Abstract
Accurate source rock characterizations via geochemical and optical methods require advanced knowledge of the processes of their formation and the factors that control their development. The current chapter starts by addressing the fundamentals of sedimentary organic matter’s origin and chemical compositions and how they interact with the atmosphere, lithosphere, and hydrosphere through comprehensive elucidations of the biogeochemical cycles of carbon, nitrogen, sulfur, and phosphorus. Then, it discusses the deposition and transportation of organic matter in different habitats and the physical and chemical factors that affect its preservations. The third part of the chapter provides insights into the kerogen formation pathways, classifications, and alteration processes. Finally, the chapter introduces the common terrestrial and marine source rock depositional environments and the processes that control the organic productivity and source rock development richness and quality. The knowledge in this chapter represents a reliable base for accurate source rocks and petroleum data interpretations. Furthermore, it can assist in explaining the changes in organofacies and thermal maturity, identifying sweet spots in unconventional resources, types of generated hydrocarbons (sweet versus sour oils), and maturing basin modeling calibrations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Source rocks are fine-grained sedimentary carbonate or siliciclastic rocks that are rich in organic matter and are expected to generate fluid hydrocarbons upon attaining sufficient thermal maturity (Littke et al., 1997). They are an essential petroleum system element whose organic matter, mineralogical compositions, and thermal maturity determine generated fluids' chemical and physical properties. The focus in the last two decades on unconventional resources, such as gas and oil shale plays, has advanced source rock characterization methods. These methods investigate controls over quantity, quality, thermal maturity, and hydrocarbon generative potential of organic matter, which, among other factors, control sweet spots and their properties. Furthermore, several new assessment parameters and techniques have advanced and matured basin modeling and petroleum systems analysis, resulting in minimizing petroleum charge risk. Examples of recent advancements in source rock characterizations methods are found in (Romero-Sarmiento et al., 2013; Inan et al., 2017; Ghassal et al., 2017; Maende et al., 2017; Al-Hajeri et al., 2020). In order to use state-of-the-art technologies effectively, a comprehensive understanding of source rock formation mechanisms is essential. The current chapter reviews the source rock development in various depositional environments by providing detailed insights into organic matter composition, types, and factors controlling their preservation and hydrocarbon productivities.
Understanding all aspects of sedimentary organic matter formation is the primary step toward advanced source rock assessment. Hence, the chapter synopses the classification, biogeochemical cycles, and the processes that produce, preserve, and transport organic matter in different geological environments. Knowing the exact chemical compositions of kerogens in potential source rocks allows for an accurate prediction of potentially generated hydrocarbons. Moreover, the kerogen chemistry characterization enables better determination of generation kinetics and thermal maturity windows for the different hydrocarbon products (Dieckmann et al., 2000; Pepper & Corvi, 1995).
Organic matter and its related hydrocarbons are composed mainly of hydrogen, carbon, oxygen, sulfur, nitrogen, phosphorous, and other elements. The cycles of these elements in nature play crucial roles in the evolution of sedimentary organic matter throughout geological history (Summons, 1993). Thus, the dynamics and the reactions of each of these elements in the different Earth pools impact the productivity and preservation of organic matter and, consequently, the generation, type (e.g., oil versus gas), and quality of hydrocarbons (e.g., sweet versus sour).
Sedimentary organic matter undergoes a series of processes that include productivity, deposition or transportation, and then preservation or decomposition. These processes are followed by kerogen formation via diagenesis, catagenesis, and, finally, metagenesis, depending upon the subsurface processes and conditions. The kerogen formation and thermal maturity pathways differ based on the depositional environments. The current review covers both the organic geochemistry and palynology aspects related to sedimentary organic matter formation and evaluation.
2 Origin of Sedimentary Organic Matter
Organic matter varies in chemical composition depending on the original biological precursor. These compositional differences, together with the syn- and post-depositional conditions, determine the levels of its preservation. Thus, the biological and biogeochemical processes in different environments throughout the Earth’s history are primary controls on the type of organic matter in any specific locality in time and space.
According to several properties, such as their morphology and physiological and biochemical capacities, the organisms are classified into main taxonomic groups (Summons, 1993). The three domains of organisms are archaea, bacteria, and eukarya (Woese et al., 1990), as in Fig. 1a.
Prokaryotes are unicellular, tiny spheroidal bodies ranging in size from 0.1 to 5 μm (Summons, 1993). They lack a membrane-bound nucleus, mitochondria, and chloroplast. Their extremely flexible metabolic and respiratory systems enable them to live and adapt to aerobic and anaerobic conditions. Some obtain their energy from chemical sources (Fig. 1b). They include the archaea and bacteria domains. These characteristics allow the prokaryotes to survive over 4 billion years (Gregory & DeSalle, 2005; Magalon et al., 2012).
Archaebacteria are prokaryotes that mostly occupy extreme environments, such as hypersaline and volcanic settings (Parker, 2001). The three main examples of archaebacteria are methanogens, halophilic, and thermophilic. First, methanogens are anaerobic archaebacteria that produce methane from the conversion of CO2, hydrogen, formate, acetate, and other simple carbon compounds (Summons, 1993). Their growth depends on the temperature and absence of oxygen. Second, the halophilic archaebacteria are aerobic and inhabit hypersaline environments (Irwin, 2020). Third, the thermophilic sulfate-dependent archaebacteria are either aerobic or anaerobic. They occupy very high-temperature or sulfide-rich environments (Summons, 1993).
Eukaryotes, on the other hand, contain a membrane-bound nucleus, mitochondria, and chloroplast. Unlike prokaryotes, they are multicellular and have larger cell sizes that range from 10 to 100 μm (Summons, 1993). They include animals, plants, fungi, and protists (Parker, 2001; Summons, 1993). The latter are unicellular or form simply organized colonies. The microorganisms, which are prokaryotes and protists, constitute the vast majority of living biomass. They are the most prevalent organisms throughout the Earth's history and extend over broad geographic areas and environments (Summons, 1993).
The availability of carbon type (e.g., organic and inorganic), energy resources, and chemical substances classifies the microorganisms based on their biochemical capacities, which in turn determine their habitats (Fig. 1c). For example, autotrophs utilize inorganic sources, such as CO2, NO3−, or HCO3−, to generate new biomass. They either reduce CO2 through energy obtained from sunlight, hence called phototrophs, or through inorganic reactions, hence called chemoautotrophs (Sage, 2008). The phototrophs use photosynthesis to convert CO2 to carbohydrates. They are the primary producer of organic matter. The prokaryotes cyanobacteria and purple and green photosynthetic bacteria are typical examples of phototrophs, while algae and higher plants represent eukaryotic phototrophs (Summons, 1993).
