Abstract
The behavior of a Si3N4 + 4 Zr reaction mixture during heat treatment in vacuum in the temperature range 750–1450 °C and subsequent nitriding of the product of vacuum treatment at temperatures 1000–1200 °C has been studied. It has been established by the XRD method that the dissociation of Si3N4 begins at 750 °C and is accompanied by the formation of zirconium nitrides, and silicon diffusion at 1000 °C is accompanied by the formation of the Zr2Si and ZrSi lower silicide phases. The nitriding process of the highly active product of vacuum treatment (precursor), aimed at obtaining a Si3N4–ZrN composite material, has been investigated. It has been established that the optimal regime of synthesis of the Si3N4–ZrN composite material in a single technological cycle is a heat treatment in vacuum at 1000 °C (stage 1) and nitriding at 1200 °C for 1 h (stage 2). The finished product contains only nitride phases of zirconium and silicon. In the established synthesis regime, composite powders have been obtained in the wide concentration range 10–40 vol.% ZrN. The synthesis product is a disperse powder of the composite material in the form of agglomerates, the particle size of which after dispersion lies in the range 0.2–3.0 μm. The spark plasma sintering has made it possible to obtain specimens with a relative density above 97% at a temperature of 1750 °C. This is connected with the formation of a continuous conductive three-dimensional network of ZrN particles.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Solid-state synthesis
- Nitride ceramics
- Composite powders
- Vacuum
- Nitrogen
- Consolidation
- Spark plasma sintering method
1 Introduction
The complex of the physicomechanical and chemical properties of porousless nanocomposites with a ceramic matrix based on Si3N4 and reinforced by nanosized particles of carbides and nitrides of transition metals makes them promising candidates for the development of next-generation ceramic bearings and seals intended for operation under conditions of severe friction. For instance, the introduction of zirconium nitride into the silicon nitride matrix makes it possible to enhance the corrosion resistance due to the formation of insoluble oxide layers, which provides the self-healing effect of the material. During contact in an aqueous medium, a gel that has a larger volume and protects grains boundaries against subsequent corrosion forms [1].
The low chemical activity of these ceramic materials, high oxidation resistance, and extremely low porosity of products manufactured from them by the spark plasma sintering (SPS) method guarantee increased operating characteristics of rolling and plain bearings intended for operation under severe conditions in corrosive-erosive media without lubrication.
In the development of new silicon nitride composite powder materials with a uniform distribution of components and a homogeneous character of development of structural transformation, it is reasonable to use precursors containing all elements required for the synthesis of the final composition. In this case, the main requirements for precursors are as follows:
-
the thermodynamic instability at relatively low temperatures as compared with the thermodynamic stability of interaction products;
-
the completeness of the reaction.
Our previous investigations in the development of methods for preparing nanopowders of nitride composite materials [2] and the literature data [3, 4] indicate the prospects of using silicides of transition metals containing all components of their nitriding products as initial components (precursors). However, the use of disilicides as precursors restricts the possibility of preparing nitride composite materials in a wide concentration range [3].
The aim of the present work is to investigate the synthesis of nanodisperse composite powders of the Si3N4–ZrN system in a wide concentration range and their consolidation.
2 Experimental Technique
During phase transformations in the process of decomposition of complex substances, high-activity ingredients, which are initial components in the synthesis of new compounds, form [4]. The interaction of a transition metal with silicon nitride in vacuum is an example of such a process. The metal accelerates the dissociation process of silicon nitride at temperatures much lower than the decomposition temperature of pure Si3N4 in vacuum. Due to the decomposition of Si3N4 into high-activity silicon and nitrogen, in the process of their interaction with the metal in vacuum, the possibility of obtaining a disperse product, namely, metal nitride or silicide, arises [5].
Zirconium and β-Si3N4 powders were used as initial materials. The commercial zirconium powder (PTsE grade) had a mean particle size of 20 µm. The β-Si3N4 powder synthesized under laboratory conditions had a specific surface of 3.8 m2/g and nitrogen content of 38.0 mass%. Reaction mixtures were calculated according to the following reaction:
To provide a uniform distribution of elements, the components were mixed in a Pulverizette–6 planetary mill (FRITSCH GmbH, Germany) in ethanol in a drum with silicon nitride grinding balls. During mixing, the particle size did not change. Homogenized reaction mixtures were heat-treated in an SNV–1.3,1/20–I1 electric vacuum furnace in a vacuum of ~1 × 10−3 Pa in the temperature range 750–1450 °C and in a nitrogen atmosphere at temperatures of 1000–1200 °C.
