Abstract
The tumor microenvironment consists of complex and dynamic networks of cytokines, growth factors, and metabolic products. These contribute to significant alterations in tissue architecture, cell growth, immune cell phenotype, and function. Increased glycolytic flux is commonly observed in solid tumors and is associated with significant changes in metabolites, generating high levels of lactate. While elevated glycolytic flux is a characteristic metabolic adaption of tumor cells, glycolysis is also a key metabolic program utilized by a variety of inflammatory immune cells. As such lactate and the pH changes associated with lactate transport affect not only tumor cells but also immune cells. Here we provide an overview of lactate metabolic pathways and the effects lactate has on tumor growth and immune cell function. This knowledge provides opportunities for synergistic therapeutic approaches that combine metabolic drugs, which limit tumor growth and support immune cell function, together with immunotherapies to enhance tumor eradication.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Lactate
- Lactic acid
- pH
- Tumor microenvironment
- Anti-tumor immunity
- Metabolism
- Glycolysis
- Immunotherapy
- Biosynthesis
- Warburg
- NK cells
- T cells
- Macrophages
- MCT
- Metabolic therapies
7.1 An Overview of the Tumor Microenvironment and Tumor Metabolism
Human tissues are a complex mixture of parenchymal cells, immune cells, stromal cells, extracellular matrix, and soluble factors cooperating, as components of a healthy microenvironment, to perform the necessary physiological and structural functions of that specific organ. Tumor cells are derived from these healthy cells through accumulation of genetic and epigenetic alterations, which lead to disruption of this finely tuned microenvironment. As a tumor develops, it constantly interacts, physically and through secreted factors, with its neighboring cells, often altering their phenotype and function [1, 2]. The interaction between malignant and non-malignant cells creates a dysregulated microenvironment that promotes tumor growth through a variety of mechanisms. A dynamic network of cytokines, growth factors, and extracellular matrix-degrading enzymes develops, which collectively result in significant alterations in the tissue architecture, dysregulated proliferation, and immune dysfunction [3, 4].
Proliferating cells require a constant supply of biomolecules to replicate cell structures and divide; these include cholesterol, glucose, glutamine, fatty acids, nucleotides, and non-essential amino acids [5]. To meet the metabolic demands of relentless cell division, tumor clones dramatically alter their metabolic activity. Biosynthesis of cellular components during cell division requires a range of carbon intermediates, which are provided primarily by the catabolism of glucose, via glycolysis (Fig. 7.1). The TCA cycle (or Kreb’s cycle) and oxidative phosphorylation are the primary sources of cellular energy in quiescent, regulatory, and non-proliferative cells. Tumor cells switch from TCA, which can efficiently generate 28 molecules of ATP per molecule of glucose, to glycolysis, which is far less efficient, but produces key carbon intermediates as by–products. By converting pyruvate to lactate, tumor cells can prevent negative feedback signals and the consumption of NAD+ during mitochondrial respiration, thereby maintaining constant biosynthesis through glycolysis intermediates [6, 7]. This phenomenon, termed the Warburg effect, was first observed in tumor cells 90 years ago by Otto Warburg [8]. Due to a large amount of glucose consumed by tumor cells during glycolysis, metabolic by-products, in particular lactate, are produced in significant quantities within tumors and released into the extracellular space (Fig. 7.1).
Glycolytic intermediates fuel biosynthesis of essential molecules for tumor cell proliferation. Tumor cells favor glycolysis due to the range of intermediates produced and the ability to produce the reducing molecule NAD+ by converting pyruvate to lactate. Detailed are the biochemical intermediates produced by glycolysis which are used for biosynthesis of essential molecules for cell proliferation. NAD+, oxidized nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; MCT, monocarboxylate transporter; TCA, tricarboxylic acid cycle; GLUT, glucose transporter
In the intervening years, additional metabolic changes in tumor cells have been identified beyond their requirement for glucose. This includes increased reliance on glutamine, which provides the building blocks of nitrogen-based compounds such as nucleotides and non-essential amino acids [9], and the ability to harvest free fatty acids from the environment [10]. In cases of extreme nutrient deprivation, tumor cells can even catabolize their proteins and lipoproteins through autophagy to liberate amino acids and fatty acids [11]. These tumor-associated metabolic alterations are maintained by altered metabolism-related gene expression, such as lactate dehydrogenase (LDH) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). This reprogramming of the metabolic circuits has significant consequences for neighboring cells within the tumor microenvironment, including tumor-associated fibroblasts, endothelial cells, and immune cells [6, 12].
