Abstract
Phytoplankton are the primary producers in aquatic ecosystem and play crucial role in the nutrient cycling, carbon fixation, and regulating the overall food-web dynamics. In addition to ensuring ecological services, phytoplankton species composition is also considered an efficient bio-indicator of the water quality. Thus, phytoplankton composition, diversity, and their distribution could be used as a biological proxy to assess the ecological health of a water body. Considering the ecological significance of phytoplankton, various studies have targeted them to understand their spatiotemporal variation and environmental drivers in the Chilika lagoon. Phytoplankton community structure of Chilika lagoon is influenced by several environmental factors (nutrients, light, and salinity) of which salinity predominantly determines the composition and distribution of phytoplankton communities. In Chilika lagoon, spatial variation in salinity regime provides a variety of habitats (e.g. oligohaline (0–5 ppt), mesohaline (5–18 ppt), and polyhaline (>18 ppt)) for the proliferation of freshwater, estuarine, and marine phytoplankton forms. Based on the published literature, a total of 739 phytoplankton species have been documented from the Chilika lagoon, which included a diverse assemblage of species spectrum represented by Bacillariophyta (270 species), Dinophyta (88 species), Cyanophyta (103 species), Chlorophyta (178 species), Euglenophyta (92 species), Chrysophyta (5 species) and Xanthophyta (3 species). Among these, Bacillariophyta has been shown to be the most diverse and abundant in the phytoplankton communities. The total inventory of 709 phytoplankton species during the post-restoration study (2000–2014) included 612 new records which were documented for the first time from Chilika lagoon. Long-term systemic monitoring of phytoplankton is essential to understand their intrinsic spatiotemporal variability and also to recover maximum species diversity in lagoon. Further, continuous and detailed observation of phytoplankton community is necessary to monitor the occurrence of toxic species and harmful algal blooms. In addition to the application of classical microscopy based taxonomic approach to document phytoplankton species diversity, efforts should also be directed to integrate the molecular tools such as high-throughput DNA sequencing to understand the genetic diversity of smaller size nano-phytoplankton and pico- phytoplankton in the lagoon ecosystem.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Background
Coastal lagoons are commonly distinguished as highly productive ecosystems due to their shallow depth and restricted water exchange with the sea (Bec et al. 2011). In addition, the productivity of coastal lagoons are influnced by development of strong boundaries and salinity gradients. Depth profiles also plays pivotal role by regulating solar insolation and controls benthic-pelagic coupling (Pérez-Ruzafa et al. 2011). Most of the coastal lagoons are simultaneously influenced by riverine and seawater influx resulting in brackish water salinity regime. The brackish water habitat hosts a wide array of biodiversity including major feeding and breeding grounds of fishes and birds. Lagoons also extends ecological services by providing food (largely fishes), stock of freshwater, maintainance of hydrobiology, climate regulation, flood protection, water purification, oxygen production, fertility, recreation and ecotourism (Newton et al. 2018). Hence, coastal lagoons have immense ecological, economic, and social values by supporting livelihoods of fisher folks and coastal communities (Newton et al. 2014). Coastal lagoons are typically characterized by shallow depth, bi-directional horizontal flows, and frequent mixing in the water column which results in a highly variable gradient in the physicochemical properties (Rakhesh et al. 2015). The variability in these properties also influences the phytoplankton community composition over the spatial and temporal scales in a lagoonal environment.
The challenge of deciphering the roles of phytoplankton community assembly remains a central problem of aquatic ecology (Cloern and Dufford 2005) and aquatic ecosystems are characterized by remarkable phytoplankton diversity (Goebel et al. 2013). Long-term changes in phytoplankton communities have been a major concern for global changes, which could be used to track ecosystem’s response to the eutrophication and climate changes (Chen et al. 2010). Therefore, knowledge of phytoplankton community structure and associated variability at larger temporal scales covering multiple years is essential to understand underlying environmental changes, caused due to various drivers and pressures, which are accentuated by climate change. Krebs (1994) has opined that phytoplankton dynamics is influenced by bottom-up and/or top-down factors. Bottom-up factors (e.g., temperature, light intensity, salinity, nutrients, nitrogen, and phosphorus) control species growth, while top-down factors (e.g., predation and competition) control their biomass (Wehr and Descy 1998).
Phytoplankton are recognized as a major entity in global biogeochemical cycles (Myklestad 2000) and supply intermittently new, potentially labile dissolved organic carbon to aquatic systems (Sondergaard et al. 1985; Kirchman et al. 1991). Phytoplankton are also an important source of primary production and determine the potential productivity of entire aquatic food webs (Wissel and Fry 2005). Some species of phytoplankton are considered to be an important food source for pelagic and benthic species (e.g. fishes, and molluscs) (Pasquaud et al. 2010). For example, larval oysters feed on smaller phytoplankton cells (Olson and Olson 1989). Further, phytoplankton are generally considered as indicators of climate change due to their short lifespan and ability to produce resting stages (Guerrero and Rodriguez 1998; McQuoid et al. 2002). Studies have also shown that besides their role as bio-indicators of climate change, phytoplankton are also reliable indicators for assessing the pollution and eutrophication in aquatic ecosystems. For instance, spatiotemporal distribution of phytoplankton composition and biomass with emphasis on harmful algal blooms as indicators of eutrophication has been studied in the Cienfuegos Bay (Cuba) (Moreira-González et al. 2014). In another study, phytoplankton species composition was applied as a bio-diagnostic tool in relation to associated pollution in the Iyagbe lagoon (South-western Nigeria) (Onyema 2013).
Phytoplankton community represents an assemblage of heterogeneous microscopic algal forms, more or less dependent upon prevailing water currents (Kudela and Peterson 2009). Phytoplankton distribution in an estuarine lagoonal ecosystem is closely linked to several physicochemical and biological factors. Of these, salinity has been recognised as an important factor in determining community composition and their distribution (Huang et al. 2004; Lionard et al. 2005; Varona-Cordero et al. 2010; Lueangthuwapranit et al. 2011; Harris and Vinobaba 2012; Canini et al. 2013). Other environmental factors such as pH, temperature, light (influenced by turbidity) and nutrients also regulate the spatiotemporal distribution of these communities. For example in Bach Dang estuary (Vietnam) phytoplankton community structure was influenced by nutrient, turbidity, and heavy metals (Rochelle-Newall et al. 2011). In another study, temperature, salinity, silicate, and total phosphorus affected phytoplankton structure in Tagus estuary (Portugal) (Brogueira et al. 2007).
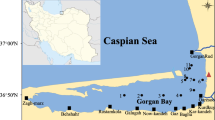
The Chilika lagoon represents a biologically diverse and ecologically unique ecosystem located along the east coast of India (19°28′–19°54′ N and 85°06′–85°35′ E). The lagoon is a shallow bar-built estuary with a surface area of 906 km2 in summer and 1165 km2 in monsoon (Mohanty et al. 2015). The lagoon experiences tropical monsoon-forced climate with average annual precipitation of 1238 mm (Gupta et al. 2008). Semi-diurnal tides facilitate seawater influx into the lagoon, mostly restricted to seawater inlet (Ganguly et al. 2015). Simultaneous mixing of river water and seawater makes the hydrological regime highly dynamic in lagoon. About 78% of the freshwater flows into the lagoon are from 12 major rivers located in the northern and western catchment of the lagoon (Srichandan et al. 2015a). Historically, based on the salinity gradient, the lagoon has been spatially delineated into four ecological sectors; northern sector, central sector, southern sector, and outer channel (Fig. 11.1).
Phytoplankton are frequently characterized in relation to discrete size classes– picoplankton (<2 μm), nanoplankton (2–20 μm), microplankton (20–200 μm) and macroplankton (>200 μm) (Brewin et al. 2010). Majority of studies on phytoplankton diversity and distribution in Indian coastal ecosystems have relied on the classical morphological identification using light microscopy (Selvaraj et al. 2003; Srichandan et al. 2015a; b). Conventional light microscopy is only suited to discriminate large size (>10 μm) phytoplankton cells and has minimal use in assessing genetic diversity of smaller size (≤ 2 μm) phytoplankton. Although, electron microscopy generally allows assignment to taxonomic classes, but most of the picoplankton do not have enough ultra-structural features for the identification at lower taxonomic level. Recent studies on natural plankton assemblages have also employed flow cytometry and photosynthetic pigment analysis which provide information on the structure and dynamics of the phototrophic and/or autotrophic behaviour of the planktonic organism, but phylogenetic information supplied by these methods is limited (Diez et al. 2001).
Recently, advances in high-throughput DNA sequencing has allowed sequencing of hundreds of environmental samples in a very cost-effective manner, generating millions of sequence reads which can provide a realistic estimate on the true extent of the genetic diversity of picophytoplankton. For example, 454 pyrosequencing of 18S rRNA genes from the Pacific coastal waters, for the first time presented a comprehensive picture of the diversity of marine picoeukaryotes (Cheung et al. 2010). The 18S/16S rRNA genes are widely used in picophytoplankton diversity studies allowing discrimination of both heterotrophic and phototrophic picophytoplankton at different taxonomic levels (Xiao et al. 2014). In contrast, sequencing of functional genes, provide direct linkages to the essential functions in carbon biogeochemistry that picophytoplankton performs. Diversity, gene abundance, and gene expression studies based on the rbcL (Ribulose-1, 5-bisphosphate carboxylase oxygenase) gene, which encodes the large subunit of the CO2-fixing enzyme Rubisco (Ribulose-1, 5-bisphosphate carboxylase oxygenase), have produced valuable insights into community composition and environmental patterns in the different aquatic ecosystem (Samanta and Bhadury 2014).
