Abstract
In Nicotiana tabacum (tobacco), nicotine and related pyridine alkaloids are produced in the roots and accumulate mainly in the leaves. Molecular analyses of nicotine biosynthesis, especially of the steps involved in pyrrolidine and pyridine formation, suggest that this specialized pathway evolved through repeated duplication of primary pathways, followed by the recruitment of the metabolic genes into a regulon. In tobacco, jasmonates elicit nicotine formation via a conserved signaling cascade anchored to the downstream nicotine biosynthesis pathway by master transcription factors of the ERF family, particularly ERF189 and its homolog ERF199. ERF transcription factors upregulate metabolic and transport genes directly involved in the pathway by recognizing cis-elements in the promoters of target genes. A pair of homologous clusters of related ERF genes, including ERF189 and ERF199, occurs in the tobacco genome. ERF189 corresponds to the nicotine-controlling NIC2 locus. A large chromosomal deletion of the cluster that includes ERF189, as found in the nic2 mutant allele, has been exploited to breed low-nicotine tobacco.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Alkaloids are a large group of nitrogen-containing specialized metabolites, typically with bioactive properties, that are produced by multiple plant species (Shoji 2016). Nicotine and its derivatives, such as nornicotine, anabasine, and anatabine, are pyridine alkaloids found in Nicotiana species, including cultivated Nicotiana tabacum (tobacco) (Saitoh et al. 1985) (Fig. 9.1). Even though smoking is detrimental to human health, the stimulatory and addictive properties of tobacco alkaloids, which act on nicotinic acetylcholine receptors essential for a range of neuronal activities, account for the widespread consumption of tobacco products.
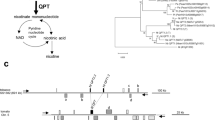
Biosynthesis pathways of nicotine and related alkaloids. Defined steps are shown with arrows and enzyme names; dashed arrows represent undefined or multiple steps. Boxes represent enzymes regulated by ethylene response factor (ERF) transcription factors and thus included in the nicotine biosynthesis regulon. Putrescine N-methyltransferase (PMT) and N-methylputrescine oxidase (MPO1) have been proposed to evolve from spermidine synthase (SPDS) and diamine oxidase (DAO), respectively (Hibi et al. 1994; Heim et al. 2007; Katoh et al. 2007). AO, aspartate oxidase; BBL, berberine bridge enzyme-like protein; NND, nicotine N-demethylase; ODC, ornithine decarboxylase; QPT, quinolinate phosphoribosyltransferase; QS, quinolinate synthase
In tobacco, pyridine alkaloids are synthesized exclusively in underground roots and are largely stored in the leaves as defenses against insects and other predators (Steppuhn et al. 2004). Herbivory can result in increased accumulation of nicotine and other toxic alkaloids (Baldwin 1989). Jasmonates are key signaling molecules that trigger nicotine formation via transcription factors that direct the coordinated expression of genes involved in the nicotine biosynthesis pathway (Shoji et al. 2010). Molecular and genomic analyses have identified genes responsible for nicotine biosynthesis, transport, and regulation (Shoji and Hashimoto 2011a; Dewey and Xie 2013; Shoji and Hashimoto 2013), providing insight into the evolution of this metabolic pathway (Kajikawa et al. 2017a. Shoji 2019).
9.2 Biosynthesis
Nicotine is composed of heterocyclic pyrrolidine and pyridine rings; these rings are formed early in the pathway and are coupled together in later steps (Fig. 9.1). A five-member pyrrolidine ring is formed from ornithine via a symmetric diamine, putrescine, through three consecutive reactions catalyzed by ornithine decarboxylase (ODC) (Imanishi et al. 1998), putrescine N-methyltransferase (PMT) (Hibi et al. 1994), and N-methylputrescine oxidase (MPO) (Heim et al. 2007; Katoh et al. 2007) (Fig. 9.1). An alternative route to putrescine from arginine exists, which includes a step catalyzed by arginine decarboxylase (ADC). However, this route is not considered to contribute greatly to nicotine biosynthesis, because transgenic suppression of ADC, but not of ODC (De Boer et al. 2011a), failed to alter nicotine content significantly (Chintapakorn and Hamill 1990). Because of their structural similarities, PMT and MPO are thought to have arisen through catalytic innovation from primary enzymes involved in related polyamine metabolism, namely, spermidine synthase and diamine oxidase, respectively (Junker et al. 2013; Naconsie et al. 2014) (Fig. 9.1). The ornithine-derived moiety is also used to produce tropane alkaloids (e.g., the clinically important hyoscyamine and scopolamine) and nortropane alkaloids (e.g., calystegines, which inhibit glycosidase) in various Solanaceae species (Shoji and Hashimoto 2015) (Fig. 9.1). The shared branch for pyrrolidine formation may have developed through duplication of the polyamine pathway. The resulting doubling of ODC genes may have enabled increased metabolic flow into this branch, and the subsequent innovative evolution of PMT and MPO1 (Kajikawa et al. 2017a). The establishment of the ring-forming extension before the Solanaceae species diversified is underlined by the common existence of PMT and MPO1 genes in Solanaceae genomes (Kajikawa et al. 2017b; Xu et al. 2017).