7.2 The Importance of Lactate Metabolism
The generation of lactate is a cellular process necessary for maintaining glycolytic flux and facilitating the removal of pyruvate from the cell. The interconversion of pyruvate to lactate is mediated by LDH and results in the oxidation of NADH to NAD+. The lactate generated within a cell is then either exported from the cell via monocarboxylate transporters (MCTs) or converted back into pyruvate to fuel oxidative phosphorylation within the mitochondria (Fig. 7.1).
Lactate levels are consistently upregulated in a wide range of solid tumors [13]. Elevated lactate levels, upregulation of LDH enzymes, and the expression of MCTs are prognostic of tumor progression and metastases [14,15,16,17]. High levels of lactate in primary tumors are predictive of metastasis risk in head and neck cancer [18] and cervical cancers [19]. Serum levels of lactate dehydrogenase in patients with solid tumors are predictive of overall survival, disease progression, and recurrence-free survival [17, 20, 21]. Furthermore, suppression of lactate production within tumor cells in murine models reduces the metastatic ability of tumor cell lines [22,23,24].
7.3 Lactate Transport and Signaling
Lactate is transported across cell membranes via MCTs. These are a family of membrane transporters (also known as solute carrier 16 proteins), of which four members are proton-linked symporters (MCT1-MCT4) with varying tissue expression [25]. Tumors and immune cells predominantly express MCT1 and MCT4, and this expression profile appears to be characteristic of highly glycolytic cells [26]. MCTs passively transport lactate and a co-transported proton across the cell membrane. In situations where extracellular concentrations of either lactate or protons are elevated, these MCTs also facilitate the transport of lactate back into the cellular cytoplasm. This facilitates cell-cell lactate shuttles, whereby a glycolytic cell produces lactate, which in turn is taken up and utilized as an energy source by a neighboring oxidative cell [27, 28].
Extracellular lactate produced by glycolytic cells can also enter the circulation through capillaries or draining lymph. This lactate is subsequently removed from the circulation in the liver and kidney via gluconeogenesis (also referred to as the Cori cycle). Circulating lactate is transported into hepatocytes and renal cortex cells via MCTs and is converted via pyruvate back into glucose [29, 30]. Gluconeogenesis results in the consumption of ATP molecules generated from oxidative phosphorylation, and the glucose produced is either stored as glycogen in hepatocytes or exported back into the circulation where it can once again be utilized as a fuel source by glycolytic cells.
In addition to its role in glycolysis, lactate also possesses signaling and suppressor functions. Lactate is able to bind to the G-protein-coupled receptor GPR81 [31], which reduces cAMP and protein kinase A signaling, reducing proinflammatory cytokine production and inducing expression of regulatory factors such as IL-10, retinoic acid, and indoleamine 2,3-dioxygenase (IDO) [32, 33]. Lactate can also directly bind to the transmembrane domain of the mitochondrial antiviral-signaling protein (MAVS). MAVS is an innate intracellular sensor of double-stranded RNA [34]. Binding of lactate to MAVS prevents type I IFN production [35]. Lactate binding to MAVS prevents protein aggregation and provides a mechanistic link between metabolism and type I interferon responses, limiting interferon production in cells undergoing anaerobic glycolysis.
7.4 Lactate Dynamics in the Tumor Microenvironment
While elevated glycolytic flux is a well-documented characteristic of tumor cells, certain tumor cell subpopulations can utilize this lactate to fuel oxidative phosphorylation [36]. Highly glycolytic tumors have been shown to share space with low glycolytic neighboring tumors, which use lactate as a fuel source for mitochondrial respiration obtained via lactate shuttling from their glycolytic neighbors [27]. In breast cancer, signals from tumor cells can also lead to increased lactate production by stromal cells [37]. This lactate is then taken up by tumor cells, converted to pyruvate, and shuttled into the TCA cycle to fuel oxidative phosphorylation. The use of lactate as a fuel source requires an intact TCA cycle and functional mitochondria to metabolize the pyruvate generated.
