Abstract
The early detection of the clinical expressions of shoulder calcific tendinopathy is fundamental to start adequate therapeutic interventions before the development of articular and tendinous damage. In this perspective, imaging techniques and particularly ultrasound (US) are important tools to demonstrate calcification pattern, location, and inflammatory phase. Shoulder calcific tendinitis is a relatively common, painful disease. The cause of calcifying tendinitis is not known. It is a dynamic and self-limited process in which the calcifications tend to resolve after a period of worsening and intense pain, through three distinct stages of the disease process: the precalcific stage, the calcific stage (subdivided into three phases: formation, resting, and resorption), and the postcalcific stage. The first-line imaging modalities are X-ray and ultrasound, as calcium deposits are readily identifiable on both. The evaluation of calcific tendinitis is based mainly on radiography, and characterizing by which the shape and contour of the calcific deposit consent to classify the pathology, in order to determine the best possible treatment for the patient. Calcifications with a well-defined, homogeneous contour are less likely to be symptomatic and may correlate with the formative or resting phase. Deposits with fluffy, hazy, ill-defined edges are often seen in patients with acute pain and may correlate with the resorptive phase of calcific tendinitis. Ultrasound is useful in both detection of rotator cuff calcium deposits and therapeutic procedures, and is also beneficial in detecting associated conditions such as rotator cuff tears, subacromial–subdeltoid bursitis, and long head of the biceps pathology. There is a correlation between the ultrasound appearance of the calcified deposit, the clinical symptoms, and the three phases of calcific stage. The combination of ultrasound and color Doppler appearance predicts more accurately formative or resorptive stage. A rare painful complication of calcifying tendinitis is the migration of calcium deposits from tendons into the subacromial–subdeltoid bursa or into the underlying bone at the tendon attachment site.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Calcific tendinitis of the shoulder is a relatively common, painful disease, estimated to occur in 2.5–7.5% of adults. Although more common in the right shoulder, at least a 10–25% incidence of bilaterality has been reported.
It predominantly affects individuals aged between 40 and 60 years, and 57–76.7% of patients are women. Calcific tendinitis is characterized by the presence of calcium salt deposits, primarily hydroxyapatite, in the substance of the rotator cuff tendons. Most calcification occurs in the supraspinatus tendon. Calcification is observed with decreasing frequency in the infraspinatus, teres minor, and subscapularis tendons. More than one tendon may be involved.
The calcific deposit usually is described as being approximately 1–2 cm proximal to the tendon insertion on the greater tuberosity. Calcifying tendinitis may be an incidental finding in 7.5–20% of asymptomatic adults, or it may be the cause of shoulder pain. Symptomatic patients usually present with impingement-type pain in the affected shoulder during overhead activity. Active and passive range of motion is painful and restricted. The pain may seem to be out of proportion to any objective physical findings. The patient may describe difficulty sleeping on the shoulder and trouble falling asleep. Symptoms may last for a few weeks or a few months. The cause of calcifying tendinitis is not known. It is generally agreed that it is not caused by trauma, and it rarely is part of a systemic disease. The pathophysiology of calcifying tendinitis is controversial, and has been attributed to cell-mediated calcification and subsequent spontaneous phagocytic resorption.
Based on the pathogenesis of histic hypoxia, the hypoxic state produces a lack of irrigation of the “critical area” near the insertion of the tendon and induces calcified deposits. It is a self-limited process in which the calcifications tend to resolve after a period of worsening and intense pain. Therefore, many cases may resolve spontaneously and require no special treatment. Thus, it is a dynamic process (Fig. 15.1) that evolves through three distinct stages of the disease process: the precalcific stage , characterized by the asymptomatic change of the tenocytes into chondrocytes, and then fibrocartilage; the calcific stage , which is subdivided into three phases—formation, resting, and resorption; and the postcalcific stage , characterized by an attempt by the tendon to self-heal. The formation phase of calcific stage is characterized by deposition of amorphous calcium phosphate, and can be relatively painless. This phase is followed by the resting phase, which tends to be quiescent and may last for months to years. The resorptive phase of calcific stage tends to be painful, as calcium crystals are resorbed, inducing regional neoangiogenesis, beginning at the margin of the calcium deposit, and infiltration of phagocytes. The postcalcific stage, which can be painless, is characterized by the collagenization of the lesion by fibroblasts.