The products of the solid-state interaction in a vacuum and a nitrogen atmosphere were investigated by the XRD method on a DRON-3 diffractometer in filtered Cu-Kα radiation. The nitrogen and zirconium contents in the final product were determined by the chemical analysis method according to standard techniques (GOST 27417-87). The evaluation of the particle size of the synthesized powder was performed in a CILAS 990 laser particle size analyzer. Microstructural studies were carried out with a Superprobe-733 scanning electron microscope.
The consolidation of the obtained Si3N4–ZrN composite powders was performed by the spark plasma sintering method using an HD25 unit (FCT Systeme GmbH, Germany) (maximal temperature of 2400 °C, maximal pressing pressure of 250 kN, maximal current of 8000 A, maximal voltage of 10 V, media are a vacuum of 5 × 10−2 mbar and nitrogen). Powder mixtures in amounts of about 8 g were loaded into a graphite die with two graphite punches. The temperature on the internal surface of the upper graphite punch was measured with a digital pyrometer.
3 Results and Discussion
The investigation of the behavior of the β-Si3N4 + 4Zr reaction mixture in a vacuum under the action of the temperature factor (Table 1) showed that already at a temperature of 750 °C, solid-state interaction occurs. Along with the β-Si3N4 phase, in the products of heat treatment at a temperature of 750 °C, the α-ZrN0.28 solid solution and ZrN phase, formed by the mechanism of contact diffusion, were recorded. This is connected with the self-diffusion rate of nitrogen in β-Si3N4 (Di = 6.8 × 102exp(–777.5/RT) m2/s). The solubility of nitrogen at this temperature in α-Zr is equal to 4.8 mass% [6].
An increase in the treatment temperature up to 1000 °C leads to the formation of nitride (α-Zr2N and ZrN) and silicide (Zr5Si3, Zr2Si, and ZrSi) phases of zirconium. Moreover, free silicon and the α-Si3N4 phase are present. The product of solid-state interaction at a temperature of 1450 °C is a mixture of ZrN, ZrSi2, and a small amount of β-Si3N4. At a temperature of 1450 °C, the complete dissociation of silicon nitride occurs, and the main phases are ZrN and lower zirconium silicides. According to the chemical analysis data, the nitrogen content in the interaction products remains practically unchanged and is nearly equal to its calculated content in the (Si3N4 + 4Zr) initial reaction mixture (9.8 mass%). This indicates that practically the whole amount of nitrogen took part in the solid-state interaction.
Thus, as a result of the dissociation of Si3N4, the solid-state interaction in vacuum occurs by the mechanism of reaction diffusion on interfaces of zirconium and silicon nitride particles. This suggests that the formation of the composite material occurs on particles of initial zirconium. The diffusion of nitrogen into zirconium at 750 °C is accompanied by the formation of zirconium nitrides, and the diffusion of silicon at 1000 °C is accompanied by the formation of the lower silicide phases Zr2Si, ZrSi, and Zr5Si3. This testifies the reasonability of using the high-activity product of vacuum solid-state interaction at 1000 °C as a precursor for subsequent nitriding. The realization of these operations in a single technological process is economically sound.
The process of subsequent nitriding in a single cycle of the product of preliminary vacuum heat treatment of the β-Si3N4 + 4Zr mixture was investigated in the temperature range 1000–1200 °C for 1 h. Characteristics of the final product according to the XRD and chemical analysis data are presented in Table 2.
The analysis of the presented data indicates that, at a nitriding temperature of 1200 °C, the phase composition of the product is ZrN and β-Si3N4.
Thus, the optimal synthesis regime of the Si3N4–ZrN composite material in a single cycle is vacuum treatment at 1000 °C (stage 1) and nitriding at 1200 °C for 1 h (stage 2). The final product contains only nitride phases of zirconium and silicon. The nitrogen content in the product is equal to its content calculated by reaction (1).