While these studies highlight the importance of increased glycolytic flux in tumor cell survival and cancer progression, the exact location of this lactate remains somewhat uncertain and further research is required to directly quantify lactate levels and pH within the tumor microenvironment [38]. Direct measurements of the interstitial fluid of tumors via both in vivo and ex vivo methods indicate only a modest increase of lactate, in contrast to the dramatically elevated levels of lactate observed in whole tumor tissues [38]. These conflicting data can be reconciled if lactate preferentially accumulates within tumor cells. The proton gradient generated by the low pH of the tumor microenvironment, and relative alkaline intracellular pH of tumor cells, may favor the transport of lactate into tumor cells, thereby limiting lactate accumulation within the extracellular microenvironment [38]. Understanding the composition of the tumor microenvironment is central to untangling the individual (and potentially synergistic) effects of lactate and pH on tumor characteristics and immune cell function.
7.5 Impact of Lactate and pH on the Tumor Microenvironment
Lactate and pH have additional impacts on the tumor microenvironment beyond providing alternative energy sources for oxidative tumor cell subpopulations. Lactate has been shown to play several roles in reorganizing the physical tumor architecture and the immune landscape of many tumor types [39]. Lactate and reduced pH can promote tumor cell survival under conditions of nutrient deprivation. Glucose deprivation of the breast tumor cell line 4T1, in the absence of lactate, results in rapid apoptosis. In contrast, high concentrations of lactate induce cell cycle arrest and autophagy, enabling 4T1 cells to survive for extended periods when deprived of glucose [40]. Lactate can act on vascular endothelial cells, activating the hypoxia-inducible factor-1α (HIF-1α) pathway to induce vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) expression, as well as stimulating autocrine NF-κB/IL-8 (CXCL8) signaling to drive angiogenesis [41, 42].
Lactate also acts on tumor-associated fibroblasts to induce the production of hyaluronic acid, which promotes the migration and extravasation of tumor cells [43]. Perhaps surprisingly, tumors can also influence sites distant from the primary tumor via metabolites. Lactate is enriched in tumor-draining lymph nodes and drives a pro-tumorigenic fibroblast phenotype in fibroblastic reticular cells by inducing activation and mitochondrial dysfunction in a pH-dependent manner [44].
7.6 The Emerging Links Between Metabolism and Effector Immune Responses
The importance of energy production and biosynthesis for the metabolic demands of activated proliferating immune cells was first documented in early studies on macrophages and neutrophils [45, 46]. However, the full extent of the links between metabolism and immune responses are only now emerging. Beyond simply meeting the energy and biosynthesis demands of activated immune cells, it is now clear that metabolic pathways directly regulate immune cell effector function, and the metabolic intermediates generated play an essential role in coordinating overall immune responses. While elevated glycolytic flux is a characteristic metabolic adaption of tumor cells, glycolysis is also a key metabolic program utilized by a variety of inflammatory immune cells, including cytotoxic lymphocytes, which migrate into the tumor microenvironment.
The upregulation of glycolytic machinery is a common feature amongst rapidly proliferating inflammatory immune cells [47,48,49]. Activated immune cells bear a striking resemblance to proliferating tumor cells. Immune cells require rapid production of carbon intermediates to fuel proliferation, production of effector molecules, and energy-intensive cell processes, such as migration and phagocytosis. While glycolysis is relatively inefficient in the generation of ATP, it enables the reduction of NAD+ to NADH as well as the generation of intermediates essential for sustaining immune cell biosynthesis [50]. Proinflammatory and effector immune cells display a dramatic upregulation of glycolysis, together with an increased use of the pentose phosphate pathway, fatty acid synthesis, and amino acid metabolic pathways [50]. This distinct metabolic program supports inflammatory cytokine production, proliferation, reactive oxygen species (ROS) production, nitric oxide production, and effector cell differentiation.
Upregulation of the TCA cycle together with increased fatty acid oxidation, which reduces intracellular lipid accumulation, is associated with suppressive immune responses, the generation of immune tolerance, and the promotion of memory cell generation and survival [51,52,53]. These metabolic pathways are upregulated in macrophages with an M2 polarization [54], regulatory T helper cells [53], and quiescent memory T cells [55].