2 Imaging of Calcifying Tendinitis
The first-line imaging modalities are X-ray and ultrasound, as calcium deposits are readily identifiable on both. The evaluation of calcific tendinitis is based mainly on radiography. It is cost effective and useful, not only for determining the presence of calcium deposits but also for assessing their size, delineation, and density. Standard radiographic evaluation of the shoulder should include internal and external rotation anteroposterior views to help visualize calcific deposits and their relationship to landmarks on the humeral head. External rotation consent to visualize the calcific tendinitis in the supraspinatus tendon profiles the greater tuberosity (Fig. 15.2a). Internal rotation of the humerus profiles the posterior aspect of the head on the lateral aspect of the radiograph and the anterior head medially. Calcification in the infraspinatus tendon profiles posteriorly on internal rotation (Fig. 15.2b). Calcification in the subscapularis profiles anteriorly on internal rotation (Fig. 15.2b). The regions most affected by calcific tendinitis are the critical zone of the supraspinatus tendon (80%), the lower side of the infraspinatus tendon (15%), and the preinsertional part of the subscapularis tendon (5%).
The radiographic appearance of calcific tendinitis is as homogeneous, amorphous densities without trabeculation, which allows for differentiation from enthesopathic spurs or accessory ossicles. Most calcifications are ovoid, and margins may be smooth or ill-defined. Characterizing the shape and contour of the calcific deposit is important to classify the pathology, in order to determine the best possible treatment for the patient. It is important to be able to reliably predict the consistency of the deposit and hereby the stage of the disease by characterizing the radiological image in one of the classification systems in clinical use at present. Several radiological classifications have been proposed, based on the size or morphology, although none of them guarantee sufficient reliability and reproducibility, or reliable correlation with the radiologic picture and clinical symptoms. Gärtner and Heyer proposed a radiographic classification based on the morphological appearance of the calcification, identifying three types (Fig. 15.3a–c): (I) sharply defined and dense, (II) ill-defined/dense or sharply defined/inhomogeneous-less radiodense, and (III) translucent and cloudy appearance with vague border.
Shoulder calcific tendinitis radiograph. (a) Type I calcification is well defined, with dense and homogeneous structure; (b) type II calcifications are depicted from less radiodense calcific deposits, with either sharp or poorly defined border (as in this case), and homogeneous or inhomogeneous structure; (c) type III calcification appearing as hazy, ill-defined globular area, more or less transparent in structure, typically seen in acute symptomatic patients
Calcifications with a well-defined, homogeneous contour are less likely to be symptomatic and may correlate with the formative or resting phase. Deposits with fluffy, hazy, ill-defined edges are often seen in patients with acute pain and may correlate with the resorptive phase of calcific tendinitis. Ultrasound is useful in both detection of rotator cuff calcium deposits and therapeutic procedures, and is also beneficial in pre- and postoperative evaluation. Its diagnostic accuracy has been reported to be similar to that of magnetic resonance imaging. Calcific plaque morphology and increased flow on power Doppler were the most useful ultrasound findings. Ultrasonography could also detect associated conditions such as rotator cuff tears, subacromial–subdeltoid bursitis, and long head of the biceps pathology and allows us to perform a dynamic evaluation to assess the subacromial impingement. All three of the main rotator cuff tendons may be involved although the supraspinatus is the most common site of calcific deposits. Tendon calcifications are visible as echogenic foci usually accompanied by acoustic shadowing. With soft deposits the echogenicity may be more subtle and acoustic shadowing more variable.
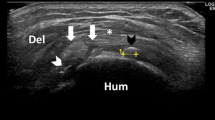
Various classifications were proposed for the calcific plaques based on their location and appearance on ultrasound. Chiou et al. proposed a classification of calcific deposits into four shapes (Fig. 15.4a–d): (1) an arc shape (echogenic arc with clear shadowing); (2) a fragmented or punctate shape (two or more echogenic plaques), with or without shadowing; (3) a nodular shape (cloudy echogenic nodule without shadowing); and (4) a cystic shape (a bold echogenic wall with an anechoic area, weak internal echoes, or layering content).
