Abstract
Glycine betaine (N,N,N-trimethyl glycine, GB) plays an important role in the response of plants to abiotic stress, mainly hydric stress. The aim of this review is to gather information about biochemical processes in which glycine betaine is involved and their impact in plant growth and development. In plants, GB is synthesized by two choline oxidation steps: the first step is choline oxidation to betaine aldehyde (BA) catalyzed by choline monooxygenase, and the second step is BA oxidation to GB catalyzed by betaine aldehyde dehydrogenase which uses NAD(P)+ as coenzyme. In plants, GB synthesis takes place in chloroplast, peroxisome, and cytoplasm. There is scarce information about GB degradation routes in plants. The role of GB as osmolyte is well known, but only until recently, the participation of GB in several metabolic processes including regulation of gene expression, regulation of the concentration and activity of enzymes, and proper protein folding and association has been studied. GB plays a role in growth and development because it increases photosynthetic capacity and protects the thylakoid membrane and increases antioxidant enzymes activity and concentration. GB synthesis provokes changes in ethylene synthesis and increases expression of auxin responsive IAA gene levels. The modulation mechanisms mediated by GB are described in this work.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Plant growth and development is affected by abiotic stress which results in important losses in plant yield and in the money spent in agriculture. The abiotic stress that has a strong impact on plant yield is the hydric stress (drought, salinity, and cold). Plants have developed strategies to contend with hydric stress, between them one of the most important is the synthesis and accumulation of osmolytes (Yancey et al. 1982). In plants, one of the most studied osmolytes is glycine betaine followed by proline and trehalose (Singh et al. 1972; Stewart and Lee 1974; Hare et al. 1998; Iordachescu and Imai 2008; Chen and Murata 2008; Paul et al. 2008; Krasenky and Jonak 2012).
Glycine betaine (N,N,N-trimethyl glycine, GB) is a quaternary amine, isolated for the first time from sugar beet (Scheibler 1869). In mammals, GB participates in homocysteine/methionine cycle [Hcy/Met cycle] as methyl donor to homocysteine to produce methionine, reaction catalyzed by betaine homocysteine methyl transferase [BHMT] (du Vigneaud et al. 1946; Finkelstein and Martin 1984; Pajares and Pérez-Sala 2006). As a consequence of GB participation in the Hcy/Met cycle, a wide set of physiological roles of GB has been found (Craig 2004; Olthof and Verhoef 2005; Lawson-Yuen and Levy 2006; Lever and Slow 2010; Ueland 2011; Figueroa-Soto and Valenzuela-Soto 2018).
Physiological functions of GB are as an osmolyte to contribute to maintaining cellular volume, as an osmoprotector to protect cells under stress, and/or as a source of methyl groups through transmethylation reactions (Takabe et al. 2006; Craig 2004; Chen and Murata 2011). However, not all plants accumulate GB in response to stress; in fact, the vast majority of plants of agricultural importance are not accumulators of GB. For this reason, attempts have been made to genetically transform those plants with the genes of GB synthesis enzymes (Takabe et al. 2006; Giri 2011; Chen and Murata 2011; Wani et al. 2013).
In plants, two routes of GB synthesis have been proposed, one from choline and the other from glycine. The first route requires two choline oxidation steps catalyzed by the choline monooxygenase [CMO ] and betaine aldehyde dehydrogenase [BADH]; the second route involves two methylation steps catalyzed by glycine sarcosine methyltransferase [GSMT] and sarcosine dimethylglycine transferase [SDMT] (Weretilnyk and Hanson 1989; Rathinasabapathi et al. 1997; Valenzuela-Soto and Muñoz-Clares 1994; Nyyssola et al. 2000; Waditee et al. 2005; Chen et al. 2008).
There are several studies about the GB accumulation in different plant species as a response to abiotic stress [drought, salinity, cold, heat, etc.] (Rhodes and Hanson 1993; Sakamoto and Murata 2002; Giri 2011; Chen and Murata 2011; Kurepin et al. 2015). These studies have demonstrated that GB plays a role in different processes in plant metabolism, e.g., there is evidence of GB direct and/or indirect participation in protein stability, protein synthesis, enzyme activity, photosynthesis, oxidative stress response, and plant growth and development (Chen et al. 2008; Khan et al. 2009; Giri 2011; Chen and Murata 2011; Wani et al. 2013). However, less is known about the enzymes that synthesize GB or transform it into other compounds, e.g., what are the structural and kinetic characteristics of that enzymes or how they are regulated. The aim of this review is to summarize the knowledge garnered about GB’s metabolism and how it impacts the growth and development of plants under abiotic stress conditions.
