of energy conservation in problem situations. Physics Education, 20(4), 171–176.
Duit, R. (1984). Learning the energy concept in school: Empirical results from the Philippines and West Germany. Physics Education, 19(1), 59–66.
Fang, Z. (2006). The language demands of science reading in middle school. International Journal of Science Education, 28(5), 491–520.
Gilbert, J., & Watts, D. (1983). Concepts, misconceptions and alternative conceptions: Changing perspectives in science education. Studies in Science Education, 10, 61–98.
Intergovernmental Panel on Climate Change [IPCC]. (2007). Contribution of Working Group III to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Jin, H., & Anderson, C. W. (2012a). A learning progression for energy in socio-ecological systems. Journal of Research in Science Teaching, 49(9), 1149–1180.
Jin, H., & Anderson, C. W. (2012b). Development of assessments for a learning progression on carbon cycling in socio-ecological systems. In A. Alonzo & A. W. Gotwals (Eds.), Learning progressions in science: Current challenges and future directions (pp. 151–182). Rotterdam: Sense Publishers.
Jin, H., Zhan, L., & Anderson, C. W. (2013). Developing a fine-grained learning progression framework for carbon-transforming processes. International Journal of Science Education, 35(10), 1663–1697.
Kaper, W. H., & Goedhart, M. J. (2002). “Forms of energy”, an intermediary language on the road to thermodynamics? Part 1. International Journal of Science Education, 24(1), 81–95.
Laburu, C. E., & Niaz, M. (2002). A Lakatosian framework to analyze situations of cognitive conflict and controversy in students’ understanding of heat energy and temperature. Journal of Science Education and Technology, 11(3), 211–219.
Lewis, E. L., & Linn, M. C. (1994). Heat energy and temperature concepts of adolescents, adults, and experts: Implications for curricular improvements. Journal of Research in Science Teaching, 31(6), 657–678.
Lin, C.-Y., & Hu, R. (2003). Students’ understanding of energy flow and matter cycling in the context of the food chain, photosynthesis, and respiration. International Journal of Science Education, 25(12), 1529–1544.
Liu, J., Dietz, T., Carpenter, S. R., Alberti, M., Folke, C., Moran, E., Pell, A. N., Deadman, P., Kratz, T., Lubchenco, J., Ostrom, E., Ouyang, Z., Provencher, W., Redman, C. L., Schneider, S. H., & Taylor, W. W. (2007). Complexity of coupled human and natural systems. Science, 317(5844), 1513–1516.
Long Term Ecological Research Network [LTER]. (2007). Integrative science for society and environment: A plan for research, education, and cyber-infrastructure in the US long-term ecological research network. Retrieved from http://www.Lternet.edu/decadalplan/
Loverude, M. E., Kautz, C. H., & Heron, P. R. L. (2002). Student understanding of the first law of thermodynamics: Relating work to the adiabatic compression of an ideal gas. American Journal of Physics, 70(2), 137–148.
McKeown, M. G. (1991). Learning word meanings from definitions: Problems and potential. In P. Schwanenflugel (Ed.), The psychology of word meanings (pp. 137–156). Hillsdale: Lawrence Erlbaum Associates.
Millar, R. (2005). Teaching about energy (Research Paper 2005/11). York: Department of Educational Studies, University of York. Retrieved October 30, 2012, from www.york.ac.uk/education/research/research-paper
Mohan, L., Chen, J., & Anderson, C. W. (2009). Developing a multi-year learning progression for carbon cycling in socio-ecological systems. Journal of Research in Science Teaching, 46(6), 675–698.
Morris, R. J. (1972). Lavoisier and the caloric theory. The British Society for the History of Science, 6(1), 1–38.
National Research Council. (1996). National Science Education Standards. Washington, DC: National Academy Press.
National Research Council. (2011). A framework for K-12 science education: Practices, crosscutting concepts, and core ideas. Washington, DC: The National Academies Press.
NGSS Consortium of Lead States. (2013). Next generation science standards: For states, by states. Washington, DC: Achieve, Inc.
Nordine, J., Krajcik, J., & Fortus, D. (2011). Transforming energy instruction in middle school to support integrated understanding and future learning. Science Education, 95(4), 670–699.
Pinker, S. (2007). The stuff of thought. New York: Penguin Group.
Pinto, R., Couso, D., & Gutierrez, R. (2005). Using research on teachers’ transformations of innovations to inform teacher education: The case of energy degradation. Science Education, 89(1), 38–55.
Schmid, G. B. (1982). Energy and its carriers. Physics Education, 17(6), 212–218.
Shanks, N. (2001). Animals and science: A guide to the debates. Santa Barbara: ABC-Clio.
Talmy, L. (1988). Force dynamics in language and cognition. Cognitive Science, 12, 49–100.
Trumper, R. (1998). A longitudinal study of physics students’ conceptions on energy in pre-service training for high school teachers. Journal of Science Education and Technology, 7(4), 311–318.
Watts, D. (1983). Some alternative views of energy. Physics Education, 18(5), 213–217.
Acknowledgement
The authors wish to thank Reinders Duit (Leibniz-Institute for Science and Mathematics Education) for his insightful comments and inspiring discussions. The authors also wish to thank Knut Neumann (Leibniz-Institute for Science and Mathematics Education) and Bob Chen (University of Massachusetts Boston) for their comments, and Kimberly Groshong and Deborah Lan (The Ohio State University) for the language editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Jin, H., Wei, X. (2014). Using Ideas from the History of Science and Linguistics to Develop a Learning Progression for Energy in Socio-ecological Systems. In: Chen, R., et al. Teaching and Learning of Energy in K – 12 Education. Springer, Cham. https://doi.org/10.1007/978-3-319-05017-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-05017-1_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05016-4
Online ISBN: 978-3-319-05017-1
eBook Packages: Humanities, Social Sciences and LawEducation (R0)