Conversely, heterotrophic organisms cannot produce their food and rely on other organic sources (Gaedke, 2021). Therefore, they return the organic carbon into the geosphere mainly through organic carbon oxidation to obtain energy through various processes. They play significant roles in biogeochemical cycles. The aerobes are heterotrophic organisms that utilize oxygen as an oxidant. Furthermore, anaerobes are organisms that use nitrates or sulfates to oxidize organic matter. Typical examples of heterotrophic organisms include heterotrophic bacteria and fungi (Ritz, 2005; Summons, 1993).
2.1 Organic Matter Compositions
Biomass is composed mainly of proteins, carbohydrates, lignin, and lipids (Fig. 2) (Selly, 1998). The proportions of these components determine the organic matter types and their stability under various geological conditions. Proteins are composed of carbon, hydrogen, oxygen, and nitrogen with little sulfur and phosphorous in mostly 20 amino acid types. They predominantly exist in animals (Selly, 1998) and account for most of the nitrogen found in organic matter (Killops & Killops, 2013). Proteins constitute a significant fraction of organic nitrogen in organisms (Killops & Killops, 2013).
Carbohydrates occur in most organisms as an essential energy source. They encompass only carbon, hydrogen, and oxygen, with the basic formula Cn(H2O)n. They include all sugars, such as glucose, and other polymers, such as cellulose, starch, and chitin (De Leeuw and Largeuw, 1993).
Lignins are dominant constituents in higher plants and are not common in animals. They constitute 20 to 30% of vascular tissue components, are composed primarily of several types of aromatic compounds (Selly, 1998), and represent the second dominant biopolymer after cellulose (Killops & Killops, 2013).
Lipids are the most significant chemical class that dominates the oil source rock formations and are present widely in most organisms (Selly, 1998). They contain carbon, hydrogen, and oxygen atoms and, in some cases, iron and magnesium (De Leeuw and Largeuw, 1993). They are insoluble in water and soluble in nonpolar organic solvents, such as hexane and chloroform. Lipids include fats, waxes, steroids, oils, and phospholipids (Selly, 1998; Killops & Killops, 2013). One of their most important examples is chlorophylls, which exist in all algae, higher plants, and cyanobacteria.
2.2 Biogeochemical Cycles
Biogeochemical cycles are defined as the interactions, movements, and cyclization of elements between the biotic (biosphere) and abiotic factors. The latter includes the atmosphere, geosphere, and hydrosphere. Six primary elements comprise the organic molecules and inhabit several biotic and abiotic reservoirs in various chemical forms: carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur (Summons, 1993). Their movements between these pools occur through spontaneous oxidizing or reducing chemical reactions or biological interactions. These cycles are primarily interlinked and fueled by sunlight through photosynthesis. In some cases, hydrothermal activities source the energy needed for carbon fixation. Understanding these processes improves the realization of the factors controlling organic matter richness, quality, and isotopic variation (Brusseau, 2019).
Oxygen is not discussed separately due to its complexity as a by-product of numerous reactions linked to other element cycles. The main processes that transfer the oxygen between the various reservoirs are respiration, photosynthesis, and the decay and combustion of organic matter. The primary oxygen producers are phytoplankton and plants. The oxygen released during the sunlight reaction with water vapor is also an essential source (Summons, 1993).
2.2.1 Carbon Cycle
Carbon is an essential element in all living organisms and acts as an energy source for most species. However, most of the carbon is preserved in the lithosphere, with fossil fuel constituting one-fifth of it (Brusseau, 2019; Vallero, 2014). The atmosphere is another primary reservoir of carbon. In these reservoirs, carbon exists in either oxidized or reduced chemical forms. The carbon cycle consists of two interlinked sub-cycles that occur at various temporal and spatial scales (Fig. 3). The first sub-cycle takes place on a short timescale (days–months) and within the biosphere. During photosynthesis, autotrophs convert CO2 into simple organic intermediates and then to carbohydrates, such as glucose. Heterotrophs that feed on these autotrophs metabolize the carbohydrate to obtain energy through respiration. The carbon returns to the atmosphere through decomposition, excretion, and respiration (Summons, 1993).
The second sub-cycle occurs over a long timescale (hundred–thousand years) through geological processes. CO2, which is the dominant form of carbon in the atmosphere, plays a critical role in regulating and maintaining Earth’s temperature and oxygen concentrations. The exchange of CO2 between the atmosphere, biosphere, and geosphere predominantly controls its concentrations in the atmosphere and influences its oxygen abundance (Summons, 1993). Phytoplankton converts CO2 through photosynthesis into nutrients for other higher-level organisms, which return the carbon to the atmosphere through respiration. The CO2 in the atmosphere dissolves in the oceans and transforms into other inorganic compounds such as HCO3− and CO3. Some organisms assimilate Ca with CO3 to form CaCO3, which composes the hard bodies of most marine shells. These taxa deposit on the seafloor after they die, contributing to the sedimentary pile. Over thousands of years, these sediments solidify to form various carbonate rocks through several geological processes (Wigley and Schimel, 2005). The carbon in the organic reservoir, on the other hand, is found in the reduced form either in living biomass, dissolved or suspended in seawater, or recently deposited on the seafloor. When deposited and preserved, this organic matter is encompassed in sediments to eventually form petroleum source rocks (Summons, 1993; Brusseau, 2019).
2.2.2 Nitrogen Cycle
Nitrogen is one of the primary nutrient sources for most living biomass in oceans and lands. Its concentration controls primary organic productivity and organic matter decay. It also plays a significant role in regulating atmospheric temperature. Moreover, nitrogen is an essential constituent in chlorophyll composition responsible for photosynthesis processes and is one of the main elements of living biomass tissues. The consecutive transfer of nitrogen by several processes between the various reservoirs (atmosphere, geosphere, and biosphere) defines its cycle, converting it into different end products (Fig. 4). These processes are fixation, nitrification, assimilation, ammonification, and denitrification, all involving reduction or oxidation of nitrogen compounds by biological, physical, or chemical processes (Brusseau, 2019; Hayes, 2018; Summons, 1993).
Nitrogen in the atmosphere is present in the inert form (N2), which is not usable by living biomass. It precipitates in soil from air or surface water. Bacteria such as cyanobacteria, phototrophic bacteria, and Rhizobiaceae convert N2 to ammonia through the nitrogen fixation process. Furthermore, nitrogen fixation can occur by the light energy in the atmosphere that breaks N2 into nitrogen oxide (Bernhard, 2010).
Nitrification is a successive biological process of converting ammonia to nitrites by bacterial oxidation. The Nitrobacter subsequently converts nitrites to nitrates. The other essential nitrogen cycle process is assimilation, which is transferring the ammonia and the nitrite, nitrate, and ammonium ions from soil to plants to form tissues and proteins. Ammonification is the decay of organic matter after burial. Bacteria and fungi decompose nitrogen compounds into ammonia. The last process of the nitrogen cycle is denitrification, where nitrogen is released back into the atmosphere. The process involves the bacterial conversion of nitrate to N2 in reduced conditions. In marine environments, nitrogen compounds are deposited with the sediments, forming sedimentary rocks over geological time (Bernhard, 2010; Brusseau, 2019).