A pyridine ring of nicotine and related alkaloids is derived from nicotinic acid, a primary metabolite in the pathway that supplies nicotinamide adenine dinucleotide (NAD), an important co-factor for oxidation–reduction reactions. In the NAD pathway, aspartate is converted to nicotinic acid mononucleotide via quinolinate via steps catalyzed by aspartate oxidase (AO), quinolinate synthase (QS), and quinolinate phosphoribosyltransferase (QPT) (Sinclair et al. 2000; Katoh et al. 2006) (Fig. 9.1). To meet increased metabolic demands to support massive downstream production of nicotine, AO and QPT genes have been duplicated in Nicotiana species, but not in other lineages (Kajikawa et al. 2017b; Xu et al. 2017). Nicotinic acid is supplied as an intermediate of a cyclic pathway for de novo and salvage production of NAD, which starts with nicotinic acid mononucleotide (Noctor et al. 2006) (Fig. 9.1). It is unclear whether nicotinic acid itself or its derivatives are directly incorporated into alkaloids (Shoji and Hashimoto 2011a). To avoid excess accumulation of toxic nicotinic acid, plants have developed mechanisms to convert nicotinic acid to less-toxic derivatives (Li et al. 2015a, b; Li et al. 2017). Using nicotinic acid for alkaloid production may have originally emerged as one such detoxification reaction.
Little is known about the late steps required to couple the heterocyclic rings. It has been proposed that two oxidoreductases, A622 (De Boer et al. 2009; Kajikawa et al. 2009) and berberine bridge enzyme-like protein (BBL) (Kajikawa et al. 2011), catalyze the late stages of the pathway (Fig. 9.1), though biochemical details of their reactions are yet to be defined. A622 and BBL are required to produce not only nicotine, but also other pyridine alkaloids (De Boer et al. 2009; Kajikawa et al. 2009, 2011), implying that A622- and BBL-dependent steps are shared between the pathways that produce the different alkaloids.
Nornicotine is formed from nicotine via demethylation mediated by nicotine N-demethylase (NND) (Fig. 9.1), an enzyme belonging to the CYP82E subfamily of cytochrome P450 monooxygenases. Three genes, CYP82E4, CYP82E5, and CYP82E10, encoding functional NND enzymes have been cloned from tobacco (Siminszky et al. 2005; Gavilano and Siminszky 2007; Lewis et al. 2010). Nornicotine typically accounts for 3–5% of the total alkaloids in mature tobacco leaves. CYP82E5 and CYP82E10 contribute to this conventional accumulation of nornicotine (Gavilano and Siminszky 2007; Lewis et al. 2010). However, a small number of plants within tobacco populations, especially in Burley cultivars, are termed converters, as they convert over 90% of their nicotine to nornicotine during leaf senescence and curing (Griffith et al. 1955). CYP82E4 is responsible for this unstable conversion phenotype, which depends on occasional reactivation of normally silenced CYP82E4 in converters (Siminszky et al. 2005). Nornicotine is reduced to nominal levels by knockout mutations of all three CYP82E genes (Lewis et al. 2010). Because N′-nitrosonornicotine is a carcinogen that is more harmful than other tobacco-specific nitrosamines and is readily formed from nornicotine during the curing process (Bush et al. 2001), reducing nornicotine levels is a desirable breeding goal.