Intriguingly several metabolic intermediates and metabolic enzymes have been shown to have secondary signaling functions in immune cells [56,57,58,59]. This additional level of complexity facilitates the direct regulation of immune responses by metabolic processes. Hexokinase 1 has been shown to directly interact with and activate the NLRP3 inflammasome, leading to caspase activation and the processing of pro-IL-1β [60]. GAPDH binds to mRNA encoding interferon γ (IFNγ) and represses its translation; the switch to glycolysis that occurs in response to T cell activation leads to the dissociation of GAPDH allowing for translation of IFNγ [61]. Metabolic intermediates are also capable of regulating immune responses. Succinate, a metabolic intermediate of the TCA cycle, is dramatically increased upon activation of pro-inflammatory macrophages. Increased succinate levels stabilize HIF-1α, which is required for maximal IL-1β production by macrophages [58]. Conversely, the metabolite itaconate is increased as part of an anti-inflammatory response upon diversion of aconitate away from the TCA cycle during pro-inflammatory macrophage activation. Itaconate alkylates KEAP1 leading to activation of the anti-inflammatory transcription factor Nrf2, which regulates inflammation and type I interferon responses [56]. In the context of these intimate links between metabolism and immune cell responses, the impact of lactate on immune cell function is of particular relevance for effective tumor immunity [62].
7.7 The Impact of Lactate on Immune Cell Function
Elevated lactate and decreased pH affect the phenotype and function of immune cells, polarizing the innate immune system toward tolerance and immunosuppression. It is important to note that lactate and pH can act both independently and synergistically to alter immune cell function.
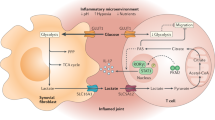
Macrophages can be broadly divided into M1-like inflammatory macrophages and M2-like regulatory macrophages [63]. Lactate acts upon macrophages, independently of pH, upregulating markers associated with an M2-like phenotype and downregulating markers associated with M1-like macrophages (Fig. 7.2). Lactate induces HIF-1α signaling and drives arginase-1 and VEGF expression [64], and synergizes with hypoxia to drive activation of MAPK signaling and arginase-1 expression in tumor-associated macrophages [15]. Lactate also signals via the GPR81 receptor on macrophages to reduce NFκB and inflammasome activation, resulting in reduced production of proinflammatory cytokines including IL-6, IL-1β, and TNFα (Fig. 7.3) [32, 33, 65]. At the same time, GPR81 signaling in macrophages drives the expression of immune suppressive factors associated with M2-like phenotypes including IL-10, retinoic acid, and IDO [32, 33].
Immunological consequences of elevated lactate and decreased pH in the tumor microenvironment. Lactate and reduced pH have differential and synergistic effects on immune cells in the tumor microenvironment. Effects mediated by lactate alone are written in blue, by pH alone in red, and combined effects in black. HIF1a, hypoxia-inducible factor 1a; IDO, indoleamine-2,3-deoxygenase; NK, natural killer; ROS, reactive oxygen species; TNFα, tumor necrosis factor α; VEGF, vascular endothelial growth factor
Intracellular effects of uptake of lactate and decreased pH in the tumor microenvironment. Lactate can signal through GPR81, resulting in decreased cAMP and loss of PKA signaling. Alternatively, lactate can be absorbed into the cell, with protons, via MCTs, causing decreased intracellular pH, mitochondrial dysfunction, and reduced metabolic output. Finally, protons can be directly internalized by proton transporters, resulting in reduced pH and mitochondrial dysfunction. cAMP, cyclic adenosine monophosphate; ATP, adenosine triphosphate; GPR81, G protein-coupled receptor 81; MCT, monocarboxylate transporter; ROS, reactive oxygen species
Macrophages are capable of shuttling lactate from the extracellular microenvironment via MCTs. The accumulation of intracellular lactate reduces RIG-I-like receptor signaling independently of pH by directly binding to the adaptor protein MAVS [35]. This blocks localization of MAVS to mitochondrial membranes and thereby inhibits RIG-I activation [35]. At a transcriptional level, changes in macrophage gene expression induced by lactate vary depending on the presence of lactate and/or reduced pH [66]. Lactate synergizes with low pH to induce IL23A transcription in monocytes, promoting the IL-23/IL-17 proinflammatory pathway [67], and likewise TNF and ROS suppression upon exposure to high levels of lactate requires the synergistic effects of both lactate and decreased pH [68] (Fig. 7.2).
A synergistic effect of lactate and decreased pH is also observed on dendritic cells [69, 70] and T cells [71]. Lactate together with a decreased pH inhibits dendritic cell differentiation as measured by CD1a, HLA-DR, and CD86 expression [69, 70]. This effect was not recapitulated by acidic pH alone (via HCl) or by the presence of lactate at pH 7.4 [69], with lactate and decreased pH acting synergistically to induce IL-10 production and suppress IL-12 production from dendritic cells [70]. In cytotoxic T lymphocytes, lactate and decreased pH induces apoptosis after 24 hours and decreases IFNγ and IL-2 production, effects not observed upon HCl treatment alone [71]. In this study, cytotoxic T lymphocyte proliferation and cytotoxic function appeared to be driven mainly by the decrease in pH associated with lactic acid treatment [71], and several other studies have highlighted the important effects of acidic microenvironments on immune cell function.
