Abstract
Series of hydrogels based on polyacrylamide and copolymer dextran-graft-polyacrylamide with various cross-linking density and various molecular weight of dextran component were prepared using N,N′-methylene-bis-acrylamide as cross-linking agent. The comparative equilibrium swelling capacity of synthesized hydrogels was studied. The swelling behavior was analyzed as a function of the polymeric composition, cross-linking degree and hydrolysis degree, ionic strength. It was found that cross-linking degree and molecular weight of polysaccharide component determines the internal structure (mesh size) of hydrogels and affects their swelling capacity. A significant increase in the swelling parameters of hydrogels was achieved due to the hydrolysis of amide groups of the polyacrylamide component of hydrogels. It was registered the high sensitivity of swelling capacity of hydrogels towards ionic strength of the external solution. The absorption and desorption of methylene blue in non-hydrolyzed hydrogels were carried out. Hydrogels were characterized by FTIR and SEM methods.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Hydrogels are three-dimensional cross-linked hydrophilic polymers that are able to swell in an aqueous environment or biological fluids. Their capacity to absorb and retain water is caused by the presence of hydrophilic groups such as –OH, –COOH, –CONH2, and –SO3H in polymer structure forming hydrogel [1]. Because of their high water-absorbing ability (sometimes more than 90 wt%) hydrogels show a unique swelling behavior in an aqueous environment and high permeability due to the presence of chemical cross-links in the hydrogels structure. Besides of covalent chemical bonds the three-dimensional network of a hydrogel can be stabilized by physical (ionic bonds, entanglements, crystallites, charge complexes, hydrogen bonding, van der Waals or hydrophobic interactions) cross-links [2, 3]. The presence of numerous functional groups along the polymer chains leads to strong depending on hydrogels properties on external environmental conditions like temperature, pH, ionic strength, etc. Due to this peculiarity, hydrogels are named as “intelligent” materials which are suitable for the design of regulated systems. Hydrogels are especially promising for bioanalytical applications because of their high water content and elastic nature similar to natural tissue [4, 5].
In hydrogel synthesis, different methods of cross-linking have been used. Stable chemically cross-linked gels can be produced by radical polymerization, chemical interaction between functional groups, high-energy irradiation and by using biological active molecules [6].
In the last few years, a lot of studies have been carried out on biomaterials based on proteins and polysaccharides. Many polymeric networks based on dextran, konjac glucomannan, guar gum with acrylic acid have been prepared [1, 7, 8]. Our previous researches [9] have shown that star-like dextran-graft-polyacrylamide copolymers (D-g-PAA) have advantages in comparison with linear polyacrylamide (PAA) because of the possibility to regulate their internal structure during the synthesis process. In water solution, dextran has the coil conformation of various sizes, which depends on dextran molecular weight. Grafting of PAA chains onto dextran coils allows realizing the certain hydrogel structures. In the current study, covalently cross-linked hydrogels PAA and D-PAA were prepared by varying the amount of cross-linking agent and dextran molecular weight.
In the present work, we focused on the study of swelling behavior of prepared hydrogels as a potential material for biomedical application. The absorption and desorption of methylene blue in hydrogels was studied as a model of drug delivery systems.
2 Experimental
2.1 Materials
Acrylamide (AA) obtained from Aldrich was twice recrystallized from chloroform and dried under vacuum at room temperature for 24 h. Cerium (IV) ammonium nitrate (CAN), N,N′-methylene-bis-acrylamide (MBA), methylene blue and potassium hydroxide were purchased from Aldrich, without additional purification. Dextran (D) and dextran sulfate sodium salt (DSNa) were purchased from Fluka, the characteristics given by the manufacturer are Mw = 2 × 104, 1 × 105, 5 × 105 g/mol (designated as D20, D100, D500 and DSNa500 throughout). Distilled water was used throughout the experiments and as polymerization medium during hydrogel synthesis.