Phytoplankton communities in Chilika are mixed assemblages consisting freshwater, estuarine, and marine species (Panigrahi et al. 2009; Srichandan et al. 2015a). Most of these species have wide salinity tolerance and are eurytopic in nature (Srichandan et al. 2015b). Literature also suggests that Chilika experiences significant seasonal changes in nutrient and salinity regime during dry and wet seasons (Srichandan et al. 2015b). Excessive nutrient loading could lead to eutrophication and may promote the development of Cyanophyta blooms (Conley et al. 2009; Stal 2012). Given the ecological significance of phytoplankton, number of studies have explored the taxonomic diversity of phytoplankton communities in the Chilika lagoon (Biswas 1932; Devasundaram and Roy 1954; Patnaik 1973; Patnaik and Sarkar 1976; Raman et al. 1990; Adhikary and Sahu 1992; Rath and Adhikary 2008; Panigrahi et al. 2009; Jha et al. 2009; Mohanty and Adhikary 2013; Mukherjee et al. 2016). In addition, environmental factors (e.g. salinity, transparency, dissolved nutrients) which drive the spatiotemporal distribution of phytoplankton communities in the Chilika lagoon have been well studied (Srichandan et al. 2015a, b).
In view of the foregoing discussions on the importance of phytoplankton, the present chapter provides a detailed overview of the current knowledge on the species diversity, spatiotemporal distribution, and environmental drivers of phytoplankton communities in the Chilika lagoon. This article also highlights consideration for a future line of phytoplankton research to enable bridging the knowledge gaps, particularly related to smaller size pico and nanophytoplankton.
11.2 Floral Classification of Phytoplankton
Phytoplankton are ususally classed in two major groups, (i) non-motile, fast-growing Bacillariophyta (diatom) and (ii) motile Dinophyta (dinoflagellates), capable of vertical migration in the water column in response to photosynthetically available solar irradiance (Moreno-Díaz et al. 2015). Other groups viz. Cyanophyta (blue-green algae), Chlorophyta (green algae), Euglenophyta (euglenoids), Chrysophyta (silicoflagellates) and Xanthophyta (yellow-green algae) are also the members of phytoplankton communities and often predominates under certain favourable circumstances.
In total, 739 phytoplankton species represented by Bacillariophyta (270 species), Dinophyta (88 species), Cyanophyta (103 species), Chlorophyta (178 species), Euglenophyta (92 species), Chrysophyta (5 species) and Xanthophyta (3) have been documented so far from the Chilika lagoon (Tables 11.1, 11.2, 11.3, 11.4, 11.5, 11.6, and 11.7). The photomicrographs of some dominant phytoplankton species recorded in Chilika lagoon are depicted in Plate 11.1. However, it should be noted that different studies have used various techniques for collection, preservation, concentration, enumeration, and taxonomic identification of phytoplankton communities. Due to this reason, the data would not be directly comparable across different studies. For example, some studies have used plankton nets of 10–20 μm (Rath and Adhikary 2008; Srichandan et al. 2015a, b) and 45 μm (Mohanty and Adhikary 2013) mesh sizes, while others have used a gravity sedimentation method without plankton net (Panigrahi et al. 2009). The studies also differ in sampling frequency and sample size (in terms of the amount of water collected and number of sampling sites). Most of the earlier studies have employed seasonal sampling in the sense that water samples were collected only once during a given season. In recent studies, comprehensive monitoring of phytoplankton communities was carried out on a monthly basis over the period of 3 years (2011–2014) covering sampling locations which spanned all four sectors of the lagoon (Srichandan et al. 2015a, b). Such studies have highlighted the importance of conducting long-term systemic monitoring of phytoplankton communities and provided a detailed understanding of their variability caused either by the intrinsic environmental forces or by the extreme events such as a cyclone.
Pictures of some dominant phytoplankton species recorded in Chilika Lagoon (a) Asterionellopsis glacialis1,2,3,4 (b) Bacteriastrum sp.2; (c) Ceratium fusus6; (d) Cocconeis pediculus4; (e) Gyrosigma fasciola6; (f) Prorocentrum micans4,6; (g) Thalassiothrix frauenfeldii2; (h) Odontell mobiliensis1,3,4; (i) Pleurosigma normanii3,4,5,6; (j) Thalassionema nitzschioides3,6; (k) Navicula transitrans6; (l) Ditylum brightwelli2; (m) Trichodesmium erythraeum2,4; (n) Pseudonitzschia sp.5,6. 1Devasundaram and Roy (1954), 2Patnaik (1973), 3Rath and Adhikary (2008), 4Panigrahi et al. (2009), 5Srichandan et al. (2015a), 6Srichandan et al. (2015b)
11.2.1 Bacillariophyta
Bacillariophyta represents unicellular and uni-nucleate algae with a size range of about 15 mm–400 mm in maximum dimension, although some smaller and a few considerably larger forms exist in the aquatic ecosystem. Bacillariophyta can be used as a suitable bioindicator for water quality assessments due to their short generation time and sensitivity to subtle environmental changes (Stevenson and Pan 1999; Goma et al. 2005). Bacillariophyta have been reported to constitute the bulk of the phytoplankton assemblages in many estuarine ecosystems. For instance, in Tagus (Portugal), Mahanadi (India), Batticaloa (Sri Lanka), and Bach Dang (Vietnam) estuaries, phytoplankton communities were dominated by Bacillariophyta (Cabeçadas 1999; Naik et al. 2009; Harris and Vinobaba 2012; Chu et al. 2014). In Chilika lagoon, Bacillariophyta has also been reported to dominate the phytoplankton community due to their eurythermal and euryhaline adaptations (Srichandan et al. 2015a). Literature also suggests that Bacillariophyta can tolerate a wide range of fluctuation in salinity and temperature (Sasamal et al. 2005). For instance, Aquino et al. (2015), while studying seasonal and spatial variation in phytoplankton community structure of Passos River estuary in Brazil remarked that Bacillariophyta are spatially affected by salinity and occurs in most estuaries in the world. However, nutrient availability and their stoichiometry regulates bacillariophytic metabolism and often results in a change in species composition in response to changing water quality (Lie et al. 2011). Molar ratios of available macronutrient concentration as dissolved silicate (16): dissolved inorganic nitrogen (16): dissolved inorganic phosphorus (1) is required for optimum growth of Bacillariophyta (Redfield et al. 1963). Apart from the nutrient availability in estuarine ecosystems, the growth and distribution of Bacillariophyta is also governed by water transparency that determines the availability of light in the water column (Resende et al. 2005; Masuda et al. 2011).
In Chilika lagoon, 270 Bacillariophyta species belonging to 95 genera have been reported (Table 11.1). Devasundaram and Roy (1954) investigated plankton community assemblages particularly in Balugaon, Kalupadaghat, Rambha, Satpara and Arkhakuda regions of Chilika lagoon and documented 31 species of Bacillariophyta, mostly marine in nature. Later, Patnaik (1973) identified 40 taxa of Bacillariophyta of which Chaetoceros sp., Coscinodiscus sp., Asterionellopsis glacialis, Rhizosolenia sp., Bacteriastrum hyalinum, Grammatophora sp. and Nitzschia sp. were found to be abundant across the outer channel of Chilika lagoon. The Bacillariophyta such as Chaetoceros perpusillus, Chaetoceros peruvianus, Cheatoeceros lorenzianus, Asterionellopsis glacialis, Thalassionema frauenfeldii, and Ditylum brightwellii were mostly represented in the central sector while Chaetoceros affinis, Chaetoceros pendulus, and Chaetoceros sp. were dominant in the northern sector. Patnaik and Sarkar (1976) documented 29 species of Bacillariophyta in Chilika lagoon out of which 18 species were new records. Raman et al. (1990) have mentioned the name of only 9 species (Cerataulus heteroceros, Chaetoceros sp., Cylindrotheca closterium, Mastogloia exigua, Pleurosigma normanii, Rhizosolenia sp., Stephanodiscus sp., Synedra affinis, and Triceratium sp.) of Bacillariophyta in their publication. Subsequently, Adhikary and Sahu (1992) did the sampling from stations spanning the entire lagoon and reported an additional two species (Tabellaria fenestrata and Thalassiosira subtilis) of Bacillariophyta. A study undertaken by Rath and Adhikary (2008) documented 57 species of Bacillariophyta in Chilika lagoon. Later, Panigrahi et al. (2009) recorded a total of 30 Bacillariophyta species during sampling between the years 2001–2003. The species such as Chaetoceros paradoxus, Coscinodiscus gigas, C. marginatus, Coscinodiscus sp., Lauderia annulata, and Cylindrotheca closterium were dominant in outer channel. Subsequently, the study of phytoplankton carried out in Chilika lagoon over the period 2003–2006 by Jha et al. (2009) led to documentation of 80 species of Bacillariophyta. Mohanty and Adhikary (2013) carried out an investigation on changes in the algal diversity subsequent to the opening of a new seawater inlet in the lagoon and recorded 20 more species (Mastogloia elliptica, Odontella litigiosa, Chaetoceros decipiens, Cyclotella maxima, Cyclotella meneghiniana, Cymbella affinis, Rhopalodia gibberula, Gomphonema micropus, Hantzschia amphioxys, Melosira decussata, Neidium affine var. amphirhynchus, Pinnularia major, Tryblionella acuta, Pinnularia subsimilis, Pinnularia nodosa, Pleurosigma naviculaceum, Synedra crystallina, Fragilaria radians, Tabularia fasciculata and Tabellaria flocculosa) to the existing Bacillariophyta species inventory of Chilika lagoon.