2.2.3 Sulfur Cycle
Sulfur is one of the Earth’s most abundant elements, which exists in different forms in all reservoirs in the ecosystem. Organic sulfur is usually amino acid found in protein, hormones, and vitamins. Sulfur abundance and cyclization within marine environments control, to a large degree, organic matter preservation and quality (Brimblecombe, 2003).
The sulfur cyclization is driven mostly by biological activities, weathering, and hydrological activities (Fig. 5). Sulfur is leached from the lithosphere to the atmosphere via weathering processes before its oxidation to sulfate (SO4). Some bacteria groups utilize sulfate in their respiration as an oxidant. Also, other plants and microorganisms reduce sulfate to produce biomass through the assimilatory sulfate reduction process. On the other hand, animals uptake sulfur from consuming amino acids from other living organisms (Brusseau, 2019; Summons, 1993). The two significant bacterial groups that play a primary role in sulfur cyclization are the sulfate-reducing bacteria (SRB) and sulfide oxidizer bacteria (Zavarzin, 2008). Benthic communities feed on the organic matter deposited on the seafloor, where oxygen is available for respiration. Moreover, this zone witnessed metal reductions, such as Fe(III) to Fe+2 and Mn(IV) to Mn+2. Beneath this zone, the oxygen becomes depleted. In these anaerobic conditions, the SRB and archaea utilize the dissimilatory sulfate reduction (DSR) process to oxidize sulfate as an oxidant to gain energy from buried organic carbon and methane to generate hydrogen sulfide (H2S), HS−, and S2− (e.g., Jørgensenet al., 2019; Simon and Kroneck, 2013). It is in this anoxic zone where pyrite is formed via bacteria by reducing the dissolved sulfates to sulfide.
The sulfide oxidizer bacteria obtain their energy from oxidizing sulfides in magmatic regions in chemolithotrophic or phototrophic processes. The sulfur returns from the biosphere to the soil via organic matter decay. Some sulfur is leached from terrestrial environments and is transferred through rivers to oceans and lakes. The sulfur cycles back to the atmosphere or moves to the marine biosphere (Summons, 1993).
2.2.4 Phosphorus Cycle
Phosphorus is another crucial element for living biomass occurring in DNA, RNA, and animal bones. Notwithstanding its vast abundance in the lithosphere, it is less abundant than other organic elements, especially in fossil organic matter. Phosphorous primarily regulates the fertility of the ecosystem and cycles between the lithosphere, hydrosphere, and biosphere. The cycle involves three main processes: geological weathering, absorption by living organisms, and organic matter decay (Fig. 6). The phosphate salts are removed from rocks during weathering processes and then deposit in soils. Note that the phosphate salts do not dissolve in water completely; therefore, aquatic living organisms absorb phosphorous from the lower water layers. In terrestrial environments, plants absorb phosphorous from the soil. Animals gain their phosphorous from consuming these plants. The phosphorous returns to the lithosphere again through the decay of organic matter. (Summons, 1993; Ruttenberg, 2003; Macdonald et al., 2018).
2.3 Primary Organic Productivity
Primary organic productivity is defined as the carbon fixation rate by converting inorganic carbon into hydrocarbons through photosynthetic organisms (Killops & Killops, 2013; Littke et al., 1997; Selley, 1998). The primary reaction that occurs in plants and algae yields water and glucose. The produced glucose acts as the onset for the formation of complex carbon compounds such as polysaccharides occurring in plants and their consuming animals (Selly, 1998). The second process that converts inorganic carbon to organic carbon is chemosynthesis—another form of bioproductivity but only significant locally (Sorokin, 1966).
Physical and chemical factors that control the occurrence and the intensity of the primary organic productivity are shown in Fig. 7. The physical parameters include the temperature and amount of light, in the case of aquatic environments, the photic zone thickness, and the water turbidity (Selly, 1998). Crucial factors that control the intensity of primary organic productivity are the Earth's climate and atmospheric green gas levels. The primary productivity intensifies with increasing CO2 and temperature and light availability (Littke et al., 1997). The chemical and physical factors are in essence, interlinked and are expected to vary temporally and spatially in different geographic locations and geological settings.
Chemical factors play integral roles in primary organic productivity. Phosphates and nitrates are essential for plant and animal growth and are considered a preferred ingredients for organic matter productivity (Selly, 1998). In addition, oxygen is a by-product of photosynthesis, allowing phytoplankton to flourish, thereby improving zooplankton productivity. These factors are maximized in two high primary productivity belts between the polar and equatorial oceans attributed to high-latitude upwelling manifested in modern days (Littke et al., 1997). In the upwelling zones, deep cold nutrient-rich oceanic currents upwell to shallower waters to enrich the shelf and coastal areas with phosphates and nitrates, consequently increasing bioproductivity (Selly, 1998). Wind direction, Earth rotation, and Earth climate control these settings.
Sunlight intensity, as a primary control over photosynthesis and bioproductivity, varies based on climate, weather, and geographic location, as well as on geological settings that can prevent access to surface water. Bioproduction is the highest in the upper part of the photic oxygen-rich zone and steadily diminishes deeper with decreasing light and temperature (Selly, 1998).
Water provides hydrogen for the photosynthesis process, which intensifies the bioproductivity. Its availability plays a primary role in terrestrial environments. For example, high rainfall environments characterize the great coal-forming forest (Killops & Killops, 2013). The bioproductivity is also controlled by geographic and climatic factors. In high-latitude or cold regions with ice cover, plant growth is limited. On the other hand, the very high temperature reduces photosynthesis as it destroys or alters enzymes and cellular components (bioproductivity). In marine environments, temperature and rainfall cause the algae breed to overgrow. Therefore, humid, subtropical, and tropical climates favor high bioproductivity (Li et al., 2015).
Water salinity has a vital role in the composition of primary producers in fresh, saline, or hypersaline waters. Greater diversity of primary producers occurs in fresh and sea waters than in hypersaline waters (Killops & Killops, 2013). The diversity may not affect bioproductivity but the composition of the preserved organic matter later on. In restricted basins, positive water balance traps nutrients, resulting in increasing bioproductivity (Li et al., 2015).
The four main contributors to the formation of sedimentary organic matter are phytoplankton, zooplankton, higher plants, and bacteria with an insignificant contribution from higher organized animals such as sharks (Mendonça Filho et al., 2012; Tissot & Welte, 1984).
2.4 Deposition and Transportation of Organic Matter
Organic matters are the black or dark-colored remains of plants and/or animals deposited during rock sedimentation. Apart from coal seams, which are nearly composed of only organic constituents, most sedimentary rocks encompass minor organic matter amounts. In marine environments, organic matter availability and generation and their preservation versus destruction in the sediment–water interface are the primary constraints that control the organic matter richness in the sedimentary rock. Marine organisms occupying ocean surfaces biosynthesize most organic matter before their deposition on the seafloor (Wakeham & Lee, 1993).