9.3 Transport
Many plant metabolites, both end products and intermediates, move within and between cells. Various membrane-localized transporters, such as those belonging to the ATP-binding cassette, multidrug and toxic compound extrusion (MATE), and purine permease families, mediate the active transport of low-molecular-weight compounds across biological membranes, including alkaloids, which are often positively charged and thus membrane-impermeable (Shoji 2014; Shitan et al. 2014a).
To prevent cytotoxicity when accumulated at high concentrations, nicotine is sequestered into storage vacuoles in tobacco cells. Tonoplast-localized MATE transporters, N. tabacum jasmonate-inducible alkaloid transporter 1 (Nt-JAT1) (Morita et al. 2009), Nt-JAT2 (Shitan et al. 2014b), NtMATE1, and NtMATE2 (Shoji et al. 2009), are proton antiporters that sequester nicotine in the vacuole. These transporters couple proton gradients across the membrane with the energy-consuming uptake of alkaloids into organelles. The Nt-JAT1 and Nt-JAT2 homologs, which are phylogenetically related to xenobiotic-transporting DTX1 from Arabidopsis, mediate the vacuolar sequestration of nicotine in tobacco leaves (Morita et al. 2009; Shitan et al. 2014b). NtMATE1 and NtMATE2, which encode homologs of flavonoid transporters, are co-expressed with nicotine biosynthesis genes, and the encoded proteins are thereby involved in the uptake of nicotine into the vacuoles in alkaloid-producing roots (Shoji et al. 2009). It is noteworthy that NtMATE2 resides near a gene encoding a late-step enzyme A622 (see “Biosynthesis” section) on chromosome (chr) 12 in the tobacco genome; this is the only example of non-homologous clustering among nicotine pathway genes reported to date (Kajikawa et al. 2017b).
Nicotine uptake permease 1 (NUP1) is a plasma membrane-localized purine permease-family transporter of the pyridine ring-bearing molecules nicotine and vitamin B6 (e.g., pyridoxine) (Hildreth et al. 2011; Kato et al. 2014, 2015). Unlike other nicotine pathway genes expressed in inner cell layers (Shoji et al. 2000, 2002, 2009; Shoji and Hashimoto 2011b; Kajikawa et al. 2017b), NUP1 is mainly expressed in root epidermal cells (Kato et al. 2014). In addition to its role in metabolite transport, NUP1 is involved in the genetic regulation of nicotine biosynthesis by way of master transcription factors (see “Regulation” section) and in the regulation of root growth (Hildreth et al. 2011; Kato et al. 2014). The mechanisms underlying this regulation, however, are unclear.
Dawson (1942) conducted a classic grafting experiment that clearly demonstrated root-to-shoot transport of tobacco alkaloids between tobacco rootstock and Solanum lycopersicum (tomato) scion. It is known that nicotine moves up through the xylem along the transpiration stream, and that xylem loading and unloading depend on nicotine efflux from root cells and influx into leaf cells, respectively, although the transporters responsible for these processes have yet to be defined. Unlike most species in the Nicotiana genus, the flowering tobacco N. alata is devoid of alkaloids in aboveground shoots, because it lacks long-distance translocation ability (Pakdeechanuan et al. 2012). Elucidating the genetic basis of this natural variation may provide mechanistic insights into alkaloid transport.
9.4 Regulation
While primary pathways are nearly constitutive, pathways for specialized metabolites are subject to dynamic regulation in developmental and environmental contexts. Metabolic flow through a long, multistep pathway relies on the coordinated expression of metabolic and transport genes, or structural genes. Transcription factors typically control this coordination at the transcriptional level, often forming a multigene network, or regulon, with downstream structural genes (Shoji 2019).
A few transcription factors in the ethylene response factor (ERF) family, particularly ERF189 and its closest homolog ERF199, are involved in the master transcriptional regulation of nicotine pathway genes in tobacco (Dewey and Xie 2013; Shoji and Hashimoto 2013a) (Fig. 9.2). ERF189 and ERF199 are expressed strongly, but not exclusively, in the roots and are induced by jasmonates along with structural genes at the transcript level (Shoji et al. 2010; Kajikawa et al. 2017b). ERF transcription factors upregulate nearly the entire series of nicotine metabolic and transport genes, including ODC2, PMT, MPO1, AO2, QS, QPT2, A622, BBL, NtMATE1, and NtMATE2 (Fig. 9.1), but not NUP1, NtJAT1, or NtJAT2, by directly recognizing specific cis-regulatory elements, termed P boxes, in their promoter regions (Fig. 9.2) (Todd et al. 2010; Shoji et al. 2010; De Boer et al. 2011b; Shoji and Hashimoto 2011b; Kajikawa et al. 2017b). The GC-rich P box resembles a canonical GCC box, and a few amino acid residues critical for its recognition have been identified within the DNA-binding domain of the ERF transcription factors (Shoji et al. 2013).
