Abstract
The acoustic behavior of minke whale populations worldwide has been a mystery for the better part of the twentieth century. Several likely biological sound sources such as the ‘boing’ recorded in the North Pacific, or the ‘bio-duck’ with its ubiquitous distribution in the Southern Ocean, had been described by seafarers since the middle of the twentieth century. However, the origin of these sounds could only be revealed once technological advances allowed scientists to simultaneously acoustically and visually track the elusive species producing them. The current data show that, like other baleen whales, most minke whale populations produce long song sequences presumably in a reproductive context. Over the past two decades, by extending our listening efforts into remote habitats, we have learned much about minke whales and can assume that many more mysteries are waiting to be unlocked.
Dedicated to Thomas F Norris
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords

Photo courtesy of Ari S. Friedlaender
Antarctic minke whale (Balaenoptera bonaerensis) with multi-sensor acoustic recording tag.
1 Introduction
Although they are one of the most widely distributed baleen whale species, often found in coastal waters during summer, most minke whale populations have been little studied. Most of our knowledge about the species’ life history originates from scattered observations in coastal habitats and whaling records (Horwood 1990), and much of their behavioral ecology remains unknown.
Common minke whales (Balaenoptera acutorostrata) inhabit all oceans from the tropics to the poles and are closely related to their sister species, the Antarctic minke whale (Balaenoptera bonaerensis), also referred to as the ‘southern minke whale’ (Rychel et al. 2004). Based on genetic evidence and geographic distribution, the common minke whale is split into three subspecies: Balaenoptera acutorostrata acutorostrata in the North Atlantic, Balaenoptera acutorostrata scammoni in the North Pacific, and an unnamed subspecies of dwarf minke whale in the Southern Hemisphere (Rice 1998). The exact placement of the dwarf minke whale in baleen whale taxonomy is still unclear. At least two different populations of dwarf minke whales appear to exist in the Southern Hemisphere, one in the South Atlantic where they were first described in the 1980s (Best 1985) and one in the South Pacific. Genetically, the South Atlantic dwarf minke whale is more closely related to the North Atlantic minke whale than to the South Pacific dwarf minke whale (Pastene et al. 2010).
The lack of data on minke whales is partly due to the difficulties in observing them. At an average adult body length of 7–9 m, minke whales are one of the smallest of the baleen whales, only larger than the pygmy right whale (Caperea marginata). Due to their small body size, inconspicuous blows, and brief surfacings, minke whales have been described as elusive, especially in their more pelagic winter habitats. In addition, except in higher latitudes, where aggregations of up to a few hundred animals have been visually observed, larger aggregations are less prevalent than in other species (Edds and Macfarlane 1987). Indeed, across much of their range minke whales are often visually encountered as solitary individuals and exhibit a wide range of individually distinctive foraging behaviors (Hoelzel et al. 1989).
In contrast to this general reclusive demeanor, in some areas, common minke whales are known for their inquisitive behavior and tendency to actively associate with vessels. This more curious behavior is, for example, commonly observed in Australian’s Great Barrier Reef region, where dwarf minke whales approach and spend extended time with swimmers and divers (Valentine et al. 2004). These interactions are particularly remarkable given that the winter distributions and behaviors of nearly all other minke whale populations are less understood than their distributions and behaviors in summer foraging habitats.
2 Mystery in All Oceans: A Brief Overview of Global Minke Whale Vocalizations
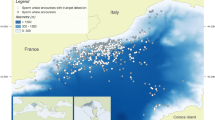
Comprehensive descriptions and behavioral functions of minke whale sounds remain incomplete. Vocalizations produced by the North Pacific, dwarf and Antarctic minke whales were only unequivocally assigned to these species in the early 2000s (Gedamke et al. 2001; Gedamke 2004; Rankin and Barlow 2005; Risch et al. 2014b), despite being recorded and described in parts of the world’s oceans (Fig. 14.1) for decades (Wenz 1964; Thompson and Friedl 1982; Matthews et al. 2004; Dolman et al. 2005). It is now clear that minke whale populations produce a variety of sounds throughout their geographic ranges. However, given that the full acoustic repertoire and functional significance of their many different types of sounds are still largely unknown, many discoveries about their vocal behaviors are yet to be made.
Global distribution of the best described Antarctic and common minke whale vocalization types (Gedamke et al. 2001; Nieukirk et al. 2004, 2016; Matthews et al. 2004; Rankin and Barlow 2005; Dolman et al. 2005; Oswald et al. 2011; Martin et al. 2013; Risch et al. 2013, 2014a, 2019; b; Delarue et al. 2013; Dominello and Širović 2016; Norris et al. 2017; Cerchio et al. 2018, 2022; Nikolich and Towers 2018; Thomisch et al. 2019; Buchan et al. 2020; Shabangu et al. 2020; Filun et al. 2020). *Nieukirk et al. 2016 recorded a sound with similarities to the ‘star-wars’ vocalizations near the Marian Trench and hypothesized that it might be produced by minke whales
New technologies are helping to fill some of the existing data gaps. For example, small, multi-sensor tags can now be equipped with acoustic recorders, allowing measurements of fine-scale movement behavior while simultaneously recording sounds from tagged and nearby animals (Risch et al. 2014b). Networks of bottom-mounted acoustic recorders, deployed for several months to years, are being used to investigate migratory routes and changes in minke whale distribution and to identify previously unknown habitats (Clark and Gagnon 2002; Risch et al. 2014a). This chapter aims to summarize what is currently known about common and Antarctic minke whale vocalizations worldwide.
2.1 North Atlantic
On a summer feeding ground in the North Atlantic, a few sounds from a minke whale were first reported in the 1970s by Beamish and Mitchell (Beamish and Mitchell 1973). These authors used a calibrated hydrophone system to record a series of clicks in the 4–8 kHz range during a very close (20–80 m) encounter with a single animal. The clicks they described were 1–5 ms in duration and repeated at 6–7 clicks/second. About a decade later, in the Gulf of St Lawrence, Canada, Edds-Walton recorded sounds in the presence of minke whales; frequency-modulated (FM) downsweeps with a median start and end frequency of 118 Hz and 80 Hz, respectively (Edds-Walton 2000). These sounds had a median duration of 0.4 s and were produced by individual animals while traveling (Edds-Walton 2000).
In 1971, based on acoustic and visual observations conducted in the Caribbean Sea region, Winn and Perkins (1976) recorded low-frequency pulse trains with varying inter-pulse intervals (IPI) and peak frequencies from 55–150 Hz. In 1993–1996, with the advent of limited scientific access to the US Navy Sound Surveillance System (SOSUS), pulse trains described as songs and attributed unequivocally to minke whales in the Caribbean and throughout the Western North Atlantic were described and reported by Mellinger et al. (2000) and Clark and Gagnon (2002) (Fig. 14.2; Chap. 2). These pulse trains appear to be the most common in the repertoire of North Atlantic minke whales and have been recorded during migration and in several summer feeding grounds (Folkow and Blix 1991; Nieukirk et al. 2004; Risch et al. 2013, 2019). Pulse trains are stereotypic, and three different pulse train types have been described (e.g., ‘speed-up’, ‘slow-down’, and ‘constant’) based on IPI and peak frequency (Fig. 14.3), while individual pulses are typically FM upsweeps and sometimes paired with a slight temporal overlap within a pair (Mellinger et al. 2000).