The alteration of organic matter composition continues at the sediment–water interface, which changes their original composition. The majority of the produced marine organic matter is recycled in the epipelagic zones, but only small fractions precipitate in the mesopelagic and bathypelagic zones (Fig. 8). In the epipelagic zones, the bacteria and zooplankton recycle more than 90% of the produced organic matter through photosynthesis to generate other biomass, degraded or dissolved organic matter. The organic matter that survives the water column recycling processes and accumulates on the seafloor (10–30%) constitutes the sedimentary organic matter records.
In terrestrial environments, mainly swamps, bog, fen moor, musky, and peatlands, only a small fraction of the original organic matter is preserved in peat deposits. Mires is the term that describes these peat-forming ecosystems. There are two types of mires, which are topogenous and ombrogenous (Taylor et al., 1998). The former refers to marshes, fen, and swamps formed in reduced rainfall climates and high groundwater levels. The ombrogenous mires are more affluent than the topogenous in organic nutrients and inorganic inputs and form in heavy rainfall systems with a water table lower than the peat surface. Three factors are responsible for the mire formation, which are flora development, geographic location, and climate (Taylor et al., 1998).
2.5 Preservation of Organic Matter
The preserved organic matter is the one that survives the biosynthesis processes by predators during transportation through the water column, escaping degradation at the sediment–water interface, and is buried in the sediments (Canfield, 1994; Piper & Calvert, 2009). Five factors principally affect organic matter preservation: primary productivity, anoxicity, mineral surface area, sedimentation rate, and wave and current energy (Fig. 9).
The deposited organic matter on the seabed is generally comparable to the primary productivity and decreases with water depth (Suess, 1980). In that case, it is evident that primary productivity is a critical factor in the amount of preserved organic matter. However, it is a vital variable but not a sole factor.
Anoxicity is the second factor that controls the organic matter preservations. Intense primary productivity and the decay of organic materials and their oxidation to CO2 tend to reduce the oxygen level and create a low-oxygen water layer called the oxygen-minimum zone, OMZ (Killops & Killops, 2013; Tissot & Welte, 1984). This zone is characterized by elevated H2S concentrations produced by sulfate-reducing bacteria (Fig. 10). The low-oxygen level and the presence of H2S reduce the rate of organic matter degradation and enhance preservation. The organic matter oxidation to CO2 is seized in this zone, but anaerobic oxidation such as sulfate reduction, denitrification, or methanogenesis occurs (Canfield et al., 2021). In this case, the destruction of organic matter occurs at a slow rate. The OMZ persists in the middle of the water column interlayered between two highly oxygenated water layers (Diaz et al., 2013; Fenchel et al., 2012).
Grain size and mineralogical composition play a vital role in organic matter preservation. The fine-grained sediments inhibit or prevent the oxygen exchange between the porewater and the free-water column. Moreover, the clays inactivate bacteria digestive enzymes, increasing organic matter preservation (Dean & Gorham, 1998). Mayer (1994) suggested that mineral surface is responsible for organic matter preservation in marine environments apart from deltas. The organic matter forms a monolayer adsorbed by the mineral surface (Killops and Killpos, 2013). This process is considered a conversion of dissolved to particulate organic matter (Tissot & Welte, 1984). This adsorption is irreversible and can protect even labile constituents such as sugar, thereby enhancing preservation (Henriches, 1992; Killops and Killpos, 2013). The length of exposure to oxygenated water during deposition is a critical factor.
The sedimentation rate has a direct impact on organic matter preservation and its types. For example, most of the preserved organic matter in deltaic systems is allochthonous (Fig. 11) (e.g., Ghassal et al., 2016a). The high sediment inputs outweigh the amount of organic matter required to form monolayers, reducing marine organic matter preservation (Henriches, 1992). Besides, terrestrial organic matter is generally more resistant to degradation compared to marine ones. Moreover, assuming the rate of organic matter input is constant, and then the sedimentation is inversely proportional to the preserved organic matter concentration (Fig. 12). The foregoing dissection explains the increase in richness and quality of organic matter in more distal and deeper settings. High-energy waves and currents cause the oxygen to circulate and destabilize the chemical and physical conditions of the water column, affecting all of the abovementioned factors.
3 Kerogen Formation, Classifications, and Alteration
Kerogen is the most common term used to describe the fossil organic matter in sedimentary rocks. It is the fraction of the organic matter in sedimentary rocks, which is insoluble in organic solvents. Chemically, it is a macromolecule made of condensed cyclic nuclei linked by heteroatomic bonds or aliphatic chains (Tissot & Welte, 1984). In the absence of migrated hydrocarbons, kerogen usually accounts for more than 95% of total organic matter in sedimentary rocks (Tyson, 1995). Humin is the early precursor of kerogen in young sediments, insoluble in the soil or marine sediments (e.g., Hayes et al., 2017). The primary distinction between humin and kerogen is the presence of hydrolyzable fractions in humin that diminish with burial (Tissot & Welte, 1984).
An array of physical and chemical analytical techniques provides reliable information on the structure and composition of kerogen. These include elemental analysis (C, H, N, and S), infrared spectroscopy, microscopic examination including palynology, degradative oxidation, and various pyrolysis techniques such as Rock–Eval, hydrous pyrolysis, laser micro-pyrolysis, and Raman spectroscopy (Mendonça Filho et al., 2012). Collectively, four types of kerogen can be identified (Figs. 13 and 14). Type-I kerogen contains many aliphatic chains and a few aromatic nuclei. The elemental H/C ratio is originally high, and the potential for oil and gas generation is also high. This kerogen type is mainly derived from algal lipid or organic matter enriched in lipids by microbial activity. It typifies kerogens of lacustrine source rocks. Type-II kerogen comprises more aromatic and naphthenic rings. The H/C ratio and the oil and gas potential are lower than observed for Type-I kerogen. It is usually related to marine organic matter deposited in reducing environments, with a low to high sulfur content. Pollen grains, spores, cuticles, and marine organic matter are important components in this type of kerogen, but their assemblages may vary depending on the proximity to the shorelines and source rock age. Type-III kerogen encompasses mostly condensed polyaromatics and oxygenated functional groups, with minor aliphatic chains. The H/C ratio is low, and oil potential is moderate, although this kerogen may still generate abundant gas at greater depths. The O/C ratio is comparatively higher than for the other types of kerogen. It is mostly derived from terrestrial higher plants. Type-IV kerogen is a secondary type of kerogen and contains practically only aromatic components. The organic matter is carbonized by oxidation before or during deposition and possesses no potential to form hydrocarbon source rocks.