A model for jasmonate (JA)-dependent regulation of nicotine biosynthesis in tobacco. Ethylene response factor (ERF) transcription factors regulate the structural genes of the nicotine pathway by binding at P box elements in their promoters. JA-induced transcription of the ERF genes is mediated by a basic helix-loop-helix (bHLH)-family MYC2 transcription factor, a direct target of upstream jasmonate ZIM-domain (JAZ) repressors. MYC2 regulates structural genes along with ERF by directly binding to G box elements. JAZ proteins are degraded when a co-receptor complex comprised of coronatine-insensitive 1 (COI1) and JAZ proteins perceives a JA signal. ERF and MYC2 transcription factors are stimulated by a phosphorylation cascade that includes the JA factor-stimulating MAPKK (JAM1). Other details of the regulation of ERF by MYC2 and the phosphorylation cascade are unclear
In the allotetraploid N. tabacum genome, there are two homologous clusters of related ERF genes, including ERF189 and ERF 199. In one cluster, ERF189 is located on chr 19, and in the other, ERF199 is located on chr 7 (Fig. 9.3). These clusters may have originated from the diploid ancestral parents of N. tabacum, N. tomentosiformis, and N. sylvestris, respectively (Shoji et al. 2010; Kajikawa et al. 2017b). The ERF189-containing cluster corresponds to the NIC2 locus, one of two genetic loci controlling nicotine content in tobacco (Shoji et al. 2010). A substantial chromosomal deletion (ca. 650 kb) encompassing a large portion of the cluster, including ERF189 (Fig. 9.3), was found in a nic2 mutant allele (Kajikawa et al. 2017b) used to breed low-nicotine tobacco cultivars (Legg and Collins 1971).
Clustered ethylene response factor (ERF) transcription factor genes in tobacco and tomato. Arrowheads indicate positions and orientations of predicted open reading frames of ERF genes. A chromosomal region of tobacco chr. 19 deleted in nic2 mutant is indicated. Boxes indicate ERF189 and ERF199 from tobacco, which are involved in nicotine regulation, and JRE4 from tomato, which is involved in steroidal glycoalkaloid regulation. Tobacco ERF genes, denoted by Δ, possibly encode non-functional transcription factors that lack full-length DNA-binding domains
Given that ERF genes exhibit low basal levels of expression and are induced by salt stress (Shoji and Hashimoto 2015; Kajikawa et al. 2017b), and considering the limited effects of overexpression on nicotine biosynthesis (Shoji et al. 2010), the contribution to nicotine regulation of clustered ERF genes other than ERF189 and ERF199 is believed to be limited. Experimental evidence contradicting this view, however, exists (De Boer et al. 2011b; Sears et al. 2014). Further work is required to functionally differentiate related and mostly clustered ERF genes in the regulation of nicotine and other pathways.
Clusters of transcription factor genes homologous to tobacco ERFs are present in the genomes of other species (Cárdenas et al. 2016; Thagun et al. 2016; Paul et al. 2017). For instance, JRE4 (also known as GAME9) from tomato (Fig. 9.3) and potato (Solanum tuberosum) (Cárdenas et al. 2016; Thagun et al. 2016, Nakayasu et al. 2018) and ORCAs from Catharanthus roseus (van der Fits and Memelink 2000; Paul et al. 2017) regulate the jasmonate-induced production of specialized metabolites, suggesting that these related factors are functionally similar (Shoji and Hashimoto 2013b). Consistent with such notion, cell type-specific and jasmonate-inducible expression of a promoter reporter of tobacco QPT2 regulated by ERF189 was found to be mediated by JRE4 in tomato (Shoji and Hashimoto 2019).