Shoulder calcific tendinitis ultrasound. (a) Arc-shaped calcification seen as well-defined echogenic arc with deep acoustic shadowing (arrowheads) (“hard” calcification within the supraspinatus); (b) fragmented shape calcification has the appearance of fragmented and punctate echogenic profile (arrows) with or without (as in this case) acoustic shadowing; (c) nodular shape calcification that appears as ill-defined cloud-like echogenic nodule (arrows) without shadowing (“soft” calcification within the supraspinatus); (d) cystic shape calcification (white asterisk) appearing as echogenic wall with weak internal echoes
There is a correlation between the ultrasound appearance of the calcified deposit, the clinical symptoms, and the three phases of histopathological findings of Uhthoff. Besides, there is an association with color Doppler ultrasonography of the rotator cuff and the calcific stage/clinical symptoms. In fact, during the resorptive phase, the deposits are surrounded by phagocytes and there was concomitant neoangiogenesis around the calcification. The combination of ultrasound and color Doppler appearance predicts more accurately formative or resorptive stage.
Severe symptoms are associated with non-arc-shape calcifications, hypervascularity, and widening of subacromial–subdeltoid bursa, suggesting resting or resorptive stage (Fig. 15.5). Identifying the resorptive phase is important for management as these deposits are nearly liquid and can be successfully aspirated. MRI is now not recommended as a first-line imaging modality, because deposits appear hypointense in all sequences, and can be missed, even though the development of new MR sequence such as susceptibility-weighted imaging (SWI) seemed to overcome this problem.
3 Complications: Subacromial Bursitis—Bone Involvement
A rare painful complication of calcifying tendinitis is the migration of calcium deposits from tendons, usually the supraspinatus, into the subacromial–subdeltoid bursa or into the underlying bone at the tendon attachment site (Fig. 15.6). The pathomechanism is still unknown, but seems to occur in the resorptive phase of the disease and seems to be mediated by aggressive inflammatory reaction and hyperemia at the tendon insertion and by rise of the intratendinous pressure. This can lead to secondary impingement resulting from the increased tendon size, and to rupture of the deposits into the subacromial space or into the bursa. Rarely, calcific tendinopathy eventually causes focal resorption of adjacent cortical bone, and intraosseous migration of calcic material might occur. These complications lead to severe shoulder pain and functional disability.
Calcium deposit migration into the sub-bursal space or into the subacromial–subdeltoid bursa appears on radiograph as ill-defined calcifications in the subacromial space (Fig. 15.7a and b). Often it is not possible to assess their exact location, whether intratendinous, sub-bursal, or intrabursal.
Shoulder extratendinous calcification migration. (a) Shoulder X-ray showing linear calcified deposit (arrows), which surrounds the profile of the humeral trochlea, indicating the location between the tendon and the bursa; (b) shoulder externally rotated X-ray clearly showing supraspinatus calcific tendinitis, and migration of calcific deposits into the subacromial–subdeltoid bursa (arrowheads)
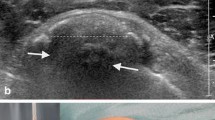
Ultrasound examination can visualize their exact location. Della Valle reported that in cases of intrabursal penetration of the calcification, at sonography and MRI examinations, the subacromial–subdeltoid bursa presents thickened walls and appears filled with inhomogeneous fluid containing calcium and debris (Fig. 15.8).
If the calcific deposit has migrated into the sub-insertional bone, standard radiographs show focal erosions of the humeral head and a rounded sclerotic intraosseous lesion in the greater tuberosity (Fig. 15.9), which could be mistaken for malignancy or infection.
Ultrasound depicts intratendinous hyperechoic focal amorphous calcification adjacent to focal bone erosions of the greater tuberosity and intraosseous calcification migration (Fig. 15.10).
Shoulder extratendinous calcification migration. Ultrasonography in the long axis of the supraspinatus tendon showing intratendinous calcification (arrows) associated with focal bone erosion and intraosseous calcification migration (arrowheads). Sonogram shows anechoic fluid within enlarged subacromial–subdeltoid bursa (white asterisk)
MRI and CT are considered the best methods to demonstrate the involvement of bone marrow in calcific tendinopathy. CT is the gold standard imaging modality to depict cortical erosion of the humeral head and a rounded well-defined lytic area located in the greater tuberosity (Fig. 15.11); it can also detect calcium deposit in its intraosseous location. MRI shows a cystic lesion in the greater tuberosity and humeral osteitis related to typical reactive bone marrow edema surrounding the lytic lesion (Fig. 15.12).