2 Glycine Betaine Metabolism
2.1 Synthesis Pathways
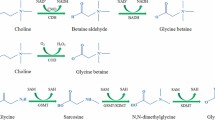
In GB accumulator plants, it is synthesized from choline, choline is oxidized to betaine aldehyde by choline monooxygenase [E.C. 1.14.15.7], and betaine aldehyde dehydrogenase [BADH EC 1.2.1.8] catalyzes the betaine aldehyde oxidation to GB (Fig. 1a) (Rathinasabapathi et al. 1997; Ling et al. 2001; Hibino 2002; Wang and Showalter 2004; Park et al. 2007; Muñoz-Clares and Valenzuela-Soto 2008). Extremely halophilic plants and microorganisms, also methanogenic organisms, synthesize GB from glycine; it is methylated by glycine sarcosine methyl transferase [GSMT] to N,N-dimethylglycine and sarcosine; furthermore, N,N-dimethylglycine is methylated by the sarcosine dimethylglycine transferase [SDMT] to GB; thus, both enzymes use S-adenosylmethionine (SAM) as methyl donor (Fig. 1b) (Nyyssola et al. 2000; Waditee et al. 2005).
Glycine betaine synthesis and degradation pathways. (a) GB synthesis in plants. (b) GB synthesis in extremely halophylic plants and microorganisms or metanogenics organisms. (c) GB catabolism in animals, some bacteria and in the cyanobacteria Aphanothece halophytica. CMO choline monooxygenase, BADH betaine aldehyde dehydrogenase, GSMT glycine sarcosine methyl transferase, and SDMT dimethylglycine transferase, BHMT betainehomocysteine methyltransferase
Choline is synthesized in the cytosol, and there are three described possible choline synthesis routes, all of them start with ethanolamine [EA], which can be N-methylated by SAM as free bases, phosphorylethanolamine bases, or phosphatidylethanolamine bases; each methylation step is catalyzed by phosphoethanolamine methyltransferase [PEAMT] (Fig. 2) (Hanson and Rhodes 1983; Datko and Mudd 1988; Nuccio et al. 1998, 2000). The three pathways can be used by plants; however, preference by one of them has been found, e.g., in Chenopodiaceae plants, choline comes from phosphoethanolamine (P-EA); in tobacco, choline originates from phosphatidyl-EA; in soy bean instead, the first step is the methylation of P-EA to phosphomonomethyl ethanolamine [P-MME] followed by a conversion to phosphatidylmonomethylethanolamine [Ptd-MME] via a cytidyl intermediate (Mudd and Datko 1989a; McNeil et al. 2000a). Later Ptd-MME is methylated to phosphatidyldimethylethanolamine [Ptd-DME], which is converted to Ptd-choline and later to P-choline (Hanson and Rhodes 1983; McNeil et al. 2000a, b; Nuccio et al. 2000). The last step in choline synthesis is the dephosphorylation of P-choline by choline phosphatase or choline kinase [CK] (Summers and Weretilnyk 1993; McNeil et al. 2000b). Choline synthesis is regulated by P-choline and S-adenosylhomocysteine [SAH]; both are inhibitors of PEAMT activity (Fig. 2) (Mudd and Datko 1989b; Nuccio et al. 2000; Sahu and Shaw 2009).
Interplay between choline, GB, ethylene, and polyamine synthesis pathways. SAMS S-adenosylmethionine synthase, PEAMT phosphoethanolamine methyltransferase, CK choline kinase, CMO choline monooxygenase, BADH betaine aldehyde dehydrogenase, ACC aminocyclopropane carboxylic acid, ACCS aminocyclopropane carboxylic acid synthase, EA ethanolamine, PEA phosphoethanolamine, Pd-EA phosphatidylethanolamine. Pink circle, choline transporter; green ellipse, chloroplast; red arrows indicate inhibition of PEAMT by P-choline
Choline is transported to the chloroplast and used as a substrate by CMO, the first enzyme in the GB synthesis. CMO is unique in plants and catalyzes the BA synthesis, its crystallographic structure has not been determined yet, the molecular mass of the monomer is ≈ 45 kDa, and it contains a Rieske-type [2Fe-2S] center and requires ferredoxin to be active (Rathinasabapathi et al. 1997). Hibino et al. (2002) found that Cys-181 is essential to the spinach CMO function, and as found in other oxygenases, a histidine [Hys-283] participates in the coordination of the [2Fe-2S] center.