In vitro studies have highlighted the important effect of pH changes associated with lactate export on T cell and NK cell function [16, 71,72,73,74] (Fig. 7.2). T cells treated with low pH display reduced activation and cytokine production [16], while NK cells exposed to acidic microenvironment display reduced granzyme B and reduced cytotoxic effector functions [73]. Acidification of the tissue microenvironment causes a drop in intracellular pH and induces the selective cell death of T cells and NK cells, by driving increased mitochondrial dysfunction and mitochondrial ROS production (Fig. 7.3) [16, 74]. Reversing tumor acidosis has been shown to restore NK cell function and improve anti-tumor activity in vivo [72], and targeted inhibition of mitochondrial ROS production can promote NK cell survival [74] highlighting the potential for therapeutic interventions targeting metabolic pathways to improve immune cell function.
7.8 Opportunities to Target Lactate Metabolism in Cancer
The availability of immunotherapies for cancer treatment is exploding, yet many cancers and/or patients are still unresponsive. Complementary immune-activating therapies are required to increase response rates. Targeting metabolic pathways in tumors has multiple potential beneficial effects. Depriving tumor cells of essential nutrients limits their biosynthetic and proliferative capacity, reducing tumor growth dramatically. This is not a new concept in oncology where therapeutics targeting metabolism, such as methotrexate, have been used in the clinic for decades [75]. Due to the importance of tumor-derived metabolites as a component of the tumor microenvironment, targeting metabolism can create a more hospitable microenvironment for the immune system to work within and induce stress response pathways in tumor cells [76].
The broad spectrum of receptors, transporters, and catalyzing enzymes involved in tumor metabolism has led to the development of an array of metabolic therapies, which are now beginning to enter the clinic, with varying degrees of success [77, 78]. While an attractive target, metabolic therapies can also have side effects, specifically on the immune system. Metabolic changes underpin many of the immune functions we associate with tumor immunity [50], in particular T cell and NK cell activation and effector function [47, 48]. Indeed, treatments targeting metabolism, such as methotrexate, are also detrimental to the immune response. One of the other major clinical indications for the use of methotrexate is in autoimmunity where it functions as an immunosuppressant [79]. Any metabolic therapeutic approach should therefore aim to target pathways differentially used by tumor and non-tumor cells.
The glycolysis pathway provides the biochemical intermediates for several essential processes required for tumor cell growth and division [7], and the glycolytic pathway has been highlighted as a potential therapeutic target in cancer [80, 81]. However, our immune response is also dependent on glycolysis for the acquisition of effector functions, especially T and NK cells, which are the main mediators of tumor immunity [47,48,49]. Clinical trials of 2-deoxyglucose (2-DG), a glucose analog that reduces the rate of glycolysis in both tumor cells and immune cells, showed limited effects on tumor progression, despite promising preclinical data [82]. More recently, preclinical studies using koningic acid to partially inhibit GAPDH induced a cytotoxic response in cancer cell lines without impacting on tumor immunity [83]. This study highlights the precision and specificity required to target this pathway without impacting on immune cell function.
The production and secretion of lactate can also be targeted via several alternative therapeutic strategies that avoid the need to completely inhibit glycolysis. These alternative strategies may hold promise in avoiding the detrimental effects of complete inhibition of glycolysis on immune cells. Targeting either lactate transport via MCTs [27, 41, 42, 84] or lactate dehydrogenase enzymes [85, 86] prevents the release of lactate from tumor cells and induces cytotoxic responses. A study using an early non-selective MCT inhibitor suggests inhibition of T cell function may still be an issue [71], and further studies are required to assess the effects of novel selective MCT and lactate dehydrogenase inhibitors on immune cell function. A specific MCT1 inhibitor, AZ3965, has shown promise in preclinical studies and is currently being trialed in solid tumors including gastric cancer and lymphoma (NCT 01791595). Furthermore, an MCT4 inhibitor is in preclinical development (AZD0095), which does not affect T cell function and when combined with checkpoint therapy improves tumor rejection in an MC-38 murine colon cancer model [87].