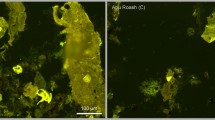
Selected images of several kerogen types seen under microscope. a-b: Organic matter (Pediastrum and Botryococcus algae) related to the kerogen Type-I. c-d: Organic matter (dinocysts, spores, pollen, and phytoclasts) related to kerogen Type-II. e-f: Organic matter (wood tissues: non-opaque phytoclasts) related to the kerogen Type-III. g-h: Organic matter (carbonized and charred wood tissues: opaque phytoclasts) related to the kerogen Type-IV
Organic matter is subjected to various compositional alterations in the sediment–water interface and during burial. The changes are caused by microbial activities, oxidations, and thermal effects at a later stage (Fig. 15). The maturation of organic matter starts with diagenesis, followed under successively higher burial temperatures by catagenesis, metagenesis, and finally metamorphism in extreme thermal regimes (Horsfield and Rullkotter, 1994).
Diagenesis includes physical, chemical, and microbiological processes occurring within a few hundred meters of overburden. It is a process through which the system approaches equilibrium under shallow burial, low temperature, and pressure, where the sediments consolidate. Catagenesis is the stage where kerogen is converted to petroleum hydrocarbons due to increasing burial and thermal stress. Tectonism and volcanism affect the geothermal regimes by intensifying catagenesis and altering geothermal gradients. Metagenesis is the last stage of thermal maturation of organic matter at very high temperatures, commonly associated with very deep burial (Tissot & Welte, 1984). At this stage, only methane is generated, and inert carbon and graphite are formed in the rock. Finally, metamorphism is the last thermal evolution stage when the sediments are deeply buried and exposed to very high pressure (>300 MPa) and temperature above 200 °C (Mendonça Filho et al., 2012; Tissot & Welte, 1984). At this stage, the remaining organic carbon converts to inorganic carbon.
4 Common Source Rock Depositional Environments
4.1 Terrestrial Environments
4.1.1 Mires
Mires are wetlands with heavy vegetation where the water table is near or above topsoil (Rydin et al., 1999). They include swamps, fen, bogs, moors, musky, and peatland (McCabe, 1991). They form peats, which are the primary precursor for coal formation. Mire characteristics are dependent on the development of flora, geographical location, climate, and structural framework (Taylor et al., 1998). The early Carboniferous period witnessed a significant flourishing of vascular plants, which resulted in extensive peat mires for the first time (Glasspool & Scott, 2005). No significant peat development was recorded before Carboniferous.
The water balance between the groundwater and rainfall must exceed evaporation to enhance organic production. The water balance maintains water depth that controls the aerobic versus anaerobic zones (Taylor et al., 1998), a condition that varies seasonally and due to climate changes. Accumulation of peat in mires requires a continued gradual increase in the water table, isolation from seawater flooding, and low fluvial sediment supply (Stach et al., 1982). Mires are classified based on the vegetation types, which depend on the geological age and the abovementioned factors related to water balance. As a result, only a small fraction of the original organic matter is developed into coal (Taylor et al., 1998). Based on their hydrological system, Taylor et al. (1998) classified mires (Fig. 16) as follows:
-
1.
Topogeneous mires: where peat formation is caused primarily by high water levels. They include marshes, fen, and swamps.
-
2.
Ombrogenous mires: form in high rainfall regions and where the groundwater level is below peat-forming layers. They include bogs.
The topogeneous mires are generally more affluent than the ombrogenous in the inorganic substance and plant nutrients.
4.1.2 Lacustrine
Lakes are landlocked water bodies isolated from marine access (Fig. 17). They constitute less than 0.1% of the hydrosphere, yet they are considered primary source rock depositional environments (Killops & Killops, 2013). They can originate from several geological and hydrological processes such as glacial, tectonic, volcanic, fluvial, coastal, and chemical. These processes generate enclosed depressions and water bodies to form lakes (Branstrator, 2009). The lake size, morphology, climate, and river inputs control the water chemistry, salinity, and thermal regime, as well as organic matter productivity and preservation (Demaison and Moor, 1980; Selly, 1998; Katz, 2012; Killops & Killops, 2013). Best organic primary productivity and preservation occur in density stratified water columns. Water density stratifications occur seasonally when warm water caps cooler water or low salinity water sets on hypersaline water in low-wind areas.
Summary of lacustrine hydrological settings in the four seasons, depositional environment follows Killops and Killops (2013)
The first condition that favors organic matter productivity and optimum preservation in lakes is water column stratification caused by temperature differences. This process is seasonal and dependent on lake depth and size as well as the sunlight and wind intensity. During the summer season, abundant light and nutrients promote phytoplanktonic bioproductivity and higher oxygen levels in the upper layer, compared to an oxygen-depleted anoxic and more dense water layer underneath. The density contrast inhibits water circulation, resulting in water stratification and improved organic preservation (Selly, 1998; Killops & Killops, 2013). Salinity variations also play a significant role in water stratification, as changes in water temperature are sensitive to its salinity.
Differences in salinity cause the second water density stratification condition. It initiates in regions where subsurface saline springs intrude the lakes or where salt diffuses from evaporites at the sediment–water interface (Killops & Killops, 2013).
Based on their access to water flows, lakes are classified as hydrologically open and closed, with some lakes alternating between the two types during their geological histories (Killops & Killops, 2013). They are also classified based on their mixing into amictic lakes, meromictic lakes, and holomictic lakes (Hutchinson & Löffler, 1956). Amictic lakes are usually located in polar regions or high mountains. Their permanent ice cover prevents water mixing. Meromictic lakes, on the other hand, partially mix, especially in the upper layer, while holomictic lakes mix annually at various degrees based on geographic locations. The differences between these types are best described in Hutchinson and Löffler (1956).
The hydrologically closed lakes are usually formed by tectonic activities, volcanism, or glaciation, primarily in open regions where evaporation exceeds precipitation (Langbein, 1961; Waiser & Robarts, 2009). The evaporation and precipitation change substantially, affecting the lake water level and shorelines, which cause sediment reworking. During high lake water levels, water stratification and anoxic conditions prevail in the central part allowing for the deposition of organic-rich layers with carbonate and evaporitic minerals (Killops & Killops, 2013; Waiser & Robarts, 2009).
The hydrologically open lakes receive a balanced water inflow from rivers and precipitation and outflow from evaporation, allowing the shorelines and water level to stabilize (Reading, 1986). Lake sedimentation is controlled by its size, depth, and river inputs that change seasonally. The river inputs are higher during the summer and decrease during the wintertime. Moreover, lakes are usually surrounded by vegetation (Killops & Killops, 2013). Therefore, the nearshore deposits are dominated by siliciclastic deposition and allochthonous organic matter sourced from plants surrounding the lakes. The siliciclastic inputs diminish toward the more distal or central part, replaced mostly by carbonates enriched with organic matter (Allen & Collinson, 1986). Similar to marine environments, the organic matter richness is greatly affected by sediment dilution. Thus, the richness of organic matter in the nearshore area is less than in the distal area due to the difference in sedimentation rates. Due to the rapid changes in fluvial inputs, the lake sedimentation and organic matter types and richness are remarkably heterogeneous. A common example of source rock deposited in a lacustrine environment is the Green River Formation in the USA.