In plants, jasmonate signals are perceived by a co-receptor complex comprised of coronatine-insensitive 1 (COI1) and jasmonate ZIM-domain (JAZ) proteins. This triggers proteasome-dependent degradation of JAZs and subsequent transcriptional activation mediated by a basic helix-loop-helix (bHLH)-family MYC2 transcription factor liberated from repression by JAZs (Wasternack and Hause 2013) (Fig. 9.2). Jasmonate-induced formation of nicotine depends on COI1, JAZ, and MYC2 proteins in tobacco (Paschold et al. 2007; Shoji et al. 2008; Todd et al. 2010; De Boer et al. 2011b; Shoji and Hashimoto 2011c; Zhang et al. 2012) (Fig. 9.2). In cooperation with ERF factors, tobacco MYC2 factors regulate a series of nicotine pathway genes directly by recognizing G box elements in their promoters (Shoji and Hashimoto 2011c; Zhang et al. 2012). These factors also act indirectly through transcriptional activation of the ERF genes (Shoji and Hashimoto 2011c) (Fig. 9.2). Similar schemes linking MYC2, a central player in general jasmonate signaling, to downstream ERFs and defense metabolism have been demonstrated in other plants (Zhang et al. 2011; Cárdenas et al. 2016; Paul et al. 2017). In addition to transcriptional regulation, ERF proteins are postulated to be regulated by protein phosphorylation in N. tabacum (De Boer et al. 2011b; Paul et al. 2017). In tobacco, a protein phosphorylation cascade involving a mitogen-activated protein kinase kinase (MAPKK), jasmonate factor-stimulating MAPKK (JAM1), has been proposed to stimulate nicotine biosynthesis via the ERF and MYC2 transcription factors (De Boer et al. 2011b) (Fig. 9.2).
A long non-coding RNA acting as an endogenous target mimicry and its corresponding microRNA predicted to target QPT2 gene were identified and found to be involved in topping-triggered induction of nicotine accumulation (Li et al. 2015a, b), presenting an example of regulation of specialized metabolism by a module consisting of non-coding RNAs.
9.5 Perspectives
Molecular and genomic studies have greatly advanced the understanding of the nicotine biosynthesis pathway in tobacco. Complementing gene cloning efforts based mainly on homologies and expression profiles, the genome sequences of tobacco (Sierro et al. 2014) and two wild Nicotiana species (Xu et al. 2017) have revealed properties of the entire suite of genes involved in nicotine biosynthesis and regulation (Kajikawa et al. 2017b; Xu et al. 2017). With several structural and regulatory genes now known, it is easier to genetically manipulate the biosynthesis of the toxic alkaloids (Sato et al. 2001; Lewis et al. 2010; De Boer et al. 2011a). Recent advances have identified a critical role for transcription factors in nicotine regulation, demonstrating conserved regulatory circuits centering on jasmonate-responsive ERF transcription factors. Molecular studies of the nicotine biosynthesis pathway have elucidated how such coordinated systems were established during plant evolution (Kajikawa et al. 2017b; Shoji 2019).
References
Baldwin IT (1989) Mechanism of damage-induced alkaloid production in wild tobacco. J Chem Ecol 25:3–30
Bush LP, Cui M, Shi H, Burton HR (2001) Formation of tobacco specific nitrosamines in air-cured tobacco. Rec Adv Tob Sci 27:23–46
Cárdenas PD, Sonawane PD, Pollier J et al (2016) GAME9 regulates the biosynthesis of steroidal alkaloids and upstream isoprenoids in the plant mevalonate pathway. Nat Commun 7:10654
Chintapakorn Y, Hamill JD (1990) Antisense-mediated reduction in ADC activity causes minor alterations in the alkaloid profile of cultured hairy roots and regenerated transgenic plants of Nicotiana tabacum. Phytochemistry 68:2465–2479
Dawson RF (1942) Accumulation of nicotine in reciprocal grafts of tomato and tobacco. Am J Bot 29:66–71
De Boer K, Lye JC, Aitken CD, Su AK, Hamill JD (2009) The A622 gene in Nicotiana glauca (tree tobacco): evidence for a functional role in pyridine alkaloid synthesis. Plant Mol Biol 69:299–312