2.2 Hydrogel Preparation
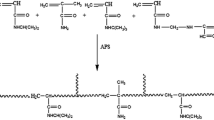
Hydrogels base on cross-linked dextran-graft-polyacrylamides (D-PAA-x) were prepared by free radical polymerization according to the procedure reported earlier [10] (Fig. 1a). The required amount of dextran (0.0005 mM) was dissolved in 25 mL of distilled water at ambient temperature (25 °C). This solution was purged by argon bubbling for 25 min and ammonium-cerium (IV) nitrate (0.01644 g, 0.03 mmol mL−1) was added as an initiator of reaction. In 2 min acrylamide (0.05 mol) and N,N′-methylene-bis-acrylamide (0.2–0.6%, w/monomer) were added. Thereafter, argon was passed for 2 min and the reaction mixture was left overnight. The formed hydrogel samples were taken out from the beaker, washed with distilled water and placed in a 400 mL beaker containing 200 mL distilled water (refilled fresh water for every 12 h for two days) to remove unreacted monomer from the hydrogel. Finally, the gels were dried at ambient temperature.
Synthesis of cross-linked hydrogels: a Dextran-graft-polyacrylamides (D-PAA-x); b polyacrylamides (PAA-x); c dextran-graft-(polyacrylamide-co-polyacrylic acid) (D-PAA-x-y), where D = D20, D100, D500 and DSNa500; x = 0.2, 0.4 or 0.6 corresponding to MBA concentration; y = 0, 3, 6 or 10, corresponding to hydrolysis time
Hydrogels base on cross-linked polyacrylamides (PAA-x) were prepared by the same method but without the addition of dextran (Fig. 1b).
Alkaline hydrolysis of synthesized cross-linked hydrogels samples was carried out as follows: 3.6 g of samples of cross-linked hydrogels were dissolved in 200 ml of distilled water. Swollen samples were placed into 20 ml 5M KOH at 58 °C. In 3; 6 and 10 min the hydrolyzed samples were obtained in the salt form. They were immersed in distilled water at room temperature for 48 h and the water was renewed several times in order to remove NaOH. As a result, the polyelectrolytes dextran-graft-(polyacrylamide-co-polyacrylic acid) hydrogels were obtained (Fig. 1c). All samples were dried and kept under vacuum.
Hydrolyzed samples with various cross-linking density and various dextran components were marked as D-PAA-x-y, were D = D20, D100, D500 and DSNa500; x = 0.2, 0.4 or 0.6 corresponding to MBA concentration; y = 0, 3, 6 or 10 corresponding to hydrolysis time.
2.3 Swelling Studies
The water absorption of initially dried hydrogels was measured gravimetrically. Dry sample of hydrogel, previously washed and weighed, was placed in distilled water at 25 °C. The sample was removed from solution at certain time intervals and was superficially dried with tissue paper, weighed on an analytic balance and placed back into the bath. The measurements were repeated until a constant weight was achieved. The absorbed water content was calculated in term of the swelling ratio (S%) using (1):
where mt—the mass of hydrogel in swollen state at time t and m0—the mass of the dry hydrogel. The dependences of swelling ratio S% versus time were analyzed. Swelling ratio in equilibrium state (Seq%) represents the maximum capacity of the hydrogel to absorb solution and corresponds to plateau of kinetic curve.
Three solutions of NaCl (pH = 7.4) with different ionic strength (10−2, 10−1, 1 M) were used to estimate the salt effect on the swelling of hydrogel.
2.4 Characterization of Hydrogel Composites
The Fourier transform infrared (FTIR) spectra of non-hydrolyzed and hydrolyzed D-PAA samples were recorded with an FTIR-Spectrophotometer (MAGNA 550, Nicolet Instruments Corporation, USA) using KBr pellet.
The morphology of hydrogel membrane was observed by SEM mod. Stereoscan 440 (LEO), Cambridge, UK instrument. The cryogenically fractured film in liquid nitrogen was mounted vertically on the SEM stub by silver adhesive paste. The specimens were sputter-coated with gold to avoid electrostatic charges and to improve image resolution before being examined by the electron microscopy.