Recently, based on monthly sampling between year 2011–2012 from 13 stations of Chilika lagoon, 138 Bacillariophyta species belonging to 54 genera have been reported (Srichandan et al. 2015a). Out of these 138 Bacillariophyta, 53 species were new reports from Chilika lagoon. Among the encountered genera, Chaetoceros and Surirella have been found to be represented by the highest number of species (8 species). Bacillariophyta such as Pleurosigma sp., P. normanii, Synedra sp., Thalassionema nitzschioides, Surirella sp., Chaetoceros sp., Coscinodiscus sp., Lithodesmium undulatum, Hemiaulas sinensis, and Paralia sulcata have been found to be dominant throughout Chilika lagoon. The diversity of Bacillariophyta was higher during pre-monsoon season which has a higher salinity. In a recent study, 136 Bacillariophyta species have been registered in Chilika lagoon (Srichandan et al. 2015b). Synedra sp., Nitzschia sp., Diploneis weissflogii, Surirella sp., Navicula sp., Pseudonitzschia sp., Thalassiosira sp., Coscinodiscus sp., Chaetoceros sp. and Cyclotella sp. were dominant species and present in a wide range of salinity (oligohaline, mesohaline, and polyhaline). Srichandan et al. (2015b) have also reported the dominance of centric Bacillariophyta over pennate as also reported from other estuarine habitats, globally (Patil and Anil 2008; Canini et al. 2013). Thus, the post-restoration status of Bacillariophyta in Chilika lagoon stood at 252 species and a total of 188 Bacillariophyta were documented as new reports.
11.2.2 Dinophyta
Dinophyta are common to abundant in fresh, marine, and estuarine environments. In general, Dinophyta occupy the second position next to Bacillariophyta in the aquatic environment (Sahu et al. 2014). Dinophyta belongs to the diverse group of unicellular eukaryotes (Leander and Keeling 2004). Some Dinophyta are autotrophs and heterotrophs (Glibert and Legrand 2006; Gaines and Elbrächter 1987) while some lead mixotrophic mode of nutrition (Burkholder et al. 2008). Further, the heterotrophic and mixotrophic Dinophyta are able to feed on diverse prey items (e.g. bacteria, picoeukaryotes, nanoflagellates, bacillariophyta, other dinophyta, heterotrophic protists, and metazoans) due to their diverse feeding mechanisms (Jeong et al. 2010). In turn they are ingested by several kinds of predators. Thus, the role of the Dinophyta in food chain and food webs are very diverse. The variation in abundance, diversity, and composition of Dinophyta depend on changes in salinity, pH, nitrogen and phosphate (Yoo 1991; Cremer et al. 2007). For instance, a study from Chapora estuary (India) has reported that the major influential agents for Dinophyta distribution were temperature and salinity (Alkawri and Ramaiah 2010). In another study, salinity, nutrient, temperature and pH were the determining factors for the growth of Dinophyta in Santo Andre lagoon (Portugal) (Macedo et al. 2001).
In Chilika lagoon, Devasundaram and Roy (1954) documented 6 species of Dinophyta: Dinophysis caudata, D. miles, Peridinium sp., Tripos furca, Ceratium trichoceros, and C. breve (Table 11.2). Patnaik (1973) investigated seasonal fluctuations of plankton in the Chilika lagoon and recorded 7 species (Tripos furca, Ceratium tripos, Tripos fusus, Tripos longipes, Noctiluca scintillans, Dinophysis caudata and Protoperidinium diabolus) of Dinophyta. Patnaik and Sarkar (1976) added two more species; Tripos minutus and Protoperidinium depressum to the previous list. Raman et al. (1990) reported Protoperidinium brevipes as the most dominant species in the lagoon. Subsequently, Adhikary and Sahu (1992) documented the occurrence of Tripos furca, Noctiluca scintillans and Dinophysis caudata. Rath and Adhikary (2008) reported the presence of five species, adding Gymnodinium heterostriatum and Tripos lineatus while Panigrahi et al. (2009) reported six species with Prorocentrum micans as new additions. A study undertaken by Jha et al. (2009) documented 33 species of Dinophyta in the Chilika lagoon, out of which 30 species were included in existing Dinophyta species list. Later, Mukherjee et al. (2016) has carried out investigation on Dinophyta diversity and distribution in Chilika lagoon with description of new records including toxic species namely Alexandrium minutum, A. ostenfeldii, A. tamarense, A.monilatum, Lingulodinium polyedrum, Dinophysis caudata, D. fortii, Prorocentrum cordatum, P. micans, P. belizeanum, P. lima, Noctiluca scintillans, and Gymnodinium catenatum. Among the reported 38 species, 12 species (Alexandrium ostenfeldii, Lingulodinium polyedrum, Protoperidinium pellucidum, Protoperidinium elegans, Protoceratium reticulatum, Tripos brevis, Tripos dens, Tripos falcatus, Tripos macroceros, Ceratium tripos var. atlanticum, Amphisolenia astragalus and Ornithocercus magnificus) were new records for Chilika lagoon which mostly prevailed in the outer channel followed by southern sector and central sector. Mukherjee et al. (2016) have also demonstrated salinity as the key factor that drives the Dinophyta distribution in the lagoon. For example, Tripos macroceros, and Prorocentrum micans were observed in a salinity range of 8.9–33.1 ppt where as, A. ostenfeldii was observed between 30.5 and 33.10 ppt (Mukherjee et al. 2016).
A survey undertaken for the period 2011–2012 on spatiotemporal distribution of phytoplankton assemblages reported 38 species of Dinophyta (Srichandan et al. 2015a). A recent study between 2012 and 2014 on interannual and cyclone-driven variability in phytoplankton communities recorded 47 species of Dinophyta (Srichandan et al. 2015b). Protoperidinium sp., Gymnodinium sp., Prorocentrum cordatum, Tripos fusus, Prorocentrum micans, Protoperidinium oceanicum, Alexandrium sp. and Gonyaulax sp. were the dominant species (Srichandan et al. 2015a, b).
An upsurge in the relative dominance of Dinophyta with concurrent increase in toxic dinophyte species namely; Alexandrium sp. (565 cells L−1), Gonyaulax sp. (999 cells L−1), and Prorocentrum cordatum (448 cells L−1) was observed in southern sector after the passage of very severe cyclonic storm Phailin (Srichandan et al. 2015b). It was suggested that several physical and physiological processes could have contributed to higher Dinophyta abundance particularly in the southern sector of the lagoon. For example, riverine run-off may contribute to the formation of low saline and nutrient-rich freshwater layer at the surface where Dinophyta could concentrate their cells due to their swimming behaviour (Srichandan et al. 2015b). Till date, 88 Dinophyta species have been reported from the Chilika lagoon. Among these species, 84 Dinophyta have been reported in inventorisation survey during post-restoration phase and 71 species were new reports.
11.2.3 Cyanophyta
Cyanophyta are unicellular or filamentous organisms that are ubiquitous in nature and are found nearly in all aquatic environments. High diversity and abundance of Cyanophyta depend on high temperature and slightly alkaline conditions. Nutrient rich freshwater discharge and high turbidity due to suspended sediments further favor their growth (Harsha and Malammanavar 2004). For example, the growth of Cyanophyta due to high temperature and water column stability has also been reported in Neuse River estuary (North Carolina), York River estuary (Virginia), Florida Bay (Florida), and San Francisco Bay (California) (Phlips et al. 1999; Ning et al. 2000; Sin et al. 2000; Valdes-Weaver et al. 2006). In Na Thap River Estuary (Thailand), turbidity was the major factor responsible for variation in Cyanophyta diversity and abundance (Lueangthuwapranit et al. 2011). In Chilika lagoon, Cyanophyta have also been reported to dominate the phytoplankton community due to their specific adaptation to survive in highly turbid freshwater part (northern sector) of the lagoon (Srichandan et al. 2015b). Further, a combination of physical upward movement, nutrient, and favorable temperature conditions promoted benthic Cyanophyta growth in Chilika lagoon (Srichandan et al. 2015b).
Biswas (1932) initially documented 11 species of Cyanophyta from Chilika lagoon (Table 11.3). Patnaik (1973) have further added 4 Cyanophyta species to the existing species inventory and found that marine species Trichodesmium erythraeum was abundant in the outer channel while freshwater species such as Anabaena sp., Nostoc sp. were largely represented in the southern and northern sectors. Patnaik and Sarkar (1976) added another 3 Cyanophyta species (Lyngbya sp., Microcystis sp., and Oscillatoria sp.) to the existing list.
Raman et al. (1990) have investigated phytoplankton species composition in the Chilika lagoon before the opening of an artificial inlet in September 2000. It was observed that freshwater Cyanophyta Pseudanabaena limnetica dominated in the central sector. Another study, reported 8 Cyanophyta species representing freshwater and marine water forms (Adhikary and Sahu 1992). A seasonal survey of phytoplankton communities was conducted in the year 2000–2001 which reported a total of 12 species, of which 8 were the first report from Chilika lagoon (Rath and Adhikary 2008). Later, Panigrahi et al. (2009), Jha et al. (2009), Mohanty and Adhikary (2013), and Srichandan et al. (2015a) have documented 15, 39, 24 and 28 Cyanophyta species, respectively from the lagoon. A recent investigation on phytoplankton community in the lagoon has reported 39 Cyanophyta species (Srichandan et al. 2015b). Among these, three species (Cylindrospermum sp., Phormidium sp. and Anabaena sp.) were found to be most abundant and consistent in central and northern sectors. Thus, in total, updated documentation of Cyanophyta species stands at 103 of which 95 species were inventorized during post-restoration period which includes 81 new records collected for the first time.