Spectrograms of North Atlantic minke whale pulse train types identified by Risch et al. (2013) and recorded in the Stellwagen Bank National Marine Sanctuary, Eastern North Atlantic. Upper panel shows three different types of ‘slow-down’ pulse trains, and lower panel shows three types of ‘constant’ pulse trains. Not shown here is an example of a speed-up pulse train, which is mostly recorded in lower latitudes (Mellinger et al. 2000). Note the different time scales for these different pulse train types. Spectrogram parameters: 2000 Hz sample rate, 512 point FFT, 75% overlap = 75%, 3.9 Hz and 64 ms frequency and time resolution, respectively
Spectrogram of a North Atlantic slow-down pulse train type recorded during the spring migration season off Cape Cod; b North Pacific ‘boing’ sound; c South Pacific ‘star-wars’ sound. The sound is comprised of three sub-units: A, B, C (Gedamke et al. 2001); d Antarctic ‘bio-duck’ sound. Spectrogram parameters: a 2000 Hz sample rate, 512 point FFT, 75% overlap, 3.9 Hz and 64 ms frequency and time resolution, respectively; b 8000 Hz sample rate, 512 point FFT, 75% overlap, 15.6 Hz and 16 ms frequency and time resolution, respectively; c 16,000 Hz sample rate, 1024 point FFT, 90% overlap, 15.6 Hz and 6.4 ms frequency and time resolution, respectively; d 2000 Hz sample rate, 512 point FFT, 95% overlap, 3.9 Hz and 12.8 ms frequency and time resolution, respectively
In addition to these stereotypic types of pulse trains, higher frequency (3–12 kHz) clicks described from initial Caribbean recordings (Winn and Perkins (1976) have more recently been confirmed to be regular components of some of these low-frequency pulse trains (Risch et al. 2015). These higher frequency clicks might also correspond to the ones originally described by Beamish and Mitchell (1973).
The durations of North Atlantic pulse trains appear to vary between pulse train types and geographically, with significantly longer pulse trains recorded in lower latitudes than in higher latitudes (Risch et al. 2014a). The average source level of 164–168 dB re 1 µPa measured by Risch et al. (2014c) gave an approximate active space, defined as the area within which a receiver might perceive the call of a signaler (Brenowitz 1982), of up to 10 km in a habitat dominated by shipping noise (Risch et al. 2014c).
2.2 North Pacific
A North Pacific sound type referred to as the ‘boing’ has been recorded in lower latitudes of the North Pacific since the late 1950s and was first described in the early 1960s (Wenz 1964). Although the sound was assumed to be biological, actual species attribution remained a mystery until 2003 when simultaneous visual observations and acoustic bearing angle localizations of individual animals were used to confirm that minke whales were the source of this mysterious sound (Rankin and Barlow 2005). Boing sounds consist of a relatively short introductory pulse followed by a longer amplitude modulation (AM) of a FM unit with decreasing amplitude over the course of the sound and a peak frequency of approximately 1.4 kHz (Fig. 14.3b; Thompson and Friedl 1982; Rankin and Barlow 2005; Oswald et al. 2011).
At least two types of the boing sound have been detected across the North Pacific, which may be indicative of geographic differences between genetically separate populations. Boings in the Eastern North Pacific have pulse repetition rates of about 92 s−1, whereas in the central part of the North Pacific, boings have pulse repetition rates of 115 s−1 (Wenz 1964; Rankin and Barlow 2005). Eastern and central boings also differ in overall durations, lasting 3.6 and 2.6 s, respectively (Rankin and Barlow 2005). Boings are primarily recorded in low-latitude presumed breeding grounds (Norris et al. 2012) and are repeated in long sequences with varying inter-boing intervals (ICI). Several studies have described a bi-modal distribution of boing repetition rates, with shorter intervals between boings (28–30 s) when several animals are presumed to be in acoustic contact with one another and longer intervals (350–600 s) when animals are further apart (Wenz 1964; Thompson and Friedl 1982; Rankin and Barlow 2005). Apart from having been recorded in lower latitudes, a few ‘boings’ have also been recorded in a summer feeding ground, the Northeastern Chukchi Sea, during late summer and autumn (Delarue et al. 2013).
In addition to boing sounds, North Pacific minke whale vocalizations from a summer feeding ground off Vancouver Island, Canada, were recently described as low-frequency downsweeps starting at 142 Hz and ending at 38 Hz, a peak frequency of 105 Hz, and a duration of 0.7 s (Nikolich and Towers 2018). At the same recording site, a series of sounds similar to North Atlantic pulse trains was attributed to a minke whale and described as “pulse chains” with variable pulse rates in the 330–1400 Hz frequency range. Overall, pulse train occurrence in this summer feeding ground was very low, despite regular visual sightings of minke whales (Nikolich and Towers 2018).
2.3 South Pacific
One of the most fascinating baleen whale sound is produced by animals in the Australian population of the dwarf minke whale. Named the ‘star-wars’ vocalization (Fig. 14.3c), due to its distinct synthetic or metallic quality, this sound type was first scientifically described and unequivocally linked to the species in the Northern Great Barrier Reef sing a combination of dedicated visual observations and acoustic localization (Gedamke et al. 2001). The reef is a winter habitat and presumed breeding ground for the species. The star-wars sound is somewhat similar in structure to the boing sound. The sound is composed of three sub-units (A, B, C; Fig. 14.3c), each of which is an amplitude modulation of a FM unit, with AM rate and FM characteristics being different for each of the three components. This complex combination results in a sound that seems oddly unworldly to the human ear, hence, the reference to artificial sounds produced for the movie ‘Star Wars’. Individual star-wars sounds are usually repeated in stereotyped sequences of equal intervals ranging from 1–2 s to 3–4 min. Source-level estimates for these sounds ranged from 150–165 dB re 1 µPa (Gedamke et al. 2001).
Similar to North Atlantic minke whales, dwarf minke whales in the Great Barrier Reef region also produce low-frequency, downswept vocalizations starting around 250 Hz and sweeping down to around 50 Hz. These sounds are typically 0.2–0.3 s in duration and have source levels of approximately 148–160 dB re 1 µPa (Gedamke et al. 2001). In addition, several ‘noisy’, broadband social sounds have been described for Great Barrier Reef dwarf minke whales (Gedamke 2004). As is the case in vocal repertoires of other baleen whales, the acoustic characteristics of these social sounds appear to be vary along a continuum, which makes their division into distinct call types difficult. They appear structurally similar to, but are generally less complex than, the typical star-wars sound (Gedamke 2004).