De Boer K, Dalton HL, Edward FJ, Hamill JD (2011a) RNAi-mediated down-regulation of ornithine decarboxylase (ODC) leads to reduced nicotine and increased anatabine levels in transgenic Nicotiana tabacum L. Phytochemistry 72:344–355
De Boer K, Tileman S, Pauwels L et al (2011b) APETALA2/ethylene response factor and basic helix-loop-helix transcription factors cooperatively mediate jasmonate-elicited nicotine biosynthesis. Plant J 66:1053–1065
Dewey RE, Xie J (2013) Molecular genetics of alkaloid biosynthesis in Nicotiana tabacum. Phytochemistry 94:10–27
Gavilano LB, Siminszky B (2007) Isolation and characterization of the cytochrome P450 gene CYP82E5v2 that mediates nicotine to nornicotine conversion in the green leaves of tobacco. Plant Cell Physiol 48:1567–1574
Griffith RB, Valleau WD, Stokes GW (1955) Determination and inheritance of nicotine to nornicotine conversion in tobacco. Science 121:343–344
Heim WG, Sykes KA, Hildreth SB et al (2007) Cloning and characterization of a Nicotiana tabacum methylputrescine oxidase transcript. Phytochemistry 68:454–463
Hibi N, Higashiguchi S, Hashimoto T, Yamada Y (1994) Gene expression in tobacco low-nicotine mutants. Plant Cell 6:723–735
Hildreth SB, Gehman EA, Yang H et al (2011) Tobacco nicotine uptake permease 1 (NUP1) affects alkaloid metabolism. Proc Natl Acad Sci USA 108:18179–18184
Imanishi S, Hashizume K, Nakakita M et al (1998) Differential induction of methyl jasmonate of genes encoding ornithine decarboxylase and other enzymes involved in nicotine biosynthesis in tobacco cell culture. Plant Mol Biol 38:1101–1111
Junker A, Fischer J, Sichhart Y, Brandt W, Dráger B (2013) Evolution of the key alkaloid enzyme putrescine N-methyltransferase from spermidine synthase. Front Plant Sci 4:260
Kajikawa M, Hirai N, Hashimoto T (2009) A PIP-family protein is required for biosynthesis of tobacco alkaloids. Plant Mol Biol 69:287–298
Kajikawa M, Shoji T, Katoh A, Hashimoto T (2011) Vacuole-localized berberine bridge enzyme-like proteins are required for a late step of nicotine biosynthesis in tobacco. Plant Physiol 155:2010–2022
Kajikawa M, Sierro N, Hashimoto T, Shoji T (2017a) A model for evolution and regulation of nicotine biosynthesis regulon in tobacco. Plant Signal Behav 12(6):e1338225
Kajikawa M, Sierro N, Kawaguchi H et al (2017b) Genomic insights into the evolution of the nicotine biosynthesis pathway in tobacco. Plant Physiol 174:999–1011
Kato K, Shitan N, Shoji T, Hashimoto T (2015) Tobacco NUP1 transports both tobacco alkaloids and vitamin B6. Phytochemistry 113:33–40
Kato K, Shoji T, Hashimoto T (2014) Tobacco nicotine uptake permease regulates the expression of a key transcription factor gene in the nicotine biosynthesis pathway. Plant Physiol 166:2195–2204
Katoh A, Shoji T, Hashimoto T (2007) Molecular cloning of N-methylputrescine oxidase from tobacco. Plant Cell Physiol 48:550–554
Katoh A, Uenohara K, Akita M, Hashimoto T (2006) Early steps in the biosynthesis of NAD in Arabidopsis start with aspartate and occur in the plastid. Plant Physiol 141:851–857
Legg PG, Collins GB (1971) Inheritance of percent total alkaloids in Nicotiana tabacum L. II. Genetic effects of two loci in Burley21 × LA Burley21 population. Can J Genet Cytol 13:287–291
Lewis RS, Bowen SW, Keogh MR, Dewey RE (2010) Three nicotine demethylase genes mediate nornicotine biosynthesis in Nicotiana tabacum L.: functional characterization of the CYP82E10 gene. Phytochemistry 71:1988–1998
Li F, Wang W, Zhao N et al (2015a) Regulation of nicotine biosynthesis by an endogenous target mimicry of microRNA in tobacco. Plant Physiol 169:1062–1071
Li W, Zhang F, Chang Y et al (2015b) Nicotine O-glucosylation is an evolutionally metabolic trait important for seed germination under stress conditions in Arabidopsis thaliana. Plant Cell 27:1907–1924