The possibility of obtaining a Si3N4–ZrN composite material in a wide concentration range in the established optimal synthesis regime was investigated.
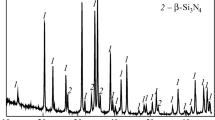
In Fig. 1, X-ray diffraction patterns of the products of the two-stage synthesis of reaction mixtures of different composition are shown. The XRD data analysis indicates that, for all investigated compositions, a two-phase product, in which the ZrN content ranged from 11 up to 33 vol.%, was obtained. It should be noted that in the X-ray diffraction patterns of the mixtures with a ZrN content of 11 and 22 vol.%, traces of ZrSi2 are also present.
The chemical analysis data of the synthesis products (Table 3) in the established regime show that the combination of heat treatment at 1000 °C in vacuum with subsequent nitriding at 1200 °C in a single technological process makes it possible to obtain powders of the composite materials, the nitrogen contents in which are maximally close to the theoretical ones.
According to the data of evaluation of the particle size of the synthesized 78 vol.% Si3N4–22 vol.% ZrN composite powder, the mean size of agglomerates is 23.71 µm (Fig. 2a). After dispersion in isopropyl alcohol, the mean particle size decreased by an order of magnitude down to 2.81 µm (Fig. 2b), and, moreover, the number of submicron and nanosized particles increased.
The analysis of the morphology testifies (Fig. 2d) that the obtained product consists of agglomerates of the Si3N4–ZrN composite powder. A comparison of the morphology of the initial mixture based on β-Si3N4 (Fig. 2c) with the morphology of the synthesized powder indicates that the shape of particles radically changes from the elongated to the rounded one.
Thus, it is established that the process of vacuum treatment of the Si3N4 + Zr reaction mixtures at a temperature of 1000 °C makes it possible to obtain precursors containing the necessary initial components of the nitride ceramics. The combination of the vacuum heat treatment and nitriding (at 1200 °C) processes in a single cycle leads to obtaining disperse powders of the Si3N4–ZrN composite materials in a wide concentration range (11–33 vol.% ZrN).
Zirconium nitride, which exhibits high electrical conductivity, strength, and melting point, meets the main requirements to the conductive hardening phase in silicon nitride [1]. Since the difference between the electrical resistivity of silicon nitride (~1013–1014 μΏ × cm) and electrical resistivity of zirconium nitride (29 μΏ × cm) is extremely large, the electrical conductivity of the composite ceramics depends primarily on the continuity of the conductive 3D network of ZrN particles, the content of ZrN, and the homogeneity of its distribution in the composite ceramics [7, 8].
The conductivity of composite ceramics of the Si3N4–ZrN system, as ceramics of a typical insulator–conductor system, must show the percolation behavior, which is characterized by an abrupt decrease in the electrical resistivity at a certain content of the conductive phase [9]. The content of the conductive phase at which a conductive network form is called the percolation concentration or percolation threshold. Since this parameter depends to a great extent on technological regimes of preparation of the material, dispersity, and shape of particles, it is most reasonable to determine it experimentally from concentration dependences of conductivity [7]. For the obtained Si3N4–ZrN composite powders, the values of the electrical resistivity were determined (Table 2).
4 Spark Plasma Sintering of Si3N4–ZrN Composites
Data on the linear shrinkage of the Si3N4–ZrN composite powders in the process of SPS consolidation were monitored as the travel distance of the upper punch (the bottom punch is fixed) and converted into the shrinkage rate of specimens. After loading of about 8 g of the composite powder into the graphite die 20 mm in diameter with a punch unit, a pressure of 50 MPa was applied to establish good contacts between the graphite tools and powder particles. Sintering experiments were performed in the nonlinear consolidation regime developed earlier [10,11,12]. The Si3N4–ZrN composites were consolidated in few-stage regimes, in which pressure was sequentially increased from 50 up to 80 MPa and the heating rate was decreased from 100 °C/min down to 20 °C/min. The temperature was measured by a pyrometer on the inner surface of the upper graphite punch. Isothermal holding for 1.5 min at high temperatures was used to fully densify the Si3N4–ZrN composites.