More recently, a complex call of unknown origin was recorded in the Mariana Trench, off the east coast of Guam and Saipan (Nieukirk et al. 2016; Fig. 14.1). This sound consisted of an AM 38 Hz moan, followed by a broadband sweep with energy up to 7.5 kHz. Due to the similarities to the star-wars sound, it was suggested that this call might be produced by either a dwarf or common minke whale (Nieukirk et al. 2016).
While dwarf minke whales are frequently visually observed in the South Atlantic (Zerbini et al. 1996; Acevedo et al. 2006), so far they have not been detected acoustically in the South Atlantic ocean basin.
2.4 Southern Ocean
For over five decades, a regular pulsed signal (Matthews et al. 2004) referred to as the ‘bio-duck’ has been recorded in the Southern Ocean, but the animal producing this mysterious sound remained unknown until recently. The signal was first described by submarine officers in the 1960s. Since then, it has been described off the west coast of Australia and in the Ross and Weddell Seas, as well as in sub-Antarctic waters (Poulter 1964; Matthews et al. 2004; McCauley 2004; Dolman et al. 2005; Klinck and Burkhardt 2008; Van Opzeeland 2010). It was not until 2013 that the ‘bio-duck’ was unequivocally linked to Antarctic minke whales based on analysis of multi-sensor acoustic recording tags deployed on two individuals of this species in Wilhelmina Bay, Western Antarctica (Risch et al. 2014b). The ‘bio-duck’ vocalization consists of one to six pulses in the 50–300 Hz frequency band (Fig. 14.3d), typically with harmonics up to 1 kHz, although recent descriptions of the different subtypes of the ‘bio-duck’ vocalization describe harmonics up to 2 kHz and beyond (Shabangu et al. 2020). These pulse trains are then often repeated in long sequences at very regular ICIs (Fig. 14.4).
Spectrogram of a partial Antarctic ‘bio-duck’ song sequence recorded during austral winter at PALAOA station, Antarctica, (acoustic sample courtesy of I. van Opzeeland). Spectrogram parameters: 48 kHz sample rate, 4096 point FFT, 75% overlap, 11.7 Hz and 21.3 ms frequency and time resolutions, respectively
Along with the first description of the ‘bio-duck’ signal from Antarctic minke whales, low-frequency downsweeps were also recorded from the same tagged individuals during several of their dives. These downsweeps were in the frequency range of 60–130 Hz with a mean duration of 0.2 s (Risch et al. 2014b). Similar downsweeps had previously been reported from the Ross Sea (Schevill and Watkins 1972; Leatherwood et al. 1981), as well as together with ‘bio-duck’ vocalizations that were recorded in the Weddell Sea and off Western Australia (Matthews et al. 2004; Klinck and Burkhardt 2008).
A recent study of minke whale pulse trains collected near the Western Antarctic Peninsula distinguished at least four different variants of the bio-duck sound and several subtypes for one variant. These variants were based on frequency content, the number of pulses and IPIs within a train, and the pulse train repetition rate (Dominello and Širović 2016). Different bio-duck sound variants have also been described to co-occur in Western Australian and South African recordings (Matthews et al. 2004; Shabangu et al. 2020).
3 Commonalities Between Minke Whale Vocal Repertoires and a Definition of Minke Whale Song
Despite a remarkable amount of variability in the vocal repertoires currently described for common and Antarctic minke whales, there are some commonalities. Most minke whale populations produce series of relatively stereotypic low-frequency sounds that are often repeated in sequences lasting several minutes to hours. Although the sex of the caller is still unknown for most of these sequences (Sect. 14.5), the dwarf and North Atlantic minke whale vocalizations have been classified as song (Gedamke et al. 2001; Clark and Gagnon 2002; Clark and Ellison 2004; Gedamke 2004). This classification is based on the definition of song, commonly used to describe the patterned sequences produced by many birds species, where song is defined as: ‘…long, complex, vocalizations produced by males in the breeding season’. (Catchpole and Slater 2008) and by the description of male humpback whale song defined as ‘…a series of notes, generally of more than one type, uttered in succession and so related as to form a recognizable sequence or pattern in time’ (Payne and McVay 1971; Chap. 11). Given that the North Pacific boing and the Antarctic bio-duck sounds are also produced in long sequences (Fig. 14.4) and both are produced on presumed breeding grounds (Sect. 14.5), they could similarly be classified as songs, making ‘song’ a common acoustic behavior among minke whale populations worldwide.
Next to humpback whale song (Payne and McVay 1971), baleen whale songs have been described for several species, including blue (Balaenoptera musculus; Chap. 9), fin (Balaenoptera physalus), Omura’s (Balaenoptera omurai; Chap. 15), and bowhead (Balaena mysticetus; Chap. 12) whales, and singing is generally thought to function in mediating reproductive behavior (Cummings et al. 1987; Croll et al. 2002; Oleson et al. 2007a; Chap. 7).
In contrast to the highly dynamic characteristics of humpback and bowhead whale song (Cholewiak et al. 2013; Stafford et al. 2018; see Chaps. 10 and 11, respectively), minke whale songs appear more typical of the stereotypic songs of other balaenopterid whales such as blue and fin whales (Oleson et al. 2007b; Širović et al. 2013; Chap. 9), consisting of patterned phrases of a few notes with limited variability between years and individuals (Clark and Ellison 2004). That being said, individual song units can be structurally complex (e.g., the star-wars sound) and some variation has recently been shown in the Western North Atlantic where at least two different calling patterns or songs, consisting of three to four stereotyped pulse train types, have been described (Risch et al. 2014c). Across all populations, minke whale song units are typically low-frequency, pulsed FM-AM signals, with most energy below 1–2 kHz. Some geographic variation in boing song production has been described in the North Pacific (Rankin and Barlow 2005), while pulse train song units in the North Atlantic appear to increase in duration in lower latitude winter breeding grounds (Risch et al. 2014a).
The second sound type in most minke whale populations for which vocalizations have been partially described, are low-frequency (<500 Hz) downsweeps. These are usually not produced in patterned sequences and have most often been recorded in presumed summer feeding habitats (Schevill and Watkins 1972; Edds-Walton 2000; Shabangu et al. 2020).
4 Seasonal Distribution and Temporal Variation in Minke Whale Vocalizations
Marine environments are generally ‘open’ environments, with few natural barriers. Driven by wind, waves and upwellings, the distribution of available resources is variable over large spatial and temporal scales. Baleen whales have adapted to these dynamic conditions by capitalizing on large home ranges, with many species’ annual migrations ranging over thousands of kilometers from high-latitude feeding to low-latitude breeding grounds (Whitehead 2001; Stevick et al. 2011; Corkeron and Connor 1999; Chaps. 4 and 7). Migratory routes are well described for some species such as humpback (Dawbin 1966; Clapham and Mead 1999; Calambokidis et al. 2001) and right whales (Kraus et al. 1986; Mate et al. 2011; Chap. 4).