Li W, Zhang F, Wu R et al (2017) A novel N-methyltransferase in Arabidopsis appears to feed a conserved pathway for nicotinate for nicotinate detoxification among land plants and is associated with lignin biosynthesis. Plant Physiol 174(3):1492–1504
Morita M, Shitan N, Sawada K et al (2009) Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc Natl Acad Sci USA 106:2447–2452
Naconsie M, Kato K, Shoji T, Hashimoto T (2014) Molecular evolution of N-methylputrescine oxidase in tobacco. Plant Cell Physiol 55:436–444
Nakayasu M, Shioya N, Shikata M et al (2018) JRE4 is a master transcriptional regulator of defense-related steroidal glycoalkaloids in tomato. Plant J 94:975–990
Noctor G, Queval G, Gakiere B (2006) NAD(P) synthesis and pyridine nucleotide cycling in plants and their potential importance in stress conditions. J Exp Bot 57:1603–1620
Pakdeechanuan P, Shoji T, Hashimoto T (2012) Root-to-shoot translocation of alkaloids is dominantly suppressed in Nicotiana alata. Plant Cell Physiol 53:1247–1254
Paschold A, Hailtschke R, Baldwin IT (2007) Co(i)-ordinating defenses: NaCOI1 mediates herbivore-induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J 51:79–91
Paul P, Singh SK, Patra B et al (2017) A differentially regulated AP2/ERF transcription factor gene cluster acts downstream of a MAK kinase cascade to modulate terpenoid indole alkaloid biosynthesis in Catharanthus roseus. New Phytol 213:1107–1123
Saitoh K, Noma M, Kawashima N (1985) The alkaloid contents of sixty Nicotiana species. Phytochemistry 24:477–480
Sato F, Hashimoto T, Hachiya A et al (2001) Metabolic engineering of plant alkaloid biosynthesis. Proc Natl Acad Sci USA 98:367–372
Sears MT, Zhang H, Rushton PJ et al (2014) NtERF32: a non-NIC2 locus AP2/ERF transcription factor required in jasmonate-inducible nicotine biosynthesis in tobacco. Plant Mol Biol 84:49–66
Shitan N, Kato K, Shoji T (2014a) Alkaloid transporters in plants. Plant Biotechnol 31:453–463
Shitan N, Minami S, Morita M et al (2014b) Involvement of the leaf-specific multidrug and toxic compound extrusion (MATE) transporter Nt-JAT2 in vacuolar sequestration of nicotine in Nicotiana tabacum. PLoS ONE 9:e108789
Shoji T (2014) ATP-binding cassette and multidrug and toxic compound extrusion transporters in plants: a common theme among diverse detoxification mechanisms. Int Rev Cell Mol Biol 309:308–346
Shoji T (2016) Alkaloid biosynthesis and regulation in plants. In: Arimura G, Maffei M (eds) Plant specialized metabolism: genomics, biochemistry, and biological functions. CRC Press, Boca Raton, pp 85–118
Shoji T (2019) The recuirtment model of metabolic evolution: jasmonate-responsive transcription factors and a conceptual model for the evolution of metabolic pathways. Front Plant Sci 10:e560
Shoji T, Hashimoto T (2011a) Nicotine biosynthesis. In: Ashihara H, Crozier A, Komamine A (eds) Plant metabolism and biotechnology, John Wiley & Sons, New York, pp 191–216
Shoji T, Hashimoto T (2011b) Recruitment of a duplicated primary metabolism gene into the nicotine biosynthesis regulon in tobacco. Plant J 67:949–959
Shoji T, Hashimoto T (2011c) Tobacco MYC2 regulates jasmonate-inducible nicotine biosynthesis genes directly and by a way of the NIC2-locus ERF genes. Plant Cell Physiol 52:1117–1130
Shoji T, Hashimoto T (2013a) Smoking out the masters: transcriptional regulators for nicotine biosynthesis in tobacco. Plant Biotechnol 30:217–224
Shoji T, Hashimoto T (2013b) Jasmonate-responsive transcription factors: new tools for metabolic engineering and gene discovery. In: Chandra S, Lata H, Varma A (eds) Biotechnology for medicinal plants: micropropagation and improvement. Springer Publishing, New York, pp 345–357
Shoji T, Hashimoto T (2015) Stress-induced expression of NICOTINE2-locus genes and their homologs encoding Ethylene Response Factor transcription factors in tobacco. Phytochemistry 113:41–49