4.2 Marine Environments
Marine source rocks are deposited in four main settings: broad flooded continental shelves (deltaic settings), oxygen-minimum zones along continental shelves, upwelling zones, and rift/barred basins (e.g., Katz, 2012; Kosters et al., 2000; Littke, 1993). The oxygen-minimum zones in aquatic environments are defined as mid-layers deficient in oxygen concentrations compared to the layers below and above in oceans and lakes. They persist over millions of km2 and over long periods of geological time (Diaz et al., 2013). They are created by the deposition of organic matter, where oxygen can, in some instances, be reduced to complete anoxia.
4.2.1 Deltaic Settings
Deltas represent transitional depositional environments between completely terrestrial and completely marine and are considered some of the favorable settings to accommodate organic-rich sediments. Several factors control the delta formation and characteristics, including climate, sediment and water discharge, wave intensities, tides, and tectonic regime (Boggs et al., 2006; Allen and Allen, 2013). There are several criteria to classify deltas, yet in this chapter, the classification of Galloway (1975) is adopted. He classified deltas based on their front regimes into (1) wave dominated, (2) tide dominated, and (3) fluvial dominated.
The wave-dominated deltas are characterized by their destructive nature, as wave force inhibits fine-grained sedimentation and casts the shorelines to form a cuspate shape (Nienhuis et al., 2015). This high-energy type of delta does not commonly host petroleum source rocks.
On the other hand, the tide- and fluvial-dominated delta types are characterized by their constructive nature and their tendency to host prolific source rock beds (Allen and Allen, 2013). Tide-dominated deltas occur on a large scale (hundreds of km) with cyclic variant energy sediments (Goodbred & Saito, 2012).
The depositional characteristics of the deltaic environments classify deltaic source rocks into offshore and onshore types. The onshore source rocks are mostly peat and mire deposits during transgressive system tract events behind the landward stepping shorelines (Kosters et al., 2000). The offshore deltaic source rocks are primarily siliciclastics and characterized by low to good total organic carbon values that range from 0.5 to 3.0% with dominated kerogen Types-III/IV and minor marine organic matter input. The kerogen in deltaic systems is mostly transported with high proportions of higher plant tissues (allochthonous) and aquatic algae (autochthonous) (Bustin, 1988; Ghassal et al., 2016a; Tissot & Welte, 1984). Due to the high-sedimentation rates and oxygen-level fluctuations, the organic matter richness and preservation are variably reduced. Examples of deltaic source rocks include the Mississippi, Niger, and Ganges River deltas, representing fluvial-dominated, wave-dominated, and tide-dominated systems, respectively.
Rivers transport terrestrial organic matter such as vitrinite, inertinite, coal particles, and freshwater algae as well as pollen and spores and other cuticles and plant tissues to coastal areas where marine algae deposit. River discharges and sea-level changes control the relative proportions of terrestrial and marine organic inputs. In addition, the intensity of water circulation modifies the oxygen level, resulting in partial oxidation of organic matter. These conditions vanish in more distal settings, as discussed below.
4.2.2 Marine Continental Shelves
Marine continental shelves are some of the most favorable depositional environments for source rock development (Fig. 18a). The source rocks are deposited over a wide area depending on the size of the shelf and the OMZ. The source rock organofacies vary from proximal to distal setting. The source rocks in the proximal settings are rich in terrestrial organic matter and siliciclastic minerals. Further to more distal settings, the carbonate to siliciclastic proportions increase with more marine organic matter. The most distal settings are rich in carbonate contents with high sulfur-rich kerogen (Ghassal et al., 2018). A common source rock deposited in a continental shelf environment is the Posidonia Shale in Germany.
4.2.3 Upwelling Zones
About half of the world’s organic-rich source rocks were formed in upwelling zone conditions (Parrish, 1987). This is due to the higher biological activities that surpass the productivity encountered in regular shelves (Katz, 2012; Koblentz-Mishke et al., 1970). During an elevated greenhouse warm climate, the warm winds move the marine coastal waters toward the ocean, and deep nutrient-rich waters rise and replace them, which intensify bioproductivity. Upwelling activities decrease during cold climates (Bakun, 1990; Parrish, 1987). Moreover, the upwelling zones mainly prevail in the western continental margins due to wind directions and the Coriolis effect caused by the Earth’s rotation affecting mid-latitude zones (Fig. 18b) (e.g., Katz, 2012; Ghassal et al., 2016b). The prominent bioproductivity increases the amount of deposited organic matter and reduces the bottom-water oxygen levels, which leads to enhanced organic matter preservation (Katz, 2012; Littke et al., 1997). The spatial distribution of preserved organic matter depends on seabed bathymetry. The source rocks formed in upwelling zones are dominated by liptinitic kerogen. The dilution of terrestrial and reworked organic matter and sediment sourced by fluvial discharges are usually reduced relative to purely marine organic matter. The cold upwelling water reduces the air humidity in the shoreline vicinity, inhibiting fluvial runoff (Robinson & Davies, 2016). A common example of source rock deposited in an upwelling setting is Tarfaya Cenomanian/Turonian marlstone section in Morocco.
4.2.4 Restricted Marine Basins
Silled or barred basins are depressions on the seafloor where water circulation is restricted (Fig. 18c). This restriction makes them good areas to trap nutrients, intensifying the bioproductivety and oxygen depletion and consequently increasing the organic matter deposition and preservation (e.g., Allen and Allen, 2013; Selley, 1998). The aquatic organic matter here is expected to be less diversified compared to open marine settings. Due to the shallow nature and proximity to shorelines, these settings receive fair terrigenous inputs. The richness of the preserved organic matter is highly variable due to the rapid fluctuation in sea level and turbidity of the water column. Source rock development in these settings is caused cussed by thermal density or salinity stratifications.
References
Al-Hajeri, M., Sauerer, B., Furmann, A., Amer, A., Akbar, H., & Abdallah, W. (2020). Maturity estimation for Type II-S kerogen using Raman spectroscopy–A case study from the Najmah and Makhul Formations in Kuwait. International Journal of Coal Geology, 217, 103317.
Allen, P. A., & Allen, J. R. (2013). Basin analysis: Principles and application to petroleum play assessment. Wiley. 632 p.
Allen, P. A., & Collinson, J. D. (1986). Lakes. In H. G. Reading (Ed.), Sedimentary environments and facies (pp. 63–94). Blackwell Scientific Publications, Oxford.
Bakun, A. (1990). Global climate change and intensification of coastal ocean upwelling. Science, 247(4939), 198–201.
Bernhard, A. (2010). The nitrogen cycle: Processes, players, and human impact. Nature Education Knowledge, 3(10), 25.
Boggs, D. A., Boggs, G. S., Eliot, I., & Knott, B. (2006). Regional patterns of salt lake morphology in the lower Yarra Yarra drainage system of Western Australia. Journal of Arid Environments, 64(1), 97–115.
Branstrator, D. K. (2009). Origins of types of lake basins. In Encyclopedia of inland waters (pp. 613–624). Elsevier Inc.