11.2.4 Chlorophyta
Chlorophyta are green colored phytoplankton with chlorophyll a and b, xanthophylls, and carotenes as the dominant photosynthetic pigments (Dawson 1966). The Chlorophyta prevails in a wide range of environments ranging from freshwater to estuarine and marine conditions. In general, Chlorophyta occur preferably in freshwater upstream regions of estuaries. For example, in upper reaches of Tapi Estuary (India), dominance of chlorophytic phytoplankton communities such as Ankistrodesmus falcatus, Chlorella vulgaris, Scenedesmus quadricauda, Spirogyra indica, Pediastrum sp. and Closterium acerosum have been observed (George et al. 2012). Chlorophyta population was numerically more abundant in the freshwater region of Chilika lagoon (Srichandan et al. 2015b). Literature also suggests that eutrophic conditions further maximize the diversity and density of Chlorophyta population (Saify et al. 1986).
Devasundaram and Roy (1954) and Patnaik (1973) investigated the entire Chilika lagoon and recorded only one species of Chlorophyta are presented by Spirogyra sp. A survey conducted between the year 2000 and 2001 on the phytoplankton communities reported 14 species of Chlorophyta (Rath and Adhikary 2008). Further, Panigrahi et al. (2009) documented 10 species of Chlorophyta. Subsequently, a study on Chilika between 2003 and 2006 reported 114 species of Chlorophyta (Jha et al. 2009). Mohanty and Adhikary (2013) studied the algal diversity of Chilika lagoon extensively in different seasons and reported 14 species of Chlorophyta. A study on Chilika between the year 2011 and 2012 recorded 32 species belonging to 25 genera. The freshwater Chlorophyta Eudorina sp. were most abundant in the northern sector of the lagoon (Srichandan et al. 2015a). Another study during the year 2012–2014 has documented a total of 54 species of Chlorophyta (Srichandan et al. 2015b). To date, 178 Chlorophyta species have been reported from the Chilika lagoon (Table 11.4). Among the encountered 178 species, 173 species were all new records and inventorized during post-restoration period.
11.2.5 Euglenophyta
The Euglenophyta is a group of unicellular flagellates found in freshwater and marine environments. The class is distinguished by solitary unicells (only one colonial genus exists) with two anteriorly inserted flagella of which one is emergent, condensed chromosomes throughout the cell cycle, a paraxial rod associated with one or both flagella, a proteinaceous pellicle composed of individual strips each of which is lined by microtubules, and a beta-1, 3 glucan storage product known as paramylum. The diversity of Euglenophyceae members in aquatic environment can be attributed to high nutrient loading from various point and non-point sources indicating organic pollution in a water body (Kumar and Hosmani 2006; Laskar and Gupta 2009). In general, Euglenophyta are known to be dominant in freshwater regimes (preferably in upper reaches) of estuarine ecosystems in comparison to middle and lower reaches. For example, members of euglenophytic phytoplankton were observed to be dominated in upper reaches of Tapi estuary (India) (George et al. 2012). Similarly, a higher abundance of Euglenophyta has been observed in the freshwater zone i.e. northern sector of the Chilika lagoon (Srichandan et al. 2015a).
In Chilika lagoon, Euglenophyta was recorded for the first time by Jha et al. (2009) and documented 53 Euglenophyta species. Subsequently, a study on Euglenophyta diversity was carried out by Mohanty and Adhikary (2013) during 2010–2011.They encountered six species (Lepocinclis acus, Euglena agilis, Euglenaria caudata, Lepocinclis playfairiana, Trachelomonas abrupta, and Trachelomonas hispida) and found that their occurrence in northern and central sectors have attributed to increased eutrophication associated with anthropogenic discharge by human habitation around Chilika lagoon. Subsequently, Srichandan et al. (2015a) investigated phytoplankton community structure including Euglenophyta from the entire Chilika lagoon and added 4 more species to the existing Euglenophyta species list. This study has also revealed that Euglenophyta formed the most dominant group in the northern sector (freshwater zone) of the lagoon. The author has opined that this group occurs preferably in the nutrient-rich freshwater zone and serves as bio-indicator of organic pollution. Recently, a survey conducted between 2012 and 2014 have added 30 more species to the list (Srichandan et al. 2015b). Thus, the total number of Euglenophyta has increased to 92 species which were all inventorized new records during the post-restoration period (Table 11.5). It was also noticed that tropical cyclone Phailin which struck the lagoon on 12th October 2013 profoundly affected the Euglenophyta community composition in Chilika lagoon. After Phailin, the recovery of freshwater euglenophytes (e.g., Strombomonas acuminata, Trachelomonas sp.) was observed for the first time from the southern sector of the lagoon. In addition, the freshwater euglenophytes such as Phacus circumflexus, Strombomonas acuminata, Trachelomonas granulata, Trachelomonas lefevrei, Trachelomonas manginii, and Lepocinclis acus were recorded for the first time from the outer channel.
11.2.6 Chrysophyta
Chrysophyta (Golden-brown algae) are a group of marine pigmented heterokonts (Daugbjerg and Henriksen 2001) with a cosmopolitan distribution. They can be a major component in coastal and estuarine waters (e.g. Jochem and Babenerd 1989; Gómez and Gorsky 2003). They are generally autotrophs (Rigual-Hernández et al. 2010) while as opined out by Martini (1977) they have mixotrophic behavior. Further, Chrysophyta have been used as indicators of productivity (Takahashi et al. 2009), atmospheric and water mass variations (Onodera and Takahashi 2005). Chrysophyta are strongly influenced by environmental parameters, particularly by temperature and salinity (Henriksen et al. 1993). In Chilika lagoon, Chrysophyta were more numerous in brackish water salinity regime (Srichandan et al. 2015b).
In Chilika lagoon, only five taxa of Chrysophyta have been reported for the first time by Srichandan et al. (Srichandan et al. 2015a, b) and among them, three taxa (Dictyocha fibula, Dictyocha sp., and Octactis octonaria) were representative of marine water environment (Table 11.6). However, this particular group of phytoplankton is largely understudied with respect to diversity and distribution in Chilika lagoon and possible application in long-term lagoonal environmental monitoring. The present work suggests more comprehensive study on the Chrysophyta taxa in Chilika lagoon in future.
11.2.7 Xanthophyta
Xanthophyta are generally known as yellow green algae. These are non-motile, unicellular or colonial eukaryotic algae exhibits unique pigmentation which gives a yellow or fresh green appearance. This group of photosynthetic algae primarily occurs in freshwater, although a substantially found in marine environments. Literature suggests that mostly yellow green algae incline to be ecologically limited to small water bodies (Sahoo and Kumar 2015). This class characteristically possesses chlorophyll-a, β carotene and xanthophylls. The diversity of Xanthophyta in aquatic environment is large, but their biology, ecology, and biogeography are known for only a few of the more common taxa.
In Chilika lagoon, only three taxa (Gloeobotrys limneticus, Tribonema bombycinum, and Ophiocytium variable) of Xanthophyta have been reported (Adhikary and Sahu 1992; Jha et al. 2009) (Table 11.7). These species have been observed in freshwater zone i.e. northern sector and brackish water zone i.e. central sector. However, Xanthophyta is mostly understudied in Chilika lagoon. Further to clarify these data gaps and uncertainties, careful efforts, longer monthly studies, and the use of modern taxonomic keys are need to be implemented in phytoplankton monitoring programs. All the three species reported from Chilika were inventorized during post-restoration period.
11.3 Spatio-Temporal Distribution of Phytoplankton
Seasonal and spatial variability in phytoplankton communities of Chilika lagoon has been broadly described by Srichandan et al. (2015a, b) over an annual and inter-annual scale (Table 11.8). The survey conducted between 2011 and 2012, reported that Bacillariophyta such as Nitzschia sp., Chaetoceros sp., and Thalassiosira subtilis were ubiquitous during monsoon, post-monsoon and pre-monsoon, respectively in outer channel of the lagoon (Srichandan et al. 2015a). In contrast, Chrysophyta (Dictyocha sp.) dominated the phytoplankton communities during monsoon and pre-monsoon in southern sector. However, Bacillariophyta (Pleurosigma normanii) was predominant during post-monsoon. High abundance of freshwater Cyanophyta (Anabaena sp., Cylindrospermum sp.) and Euglenophyta (Trachelomonas sp.) was recorded in the northern sector of the lagoon. Central sector was dominated by species of Bacillariophyta (Pleurosigma normanii), Cyanophyta (Anabaena sp.) and Dinophyta (Prorocentrum cordatum) during monsoon, post-monsoon, and pre-monsoon seasons, respectively.
Subsequently, the survey conducted between 2012 and 2013 have shown that the phytoplankton communities in the central sector of the lagoon were dominated by Prorocentrum micans, Dictyocha sp. and Synedra sp. during monsoon, post-monsoon and pre-monsoon, respectively (Srichandan et al. 2015b). In the northern sector, the species such as Euglena sp., Trachelomonas sp. and Actinastrum sp. thrived well during monsoon period while Anabaena sp. and Trachelomonas sp. dominated during post-monsoon and pre-monsoon seasons. In the southern sector, Prorocentrum micans was predominant during monsoon while Dictyocha sp. during both post-monsoon and pre-monsoon seasons. However, phytoplankton flora of outer channel was mainly represented by Thalassionema nitzschioides in monsoon, Surirella sp. in post-monsoon and Nitzschia sp. in pre-monsoon.