Shoji T, Hashimoto T (2019) Expression of a tobacco nicotine biosynthesis gene depends on the JRE4 transcription factor in heterogonous tomato. J Plant Res 132:173–180
Shoji T, Inai K, Yazaki Y et al (2009) Multidrug and toxic compound extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots. Plant Physiol 149:708–718
Shoji T, Kajikawa M, Hashimoto T (2010) Clustered transcription factor genes regulate nicotine biosynthesis in tobacco. Plant Cell 22:3390–3409
Shoji T, Mishima M, Hashimoto T (2013) Divergent DNA-binding specificities of a group of ETHYLENE RESPONSE FACTOR transcription factors involved in plant defense. Plant Physiol 162:977–990
Shoji T, Ogawa T, Hashimoto T (2008) Jasmonate-induced nicotine formation in tobacco is mediated by tobacco COI1 and JAZ1 genes. Plant Cell Physiol 49:1003–1012
Shoji T, Winz R, Iwase T et al (2002) Expression patterns of two tobacco isoflavone reductase-like genes and their possible roles in secondary metabolism in tobacco. Plant Mol Biol 50:427–440
Shoji T, Yamada Y, Hashimoto T (2000) Jasmonate induction of putrescine N-methyltransferase genes in the root of Nicotiana sylvetris. Plant Cell Physiol 41:831–839
Sierro N, Battey JN, Ouadi S et al (2014) The tobacco genome sequence and its comparison with those of tomato and potato. Nat Commun 5:3833
Siminszky B, Gavilano L, Bowen SW, Dewey RE (2005) Conversion of nicotine to nornicotine in Nicotiana tabacum is mediated by CYP82E4, a cytochrome P450 monooxygenase. Proc Natl Acad Sci USA 102:14919–14924
Sinclair SJ, Murphy KJ, Birch CD, Hamill JD (2000) Molecular characterization of qunolinate phosphoribosyltransferase (QPRtase) in Nicotiana. Plant Mol Biol 44:603–617
Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT (2004) Nicotine’s defensive function in nature. PLoS Biol 2:1074–1080
Thagun C, Imanishi S, Kudo T et al (2016) Jasmonate-responsive ERF transcription factors regulate steroidal glycoalkaloid biosynthesis in tomato. Plant Cell Physiol 57:961–975
Todd AT, Liu E, Polvi SL, Pammett RT, Page JE (2010) A functional genomics screen identifies diverse transcription factors that regulate alkaloid biosynthesis in Nicotiana benthamiana. Plant J 62:589–600
van der Fits L, Memelink J (2000) ORCA3, a jamonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perpection, signal transdaction and action in plant stress response, growth and development: an update to the 2007 review in Annals of Botany. Ann Bot 11:1021–1058
Xu S, Brockmöller T, Navarro-Quezada A et al (2017) Wild tobacco genomes reveal the evolution of nicotine biosynthesis. Proc Natl Acad Sci USA 114:6133–6138
Zhang H, Hedhili S, Montiel G et al (2011) The basic helix-loop-helix transcription factor CrMYC2 controls the jasmonate-responsive expression of the ORCA genes regulating alkaloid biosynthesis in Catharanthus roseus. Plant J 67:61–71
Zhang HB, Bokowiec MT, Rushton PJ, Han SC, Timko MP (2012) Tobacco transcription factors NtMYC2a and NtMYC2b form nuclear complexes with the NtJAZ1 repressor and regulate multiple jasmonate-inducible steps in nicotine biosynthesis. Mol Plant 5:73–84
Acknowledgements
Thanks to Dr. Takashi Hashimoto of Nara Institute of Science and Technology for his collaboration and support. Research in the author’s group was supported in part by grants from the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research No. 17K07447) to TS.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shoji, T. (2020). Nicotine Biosynthesis, Transport, and Regulation in Tobacco: Insights into the Evolution of a Metabolic Pathway. In: Ivanov, N.V., Sierro, N., Peitsch, M.C. (eds) The Tobacco Plant Genome. Compendium of Plant Genomes. Springer, Cham. https://doi.org/10.1007/978-3-030-29493-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-29493-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29492-2
Online ISBN: 978-3-030-29493-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)