All investigated Si3N4–ZrN composites were densified at a temperature of 1750 °C withholding for 5 min. The analysis of the sintering curves shows that the densification process proceeds in two stages (Figs. 3 and 4). The first stage of densification takes place in the temperature range of 400–800 °C. This can be connected with the increase in the pressure, destruction of strong agglomerates in the powder mixture, and rearrangement of particles.
As expected, the composite with 33 vol.% ZrN demonstrates the highest sinterability (Fig. 3, curve 1). This is connected with the large content of the highly conductive phase in the mixture, which forms a branched continuous network of ZrN particles. The densification of the 67 vol.% Si3N4–33 vol.% ZrN composite starts at a temperature of 1200 °C and proceeds quite intensively at a shrinkage rate of 1 mm/min up to a temperature of 1600 °C (Fig. 4, curve 1). This agrees well with the character of SPS densification curves of ZrN nanogranular powder (Fig. 5) synthesized by the method of carbothermal reduction-nitriding of a precursor obtained by the sol–gel method (the size of the coherent scattering region is 48 ± 2 nm). The comparison of the values of the electrical resistivity of ZrN (29 μΏ × cm) and the composite material with 33 vol.% ZrN (104 × 105 μΏ cm) enables us to argue that, in the process of consolidation by the SPS method, due to the formation of the continuous 3D network of ZrN particles, the run of the densification curve of the composite is analogous to that for zirconium nitride. In holding at a temperature of 1750 °C, minor densification occurs, and the density attains 97% of the theoretical one.
The composites with 22 and 11 vol.% ZrN demonstrate similar conditions of densification during SPS (Fig. 3, curves 2 and 3). For the composites, densification begins at a temperature about 1400 °C and the most intensive phase of shrinkage with a shrinkage rate of 0.9 mm/min is observed during holding at 1750 °C. This can be explained by the low content of the conductive phase (ZrN), which is below the percolation threshold. In contrast to the composite with 33 vol.% ZrN, where densification process is controlled by the ZrN phase, for the composites with 22 and 11 vol.% ZrN, where traces of zirconium disilicide are present (Fig. 1, curves 2 and 3), the sintering process is activated by its phase transformation into zirconium nitride.
The microstructure of the sintered 78 vol.% Si3N4–22 vol.% ZrN specimen shown in Fig. 6, indicates the size inhomogeneity of both the ZrN phase (light grains) and Si3N4 phase (dark grains). The ZrN phase has the relatively narrow grain size range 200–400 nm, whereas the Si3N4 phase, whose fraction is about 80 vol.%, is characterized by bimodality. The wide particle size range 0.2–2 µm is connected with the strong agglomeration of nanograins in the process of consolidation.
In Fig. 6, the microstructure of the sintered 78 vol.% Si3N4–22 vol.% ZrN specimen is shown. The structure of this composite is characterized by a bimodal grain size distribution. Zirconium nitride grains (light) have a relatively narrow size range of the order of 100–300 nm. At the same time, the Si3N4 phase (dark), whose content is about 80 vol.%, is characterized by a bimodal grain size distribution. Grains with a size of 100–300 nm and needle-like grains with a length up to several microns are present.
The mechanical characteristics of these materials are the following: the hardness is 22.5 ± 1.8 GPa, and the fracture toughness is 6.2 MPa. The high mechanical properties of the obtained material can be explained by the structural features of the composite. The high hardness is achieved due to the decrease in the grain size down to the nanolevel, and the presence of needle-like Si3N4 grains enhances the fracture toughness of the composite.
5 Conclusions
The two-stage synthesis process of composite nanopowders in the Si3N4–ZrN system is proposed. It has been established that the vacuum treatment process of the Si3N4 + Zr reaction mixtures makes it possible to obtain a precursor containing all necessary initial components of the nitride ceramics in the first stage of synthesis at a temperature of 1000 °C. The performance of synthesis in a single cycle that combines the processes of vacuum treatment (1000 °C) and nitriding (1200 °C) leads to obtaining disperse powders of Si3N4–ZrN composite materials in the wide concentration range 11–33 vol.% ZrN. The obtained powder mixtures are characterized by the presence of agglomerates up to several microns and nanosized particles.