Another survey undertaken during the period 2013–2014 have shown that in the central sector, freshwater Cyanophyta, i.e. Phormidium sp. was mostly represented in post-monsoon and pre-monsoon period while Cylindrospermum sp. was largely represented during monsoon (Srichandan et al. 2015b). In the northern sector, Cylindrospermum sp. was the significant species during post-monsoon and pre-monsoon period while Trachelomonas sp. was more abundant during monsoon season. In outer channel, the most abundant species encountered during monsoon, post-monsoon and pre-monsoon were Phormidium sp., Gyrosigma fasciola, and Pseudonitzschia sp. respectively. In southern sector, epipelic Bacillariophyta (Diploneis sp.) dominated during monsoon period where as toxic Dinophyta (Gonyaulax sp.) and Cyanophyta (Gloeocapsa alpina) dominated during post-monsoon and pre-monsoon season, respectively.
In addition to the general spatio-temporal trends with respect to physico-chemical forcing the phytoplankton community of the lagoon well responded to the extreme climatic events such as tropical cyclone Phailin (Srichandan et al. 2015b). An increase in freshwater Cyanophyta Cylindrospermum sp., have been observed in central sector and outer channel of Chilika lagoon during post-Phailin period. Further, it was also suggested that the enhanced growth of Cylindrospermum sp. was attributed to the sediment-resuspension along with physical upward movement. Tropical cyclone Phailin had a significant impact on the phytoplankton community composition of southern sector of the lagoon. Toxic dinophytes (e.g. Alexandrium sp., Gonyaulax sp., Prorocentrum cordatum) have been observed in considerably higher number during post-Phailin period.
11.4 Phytoplankton Population Density
Typically, in an estuarine ecosystem, phytoplankton abundance is highest during dry season (pre-monsoon) while lowest abundance is recorded during wet (monsoon) season (Perumal et al. 2009; Prabhahar et al. 2011). The pre-monsoon season is usually characterized with increase in salinity, enhanced temperature, sufficient solar irradiance and stable environmental conditions (Saravanakumar et al. 2008). In contrast, heavy rainfall, cloudy sky, river/terrestrial run-off, induced high turbidity limit the light availability in water column and reduce salinity causes reduction in phytoplankton density during monsoon (Perumal et al. 2009). Although in Chilika lagoon several studies deciphered the time scale phytoplankton community structure. However, a well marked spatial and temporal variations in phytoplankton population density has been reported by Srichandan et al. (2015a, b) (Fig. 11.2). In Chilika lagoon, overwhelming dominance of a benthic Bacillariophyta i.e. Pleurosigma normanii was observed during monsoon season (Srichandan et al. 2015a). It was suggested that disturbance of benthic habitat by wind and water current was the main factor for the occurrence of large number of this benthic pennate phytoplankton in the surface water. Other factors could be use of mechanized boat for fishing and dredging operations which also cause re-suspension of bottom sediments in water column.
11.5 Spatial and Seasonal Variation in Phytoplankton Abundance
Srichandan et al. (Srichandan et al. 2015a, b) have reported a clear spatial variation in phytoplankton density with respect to four ecological sectors of the Chilika lagoon (Fig. 11.3). Euglenophyta dominated the phytoplankton communities at lower salinity zone (i.e. northern sector), while Bacillariophyta were ubiquitous throughout the higher salinity zones (i.e., southern, central and outer channel) (Srichandan et al. 2015a). When tropical cyclone Phialin hit the lagoon in October 2013, it caused a drastic reduction in salinity (avg. 1.9 ppt) resulting proliferation of Cyanophyta in central sector besides northern sector (Srichandan et al. 2015b).
A marked temporal variation in phytoplankton density with respect to seasons has also been described by Srichandan et al. (Srichandan et al. 2015a, b) (Fig. 11.4). Seasonal changes in freshwater influx during monsoon appeared to be a controlling factor in determining the phytoplankton species composition and their abundances. The survey conducted between year 2011 and 2012 have shown that Bacillariophyta were the most dominant group in the lagoon irrespective of the season albeit with varying cell densities (Srichandan et al. 2015a). Bacillariophyta were more abundant in monsoon season with mean cell density of 1879 cells L−1, which subsequently decreased to 710 cells L−1 in post-monsoon season and further increased to 1134 cells L−1 in pre-monsoon season.
11.6 Phytoplankton and Environmental Variables
Phytoplankton communities in a lagoon are largely determined by a series of environmental parameters such as temperature, light, pH, salinity, dissolved oxygen, wind force, and tidal rhythm. In many estuaries, salinity has been considered as a key environmental variable for controlling the distribution and phytoplankton community composition. For example, in Schelde estuary in Belgium and Netherlands (Lionard et al. 2005), Suwannee River estuary in Florida (Quinlan and Phlips 2007), Bach Dang estuary in Vietnam (Rochelle-Newall et al. 2011), Pearl River Estuary in South China (Zhang et al. 2014) and Passos River estuary in Northeast Brazil (Aquino et al. 2015) salinity determined the spatial and temporal distribution of phytoplankton. In Chilika lagoon, salinity played a crucial role by governing the abundance and distribution of phytoplankton (Patnaik 1973; Patnaik and Sarkar 1976; Panigrahi et al. 2009; Srichandan et al. 2015a, b). Patnaik (1973) have determined that appearance and disappearance of freshwater, brackishwater and marine forms of phytoplankton mostly depended on the salinity conditions of the lagoon. Further, Raman et al. (1990) and Srichandan et al. (2015a) have determined that salinity was the predominant factor in controlling the distribution of phytoplankton in the Chilika lagoon. For instance, Srichandan et al. (2015a) have observed Dinophyta and Chrysophyta as the dominant phytoplankton groups in southern sector due to stable salinity regime. In outer channel, marine phytoplankton forms were prevalent due to higher salinity regime because of direct connectivity to the Bay of Bengal. Due to high freshwater discharge from rivers, northern sector was mostly represented by freshwater phytoplankton forms. Further, due to inter-mixing of freshwater and seawater, central sector was represented by both freshwater and marine phytoplankton taxa.
Depending upon the salinity preference and according to the biotic categories in the ecological classification, the phytoplankton communities have been classified into 3 different groups; oligohaline (0–5 ppt), mesohaline (5–18 ppt), and polyhaline (>18 ppt) (Marshall 1993). The dominant phytoplankton species Amphiprora sp., Amphora sp., Cocconeis placentula, Coscinodiscus sp., Cyclotella sp., Diploneis sp., Diploneis weissflogii, Navicula transitans, Navicula sp., Nitzschia sp., Pleurosigma normanii, Pleurosigma sp., Surirella sp., Synedra sp., Alexandrium sp., Gonyaulax sp., Prorocentrum micans, Prorocentrum cordatum, Protoperidinium sp., Anabaena sp., Cylindrospermum sp., Phormidium sp., Spirogyra sp., Euglena sp., Trachelomonas sp., and Dictyocha sp. had a wide salinity preference ranging from oligohaline, mesohaline and polyhaline in Chilika lagoon (Srichandan et al. 2015b). Few species such as Gomphosphaeria sp., Actinastrum sp., Trachelomonas lefevrei, and Strombomonas tambowika were found only at oligohaline regions while Thalassiosira sp. was restricted only to polyhaline regions. Some species (Pseudonitzschia sp., Thalassionema nitzschioides, Tripos fusus, and Protoperidinium oceanicum) were observed both at mesohaline and polyhaline regions but were entirely absent in oligohaline regions. Species viz. Gyrosigma fasciola, Gloeocapsa alpina were distributed only in the oligohaline and mesohaline regions of the Chilika lagoon.
Nitrate and phosphate has been considered limiting nutrient to algal growth (Mukhopadhyay et al. 2006; Gle et al. 2008). Chu et al. (2014) observed in a highly turbid estuary of Southeast Asia that inorganic nutrient concentrations and their respective ratios were found to be principal factors that structured phytoplankton diversity and influenced the emergence of potentially toxic species. In Chilika lagoon, maximum nitrate and phosphate concentrations were recorded during pre-monsoon season. It was also suggested that higher nitrogenous nutrient concentration during pre-monsoon could be related to higher residence time of the water in the lagoon during the low-flow period (pre-monsoon) (Srichandan et al. 2015b). Besides seasonal variability, nitrate and phosphate concentrations also show distinct spatial variability. For instance, freshwater head of tropical estuaries such as Tagus (Portugal) and Bach Dang (Vietnam) estuaries are greatly influenced by direct riverine inputs which reflect higher nutrient loading (Brogueira et al. 2007; Chu et al. 2014). Similarly, Srichandan et al. (2015b) have observed that freshwater zone (i.e. northern sector) of the lagoon displayed the higher amount of nitrate and phosphate due to riverine inputs compared to other three sectors of the lagoon. In Chilika lagoon it has been shown that nitrate (r = −0.295, p < 0.05) and phosphate (r = −0.284, p < 0.05) has great influence on phytoplankton communities especially on the Dinophyta abundance and diversity (Srichandan et al. 2015b). Similar to nitrate and phosphate concentrations, marked spatio-temporal variation in silicate concentration has also been observed in estuarine ecosystems. For instance, persistently higher silicate concentration was reported in monsoon season in Zuari estuary (India), (Patil and Anil 2011). Similarly in Chilika lagoon, maximum silicate concentration has been observed during monsoon (Srichandan et al. 2015a, b). It was suggested that decreased silicate concentration in pre-monsoon could be due to the utilization of silicate by a large number of Bacillariophyta for the synthesis of their shells. This was evident from a strong negative correlation between chlorophyll (Chl-a) and silicate during pre-monsoon (r = −0.331, p < 0.05). The source of silicate in lagoon is mainly the heavy inflow of freshwater from riverine distributaries and land drainage of catchment area.