Long longitudinal migrations have also been documented for common minke whales (Kasamatsu et al. 1995; Skaug et al. 2004; Risch et al. 2014a). It is during these migrations and in their presumed winter breeding habitats that minke whale songs are most often recorded (Gedamke et al. 2001; Nieukirk et al. 2004; Oswald et al. 2011; Delarue et al. 2013; Risch et al. 2014a). In contrast, while low-frequency downsweeps have been described from summer feeding and winter breeding grounds, they have been more commonly recorded in higher latitude feeding grounds (Schevill and Watkins 1972; Edds-Walton 2000; Gedamke et al. 2001; Dominello and Širović 2016; Shabangu et al. 2020).
As is the case in other baleen whales, results from long-term passive acoustic recordings of minke whales are increasingly providing evidence for complex migration and seasonal distribution patterns. For example, the bio-duck song of the Antarctic minke whale has been detected simultaneously in high and low latitudes during austral winter (Matthews et al. 2004; Buchan et al. 2020; Filun et al. 2020; Shabangu et al. 2020). This indicates that while part of the population migrates to warmer waters during winter, a large part of the population stays in high latitudes year-round. This finding was corroborated by satellite tag data, showing animals staying south of the Antarctic Circumpolar Current year-round (Lee et al. 2017). To understand these distribution patterns and migratory pathways, it is important to better describe the seasonality and behavioral context of minke whale vocalizations in different populations and habitats, by using long-term passive acoustic data in combination with direct visual observation and multi-sensor tags.
Distinct diel patterns in vocalizations have been described for several minke whale populations. In the North Atlantic, minke whale pulse trains are produced primarily at night (Risch et al. 2013, 2019). The same pattern was found in South African waters and near the Greenwich meridian in the Southern Ocean for ‘bio-duck’ song (Menze et al. 2017; Shabangu et al. 2020). The authors hypothesized that the bio-duck song might be related to feeding, as the observed diel pattern was closely linked to diel vertical migration of zooplankton, with high concentrations near the surface at night (Menze et al. 2017). However, in contrast to these findings, in the Western Antarctic Peninsula and other parts of Antarctica, the bio-duck song either showed no distinct diel pattern or was more commonly detected during daylight hours (Dominello and Širović 2016; Shabangu et al. 2020). Similarly, no significant diel patterns were found in Hawaii for North Pacific minke whale ‘boing’ song (Oswald et al. 2011).
Site-specific diel patterns are not uncommon in baleen whale vocalization behavior. For example, in North Atlantic right whales (Eubalaena glacialis), differences in diel patterns between two recording sites have been linked with prey availability (Mellinger et al. 2007), and the observed variation in diel patterns of the bio-duck song in different parts of Antarctica might be related to prey distribution as well. Since prey behavior and distribution will also vary seasonally, some of these diverging patterns might be linked to seasonal shifts in prey distribution. More concurrent passive acoustic and prey distribution data are needed to fully explain these patterns.
5 Behavioral Contexts and Potential Function of Minke Whale Vocalizations
Acoustic communication using high-amplitude, low-frequency (<1 kHz) signals with the potential to propagate over large distances is a common feature of baleen whale social systems, where individual whales are dispersed over a wide area. In such large acoustically mediated networks, individuals may employ eavesdropping to obtain information about conspecifics and maintain social cohesion (McGregor and Dabelsteen 1996). Although difficult to demonstrate in the field (McComb and Reby 2005), eavesdropping has been suggested to occur in humpback whales, where females could listen to vocal interactions between competing males (Cholewiak 2008; Chap. 11). In addition to true eavesdropping, it has been shown that many mammals attend to vocalizations even if these are not directed at them. Baleen whales might use passive listening to obtain information about a productive food source by attending to calls from conspecifics, and perhaps from heterospecifics, sharing the same acoustic habitat and possibly over very large distances. Finally, baleen whales might be alerted to the presence of predators such as killer whales by passive listening.
In the North Atlantic, minke whale song patterns, consisting of three to four different pulse train types, have been described through localization of individual animals. The localized individuals were in audible range of one another which suggests that these song patterns might play an important role in conspecific interactions. However, due to a lack of visual observation data, the actual behavioral function of these sequences remains unknown (Risch et al. 2014c). Uncertainty regarding the function of baleen whale vocalizations is a common feature across the different species, subspecies, and populations. Given that most known minke whale sounds have only been identified and described within the past 20 years, the biological significance of these sounds any associated acoustic behavior of these elusive species is still in its infancy.
In other baleen whales such as humpback, fin, and blue whales, only males produce song in a reproductive context (Glockner 1983; Croll et al. 2002; Oleson et al. 2007a). Although it is currently unknown whether this is similar in minke whales, the absence of North Atlantic minke whale song in habitats with a high proportion of females, such as the Gulf of St Lawrence, Canada, and west of Greenland, provides circumstantial evidence that it might be (Risch et al. 2014a). It has, therefore, been suggested that minke whale song, which has now been described to occur in all ocean basins (14.3), might also play a role in the reproductive behavior in this species complex (Risch et al. 2013). Another indication that minke whale song in the North Atlantic might be related to reproductive behavior is based on the observation that pulse trains recorded in presumed Caribbean winter breeding grounds are about 20 s longer and exhibit more than twice as many pulses compared to those recorded in the Gulf of Maine during spring and autumn when the whales are on migration (Mellinger et al. 2000; Risch et al. 2013). In other mammal species, such as baboons and giant pandas, it has been shown that call duration can increase with heightened arousal state during the breeding season (Rendall 2003; Charlton et al. 2011).
Minke whale boing sounds recorded in the North Pacific have so far mostly been recorded in the lower latitudes around Hawaii and the Mariana Trench region, and their occurrence appears to be seasonal, with most boings being recorded from October to February, which coincides with minke whale visual sightings in those areas and the presumed breeding period for the population (Rankin et al. 2007; Oswald et al. 2011; Norris et al. 2017). Similar to the situation in the North Atlantic, direct data on age or sex of calling animals are still missing. Based on analogous lines of evidence regarding the stereotypy of the signals, their seasonal and spatial distribution, and in comparison to song and its function described for other baleen whale species, it has been suggested that the boing song might be produced by males in a reproductive context (Norris et al. 2017).
In the South Pacific, given the stereotypy and regular repetition of the star-wars sound recorded on the Great Barrier Reef and its primary production during the breeding season, it has also been speculated that these sounds function as a reproductive advertisement display, although comparable data gaps to those for North Atlantic pulse trains and North Pacific boing sounds exist (Gedamke et al. 2001).
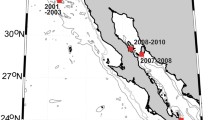
Compared to common and dwarf minke whale songs, the seasonal and spatial distribution patterns of occurrence of Antarctic minke whale bio-duck song appear to be more complex, with songs being produced in high- and low-latitude habitats (Fig. 14.4). It has been shown that bio-duck song in most areas peaks during austral spring and winter (June to August), although some sites show another peak later in the year (December). In addition, bio-duck song units appear to increase in duration during winter (Dominello and Širović 2016). Based on these data and reports of visually observed breeding behavior between August and October, it has been hypothesized that bio-duck song functions in a reproductive context, and mating in this species might take place across a wide range of habitats (Filun et al. 2020).