The hydrogen ions co-transported with lactate, which act to decrease the pH of the tumor microenvironment and suppress immune cell function, can also be therapeutically targeted. Significant clinical improvement has been reported with the use of systemic bicarbonate buffering, which neutralizes tumor acidity, reduces tumor invasiveness, and improves the immune response [72, 88, 89]. Despite these positive results from preclinical studies, translation of these strategies into clinical trials is limited by the potential for adverse events, including electrolyte imbalance, respiratory depression, and progressive vascular calcification [90]. The targeted use of bicarbonate buffering has been trialed in patients receiving trans-arterial chemoembolization for hepatocellular carcinoma, which improved tumor response rates, although had minimal effect on overall survival [91].
Decreasing intracellular pH is a consequence of the acidic microenvironment tumor-infiltrating immune cells migrate into. This decrease in pH is associated with increased mitochondrial ROS production and immune cell apoptosis [16, 74]. Reducing the accumulation of mitochondrial ROS using ROS scavengers can protect immune cells from pH-induced apoptosis ex vivo [74]. The use of mitochondria-targeted scavengers has shown some efficacy in murine models of cancer, although in these studies the effect was attributed to a direct effect on tumor cell survival [92, 93]. It remains to be seen if some of these anti–tumor effects of mitochondria–targeted scavengers in vivo are also mediated by improvements in immune cell function.
The availability of immunotherapies for cancer treatment has revolutionized the field of oncology. However, many cancers and/or patients fail to respond to these immune-activating therapies. This could be due to the inhospitable environment created by tumor metabolism, creating a toxic microenvironment for even engineered immune cells. Immunotherapies, either checkpoint inhibitors or cellular therapies, rely on the ability of immune cells to alter and maintain their metabolism to carry out effector functions. As discussed in this chapter, tumors have adapted to avoid just this. Therefore, complementary metabolic therapies are required to enhance immune-based treatments and improve patient response in solid tumors. Therapeutic approaches taking into consideration the metabolic heterogeneity of the tumor microenvironment and the metabolic demands of tumor-infiltrating immune cells in personalized models hold much promise. By harnessing the synergistic anti-tumor effects of limiting tumor growth as well as augmenting local immune cells, these metabolic approaches can complement immunotherapy and enhance tumor eradication and patient survival.
References
Liotta LA, Kohn EC (2001) The microenvironment of the tumour–host interface. Nature 411:375–379
Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci 125:5591–5596
Hanahan D, Weinberg RA (2011) Hallmarks of cancer the next generation. Cell 144:646–674
Chen DS, Mellman I (2017) Elements of cancer immunity and the cancer–immune set point. Nature 541:321–330
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 324:1029–1033
Pavlova NN, Thompson CB (2016) The emerging hallmarks of cancer metabolism. Cell Metab 23:27–47
Xie J et al (2015) Beyond Warburg effect – dual metabolic nature of cancer cells. Sci Rep 4:4927
Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J Gen Physiol 8:519–530
Altman BJ, Stine ZE, Dang CV (2016) From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer 16:619–634
Kamphorst JJ et al (2013) Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci 110:8882–8887
Boya P, Reggiori F, Codogno P (2013) Emerging regulation and functions of autophagy. Nat Cell Biol 15:713–720
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21:309–322
Goveia J et al (2016) Meta-analysis of clinical metabolic profiling studies in cancer: challenges and opportunities. EMBO Mol Med 8:1134–1142
Payen VL et al (2017) Monocarboxylate transporter MCT1 promotes tumor metastasis independently of its activity as a lactate transporter. Cancer Res 77:5591–5601
Carmona-Fontaine C et al (2017) Metabolic origins of spatial organization in the tumor microenvironment. Proc Natl Acad Sci 114:2934–2939
Brand A et al (2016) LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab 24:657–671
Zhang J et al (2015) Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: a systematic review and meta-analysis. Sci Rep 5:9800
Walenta S et al (1997) Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol 150:409–415
Schwickert G, Walenta S, Sundfør K, Rofstad EK, Mueller-Klieser W (1995) Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res 55:4757–4759