UV–visible spectra of hydrogels were recorded by using a Lambda 35 UV-Vis spectrophotometer (Perkin-Elmer, CA) in the absorbance mode (range 200–1000 nm).
2.5 Absorption and Desorption of Low Molecular Weight Compound Studies
Absorption and desorption of methylene blue were studied using non-hydrolyzed hydrogels based on PAA and D20-PAA. Dried samples of PAA-x-0 and D20-PAA-x-0 (where x = 0.2; 0.4; 0.6) were placed in 7.8 × 10−6 M dye solution up to reaching an equilibrium state. The optical density of saturated hydrogels at wavelength 670 nm was measured. Desorption of methylene blue from the hydrogels was investigated by keeping off the dye-saturated samples in distilled water until equilibrium state. For determination of methylene blue concentration in hydrogel or solutions, the calibration curve within the concentration range of 10−5–10−6 M was used. The dye concentration changes in hydrogels at absorption and desorption were comprised regard to 1.56 × 10−6 M methylene blue solution.
To calculate the rate of the dye release, the first derivative curve of the concentration versus time was calculated. The curves are described by (2):
where y = optical absorbance, D; x—time.
An obtained curve was linearized in logarithmic time (ln t) and optical absorption (ln D) coordinates with subsequent adjustment according to linear law (r > 0.98). The curve slope corresponds to the rate of function increase or its first derivative. Desorption curves of methylene blue from hydrogels linearized at coordinates (−ln t) and (−ln D). The mathematical data processing was performed using OriginLab 8.0.
3 Results and Discussion
3.1 Hydrogels Synthesis and Characterization
Hydrogels as inert matrices with three-dimensional structure were synthesized. Varying the concentration of the cross-linking agent the polymeric networks with different free space between polymer chains were obtained. The porosity affords an opportunity for the rapid diffusion of water molecules into and out of the polymers and this property may be important for antibacterial applications. Therefore, the mesh size of hydrogels appears to be a key factor for transporting of bioactive molecules such as antibiotics or other pharmaceuticals to the destination.
For modification of hydrogel network, polyacrylamide chains were grafted on dextran macromolecules of different molecular weight. It leads to a more compact structure polymer network than in the case of linear polyacrylamide. As was shown in our previous studies [11] depending on the size of dextran coils in solution the branched copolymers with different macromolecular architecture may be formed. The conformation of grafts depends on the distance between grafts and can be varied from worm-like to mushroom. The conformation of grafted PAA chains determined the internal structure of hydrogels.
We have shown [12], that after grafting on dextran with Mw = 2 × 104 g/mol mainly the worm-like structure of grafted PAA chains is formed, whereas grafting on dextran with Mw = 1 × 105 g/mol predominantly causes the formation a mushroom structure at the same PAA concentration.
The synthesized hydrogel samples are represented in Table 1.
For higher water diffusion in hydrogels, PAA was converted into polyelectrolyte. In the present study, the synthesized hydrogels were subjected to alkaline hydrolysis, and samples with different degrees of hydrolysis were obtained depending on the time of the reaction.
For identification and characterization of the PAA-x-y and D-PAA-x-y hydrogels, FTIR spectroscopy and SEM micrograph were used. In the FTIR spectra of the non-hydrolyzed hydrogels PAA-0.4-0 and D20-PAA-0.4-0 (Fig. 2, black and red curves), the peaks between at 1657 and 1611 cm−1 can be attributed to-carbonyl valence stretching in carboxamide functional groups and NH deformation stretching, respectively [13]. In the spectrum of hydrolyzed hydrogel D20-PAA-0.4-10 (Fig. 2, green curve), the intense characteristics and at 1550 cm−1 is due to –C=O asymmetric stretching in carboxylate anion that is reconfirmed by another sharp peak at 1420 cm−1 which is related to the symmetric stretching mode of the carboxylate anion. This confirmed the conversion of amide groups (–CONH2) to the carboxylate functional groups (–COO−).