Turbidity has been frequently cited as a key factor controlling the distribution, abundance and diversity of phytoplankton in estuaries. For instance, in Dhamra River Estuary (India) and Na Thap River Estuary (Thailand), distributions and compositions of phytoplankton have been reported to have relationship with changes in turbidity (Palleyi et al. 2011; Lueangthuwapranit et al. 2011). In Chilika lagoon, several studies have mentioned turbidity as the major controlling factor of primary producer (Patnaik 1973; Srichandan et al. 2015a, b). It was also observed that passage of tropical cyclone Phailin increased the turbidity (221.4 NTU (nephelometric turbidity units)) via influx of exogenous material of terrestrial origin and restricted the development of phytoplankton bloom after Phailin. In addition, satellite remote sensing imagery has also revealed that the phytoplankton biomass did not change much due to high turbidity prevailing in the lagoon after Phailin (Srichandan et al. 2015b). Furthermore, many studies have shown that phytoplankton community structure is highly correlated with pH. For example, a positive correlation between Cyanophyta abundance and pH has been noted in the estuarine region of southeastern coast of Tamilnadu, India (Ramanathan et al. 2013). Similarly, a strong positive correlation between Cyanophyta abundance and pH (r = 0.450, p < 0.01) has been observed in Chilika lagoon (Srichandan et al. 2015b). Thus phytoplankton flora of Chilika lagoon is susceptible to change under the influence of mainly salinity, light availability, pH, and nutrients resulting heterogeneity in species composition, and population size of phytoplankton.
11.7 Future Directions
Compared to understanding on the microplankton (20–200 μm), there are significant knowledge gaps regarding the species composition of picoplankton and nanoplankton in Chilika lagoon. Detailed literature search indicated that the genetic diversity of picophytoplankton community of Chilika lagoon is completely unexplored and warrants a thorough investigation using high-throughput DNA sequencing. In fact, the molecular genetic diversity of picophytoplankton, as such, from any of Indian coastal ecosystems remains poorly understood. This necessitates the application of high-throughput DNA sequencing in the area of phytoplankton ecology to understand the diversity and distribution of smaller size phytoplankton. Further, intense monitoring is necessary to study the dynamics of phytoplankton population with respect to tidal and diurnal variation in Chilika lagoon. Further, climate change is recognized as a major threat to the survival of species and integrity of ecosystems world wide. In Chilika lagoon, rise in water temperatures by 0.39 °C in a decade have already been observed (Pandey 2015). Changes in the size-structure of phytoplankton communities in response to warming are now being documented across a range of ecosystem types and spatial scales. Therefore, further intensive studies on phytoplankton dynamics in Chilika lagoon in the context of climate change assumes greater importance. Apart from response to varying temperature, phytoplankton plays an important role in cloud formation by producing dimethyle sulfide which acts as cloud condensation nuclei. Hence, role of lagoon phytoplankton in such aspects need to be investigated. Since the lagoon is prone to anthropogenic pollution and deterioration of water quality, possibilities and scope of phyto-remediation strategies should be explored. As the lagoon supports livelihood of millions of fisher folk who depend on the capture fisheries, the feeding habit of planktivore fishes should be explored for possible implementation of production enhancement strategies.
References
Adhikary SP, Sahu JK (1992) Distribution and seasonal abundance of algal forms in Chilika Lake, east coast of India. Jpn J Limnol 53:197–205
Alkawri AAS, Ramaiah N (2010) Spatio-temporal variability of dinoflagellate assemblages in different salinity regimes in the West Coast of India. Harmful Algae 9:153–162
Aquino EP, Figueiredo LGP, Borges GCP, Ferreira LC, Passavante JZDO, Gloria MD, Silva-Cunha GD (2015) Seasonal and spatial variation in phytoplankton community structure of an estuary in Northeastern Brazil. Trop Ecol 56(1):125–131
Bec B, Collos Y, Souchu P, Vaquer A, Lautier J, Fiandrino A, Benau L, Orsoni V, Laugier T (2011) Distribution of picophytoplankton and nanophytoplankton along an anthropogenic eutrophication gradient in French Mediterranean coastal lagoons. Aquat Microb Ecol 63:29–45. http://sci-hub.tw/10.3354/ame01480
Biswas K (1932) Algal flora of the Chilika lake. Mem Asiat Soc Bengal 11:65–198
Brewin RJW, Lavender SJ, Hardman-Mountford NJ, Hirata T (2010) A spectral response approach for detecting dominant phytoplankton size class from satellite remote sensing. Acta Oceanol Sin 29(2):14–32. http://sci-hub.tw/10.1007/s13131-010-0018-y
Brogueira MJ, Oliveira MDR, Cabeçadas G (2007) Phytoplankton community structure defined by key environmental variables in Tagus estuary, Portugal. Mar Environ Res 64(5):1–29. http://sci-hub.tw/10.1016/j.marenvres.2007.06.007
Burkholder JM, Glibert PM, Skelton HM (2008) Mixotrophy, a major mode of nutrition for harmful algal species in coastal waters. Harmful Algae 8:77–93
Cabeçadas L (1999) Phytoplankton production in Tagus estuary (Portugal). Oceanol Acta 22:51–65
Canini ND, Metillo EB, Azanza RV (2013) Monsoon influenced phytoplankton community structure in a Philippine mangrove estuary. Trop Ecol 54(3):331–343
Chen B, Xu Z, Zhou Q, Chen C, Gao Y, Yang S, Ji W (2010) Long-term changes of phytoplankton community in Xiagu waters of Xiamen, China. Acta Oceanol Sin 29(6):104–114. http://sci-hub.tw/10.1007/s13131-010-0081-4
Cheung MK, Au CH, Chu KH, Kwan HS, Wong CK (2010) Composition and genetic diversity of picoeukaryotes in subtropical coastal waters as revealed by 454 pyrosequencing. ISME J 4:1053–1059. http://sci-hub.tw/10.1038/ismej.2010.26
Chu TV, Torréton J-P, Mari X, Nguyen HMT, Pham KT, Pham TT, Bouvier T, Bettarel Y, Pringault O, Bouvier C, Rochelle-Newall E (2014) Nutrient ratios and the complex structure of phytoplankton communities in a highly turbid estuary of Southeast Asia. Environ Monit Assess 186(12):8555–8572. http://sci-hub.tw/10.1007/s10661-014-4024-y
Cloern JE, Dufford R (2005) Phytoplankton community ecology: principles applied in San Francisco Bay. Mar Ecol Prog Ser 285:11–28
Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, Likens GE (2009) Controlling eutrophication: nitrogen and phosphorus. Science 323:1014–1015
Cremer H, Sangiorgi F, Wagner-Cremer F, Mcgee V, Lotter AF, Visscher H (2007) Diatoms (Bacillariophyceae) and Dinoflagellate Cysts (Dinophyceae) from Rookery Bay, Florida, USA. Caribb J Sci 43:23–58
Daugbjerg N, Henriksen P (2001) Pigment composition and rbcL sequence data from the silicoflagellate Dictyocha speculum: a heterokont alga with pigments similar to some haptophytes. J Phycol 37:1110–1120
Dawson EY (1966) Marine botany: an introduction. Holt, Rinehart and Winston, New York. 371p
Devasundaram MP, Roy JC (1954) A preliminary study of the plankton of the Chilka Lake for the year 1950–1951. In: Symposium on marine and fresh-water plankton in the Indo-Pacific, Indo-Pacific, Fish. Com. Publication, pp 1–7
Diez B, Pedros-Alio C, Massana R (2001) Study of genetic diversity of eukaryotic picoplankton in different oceanic regions by small-subunit rRNA gene cloning and sequencing. Appl Environ Microbiol 67(7):2932–2941
Gaines G, Elbrächter M (1987) Heterotrophic nutrition. In: Taylor FJR (ed) The biology of dinoflagellates. Botanical monograph, vol 21. Blackwell Scientific Publications, Oxford, pp 224–268
Ganguly D, Patra S, Muduli PR, Vishnu Vardhan K, Abhilas KR, Robin RS, Subramanian BR (2015) Influence of nutrient input on the trophic state of a tropical brackish water lagoon. J Earth Syst Sci 124(5):1005–1017
George B, Nirmal Kumar JI, Kumar RN (2012) Study on the influence of hydro-chemical parameters on phytoplankton distribution along Tapi estuarine area of Gulf of Khambhat, India. Egypt J Aqua Res 38:157–170
Gle G, Amo YD, Sautour B, Laborde P, Chardy P (2008) Variability of nutrients and phytoplankton primary production in a shallow macrotidal coastal ecosystem (Arcachon Bay, France). Estuar Coast Shelf Sci 76:642–656
Glibert PM, Legrand C (2006) The diverse nutrient strategies of harmful algae: focus on osmotrophy. In: Granéli E, Turner J (eds) The ecology of harmful algae. Springer, New York, pp 163–176