Compared to the song sequences, low-frequency downsweeps produced by most minke whale populations are less well described and their behavioral function remains also unclear. The most comprehensive study of minke whale downsweeps was carried out in the Gulf of St Lawrence, Canada, where no distinct temporal pattern of downsweep production was found (Edds-Walton 2000). For this summer foraging ground, it was suggested that downsweeps might function to maintain spacing between feeding individuals (Edds-Walton 2000). Downsweeps produced by Antarctic minke whales are positively correlated with sea ice presence, but more data from direct observation or multi-sensor acoustic tags are necessary to confirm the behavioral context of these downsweep vocalizations (Dominello and Širović 2016).
6 Minke Whale Hearing and Impacts of Noise Pollution
While information about minke whale hearing capabilities is limited, we assume they can hear one another. In the absence of field measurements, minke whale hearing sensitivity can either be inferred from species-specific vocalizations, behavioral responses to sounds, or anatomical measurements on which model predictions can be based (Mellinger et al. 2000; Tubelli et al. 2012; Yamato et al. 2012; Boisseau et al. 2021; Chap 3). While general estimates of audiograms for baleen whales assume highest sensitivities between 200 and 19,000 Hz (Southall et al. 2019), simulations of the minke whale auditory pathway suggests that the most sensitive frequency range is between approximately 30 and 25,000 Hz (Tubelli et al. 2012).
There are numerous natural biotic and abiotic sound sources in the world’s oceans, including weather events such as lightening/thunder and waves, sediment movement due to tidal currents, ice noise in the Arctic and Antarctic, and earthquakes, as well as biological sounds from a variety of taxa from invertebrates to the large whales (Bass and Clark 2003). Like other baleen whales, minke whales have adapted to these natural soundscapes by producing sounds with lower fundamental frequencies and higheramplitudes that increase sound transmission range (Clark 1983).
Since the beginning of the twentieth century, noise levels in some ocean regions have been steadily increasing and have remained high since at least the 1960s (Andrew et al. 2002; McDonald et al. 2006). These increases in and continuing high levels of anthropogenic noise have been driven primarily by shipping noise and noise from seismic airgun arrays for offshore oil exploration. Other sources of man-made noise include offshore construction work, multi-beam echosounders, fish finders, and naval sonar (Duarte et al. 2021).
An 80% loss of communication space for minke whale pulse trains relative to historical ‘quiet’ conditions has recently been modeled for minke whale habitat in the Northwest Atlantic (Cholewiak et al. 2018). In other parts of the world, minke whale densities have been observed to decrease during naval exercises, and minke whale strandings occurred during at least two mass stranding events linked to military sonar activity (Parsons et al. 2000; Balcomb and Claridge 2001). Another study showed minke whales responding to mid-frequency active sonar by a reduced calling rate and avoidance of the sound source. This behavior was observed even at relatively low estimated received sound levels (Sivle et al. 2015; Martin et al. 2015; Kvadsheim et al. 2017). Minke whales have also been shown to strongly avoid acoustic deterrent devices (ADDs) that are frequently used to mitigate underwater noise of offshore construction activities (Boisseau et al. 2021).
North Pacific minke whales off Hawaii increase the amplitude of their boing sounds in association with increased background noise. However, despite this compensation, the detected increases in sound intensity were not of the same magnitude as increases in background noise, and thus, reductions of communication space with potentially negative impacts would still be expected (Helble et al. 2020).
It is likely that chronically elevated noise levels such as those found in increasingly industrialized ocean regions as a result of human activities can also increase stress levels in minke whales, as has been documented in right whales (Rolland et al. 2012), with unknown consequences for long-term health and reproduction. More research is needed to fully understand the impacts of anthropogenic noise on all baleen whale species, but it is likely that aggregate (e.g., vessel noise, seismic surveys, and sonar) and cumulative effects due to a variety of stressors including underwater noise, habitat loss, and pollution will eventually reach levels that could impact population survival. It is, therefore, important to address known and potential impacts as early as possible using effective mitigation and management actions (Andersen and Clubb 2013).
7 Conclusions
Minke whales live in large-scale, acoustically mediated social systems, and similar to other baleen whales, the combination of advanced vocal learning has given rise to some unique and intriguing social behaviors, much of which have yet to be discovered for the different minke populations and subspecies. Future research should focus on elucidating the functions of the different minke whale call and song types in different contexts, differences in age and sex of acoustically active individuals, and changes in their acoustic behaviors in relation to both natural and anthropogenically modified noise conditions. Effort should also be directed toward the identification and description of sounds of unknown but presumed biological origin, especially in regions, such as the South Atlantic, for which data are currently lacking. Finally, new machine learning techniques to enhance automated detection and classification of baleen whale vocalization (Bergler et al. 2019; Allen et al. 2021), coupled with a better description of individual calling rates and source levels using localization techniques, will enhance the application of density estimation methods to assess and monitor population size using passive acoustics (Marques et al. 2013; Martin et al. 2013; Norris et al. 2017).