Brimblecombe, P. (2003). 8.14—The global sulfur cycle. Treatise on Geochemistry, 8, 645–682.
Brusseau M. L. (2019). Ecosystems and ecosystem services (Chap. 6). In Environmental and Pollution Science (3rd ed., pp. 89–102).
Bustin, R. M. (1988). Sedimentology and characteristics of dispersed organic matter in Tertiary Niger Delta: Origin of source rocks in a deltaic environment. AAPG Bulletin, 72(3), 277–298.
Canfield, D. E. (1994). Factors influencing organic carbon preservation in marine sediments. Chemical Geology, 114(3–4), 315–329.
Canfield, D. E., van Zuilen, M. A., Nabhan, S., Bjerrum, C. J., Zhang, S., Wang, H., & Wang, X. (2021). Petrographic carbon in ancient sediments constrains Proterozoic Era atmospheric oxygen levels. Proceedings of the National Academy of Sciences, 118(23).
De Leeuw, J. W., & Largeau, C. (1993). A review of macromolecular organic compounds that comprise living organisms and their role in kerogen, coal, and petroleum formation. In Organic Geochemistry (pp. 23–72).
Dean, W. E., & Gorham, E. (1998). Magnitude and significance of carbon burial in lakes, reservoirs, and peatlands. Geology, 26(6), 535–538.
Demaison, G. J., & Moore, G. T. (1980). Anoxic environments and oil source bed genesis. AAPG Bulletin, 64(8), 1179–1209.
Diaz, R. J., Eriksson-Hägg, H., & Rosenberg, R. (2013). Hypoxia (Chap. 4). In Managing Ocean Environments in a Changing Climate. Sustainability and Economic Perspectives, (pp. 67–96). Elsevier.
Dieckmann, V., Horsfield, B., & Schenk, H. J. (2000). Heating rate dependency of petroleum-forming reactions: Implications for compositional kinetic predictions. Organic Geochemistry, 31(12), 1333–1348.
Fenchel, T., King, G. M., & Blackburn, T. H. (2012). The water column. In Bacterial Biogeochemistry (pp. 67–88).
Gaedke, U. (2021). Trophic dynamics and food webs in aquatic ecosystems. Reference Module in Earth Systems and Environmental Sciences. https://doi.org/10.1016/B978-0-12-819166-8.00009-8
Galloway, W. E. (1975). Process framework for describing the morphologic and stratigraphic evolution of deltaic depositional systems. In M. L. Broussard (Ed.), Deltas: Models for Exploration. Houston Geological Society; 87–98.
Ghassal, B. I., El Atfy, H., Sachse, V., & Littke, R. (2016a). Source Rock Potential of the Middle Jurassic to Middle Pliocene, Onshore Nile Delta Basin. Egypt. Arabian Journal of Geosciences, 9, 744.
Ghassal, B. I., Littke, R., Sachse, V., Sindern, S., & Schwarzbauer, J. (2016b). Depositional environment and source rock potential of upper Albian to Turonian sedimentary rocks of the Tarfaya Basin, Southwest Morocco. Geologica Acta, 14, 419–441.
Ghassal, B. I. H., Littke, R., & Sindern, S. (2017). Source rock depositional processes in different marine settings: examples from North African basins (No. RWTH-2017-06977). Fachgruppe für Geowissenschaften und Geographie.
Ghassal, B. I., Littke, R., El Atfy, H., Sindern, S., Scholtysik, G., El Beialy, S., & El Khoriby, E. (2018). Source rock potential and depositional environment of Upper Cretaceous sedimentary rocks, Abu Gharadig Basin, Western Desert, Egypt: An integrated palynological, organic and inorganic geochemical study. International Journal of Coal Geology, 186, 14–40.
Glasspool, I. J., & Scott, A. C. (2005). An early Carboniferous (Mississippian), Tournaisian, megaspore assemblage from Three Mile Plains, Nova Scotia, Canada. Review of Palaeobotany and Palynology, 134, 219–236.
Goodbred, S. L., & Saito, Y. (2012). Tide-dominated deltas. Principles of tidal sedimentology (pp. 129–149). Springer.
Gregory, T. R., & DeSalle, R. (2005). Comparative genomics in prokaryotes. In T. R. Gregory (Ed.), The Evolution of the Genome (pp. 585–675). Elsevier.
Hallam, A., & Bradshaw, M. J. (1979). Bituminous shales and oolithic ironstones as indicators of transgressions and regressions. Geological Society of London Journal, 136, 157–164.
Hayes, M. H., Mylotte, R., & Swift, R. S. (2017). Humin: Its composition and importance in soil organic matter. Advances in Agronomy, 143, 47–138.
Hayes, W. (2018). Genetics as a tool to understand structure and function. In Molecular Biology of Symbiotic Nitrogen Fixation (pp. 1–11). CRC Press.
Henrichs, S. M. (1992). Early diagenesis of organic matter in marine sediments: Progress and perplexity. Marine Chemistry, 39(1–3), 119–149.
Horsfield B., & Rullkötter J. (1994). Diagenesis, catagenesis and metagenesis. In L.Magoon, W. G. Dow (Eds.), The Petroleum System from Source to Trap (pp. 189–199). AAPG Memoir 60.
Hutchinson, G. E., & Löffler, H. (1956). The thermal classification of lakes. Proceedings of the National Academy of Sciences of the United States of America, 42(2), 84.
Inan, S., Henderson, S., & Qathami, S. (2017). Oxidation Tmax: A new thermal maturity indicator for hydrocarbon source rocks. Organic Geochemistry, 113, 254–261.
Irwin, J. A. (2020). Overview of extremophiles and their food and medical applications (Chap. 6). In Physiological and Biotechnological Aspects of Extremophiles (pp. 65–87).
Jørgensen, B. B., Findlay, A. J., & Pellerin, A. (2019). The biogeochemical sulfur cycle of marine sediments. Frontiers in Microbiology, 10, 849.
Katz, B. (2012). Petroleum source rocks (p. 325). Springer Science & Business Media.
Killops, S. D., Killops, V. J. (2013). Introduction to Organic Geochemistry (p. 408). Wiley.
Koblentz-Mishke, O. J., Volkovinsky, V. V., & Kabanova, J. G. (1970). Plankton primary production of the World Ocean. In W. S. Wooster (Ed.), Scientific Exploration of the South Pacific (pp. 183–193).
Kosters, E. C., VanderZwaan, G. J., & Jorissen, F. J. (2000). Production, preservation and prediction of source-rock facies in deltaic settings. International Journal of Coal Geology, 43, 13–26.
Langbein, W. B. (1961). Salinity and hydrology of closed lakes: A study of the long-term balance between input and loss of salts in closed lakes (Vol. 412). US GovernmentPrinting Office.
Li, Y. F., Lu, H., Zhang, Y., Zhang, X. L., Shao, D. Y., Yan, J. P., & Zhang, T. W. (2015). U-Mo covariation in marine shales of Wufeng-Longmaxi Formations in Sichuan Basin, China and its implication for identification of watermass restriction. Geochimica (beijing), 44(2), 109–116.