Goebel NL, Edwards CA, Zehr JP, Follows MJ, Morgan SG (2013) Modeled phytoplankton diversity and productivity in the California current system. Ecol Model 264:37–47. http://sci-hub.tw/10.1016/j.ecolmodel.2012.11.008
Goma J, Rimet F, Cambra J, Hoffmann L, Ector L (2005) Diatom communities and water quality assessment in Mountain Rivers of the upper Segre basin (La Cerdanya, Oriental Pyrenees). Hydrobiologia 551:209–225
Gómez F, Gorsky G (2003) Annual microplankton cycles in Villefranche Bay, Ligurian Sea, NW Mediterranean. J Plankton Res 25(4):323–339
Guerrero F, Rodriguez V (1998) Existence and significance of Acartia grani resting eggs (Copepoda: Calanoida) in sediments of a coastal station in the Alboran Sea (SE Spain). J Plankton Res 20:305–314
Gupta GVM, Sarma VVSS, Robin RS, Raman AV, Kumar M, Rakesh M, Subramanian BR (2008) Influence of net ecosystem metabolism in transferring riverine organic carbon to atmospheric CO2 in a tropical coastal lagoon (Chilka Lake, India). Biogeochemistry 87(3):265–285
Harris JM, Vinobaba P (2012) Impact of water quality on species composition and seasonal fluctuation of plankton of Batticaloa lagoon, Sri Lanka. J Ecosyst Ecogr 2(4):1–6. http://sci-hub.tw/10.4172/2157-7625.1000117
Harsha TS, Malammanavar SG (2004) Assessment of phytoplankton density in relation to environmental variables in Gopalaswamy pond at Chitradurga, Karnataka. J Environ Biol 25:113–116
Henriksen P, Knipschildt F, Moestrup Ø, Thomsen HA (1993) Autoecology, life history and toxicology of the silicoflagellate Dictyocha speculum (Silicoflagellata, Dictyochophyceae). Phycologia 32:29–39
Huang L, Jian W, Song X, Huang X, Liu S, Qian P, Yin K, Wu M (2004) Species diversity and distribution for phytoplankton of the Pearl River estuary during rainy and dry seasons. Mar Pollut Bull 49:588–596
Jeong HJ, Yoo YD, Kim JS, Seong KA, Kang NS, Kim TH (2010) Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci J 45(2):65–91. http://sci-hub.tw/10.1007/s12601-010-0007-2
Jha BC, Vass KK, Panda S, Bhatta KS (2009) Algal biodiversity of Chilika lagoon. CIFRI, Barrackpore and CDA, Bhubaneswar, pp 1–144. ISSN 0970-616X
Jochem F, Babenerd B (1989) Naked Dictyocha speculum—a new type of phytoplankton blooms in the Western Baltic. Mar Biol 103:373–379
Kirchman DL, Suzuki Y, Garside C, Ducklow HW (1991) High turnover rates of dissolved organic carbon during a spring phytoplankton bloom. Nature, London 352:612–614
Krebs CJ (1994) Ecology: the experimental analysis of distribution and abundance, 4th edn. Harper Collins College Publishers, New York, p 801
Kudela RM, Peterson TD (2009) Influence of a buoyant river plume on phytoplankton nutrient dynamics: what controls standing stocks and productivity? J Geophys Res 114:15
Kumar NSV, Hosmani SP (2006) Algal biodiversity in fresh water and related physic chemical factors. J Nat Environ Pollut Technol 5:37–40
Laskar HS, Gupta S (2009) Phytoplankton diversity and dynamics of Chatla floodplain lake, Barak Valley, Assam, North East India – a seasonal study. J Environ Biol 30:1007–1012
Leander BS, Keeling PJ (2004) Early evolutionary history of dinoflagellates and apicomplexans (Alveolata) as inferred from HSP90 and actin phylogenies. J Phycol 40:341–350
Lie AAY, Wong CK, Lam JYC, Liu JH, Yung YK (2011) Changes in the nutrient ratios and phytoplankton community after declines in nutrient concentrations in a semi-enclosed bay in Hong Kong. Mar Environ Res 71(3):178–188. http://sci-hub.tw/10.1016/j.marenvres.2011.01.001
Lionard M, Muylaert K, Gansbeke DV, Vyverman W (2005) Influence of changes in salinity and light intensity on growth of phytoplankton communities from the Schelde river and estuary (Belgium/The Netherlands). Hydrobiologia 540:105–115. http://sci-hub.tw/10.1007/S10750-004-7123-X
Lueangthuwapranit C, Sampantarak U, Wongsai S (2011) Distribution and abundance of phytoplankton: influence of salinity and turbidity gradients in the Na Thap River, Songkhla Province, Thailand. J Coast Res 27(3):585–594. http://sci-hub.tw/10.2112/JCOASTRES-D-10-00123.1
Macedo MF, Duarte P, Mendes P, Ferreira JG (2001) Annual variation of environmental variables, phytoplankton species composition and photosynthetic parameters in a Costal lagoon. J Plankton Res 23(7):719–732
Marshall HG (1993) Monitoring phytoplankton populations in the Chesapeake Bay, USA. Idee Ekologii Tom 3, Ser Szkice nr 2:45–60
Martini E (1977) Systematics, distribution and stratigraphical application of silicoflagellates. In: Ramsey ATSE (ed) Oceanic micropaleontology. Academic, London, pp 1327–1343
Masuda LSM, Moser GAO, Barrera-Alba JJ (2011) Variação temporal do fitoplâncton no estuarino de Santos (SP) [Temporal variation of phytoplankton in the Santos estuary]. Braz J Aquat Sci Technol 15:79–93
McQuoid MR, Godhe A, Nordberg K (2002) Viability of phytoplankton resting stages in the sediments of a coastal Swedish fjord. Eur J Phycol 37:191–201
Mohanty D, Adhikary SP (2013) Assessment of changes in the algal diversity of Chilika lagoon after opening of New Mouth to Bay of Bengal. J Water Resour Prot 5:611–623
Mohanty SK, Mishra SS, Khan M, Mohanty RK, Mohapatra A, Pattnaik AK (2015) Ichthyofaunal diversity of Chilika Lake, Odisha, India: an inventory, assessment of biodiversity status and comprehensive systematic checklist (1916–2014). Check List 11(6):1–19. http://sci-hub.tw/10.15560/11.6.1817
Moreira-González A, Seisdedo-Losa M, Muñoz-Caravaca A, Comas-González A, Alonso-Hernández C (2014) Spatial and temporal distribution of phytoplankton as indicator of eutrophication status in the Cienfuegos Bay, Cuba. J Integr Coast Zone Manag 14(4):597–609
Moreno-Díaz G, Rojas-Herrera AA, Violante-González J, González-González J, Acevedo JLR, Ibáñez SG (2015) Temporal variation in composition and abundance of phytoplankton species during 2011 and 2012 in Acapulco Bay, Mexico. Open J Mar Sci 5:358–367
Mukherjee M, Karna SK, Suresh VR, Manna RK, Panda D, Sharma AP, Pati MK, Mandal S, Ali Y (2016) Dinoflagellate diversity and distribution in Chilika Lagoon with description of new records. Indian J Geo Mar Sci 45:999–1009
Mukhopadhyay SK, Biswas H, De TK, Jana TK (2006) Fluxes of nutrients from the tropical river Hooghly at the land-ocean boundary of Sundarbans, NE Coast of Bay of Bengal. Indian J Mar Sys 6:9–21
Myklestad SM (2000) Dissolved organic carbon from phytoplankton. In: Wangersky P (ed) The handbook of environmental chemistry [D], Marine chemistry. Springer, Berlin, pp 111–148
Naik S, Acharya BC, Mohapatra A (2009) Seasonal variation of phytoplankton in Mahanadi estuary, east coast of India. Indian J Mar Sci 38:184–190
Newton A, Icely JD, Cristina S, Brito A, Cardoso AC, Colijn F, Riva SD, Gertz F, Hansen JW, Holmer M, Ivanova K, Leppäkoski E, Canu DM, Mocenni C, Mudge S, Murray N, Pejrup M, Razinkovas A, Reizopoulou S, Pérez-Ruzafa A, Schernewski G, Schubert H, Carr L, Solidoro C, Viaroli P, Zaldívar JM (2014) An overview of ecological status, vulnerability and future perspectives of European large shallow, semi-enclosed coastal systems, lagoons and transitional waters. Estuar Coast Shelf Sci 140:95–122. http://sci-hub.tw/10.1016/j.ecss.2013.05.023
Newton A, Brito AC, Icely JD, Derolez V, Clara I, Angus S, Schernewski G, Inácio M, Lillebø AI, Sousa AI, Béjaoui B (2018) Assessing, quantifying and valuing the ecosystem services of coastal lagoons. J Nat Conserv 44:50–65
Ning X, Cloern JE, Cole BE (2000) Spatial and temporal variability of picocyanobacteria Synechococcus sp. in San Francisco Bay. Limnol Oceanogr 45:695–702
Olson RR, Olson MH (1989) Food limitation of planktotrophic marine invertebrate larvae: does it control recruitment success. Annu Rev Ecol Evol Syst 20:225–247
Onodera J, Takahashi K (2005) Silicoflagellate fluxes and environmental variations in the northwestern North Pacific during December 1997–May 2000. Deep-Sea Res I 52:371–388
Onyema IC (2013) Phytoplankton bio-indicators of water quality situations in the Iyagbe Lagoon, South-Western Nigeria. J Life Phy Sci 4(2):93–107
Palleyi S, Kar RN, Panda CR (2011) Influence of water quality on the biodiversity of phytoplankton in Dhamra River Estuary of Odisha Coast, Bay of Bengal. J Appl Sci Environ Manag 15(1):69–74
Pandey K (2015) Lakes warming faster than oceans and India’s Chilika is no exception: global study. http://www.downtoearth.org.in/news/lakes-warming-faster-than-oceans-and-india-s-chilika-is-no-exception-global-study-52243