The SEM micrographs of hydrogel networks of linear PAA and PAA grafted on dextran macromolecules are shown in Fig. 3. The hydrogels of modified PAA by dextran show a more porous surface (Fig. 3). It confirms the possibility to affect the internal structure of hydrogels by means of grafting of PAA on dextran.
3.2 Swelling Studies
3.2.1 Effect of the Cross-Linking Density on the Swelling Capacity
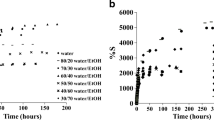
Swelling behaviors of cross-linked hydrogels at different cross-linking density are shown in Fig. 4a (Table 1). As seen in Fig. 4a, the swelling values initially increase rapidly, and then the increase slows down and finally levels off to a constant value. Figure 4b and Table 1 illustrate the effect of concentration of cross-linking agent on the equilibrium swelling percentage (Seq%) while keeping D/PAA composition constant. It was observed in Fig. 4a,b that the swelling of gels decreased both at linear PAA-x-0 and branched D-PAA-x-0 polymers as the cross-linking agent N,N′-methylene-bis-acrylamide concentration increases. This decrease in equilibrium swelling percentage with the increasing concentration of MBA in the gel is mainly caused by the growing cross-linked density of polymer chains. So the swelling of hydrogels is decreased with increase in cross-linking ratio due to tighter hydrogel structure.
3.2.2 Effect of the Dextran Component on the Swelling Capacity
The effect of the dextran component on the swelling capacity of hydrogels was studied. Samples of branched polymers based on polysaccharide dextran with different molecular weight were used for swelling investigation in distilled water. In our previous works [14], it was shown, that internal macromolecular structure of grafted polymers strongly depends on the size of polysaccharide and after cross-linking leads to the formation of more compact polymer network. In this case, the obtained networks have higher porosity and ability to swell a larger amount of water. Swelling ratios in equilibrium state (Seq%) of cross-linked polymers based on PAA, D20-PAA, D100-PAA, D500-PAA, DSNa500-PAA are presented in Fig. 5. As seen in Fig. 5 and general Table 1, an increase of molecular weight of dextran does not directly affect the swelling behavior. Obviously, it can be explained by different internal mesh structure formed at certain size of macromolecular of dextran.
While the data obtained shows that all hydrogels containing dextran have higher swelling parameters caused by their improved porosity than the hydrogel which based only on linear polyacrylamide. In addition, the hydrophilic nature of these gels may be enhanced due to the presence of free –OH groups of dextran. And moreover, the dextran sulfate, of cause, has an excellent absorption property as polyelectrolyte.
The equilibrium swelling percentage (Seq%) of PAA-x-0 and D-PAA-x-0 hydrogels, as expected, decreases with increasing of cross-linking density.
3.2.3 Effect of the Hydrolysis Degrees on the Swelling Capacity
To achieve hydrogels with high swelling capacity, cross-linked PAA-x-0 and D-PAA-x-0 were saponified. During saponification, the amide groups convert to carboxylate groups.
As shown in Fig. 6, swelling of the hydrogels increased by increasing the time of hydrolysis from 3 to 10 min. The increase of water absorbency after hydrolysis is attributed to an increase of carboxyl groups in polymer matrices. Wu et al. have reported a similar conclusion in the hydrolysis of starch-g-PAAm [15]. It was demonstrated that with increasing time of hydrolysis, the concentration of carboxyl groups is increased. In the presence of alkaline, amide groups are attacked by hydroxyl groups of alkali convert them into carboxyl groups. The transformation of polymers polyacrylamide (PAA-x-0) and dextran-graft-polyacrylamide (D-PAA-x-0) into polyelectrolytes polyacrylamide-co-polyacrylic acid (PAA-x-y) and dextran-graft-(polyacrylamide-co-polyacrylicacid) (D-PAA-x-y) causes the high swelling capacity (Table 1 and Fig. 7). When the time of hydrolysis increases to more than 10 min, the water absorbency almost does not increase.