Littke, R. (1993). Migration of oil and gas coals. In Deposition, Diagenesis and Weathering of Organic Matter-Rich Sediments (pp. 135–169)
Littke, R., Baker, D. R., & Rullkötter, J. (1997). Deposition of petroleum source rocks. In D. H. Welte, B. Horsfield, & D. R. Bake (Eds.), Petroleum and Basin Evolution (pp. 271–333). Springer.
Macdonald, C. A., Delgado-Baquerizo, M., Reay, D. S., Hicks, L. C., & Singh, B. K. (2018). Soil nutrients and soil carbon storage: Modulators and mechanisms (Chap. 6). In Soil Carbon Storage. Modulators, Mechanisms and Modeling (pp. 167–205).
Maende, A., Pepper, A., Jarvie, D., & Weldon, D. (2017). Advanced pyrolysis data and interpretation methods to identify unconventional reservoir sweet spots in fluid phase saturation and fluid properties (API gravity) from drill cuttings and cores. Petroleum Geology, 12(4), 417–452.
Magalon, A., Arias-cartin, R., & Walburger, A. (2012). Supramolecular organization in prokaryotic respiratory systems. In R. K. Poole (Ed.), Advances in Microbial Physiology (Vol. 61, pp. 217–266). Elsevier.
Mayer, L. M. (1994). Surface area control of organic carbon accumulation in continental shelf sediments. Geochimica Et Cosmochimica Acta, 58(4), 1271–1284.
McCabe, P. J. (1991). Tectonic controls on coal accumulation. Bulletin De La Societe Geologique De France, 162(2), 277–282.
Mendonça Filho, J. G., Menezes, T. R., Mendonça, J. O., de Oliveira, A. D., da Silva, T. F., Rondon, N. F., & da Silva, F. S. (2012). Organic facies: Palynofacies and organic geochemistry approaches. In Geochemsitry (pp. 211–248). www.intechopen.com. https://doi.org/10.5772/47928
Nienhuis, J. H., Ashton, A. D., & Giosan, L. (2015). What makes a delta wave-dominated? Geology, 43(6), 511–514.
Parker, J. (2001). Bacteria. In Encyclopedia of Genetics (pp. 146–151).
Parrish, J. T. (1987). Palaeo-upwelling and the distribution of organic-rich rocks. Geological Society, London, Special Publications, 26(1), 199–205.
Pepper, A. S., & Corvi, P. J. (1995). Simple kinetic models of petroleum formation. Part I: Oil and gas generation from kerogen. Marine and Petroleum Geology, 12, 291–319.
Piper, D. Z., & Calvert, S. E. (2009). A marine biogeochemical perspective on black shale deposition. Earth-Science Reviews, 95(1–2), 63–96.
Pompeckj, J. F. (1901). Der Jura Zwischen Regensburg Und Regenstauf: Geognostische Jahreshefte, 14, 139–220.
Reading, H. G. (1986). Sedimentary Environments and Facies (p. 615).
Ritz, K. (2005). Fungi. In Encyclopedia of Soils in the Environment (pp. 110–119).
Robinson, L., & Davies, A. (2016). Upwelling zones as controls on source rock deposition-is the present the key to the past? Neftex Exploration Insights magazine (March Edition), 15.
Romero-Sarmiento, M. F., Ducros, M., Carpentier, B., Lorant, F., Cacas, M. C., Pegaz-Fiornet, S., Wolfa, S., Rohaisa, S., & Moretti, I. (2013). Quantitative evaluation of TOC, organic porosity and gas retention distribution in a gas shale play using petroleum system modeling: Application to the Mississippian Barnett Shale. Marine and Petroleum Geology, 45, 315–330.
Ruttenberg, K. C. (2003). 8.13 - The Global Phosphorus Cycle. Treatise on Geochemistry, 8, 585–643.
Rydin, H., Sjörs, H., & Löfroth, M. (1999). 7. Mires. In Swedish Plant Geography, Acta Phytogeographica Suecica (pp. 91–112). Svenska Växtgeografiska Sällskapet, Uppsala.
Sage, R. F. (2008). Autotrophs. In Encyclopedia of Ecology (pp. 291–300)
Selley, R. C. (1998). Elements of petroleum geology (2nd ed., p. 470). Academic Press.
Simon, J., & Kroneck, P. M. (2013). Microbial sulfite respiration. Advances in Microbial Physiology, 62, 45–117.
Sorokin, Yu. I. (1966). On the trophic role of chemosynthesis and bacterial biosynthesis in water bodies,. In C. R. Goldman (Ed.), Primary productivity in aquatic environments (pp. 187–250). Univ. California Press.
Stach, E., Mackowsky, M. T., Teichmuller, M., Taylor, G. H., Chandra, D., & Teichmuller, R. (1982). Coal petrology. Gebruder Borntraeger.
Suess, E. (1980). Particulate organic carbon flux in the oceans—surface productivity and oxygen utilization. Nature, 288(5788), 260–263.
Summons, R. E. (1993). Biogeochemical cycles. In Organic Geochemistry (pp. 3–21). Springer.
Taylor, G. H., Teichmüller, M., Davis, A. C. F. K., Diessel, C. F. K., Littke, R., & Robert, P. (1998). Organic petrology.
Tissot, B., & Welte, D. H. (1984). Petroleum Formation and Occurrence (p. 699). Springer.
Tyson, R. V. (1995). Sedimentary organic Matter—Organic Facies and Palynofacies (p. 615). Chapman and Hall.
Vallero, D. (2014). Fundamentals of Air Pollution (p. 996). Academic Press, Technology & Engineering.
Waiser, M. J., & Robarts, R. D. (2009). Saline inland waters. In: Gene E. Likens, (Editor) Encyclopedia of Inland Waters. volume 2, pp. 634–644 Oxford: Elsevier.
Wakeham, S. G., & Lee, C. (1993). Production, transport, and alteration of particulate organic matter in the marine water column. In Organic Geochemistry (pp. 145–169). Springer.
Wigley, T. M., & Schimel, D. S. (Eds.). (2005). The carbon cycle (Vol. 6). Cambridge University Press. 312 p.
Woese, C. R., Kandler, O., & Wheelis, M. L. (1990). Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proceedings of the National Academy of Sciences of the United States of America, 87, 4576–4579.
Zavarzin, G. A. (2008). A planet of bacteria. Herald of the Russian Academy of Sciences, 78, 144–151.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ghassal, B.I., El Atfy, H. (2023). Sedimentary Organic Matter: Origin, Productivity, Preservation, and Role in Source Rock Development. In: El Atfy, H., Ghassal, B.I. (eds) Advances in Petroleum Source Rock Characterizations: Integrated Methods and Case Studies. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-031-16396-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-16396-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-16395-1
Online ISBN: 978-3-031-16396-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)