Shoulder extratendinous calcification migration. Coronal T2-weighted sequence shows low signal intensity of the ovoid lesion in the greater tuberosity in keeping with sclerosis (arrowheads). There is a superficial focus of fluid signal (white arrow) traversing the region of cortical erosion. Ill-defined hyperintensity consistent with marrow edema (black arrows) surrounds the lesion
Further Readings
Bosworth BM. Calcium deposits in shoulder and subacromial bursitis: survey of 12,122 shoulders. JAMA. 1941;116:2477–82.
Chianca V, Albano D, Messina C, Midiri F, Mauri G, Aliprandi A, et al. Rotator cuff calcific tendinopathy: from diagnosis to treatment. Acta Biomed. 2018;89(1-S):186–96.
Chiou HJ, Chou YH, Wu JJ, Hsu CC, Huang DY, Chang CY. Evaluation of calcific tendonitis of the rotator cuff: role of color Doppler ultrasonography. J Ultrasound Med. 2002;21(3):289–95.
Codman EA. The Shoulder. 3rd ed. Boston: Thomas Todd; 1934.
Della Valle V, Bassi EM, Calliada F. Migration of calcium deposits into subacromial–subdeltoid bursa and into humeral head as a rare complication of calcifying tendinitis: sonography and imaging. J Ultrasound. 2015;18:259–63.
De Palma AF, Kruper JS. Long-term study of shoulder joints afflicted with and treated for calcific tendinitis. Clin Orthop. 1961;20:61–72.
Flemming DJ, Murphey MD, Shekitka KM, et al. Osseous involvement in calcific tendinitis: a retrospective review of 50 cases. AJR Am J Roentgenol. 2003;181:965–72.
Gärtner J, Heyer A. Calcific tendinitis of the shoulder [in German]. Orthopade. 1995;24(3):284–302.
Hamada J, Ono W, Tamai K, Saotome K, Hoshino T. Analysis of calcium deposits in calcific periarthritis. J Rheumatol. 2001;28:809–13.
Le Goff B, Berthelot JM, Guillot P, Glèmarec J, Maugars Y. Assessment of calcific tendonitis of rotator cuff by ultrasonography: comparison between symptomatic and asymptomatic shoulders. Joint Bone Spine. 2010;77(3):258–63.
Molè D, Kempf JF, Gleyze P, et al. Results of endoscopic treatment of non-broken tendinopathies of the rotator cuff. Calcifications of the rotator cuff. Rev Chir Orthop. 1993;79:532–41.
Moseley HF, Goldie I. The arterial pattern of the rotator cuff of the shoulder. J Bone Joint Surg Br. 1963;45(4):780–9.
Nogueira-Barbosa MH, Gregio-Junior E, Lorenzato MM. Retrospective study of sonographic findings in bone involvement associated with rotator cuff calcific tendinopathy: preliminary results of a case series. Radiol Bras. 2015;48(6):353–7.
Nörenberg D, Ebersberger HU, Walter T, Ockert B, Knobloch G, Diederichs G, Hamm B, Makowski MR. Calcific tendonitis of the rotator cuff: susceptibility-weighted MR imaging. Radiology. 2016;278(2):475–84.
Patte D, Goutallier D. Periarthritis of the shoulder. Calcifications. Rev Chir Orthop. 1988;74:277–8.
Porcellini G, Paladini P, Campi F, Paganelli M. Arthroscopic treatment of calcifying tendonitis of the shoulder: clinical and ultrasonographic findings at two to five years. J Shoulder Elb Surg. 2004;13:503–8.
Speed CA, Hazelman BL. Calcific tendinitis of the shoulder. N Engl J Med. 1999;340:1582–4.
Uhthoff HK, Sarkar K, Maynard JA. Calcifying tendinitis: a new concept of its pathogenesis. Clin Orthop Relat Res. 1976;118:164–8.
Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis, and management. J Am Acad Orthop Surg. 1997;5(4):183–91.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Martino, G., Silvestri, E., Orlandi, D., Muda, A., Martino, F. (2022). Shoulder Calcific Tendinopathy. In: Martino, F., Silvestri, E., Orlandi, D. (eds) Musculoskeletal Ultrasound in Orthopedic and Rheumatic disease in Adults. Springer, Cham. https://doi.org/10.1007/978-3-030-91202-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-91202-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91201-7
Online ISBN: 978-3-030-91202-4
eBook Packages: MedicineMedicine (R0)