3.2.4 Effect of the Ionic Strength on the Swelling Capacity
The effect of the ionic strength on the swelling capacity is shown on Fig. 8. It shows that an increase in the ionic strength within the range of 0.01–1 M yields leads to a significant decrease in the swelling ratio of hydrogels. For both non-hydrolyzed (y = 0) cross-linked polymers PAA-0.4-0 and D20-PAA-0.4-0 the effect of the ionic strength on swelling capacity is negligible. At the same time, the high sensitivity of swelling of the anionic PAA-0.4-10 and D20-PAA-0.4-10 towards ionic strength is attributed to the change in the charge distribution on the surface of the gel network. As the concentration of Na+ ions in the swelling medium increased, a stronger “charge screening effect” of the additional cations is achieved and anion-anion electrostatic repulsion is appeared [16, 17]. Caused by a decreased osmotic pressure difference between the polymer network and the external solution, the swelling of hydrolyzed hydrogels strongly decreased in 1 M NaCl solution.
Generally, the swelling capacity of hydrogels in the salt solution is decreased compared to the values in distilled water. This peculiarity is important for understanding of swelling behavior of hydrogels in physiological solutions or liquids which imitate the plasma blood.
When the swollen hydrogel is put in the salt solution, the presence of Na+ ions in the outer medium causes a decrease in osmotic swelling pressure and equilibrium water absorption of the hydrogel sample. Therefore, the swelling is drastically reduced.
3.3 Absorption and Desorption of Methylene Blue
Absorption and desorption of methylene blue by non-hydrolyzed hydrogels was studied. The concentration of methylene blue in hydrogels is inversely proportional to the concentration of the dye in solution. On Fig. 9a optical spectra of the remainder quantity of methylene blue on the solution during its absorption are presented. It was found that dye absorbance values in hydrogels depend on their cross-linking density that correlates with swelling behavior (Fig. 4a). Absorption rate for D20-PAA-x-0 at x = 0.2, 0.4 and 0.6 was 0.1012, 0.0762, 0.0693 min−1, correspondingly. For PAA-x-0 hydrogels, the rate is increased: for x = 0.2, 0.4 and 0.6 the absorption rates were 0.1232, 0.1054 and 0.0932 min−1 correspondingly. These differences may be caused by interactions of the dye with dextran. Due to the positive charge of the methylene blue molecule, dye can form anionic bonds with hydroxyl groups of polysaccharides.
The diffusion rate of methylene blue from the PAA-x-0 and D20-PAA-x-0 with different cross-linking density is presented in Fig. 9b. An equilibrium state after 150 min for all hydrogel samples saturated with 7.8 × 10−6 M dye was observed. The increase of the desorption rate caused by the decrease of hydrogel density. For PAA-x-0 hydrogels for x = 0.2, 0.4 and 0.6 desorption rate were 0.4232, 0.3976, 0.3624 min−1 correspondingly. For D20-PAA-x-0 hydrogels the calculated desorption rates were lower: for x = 0.2, 0.4 and 0.6 desorption rates were 0.3956, 0.3518 and 0.3255 min−1 correspondingly. Thus, hydrogels based on branched copolymer provide a more prolonged release and action of the drug.
4 Conclusion
The chemically cross-linked PAA and D-PAA hydrogels were prepared by radical polymerization using dextrans of different molecular weight and various amount of cross-linking agent. For higher water diffusion in hydrogels, PAA chains of hydrogels were converted into polyelectrolyte. It was demonstrated that the internal structure of hydrogels can be regulated by means of grafting of PAA on dextran.