References
Acevedo J, Aguayo-Lobo A, Acuna P, Pastene L (2006) A note on the first record of the dwarf minke whale (Balaenoptera acutorostrata) in Chilean waters. J Cetacean Res Manag 8:293
Allen AN, Harvey M, Harrell L et al (2021) A convolutional neural network for automated detection of humpback whale song in a diverse, long-term passive acoustic dataset. Front Mar Sci. https://doi.org/10.3389/fmars.2021.607321
Andersen MS, Clubb DO (2013) Understanding and accounting for the costs of inaction. In: European Environment Agency (ed) Late lessons from early warnings: science, precaution, innovation. Rosendahls-Schultz Grafisk, Copenhagen, p 33
Andrew RK, Howe BM, Mercer JA, Dzieciuch MA (2002) Ocean ambient sound: Comparing the 1960s with the 1990s for a receiver off the California coast. Acoust Res Lett Online 3:65. https://doi.org/10.1121/1.1461915
Balcomb K, Claridge DE (2001) A mass stranding of cetaceans caused by naval sonar in the Bahamas. Bahams J Sci 3:2–12
Bass AH, Clark CW (2003) The physical acoustics of underwater sound communication. In: Simmons AM, Fay RR, Popper AN (eds) Acoustic communication. Springer, New York, pp 15–64
Beamish P, Mitchell E (1973) Short pulse length audio frequency sounds recorded in the presence of a Minke whale (Balaenoptera acutorostrata). Deep Sea Res Oceanogr Abstr 20:375–386. https://doi.org/10.1016/0011-7471
Bergler C, Schröter H, Cheng RX et al (2019) ORCA-SPOT: an automatic killer whale sound detection toolkit using deep learning. Sci Rep 9:10997. https://doi.org/10.1038/s41598-019-47335-w
Best PB (1985) External characters of southern minke whales and the existence of a diminutive form. Sci Rep Whales Res Inst 36:1–33
Boisseau O, McGarry T, Stephenson S et al (2021) Minke whales avoid a 15 kHz acoustic deterrent device. Mar Ecol Prog Ser. https://doi.org/10.3354/meps13690
Brenowitz EA (1982) The active space of red-winged blackbird song. J Comp Physiol 147:511–522. https://doi.org/10.1007/BF00612017
Buchan SJ, Balcazar-Cabrera N, Stafford KM (2020) Seasonal acoustic presence of blue, fin, and minke whales off the Juan Fernández Archipelago, Chile (2007–2016). Mar Biodivers 50:1–10. https://doi.org/10.1007/s12526-020-01087-3
Calambokidis J, Steiger G, Straley J et al (2001) Movements and population structure of humpback whales in the North Pacific. Mar Mammal Sci 17:769–794. https://doi.org/10.1111/j.1748-7692.2001.tb01298.x
Catchpole CK, Slater PJB (2008) Bird song: biological themes and variations, 2nd edn. Cambridge University Press, Cambridge
Cerchio S, Rasoloarijao T, Cholewiak DM (2018) Acoustic monitoring of blue whales (Balaenoptera musculus) and other baleen whales in the mozambique channel off the Northwest coast of madagascar. International Whaling Commission
Cerchio S, Laran S, Andrianarivelo N, et al (2022) Cetacean species diversity in Malagasy waters. In: Goodman S (ed) The new natural history of Madagascar. Princeton University Press, Princeton
Charlton BD, Keating JL, Kersey D et al (2011) Vocal cues to male androgen levels in giant pandas. Biol Lett 7:71–74. https://doi.org/10.1098/rsbl.2010.0582
Cholewiak D (2008) Evaluating the role of song in the humpback whale (Megaptera novaeangliae) breeding system with respect to intra sexual interactions. Cornell University
Cholewiak DM, Sousa-Lima RS, Cerchio S (2013) Humpback whale song hierarchical structure: historical context and discussion of current classification issues. Mar Mammal Sci 29:E312–E332. https://doi.org/10.1111/mms.12005
Cholewiak D, Clark CW, Ponirakis D et al (2018) Communicating amidst the noise: modeling the aggregate influence of ambient and vessel noise on baleen whale communication space in a national marine sanctuary. Endanger Species Res 36:59–75. https://doi.org/10.3354/esr00875
Clapham PJ, Mead JG (1999) Megaptera novaeangliae. Mamm Species 604:1–9
Clark CW (1983) Acoustic Communication and Behavior of the Southern right whale (Eubalaena australis). In: Payne R (ed) Communication and behavior of whales, vol. 76. Avalon Publishing, pp 163–198
Clark CW, Ellison WT (2004) Potential use of low-frequency sounds by baleen whales for probing the environment: evidence from models and empirical measurements. In: Thomas J, Moss CF, Vater M (eds) Advances in the study of echolocation in bats and dolphins. The University of Chicago Press, Chicago, pp 564–582
Clark CW, Gagnon GJ (2002) Low-frequency vocal behaviors of baleen whales in the North Atlantic: insights from integrated undersea surveillance system detections, locations, and tracking from 1992 to 1996. US Navy J Underw Acoust 52:609–640
Corkeron PJ, Connor RC (1999) Why do baleen whales migrate? Mar Mammal Sci 15:1228–1245. https://doi.org/10.1111/j.1748-7692.1999.tb00887.x
Croll D, Clark CW, Acevedo A et al (2002) Only male fin whales sing loud songs. Nature 417:809. https://doi.org/10.1038/417809a
Cummings WC, Diego S, Holliday DV (1987) Sounds and source levels from bowhead whales off Pt. Barrow, Alaska in the Canadian. 82
Dawbin WH (1966) The seasonal migratory cycle of humpback whales. In: Norris KS (ed) Whales, dolphins, and porpoises. University of California Press, Los Angeles, pp 145–170
Delarue J, Martin B, Hannay D (2013) Minke whale boing sound detections in the northeastern Chukchi Sea. Mar Mammal Sci 29:E333–E341. https://doi.org/10.1111/j.1748-7692.2012.00611.x
Dolman SJ, Swift RJ, Asmus K, Thiele D (2005) Preliminary analysis of passive acoustic recordings made in the Ross Sea during ANSLOPE III in 2004. Pap SC/57/E10 Present to Sci Comm Int Whal Com 1–8
Dominello T, Širović A (2016) Seasonality of antarctic minke whale (Balaenoptera bonaerensis) calls off the western antarctic Peninsula. Mar Mammal Sci 32:826–838. https://doi.org/10.1111/mms.12302
Duarte CM, Chapuis L, Collin SP et al (2021) The soundscape of the anthropocene ocean. Science (80- )371. https://doi.org/10.1126/science.aba4658
Edds PL, Macfarlane JAF (1987) Occurrence and general behavior of balaenopterid cetaceans summering in the St. Lawrence Estuary, Canada. Can J Zool 65:1363–1376. https://doi.org/10.1139/z87-216
Edds-Walton P (2000) Vocalizations of minke whales Balaenoptera acutorostrata in the St. Lawrence Estuary. Bioacoustics 11:31–50. https://doi.org/10.1080/09524622.2000.9753448
Filun D, Thomisch K, Boebel O et al (2020) Frozen verses: Antarctic minke whales (Balaenoptera bonaerensis) call predominantly during austral winter. R Soc Open Sci 7:192112. https://doi.org/10.1098/rsos.192112
Folkow LP, Blix AS (1991) Norwegian whale sighting and acoustic surveys in the Atlantic Ocean during the winter of 1989/90. Rep Int Whal Comm 41:531–538