Sagman U et al (1991) The prognostic significance of pretreatment serum lactate dehydrogenase in patients with small-cell lung cancer. J Clin Oncol 9:954–961
Malhotra P, Sidhu LS, Singh SP (1986) Serum lactate dehydrogenase level in various malignancies. Neoplasma 33:641–647
Rizwan A et al (2013) Relationships between LDH-A, lactate, and metastases in 4T1 breast tumors. Clin Cancer Res 19:5158–5169
Dhup S, Dadhich RK, Porporato PE, Sonveaux P (2012) Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des 18:1319–1330
Hirschhaeuser F, Sattler UGA, Mueller-Klieser W (2011) Lactate: a metabolic key player in cancer. Cancer Res 71:6921–6925
Halestrap AP (2013) The SLC16 gene family - structure, role and regulation in health and disease. Mol Asp Med 34:337–349
Pinheiro C et al (2010) Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J Biomed Biotechnol 2010:1–7
Sonveaux P et al (2008) Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 118:3930–3942
Draoui N, Feron O (2011) Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech 4:727–732
Roef MJ et al (2003) Gluconeogenesis in humans with induced hyperlactatemia during low-intensity exercise. Am J Physiol Endocrinol Metab 284:E1162–E1171
Gerich JE, Meyer C, Woerle HJ, Stumvoll M (2001) Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24:382–391
Cai T-Q et al (2008) Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem Biophys Res Commun 377:987–991
Ranganathan P et al (2018) GPR81, a cell-surface receptor for lactate, regulates intestinal homeostasis and protects mice from experimental colitis. J Immunol 200:1781–1789
Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ (2014) Lactate reduces liver and pancreatic injury in toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 146:1763–1774
Seth RB, Sun L, Ea C-K, Chen ZJ (2005) Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell 122:669–682
Zhang W et al (2019) Lactate is a natural suppressorof RLR signaling by targeting MAVS.Cell 178:176--189.e15
Hui S et al (2017) Glucose feeds the TCA cycle via circulating lactate. Nature 551:115–118
Whitaker-Menezes D et al (2011) Evidence for a stromal-epithelial “lactate shuttle” in human tumors. Cell Cycle 10:1772–1783
García-Cañaveras JC, Chen L, Rabinowitz JD (2019) The tumor metabolic microenvironment: lessons from lactate. Cancer Res 79:3155–3162
Romero-Garcia S, Moreno-Altamirano MMB, Prado-Garcia H, Sánchez-García FJ (2016) Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front Immunol 7:52
Wu H et al (2012) Central role of lactic acidosis in cancer cell resistance to glucose deprivation-induced cell death. J Pathol 227:189–199
Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O (2011) Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF- B/IL-8 pathway that drives tumor angiogenesis. Cancer Res 71:2550–2560
Sonveaux P et al (2012) Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One 7:e33418
Stern R, Shuster S, Neudecker BA, Formby B (2002) Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Exp Cell Res 276:24–31
Riedel A et al (2018) Tumor pre-conditioning ofdraining lymph node stroma by lactic acid. bioRxiv442137 https://doi.org/10.1101/442137
Newsholme P, Curi R, Gordon S, Newsholme EA (1986) Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem J 239:121–125
Alonso D, Nungester WJ (1956) Comparative study of host resistance of Guinea pigs and rats V. the effect of pneumococcal products on glycolysis and oxygen uptake by polymorphonuclear leucocytes J Infect Dis 99:174–181
Buck MD, O’Sullivan D, Pearce EL (2015) T cell metabolism drives immunity. J Exp Med 212:1345–1360
Assmann N et al (2017) Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat Immunol 18:1197–1206
MacIver NJ et al (2008) Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol 84:949–957
O’Neill LAJ, Kishton RJ, Rathmell J (2016) A guide to immunometabolism for immunologists. Nat Rev Immunol 16:553–565
Yaqoob P (2003) Fatty acids as gatekeepers of immune cell regulation. Trends Immunol 24:639–645
Pearce EL et al (2009) Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460:103–107
Michalek RD et al (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4 + T cell subsets. J Immunol 186:3299–3303
Jha AK et al (2015) Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42:419–430
van der Windt GJW et al (2013) CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc Natl Acad Sci USA 110:14336–14341
Mills EL et al (2018) Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556:113–117
Mills EL et al (2018) Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560:102–106
Tannahill GM et al (2013) Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496:238–242
Ryan DG et al (2019) Coupling Krebs cycle metabolitesto signalling in immunity and cancer. NatMetab 11(1):16