The consolidation of the synthesized powders of the Si3N4–ZrN system has been performed by spark plasma sintering at a temperature of 1750 °C under a pressure of 60 MPa. The composition with 33 vol.% ZrN has shown the best sinterability, which is connected with the formation of a continuous conductive 3D network of ZrN particles. Compact specimens made of the 67 vol.% Si3N4–33 vol.% ZrN composite is characterized by a relative density above 97% and a bimodal structure, where grains with a size of ~200 nm and needle-like silicon nitride grains with a size up to several microns are present. The mechanical properties of this material are as follows: the hardness is 22.5 ± 1.8 GPa, and fracture toughness is 6.2 MPa.
References
Harrison RW, Lee WE (2016) Processing and properties of ZrC, ZrN and ZrCN ceramics: a review. Adv Appl Ceram 115:294–307
Krushinskaya LА, Makarenko GN, Oleynik GS, Uvarova IV, Fedorus VB (2007) Preparation of highly disperse composite powders of the Si3N4–ТiN system. II. Structural-phase transformations during nitriding of titanium silicide powder. Nanostrukturnoe Materialovedenie (Nanostruct Mater Sci) 1:84–90 (in Russian)
Ade M, Haußelt J (2003) Electroconductive ceramic composites with low–to–zero shrinkage during sintering. J Eur Ceram Soc 23:1979–1986
Kurnetsov NT (1999) Precursors for carbide, nitride and boride synthesis. In: Gogotsi YG, Andrievski RA (eds) Materials science of carbides, nitrides and borides. NATO science series. Kluwer Academic Publishers, Dordrecht (Netherlands), pp 223–247
Kharlamov АI, Bondarenko МE, Rafal АN (1990) Kinetics of the interaction of transition metals with silicon nitride. In Collection of scientific works: silicides and their application in engineering. Institute for Problems of Materials Science of the Academy of Sciences of the Ukrainian SSR, Kiev (Ukrainian SSR), pp 35–40 (in Russian)
Polishchuk VS (2003) Intensification of the manufacturing processes of carbides, nitrides, and composite materials based on them. Veber, Sevastopol (Ukraine) (Polishchuk 2003) 7 (in Russian)
Zivkovic Lj, Nikolic Z, Boskovic S, Miljkovic M (2004) Microstructural characterization and computer simulation of conductivity in Si3N4–TiN composites. J Alloy Compd 373:231–236
Zivkovic LjM, Nikolic ZS, Boskovic SM (2002) Electrical properties and percolation concentration in Si3N4–TiN based composites. Key Eng Mater 206–213:1489–1492
Lux F (1993) Models proposed to explain the electrical conductivity of mixtures made of conductive and insulating materials. J Mater Sci 28:285–301
Zgalat-Lozynskyy OB, Herrmann M, Ragulya AV (2011) Spark plasma sintering of TiCN nanopowders in non-linear heating and loading regimes. J Eur Ceram Soc 31:809–813
Zgalat-Lozynskyy OB, Ragulya AV, Herrmann M, Andrzejczuk M, Polotai A (2012) Structure and mechanical properties of spark plasma sintered TiN-based nanocomposites. Arch Metall Mater 57:853–858
Zgalat-Lozinskii OB (2014) Nanocomposites based on refractory compounds, consolidated by rate-controlled and spark-plasma sintering. Powder Metall Met Ceram 53:19–30
Acknowledgements
The authors express their heartfelt thanks and deep gratitude to the Material Lab Ltd. for fruitful cooperation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Kud, I., Ieremenko, L.I., Krushynska, L.A., Zyatkevych, D.P., Zgalat-Lozynskyi, O.B., Shyrokov, O.V. (2020). Synthesis and Consolidation of Powders Based on Si3N4–Zr. In: Fesenko, O., Yatsenko, L. (eds) Nanooptics and Photonics, Nanochemistry and Nanobiotechnology, and Their Applications . Springer Proceedings in Physics, vol 247. Springer, Cham. https://doi.org/10.1007/978-3-030-52268-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-52268-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-52267-4
Online ISBN: 978-3-030-52268-1
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)