Gedamke J, Costa DP, Dunstan A (2001) Localization and visual verification of a complex minke whale vocalization. J Acoust Soc Am 109:3038–3047. https://doi.org/10.1121/1.1371763
Gedamke J (2004) Minke whale song, spacing, and acoustic communication on the Great Barrier Reef, Australia
Glockner D (1983) Determining the sex of humpback whales (Megaptera novaeangliae) in their natural environment. In: Payne RS (ed) Communication and behavior of whales. Westview Press, Boulder, pp 447–464
Helble TA, Guazzo RA, Martin CR et al (2020) Lombard effect: Minke whale boing call source levels vary with natural variations in ocean noise. J Acoust Soc Am 147:698–712. https://doi.org/10.1121/10.0000596
Hoelzel AR, Dorsey EM, Stern SJ (1989) The foraging specializations of individual minke whales. Anim Behav 38:786–794. https://doi.org/10.1016/S0003-3472(89)80111-3
Horwood JW (1990) Biology and exploitation of the minke whale. CRC Press, Boca Raton
Kasamatsu F, Nishiwaki S, Ishikawa H (1995) Breeding areas and southbound migrations of southern minke whales Balaenoptera acutorostrata. Mar Ecol Prog Ser 119:1–10. https://doi.org/10.3354/meps119001
Klinck H, Burkhardt E (2008) Marine mammal automated perimeter surveillance—MAPS. Rep Polar Mar Res 580:114–121
Kraus SD, Prescott JH, Knowlton AR, Stone GS (1986) Migration and calving of right whales (Eubalaena glacialis) in the western North Atlantic. Rep Int Whal Comm 10:139–144
Kvadsheim PH, DeRuiter S, Sivle LD et al (2017) Avoidance responses of minke whales to 1–4kHz naval sonar. Mar Pollut Bull 121:60–68. https://doi.org/10.1016/j.marpolbul.2017.05.037
Leatherwood S, Thomas JA, Awbrey FT (1981) Minke whales oft northwestern ross Island. Antarct J 154–156
Lee JF, Friedlaender AS, Oliver MJ, DeLiberty TL (2017) Behavior of satellite-tracked Antarctic minke whales (Balaenoptera bonaerensis) in relation to environmental factors around the western Antarctic Peninsula. Anim Biotelemetry 5:23. https://doi.org/10.1186/s40317-017-0138-7
Marques TA, Thomas L, Martin SW et al (2013) Estimating animal population density using passive acoustics. Biol Rev 88:287–309. https://doi.org/10.1111/brv.12001
Martin SW, Marques TA, Thomas L et al (2013) Estimating minke whale (Balaenoptera acutorostrata) boing sound density using passive acoustic sensors. Mar Mammal Sci 29:142–158. https://doi.org/10.1111/j.1748-7692.2011.00561.x
Martin SW, Martin CR, Matsuyama BM, Henderson EE (2015) Minke whales (Balaenoptera acutorostrata) respond to navy training. J Acoust Soc Am 137:2533–2541. https://doi.org/10.1121/1.4919319
Mate BR, Best PB, Lagerquist BA, Winsor MH (2011) Coastal, offshore, and migratory movements of South African right whales revealed by satellite telemetry. Mar Mammal Sci 27:455–476. https://doi.org/10.1111/j.1748-7692.2010.00412.x
Matthews D, Macleod R, McCauley RD (2004) Bio-duck activity in the perth canyon. An Automatic Detection Algorithm. Proc Acoust 1–4
McCauley R (2004) Western Australian exercise area blue whale project. C Rep R2004–29. Proj - 350:1–73
McComb K, Reby D (2005) Vocal communication networks in large terrestrial mammals. In: McGregor PK (ed) Animal communication networks. Cambridge University Press, Cambridge, pp 372–389
McDonald MA, Hildebrand JA, Wiggins SM (2006) Increases in deep ocean ambient noise in the Northeast Pacific west of San Nicolas Island, California. J Acoust Soc Am 120:711. https://doi.org/10.1121/1.2216565
McGregor PK, Dabelsteen T (1996) Communication networks. In: Kroodsma DE, Miller HE (eds) Ecology and evolution of acoustic communication in birds. Cornell University Press, Ithaca, pp 409–425
Mellinger DK, Carson CD, Clark CW (2000) Characteristics of minke whale (Balaenoptera acutorostrata) pulse trains recorded near Puerto Rico. Mar Mammal Sci 16:739–756. https://doi.org/10.1111/j.1748-7692.2000.tb00969.x
Mellinger DK, Nieukirk SL, Matsumoto H et al (2007) Seasonal occurrence of North Atlantic right whale (Eubalaena glacialis) vocalizations at two sites on the Scotian Shelf. Mar Mammal Sci 23:856–867. https://doi.org/10.1111/j.1748-7692.2007.00144.x
Menze S, Zitterbart DP, van Opzeeland I, Boebel O (2017) The influence of sea ice, wind speed and marine mammals on Southern Ocean ambient sound. R Soc Open Sci 4
Nieukirk SL, Stafford KM, Mellinger DK et al (2004) Low-frequency whale and seismic airgun sounds recorded in the mid-Atlantic Ocean. J Acoust Soc Am 115:1832–1843. https://doi.org/10.1121/1.1675816
Nieukirk SL, Fregosi S, Mellinger DK, Klinck H (2016) A complex baleen whale call recorded in the Mariana Trench Marine National Monument. J Acoust Soc Am 140:EL274–EL279. https://doi.org/10.1121/1.4962377
Nikolich K, Towers JR (2018) Vocalizations of common minke whales (Balaenoptera acutorostrata) in an eastern North Pacific feeding ground. Bioacoustics 29:97–108. https://doi.org/10.1080/09524622.2018.1555716
Norris TF, Dunleavy KJ, Yack TM, Ferguson EL (2017) Estimation of minke whale abundance from an acoustic line transect survey of the Mariana Islands. Mar Mammal Sci 33:574–592. https://doi.org/10.1111/mms.12397
Norris TF, Martin S, Thomas L et al (2012) Acoustic ecology and behavior of minke whales in the Hawaiian and marianas Islands: localizations, abundance estimation, and characterization of minke whale “Boings.” In: Popper AN, Hawkins T (eds) The effects of noise on aquatic life. pp 149–153
Oleson EM, Calambokidis J, Burgess WC et al (2007a) Behavioral context of call production by eastern North Pacific blue whales. Mar Ecol Prog Ser 330:269–284. https://doi.org/10.3354/meps330269
Oleson EM, Wiggins SM, Hildebrand JA (2007b) Temporal separation of blue whale call types on a southern California feeding ground. Anim Behav 74:881–894. https://doi.org/10.1016/j.anbehav.2007.01.022
Oswald JN, Au WWL, Duennebier F (2011) Minke whale (Balaenoptera acutorostrata) boings detected at the Station ALOHA Cabled Observatory. J Acoust Soc Am 129:3353–3360. https://doi.org/10.1121/1.3575555
Parsons ECM, Birks I, Evans PGH et al (2000) The possible impacts of military activity on cetaceans in West Scotland. Eur Res Cetaceans 14:185–190
Pastene LA, Acevedo J, Goto M et al (2010) Population structure and possible migratory links of common minke whales, Balaenoptera acutorostrata, in the Southern Hemisphere. Conserv Genet 11:1553–1558. https://doi.org/10.1007/s10592-009-9944-7
Payne RS, McVay S (1971) Songs of humpback whales. Science (80- ) 173:585–597. https://doi.org/10.1126/science.173.3997.585
Poulter T (1964) Recording # 120342 (December 26th, 1964). Macaulay Libr
Rankin S, Barlow J (2005) Source of the North Pacific “boing” sound attributed to minke whales. J Acoust Soc Am 118:3346–3351. https://doi.org/10.1121/1.2046747