Moon J-S et al (2015) mTORC1-induced HK1-dependent glycolysis regulates NLRP3 inflammasome activation. Cell Rep 12:102–115
Chang C-H et al (2013) Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153:1239–1251
Mills EL, Kelly B, O’Neill LAJ (2017) Mitochondria are the powerhouses of immunity. Nat Immunol 18:488–498
Murray PJ, Wynn TA (2011) Protective andpathogenic functions of macrophage subsets. NatRev Immunol 11:723--737
Colegio OR et al (2014) Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513:559–563
Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W (2011) Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol 39:453–463
Peter K, Rehli M, Singer K, Renner-Sattler K, Kreutz M (2015) Lactic acid delays the inflammatory response of human monocytes. Biochem Biophys Res Commun 457:412–418
Shime H et al (2008) Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol 180:7175–7183
Dietl K et al (2010) Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J Immunol 184:1200–1209
Gottfried E et al (2006) Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 107:2013–2021
Nasi A et al (2013) Dendritic cell reprogramming by endogenously produced lactic acid. J Immunol 191:3090–3099
Fischer K et al (2007) Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109:3812–3819
Pötzl J et al (2017) Reversal of tumor acidosis by systemic buffering reactivates NK cells to express IFN-γ and induces NK cell-dependent lymphoma control without other immunotherapies. Int J Cancer 140:2125–2133
Husain Z, Huang Y, Seth P, Sukhatme VP (2013) Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol 191:1486–1495
Harmon C et al (2019) Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol Res 7:335–346
Chabner BA, Roberts TG (2005) Chemotherapy and the war on cancer. Nat Rev Cancer 5:65–72
Renner K et al (2017) Metabolic hallmarks of tumor and immune cells in the tumor microenvironment. Front Immunol 8:248
Tennant DA, Durán RV, Gottlieb E (2010) Targeting metabolic transformation for cancer therapy. Nat Rev Cancer 10:267–277
Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G (2013) Metabolic targets for cancer therapy. Nat Rev Drug Discov 12:829–846
Cutolo M, Sulli A, Pizzorni C, Seriolo B, Straub RH (2001) Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis 60:729–735
Doherty J, Cleveland J (2013) Targeting lactate metabolism for cancer therapeutics. J Clin Invest 123:3685–3692
Ganapathy-Kanniappan S, Geschwind J-FH (2013) Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer 12:152
Raez LE et al (2013) A phase I dose-escalation trial of 2-deoxy-d-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 71:523–530
Liberti MV et al (2017) A predictive model forselective targeting of the Warburg effect throughGAPDH inhibition with a natural product. CellMetab 26:648--659.e8
Noble RA et al (2017) Inhibition of monocarboxyate transporter 1 by AZD3965 as a novel therapeutic approach for diffuse large B-cell lymphoma and burkitt lymphoma. Haematologica 102:1247–1257
Manerba M et al (2012) Galloflavin (CAS 568-80-9): a novel inhibitor of lactate dehydrogenase. ChemMedChem 7:311–317
Le A et al (2010) Inhibition of lactate dehydrogenase a induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci 107:2037–2042
Critchlow SE et al (2019) Abstract 1207: Reversinglactate-driven immunosuppression using the novel,potent and selective MCT4 inhibitor AZD0095. In:Experimental and molecular therapeutics, pp 1207--1207
Ibrahim-Hashim A et al (2017) Tris-base buffer: a promising new inhibitor for cancer progression and metastasis. Cancer Med 6:1720–1729
Corbet C, Feron O (2017) Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer 17:577–593
Adeva-Andany MM, Fernández-Fernández C, Mouriño-Bayolo D, Castro-Quintela E, Domínguez-Montero A (2014) Sodium bicarbonate therapy in patients with metabolic acidosis. Sci World J 2014:627673
Chao M et al (2016) A nonrandomized cohort and a randomized study of local control of large hepatocarcinoma by targeting intratumoral lactic acidosis. Elife 5:e15691
Nazarewicz RR et al (2013) Does scavenging of mitochondrial superoxide attenuate cancer prosurvival signaling pathways? Antioxid Redox Signal 19:344–349
Porporato PE et al (2014) A mitochondrial switch promotes tumor metastasis. Cell Rep 8:754–766
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Harmon, C., O’Farrelly, C., Robinson, M.W. (2020). The Immune Consequences of Lactate in the Tumor Microenvironment. In: Birbrair, A. (eds) Tumor Microenvironment. Advances in Experimental Medicine and Biology, vol 1259. Springer, Cham. https://doi.org/10.1007/978-3-030-43093-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-43093-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-43092-4
Online ISBN: 978-3-030-43093-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)