Panigrahi S, Wikner J, Panigrahy RC, Satapathy KK, Acharya BC (2009) Variability of nutrients and phytoplankton biomass in a shallow brackish water ecosystem Chilika Lagoon, India. Limnology 10:73–85
Pasquaud S, David V, Lobry J, Girardin M, Sautour B, Elie P (2010) Exploitation of trophic resources by fish under stressful estuarine conditions. Mar Ecol Prog Ser 400:207–219
Patil JS, Anil AC (2008) Temporal variation of diatom benthic propagules in a monsoon influenced tropical estuary. Cont Shelf Res 28(17):2404–2416. http://sci-hub.tw/10.1016/j.csr.2008.06.001
Patil JS, Anil AC (2011) Variations in phytoplankton community in a monsoon influenced tropical estuary. Environ Monit Assess 182:291–300
Patnaik S (1973) Observation on the seasonal fluctuating of plankton in the Chilika lake. Ind J Fish 20:43–45
Patnaik S, Sarkar SK (1976) Observations on the distribution of phytoplankton in Chilika lake. J Inland Fish Soc India 8:38–48
Pérez-Ruzafa A, Marcos C, Pérez-Ruzafa IM, Pérez-Marcos M (2011) Coastal lagoons: “transitional ecosystems” between transitional and coastal waters. J Coast Conserv 15(3):369–392
Perumal NV, Rajkumar M, Perumal P, Rajasekar KT (2009) Seasonal variations of plankton diversity in the Kaduviyar estuary, Nagapattinam, southeast coast of India. J Environ Biol 30(6):1035–1046
Phlips EJ, Badylak S, Lynch TC (1999) Blooms of the picoplanktonic cyanobacterium Synechococcus in Florida Bay, a subtropical inner-shelf lagoon. Limnol Oceanogr 44:1166–1175
Prabhahar C, Saleshrani K, Enbarasan R (2011) Studies on the ecology and distribution of phytoplankton biomass in Kadalur coastal zone, Tamilnadu, India. Curr Bot 2(3):26–30
Quinlan EL, Phlips EJ (2007) Phytoplankton assemblages across the marine to lowsalinity transition zone in a blackwater dominated estuary. J Plankton Res 29(5):401–416. http://sci-hub.tw/10.1093/plankt/fbm024
Rakhesh M, Madhavirani KSVKS, Charan Kumara B, Raman AV, Kalavati C, Prabhakara Rao Y, Rosamma S, Ranga Rao V, Gupta GVM, Subramanian BR (2015) Trophic–salinity gradients and environmental redundancy resolve mesozooplankton dynamics in a large tropical coastal lagoon. Reg Stud Mar Sci 1:72–84
Raman AV, Satyanarayana C, Adiseshasai K, Phani Prakash K (1990) Phytoplankton characteristics of Chilka lake, a brackish water lagoon along east coast of India. Ind J Mar Sci 19(4):274–277
Ramanathan G, Sugumar R, Jeevarathinam A, Rajarathinam K (2013) Studies of Cyanobacterial distribution in estuary region of southeastern coast of Tamilnadu. Indian J Algal Biomass Util 4(3):26–34
Rath J, Adhikary SP (2008) Biodiversity assessment of algae in Chilika Lake, East Coast of India. In: Monitoring and modelling lakes and coastal environments. Springer, Dordrecht, pp 22–33
Redfield AC, Ketchum BH, Richards FA (1963) The influence of organisms on the composition of sea-water. In: Hill MN (ed) The sea, vol 2. Wiley, New York, pp 26–77
Resende P, Azeiteiro U, Pereira MJ (2005) Diatom ecological preferences in a shallow temperate estuary (Ria de Aveiro, Western Portugal). Hydrobiologia 544:77–88
Rigual-Hernández AS, Bárcena MA, Sierro FJ, Flores JA, Hernnández-Almeida I, Sanchez-Vidal A, Palanquez A, Heussner S (2010) Seasonal to interannual variability and geographic distribution of the silicoflagellate fluxes in the Western Mediterranean. Mar Micropaleontol 77:46–57
Rochelle-Newall EJ, Chu VT, Pringault O, Amouroux D, Arfi R, Bettarel Y, Bouvier T, Bouvier C, Got P, Nguyen TMH, Mari X, Navarro P, Duong TN, Cao TTT, Pham TT, Ouillon S, Torréton JP (2011) Phytoplankton distribution and productivity in a highly turbid, tropical coastal system (Bach Dang Estuary, Vietnam). Mar Pollut Bull 62:2317–2329. http://sci-hub.tw/10.1016/j.marpolbul.2011.08.044
Sahoo D, Kumar S (2015) Xanthophyceae, euglenophyceae and dinophyceae. In: The algae world, volume 26 of the series cellular origin, life in extreme habitats and astrobiology, pp 259–305. http://sci-hub.tw/10.1007/978-94-017-7321-8_9
Sahu G, Mohanty AK, Samantara MK, Satpathy KK (2014) Seasonality in the distribution of dinoflagellates with special reference to harmful algal species in tropical coastal environment, Bay of Bengal. Environ Monit Assess 186:6627–6644. http://sci-hub.tw/10.1007/s10661-014-3878-3
Saify T, Chaghtai SA, Parveen A, Durrani A (1986) Hydrology and periodicity of phytoplankton in the sewage fed Motia pond, Bhopal (India). Geobios 13(5):199–203
Samanta B, Bhadury P (2014) Analysis of diversity of chromophytic phytoplankton in a mangrove ecosystem using rbcL gene sequencing. J Phycol 50(2):328–340. http://sci-hub.tw/10.1111/jpy.12163
Saravanakumar A, Rajkumar M, Thivakaran GA, Serebiah JS (2008) Abundance and seasonal variations of phytoplankton in the creek waters of western mangrove of Kachchh-Gujarat. J Environ Biol 29:271–274
Sasamal SK, Panigrahy RC, Misra S (2005) Asterionella blooms in the northwestern Bay of Bengal during 2004. Int J Remote Sens 26(17):3853–3858
Selvaraj GSD, Thomas VJ, Khambadkar LR (2003) Seasonal variation of phytoplankton and productivity in the surf zone and backwater at Cochin. J Mar Biol Assoc India 45(1):9–19
Sin Y, Wetzel RL, Anderson IC (2000) Seasonal variations of size-fractionated phytoplankton along the salinity gradient in the York River Estuary, Virginia (USA). J Plankton Res 22:1945–1960
Sondergaard M, Riemann BJ, Orgensen NOG (1985) Extracellular organic carbon (EOC) released by phytoplankton and bacterial production. Oikos 45:323–332
Srichandan S, Kim JY, Bhadury P, Barik SK, Muduli PR, Samal RN, Pattnaik AK, Rastogi G (2015a) Spatiotemporal distribution and composition of phytoplankton assemblages in a coastal tropical lagoon: Chilika, India. Environ Monit Assess 187:47. http://sci-hub.tw/10.1007/s10661-014-4212-9
Srichandan S, Kim JY, Kumar A, Mishra DR, Bhadury P, Muduli PR, Rastogi G (2015b) Interannual and cyclone-driven variability in phytoplankton communities of a tropical coastal lagoon. Mar Pollut Bull 101(1):39–52
Stal LJ (2012) Cyanobacterial mats and stromatolites. In: Whitton BA (ed) The ecology of cyanobacteria. Springer, London, pp 65–125
Stevenson RJ, Pan Y (1999) Assessing environmental conditions in rivers and streams with diatoms. In: Stoermer EF, Smol JP (eds) The diatoms: applications for the environment and earth science. Cambridge University Press, Cambridge
Takahashi K, Onodera J, Katsuki K (2009) Significant populations of seven-sided Distephanus (Silicoflagellata) in the sea-ice covered environment of the central Arctic Ocean, summer 2004. Micropaleontology 55:313–325
Valdes-Weaver LM, Piehler MF, Pinckney JL, Howe KE, Rossignol K, Paerl HW (2006) Long-term temporal and spatial trends in phytoplankton biomass and class level composition in the hydrologically variable Neuse-Pimlico estuarine continuum, North Carolina, USA. Limnol Oceanogr 51(3):1410–1420
Varona-Cordero F, GutiErrez-mendieta FJ, Castillo MEMd (2010) Phytoplankton assemblages in two compartmentalized coastal tropical lagoons (Carretas-Pereyra and Chantuto-Panzacola, Mexico). J Plankton Res 32(9):1283–1299. http://sci-hub.tw/10.1093/plankt/fbq043
Wehr JD, Descy JP (1998) Use of phytoplankton in large river management. J Phycol 34:741–749
Wissel B, Fry B (2005) Tracing Mississippi River influences in estuarine food webs of coastal Louisiana. Oecology 144:659–672
Xiao X, Sogge H, Lagesen K, Tooming-Klunderud A, Jakobsen KS, Rohrlack T (2014) Use of high throughput sequencing and light microscopy show contrasting results in a study of phytoplankton occurrence in a freshwater environment. PLoS One 9(8):e106510
Yoo KI (1991) Population dynamics of dinoflagellate community in Masan bay with a note on the impact of environmental parameters. Mar Pollut Bull 23:185–188
Zhang X, Zhang J, Huang X, Huang L (2014) Phytoplankton assemblage structure shaped by key environmental variables in the Pearl River Estuary, South China (2014). J Ocean Univ China 13:73–82
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Srichandan, S., Rastogi, G. (2020). Spatiotemporal Assessment of Phytoplankton Communities in the Chilika Lagoon. In: Finlayson, C., Rastogi, G., Mishra, D., Pattnaik, A. (eds) Ecology, Conservation, and Restoration of Chilika Lagoon, India. Wetlands: Ecology, Conservation and Management, vol 6. Springer, Cham. https://doi.org/10.1007/978-3-030-33424-6_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-33424-6_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33423-9
Online ISBN: 978-3-030-33424-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)