Rankin S, Norris TF, Smultea MA et al (2007) A visual sighting and acoustic detections of minke whales, balaenoptera acutorostrata (Cetacea: Balaenopteridae), in Nearshore Hawaiian Waters. Pacific Sci 61:395–398. https://doi.org/10.2984/1534-6188
Rendall D (2003) Acoustic correlates of caller identity and affect intensity in the vowel-like grunt vocalizations of baboons. J Acoust Soc Am 113:3390–3402. https://doi.org/10.1121/1.1568942
Rice DW (1998) Marine mammals of the world: systematics and distribution. In: Special publication number 4, the society for marine mammology. Lawrence, Kansas
Risch D, Clark CW, Dugan PJ et al (2013) Minke whale acoustic behavior and multi-year seasonal and diel vocalization patterns in Massachusetts Bay, USA. Mar Ecol Prog Ser 489:279–295. https://doi.org/10.3354/meps10426
Risch D, Castellote M, Clark C et al (2014a) Seasonal migrations of North Atlantic minke whales: novel insights from large-scale passive acoustic monitoring networks. Mov Ecol 2:24. https://doi.org/10.1186/s40462-014-0024-3
Risch D, Gales NJ, Gedamke J et al (2014b) Mysterious bio-duck sound attributed to the Antarctic minke whale (Balaenoptera bonaerensis). Biol Lett 10:20140175. https://doi.org/10.1098/rsbl.2014.0175
Risch D, Siebert U, van Parijs SM (2014c) Individual calling behaviour and movements of North Atlantic minke whales (Balaenoptera acutorostrata). Behaviour 151:1335–1360. https://doi.org/10.1163/1568539X-00003187
Risch D, Baumann-Pickering S, Norris T et al (2015) High-frequency clicks associated with mink whale pulse trains at three locations in the Western North Atlantic. In: 21st Biennial conference on the biology of marine mammals. San Francisco
Risch D, Wilson SC, Hoogerwerf M et al (2019) Seasonal and diel acoustic presence of North Atlantic minke whales in the North Sea. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-39752-8
Rolland RM, Parks SE, Hunt KE et al (2012) Evidence that ship noise increases stress in right whales. Proc R Soc B Biol Sci 279:2363–2368. https://doi.org/10.1098/rspb.2011.2429
Rychel AL, Reeder TW, Berta A (2004) Phylogeny of mysticete whales based on mitochondrial and nuclear data. Mol Phylogenet Evol 32:892–901. https://doi.org/10.1016/j.ympev.2004.02.020
Schevill WE, Watkins WA (1972) Intense low-frequency sounds from an antarctic minke whale, Balaenoptera acutorostrata. Breviora 388:1–8
Shabangu FW, Findlay K, Stafford KM (2020) Seasonal acoustic occurrence, diel-vocalizing patterns and bioduck call-type composition of Antarctic minke whales off the west coast of South Africa and the Maud Rise, Antarctica. Mar Mammal Sci 36:658–675. https://doi.org/10.1111/mms.12669
Širović A, Williams LN, Kerosky SM et al (2013) Temporal separation of two fin whale call types across the eastern North Pacific. Mar Biol 160:47–57. https://doi.org/10.1007/s00227-012-2061-z
Sivle LD, Kvadsheim PH, Curé C et al (2015) Severity of expert-identified behavioural responses of humpback whale, minke whale, and Northern Bottlenose whale to Naval Sonar. Aquat Mamm 41:469–502. https://doi.org/10.1578/AM.41.4.2015.469
Skaug HJ, Øien N, Schweder T, Bøthun G (2004) Abundance of minke whales (Balaenoptera acutorostrata) in the Northeast Atlantic: variability in time and space. Can J Fish Aquat Sci 61:870–886. https://doi.org/10.1139/F04-020
Southall BL, Finneran JJ, Reichmuth C et al (2019) Marine mammal noise exposure criteria: updated scientific recommendations for residual hearing effects. Aquat Mamm 45:125–232. https://doi.org/10.1578/AM.45.2.2019.125
Stafford KM, Lydersen C, Wiig Ø, Kovacs KM (2018) Extreme diversity in the songs of Spitsbergen’s bowhead whales. Biol Lett 14:20180056. https://doi.org/10.1098/rsbl.2018.0056
Stevick PT, Neves MC, Johansen F et al (2011) A quarter of a world away: female humpback whale moves 10,000 km between breeding areas. Biol Lett 7:299–302. https://doi.org/10.1098/rsbl.2010.0717
Thomisch K, Boebel O, Bachmann J et al (2019) Temporal patterns in the acoustic presence of baleen whale species in a presumed breeding area off Namibia. Mar Ecol Prog Ser 620:201–214. https://doi.org/10.3354/meps12952
Thompson PO, Friedl WA (1982) A long term study of low frequency sounds from several species of whales off Oahu, Hawaii. Cetology 45:1–19
Tubelli AA, Zosuls A, Ketten DR et al (2012) A prediction of the minke whale (Balaenoptera acutorostrata) middle-ear transfer function. J Acoust Soc Am 132:3263. https://doi.org/10.1121/1.4756950
Valentine PS, Birtles A, Curnock M et al (2004) Getting closer to whales—passenger expectations and experiences, and the management of swim with dwarf minke whale interactions in the Great Barrier Reef. Tour Manag 25:647–655. https://doi.org/10.1016/j.tourman.2003.09.001
Van Opzeeland IC (2010) Acoustic ecology of marine mammals in polar oceans. Reports Polar Mar Res 619:332
Wenz GM (1964) Curious noises and the sonic environment in the ocean. In: Tavolga WN (ed) Marine bio-acoustics. Pergamon, New York, pp 101–119
Whitehead H (2001) Analysis of animal movement using opportunistic individual identifications: application to sperm whales. Ecology 82:1417–1432. https://doi.org/10.1890/0012-9658
Winn HE, Perkins PJ (1976) Distribution and sounds of the minke whale, with a review of mysticete sounds. Cetology 19:1–12
Yamato M, Ketten DR, Arruda J et al (2012) The auditory anatomy of the minke whale (Balaenoptera acutorostrata): a potential fatty sound reception pathway in a baleen whale. Anat Rec Adv Integr Anat Evol Biol 295:991–998. https://doi.org/10.1002/ar.22459
Zerbini AN, Secchi ER, Siciliano S, Simoes-Lopes P (1996) The dwarf form of the minke whale Balaenoptera acutorostrata Lacépède, 1804, in Brazil. Report: International Whaling Commission 46
Acknowledgements
In memory of Thomas F. Norris, whose dedication and enthusiasm for studying marine mammal acoustics and minke whales were so infectious. Many shared discussions of minke whales sounds and their potential function have inspired large parts of this review. I thank Sofie van Parijs for her mentorship, support, and encouragement over the years, which ultimately enabled me to write this review. Finally, many thanks to Ellen Garland and Christopher W. Clark for their helpful comments on drafts of this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Risch, D. (2022). Mysterious Minke Whales: Acoustic Diversity and Variability. In: Clark, C.W., Garland, E.C. (eds) Ethology and Behavioral Ecology of Mysticetes . Ethology and Behavioral Ecology of Marine Mammals. Springer, Cham. https://doi.org/10.1007/978-3-030-98449-6_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-98449-6_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-98448-9
Online ISBN: 978-3-030-98449-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)