The effect of the cross-linking density, dextran component, hydrolysis degree and ionic strength on the swelling capacity of hydrogels was analyzed. It was found that with increasing cross-linking density of hydrogels the swelling capacity decreases. The dynamic and equilibrium swelling ratio of the hydrogels are directly proportional to hydrolysis degree and are inverse to the ionic strength. All hydrogels containing dextran have higher swelling parameters caused by higher porosity than the hydrogel which based on linear polyacrylamide. It was demonstrated, that hydrogels based on branched copolymers dextran-graft-polyacrylamide can be used due to its potential swelling capacity as a promising candidate for developing materials for medical application. Investigation of absorption and desorption of methylene blue by hydrogels confirms the potential their application as drug delivery system.
References
Rodigues IR, Forte MMC, Azambuja DS, Castagno KRL (2007) Synthesis and characterization of hybrid polymeric networks (HPN) based on poly (vinyl alcohol)/chitosan. React Funct Polym 67:708–715
Cui J, Campo A (2012) Multivalent H-bonds for self-healing hydrogels. Chem Commun 48:9302–9304
Picchioni F, Muljana H (2018) Hydrogels based on dynamic covalent and non covalent bonds: a chemistry perspective. Gels 4:21–31
Peppas NA, Hilt ZJ, Khademhosseini A, Langer R (2006) Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater 18:1345–1360
Allan SH (2002) Hydrogels for biomedical applications. Adv Drug Deliver Rev 43:3–12
Mathur AM, Moorjani ShK, Scranton AB (1996) Methods for synthesis of hydrogel networks: a review. J Macromol Sci C 36(2):405–430
Cascone MG, Barbani N, Cristallini C, Giusti P, Ciardelli G, Lazzeri L (2001) Bioartificial polymeric materials based on polysaccharides. J Biomater Sci Polym Edn 12(3):267–281
Kim IS, Oh IJ (2005) Drug release from the enzyme-degradable and pH-sensitive hydrogel composed of glycidyl methacrylate dextran and poly(acrylic acid). Arch Pharmacal Res 28:983–988
Kutsevol N, Chumachenko V, Rawiso M, Shyichuk A (2016) Micro Nano Lett 11(5):256–260
Nadtoka O, Kutsevol N, Krysa V, Krysa B (2018) Hybrid polyacryamide hydrogels: synthesis, properties and prospects of application. Mol Cryst Liquid Cryst 672(1):1–10
Kutsevol N, Bezugla T, Bezuglyi M, Rawiso M (2012) Branched dextran-graft-polyacrylamide copolymers as perspective materials for nanotechnology. Macromol Symp 317–318:82–90
Kutsevol N, Guenet JM, Melnyk N, Sarazin D, Rochas C (2006) Solution properties of dextran-polyacrylamide graft copolymers. Polymer 47:2061–2068
Silverstein RM, Webster FX (1998) Spectrometric identification of organic compounds, 6th edn. Wiley, NewYork
Kutsevol NV, Chumachenko VA, RawisoM Shkodich VF, Stoyanov OV (2015) Star-like polymers dextran-polyacrylamide: the prospects of application for nanotechnology. J Struct Chem 56(5):1016–1023
Wu J, Wei Y, Lin J, Lin S (2003) Study on starchg-acrylamide/mineral powder superabsorbent composite. Polymer 44:6513–6520
Zhao Y, Su H, Fang L, Tan T (2005) Superabsorbent hydrogels from poly(asparticacid) with salt, temperature and pH-responsiveness properties. Polymer 46:5368–5376
Vishal Gupta N, Shivakumar HG (2012) Investigation of swelling behavior and mechanical properties of a pH-sensitive superporous hydrogel composite. Iran J Pharm Res 11(2):481–493
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Nadtoka, O., Virych, P., Kutsevol, N. (2020). Investigation of Swelling Behavior of PAA and D-PAA Hydrogels. In: Fesenko, O., Yatsenko, L. (eds) Nanooptics and Photonics, Nanochemistry and Nanobiotechnology, and Their Applications . Springer Proceedings in Physics, vol 247. Springer, Cham. https://doi.org/10.1007/978-3-030-52268-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-52268-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-52267-4
Online ISBN: 978-3-030-52268-1
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)