The genes coding CMO have been studied in plant accumulator species and plant non-accumulator species. CMO gene sequences from spinach and sugar beet share 78% identity between them, while the sequence of CMO from Arabidopsis shares 51% identity with that of spinach and sugar beet (Hibino et al. 2002). Instead, Amaranthus tricolor CMO shares 69.4% and 69.5% identity with spinach and sugar beet CMOs and Atriplex prostrate, while rice shares 82.9% and 63% identity with deduced amino acid sequence of spinach and sugar beet, respectively (Ling et al. 2001; Wang and Showalter 2004; Luo 2007).
Amino acid sequence analysis of the CMO from Amaranthus tricolor, Arabidopsis, barley, rice, sugar beet, and spinach showed that all of them contained consensus sequences for coordination of the Rieske-type [2Fe-2S] cluster, CXHX15–17CX2H, and for coordination of mononuclear non-heme Fe, G/DX3–4 DX2HX4–5H [X equal to any amino acid] (Russell et al. 1998; Rathinasabapathi et al. 1997; Meng et al. 2001; Ling et al. 2001; Hibino et al. 2002; Wang and Showalter 2004; Luo et al. 2007; Mitsuya et al. 2011). In addition, the modeling of Spinacia oleracea CMO showed that in the active site there is an aromatic box conformed by Tyr281, Tyr295, and Phe301 and by a Glu residue [Glu346] (Carrillo-Campos et al. 2018). Aromatic box is involved in the choline’s trimethylammonium group, while the side chain carboxyl group of Glu346 participates in an ionic interaction with that group (Carrillo-Campos et al. 2018).
An analysis of the promoter of Amaranthus tricolor CMO gene allowed identifying a fragment of 410 pb upstream of the translation start codon that contains the sequence responsive to salt stress (Bhuiyan et al. 2007). In addition, Xu et al. (2018) found that the CMO gene from watermelon [Citrullus lanatus] suspension cells contained responsive elements to light, plant hormone-responsive cis-elements, and cis-elements responsive to biotic and abiotic stresses.
To this date, it seems that plants that do not accumulate GB possess the CMO genes, but those genes were proposed as not functional, as it has been found in rice and maize (Peel et al. 2010; Luo et al. 2012). However, there is other possible explanation to that results, for a recent phylogenetic study in Amaranthaceae plants showed that plant CMO evolved to two kinds of CMO proteins grouped in two clades called CMO1 and CMO2 and CMO2 diverged from CMO1 (Carrillo-Campos et al. 2018). From 167 plant CMO sequences analyzed, Carrillo et al. (2018) found that CMO1 and CMO2 proteins share 30% identity, CMO1 proteins share 50% identity between them, and otherwise CMO2 proteins share more than 85% identity. CMO1 and CMO2 modelling results showed that neither the CMO1 active site nor the Glu346 as found in CMO2 has the aromatic box; this would explain why CMO1 does not catalyze the oxidation of choline to betaine aldehyde (Carrillo-Campos et al. 2018). In addition, the chloroplast signal peptide is not conserved in CMO1 amino acid sequences (Carrillo-Campos et al. 2018).
The second step in GB synthesis is catalyzed by betaine aldehyde dehydrogenase [BADH]. Plant BADHs belong to ALDH superfamily, and they are grouped in the family ten [ALDH10] (Sophos and Vasiliou 2003). Within the ALDH10 family, there are proteins that use as substrate ω-aminoaldehydes [3-amino propionaldehyde or 4-aminobutyraldehyde] and ω-quaternary amino group [trimethylammonium] and as betaine aldehyde, trimethylaminobutiraldehyde or dimethylsulfoniopropionaldehyde (Trossat et al. 1997; Vojtechová et al. 1997; Ŝebela et al. 2000; Brauner et al. 2003; Livingstone et al. 2003; Oishi and Ebina 2005; Bradbury et al. 2008; Fujiwara et al. 2008). It has been proposed that BADH activity depends on only one amino acid residue at position 441 [SoBADH numbering] (Muñoz-Clares et al. 2014). The ability to oxidize betaine aldehyde by the BADH is related to the presence of an Ala or Cys in the 441 position in the protein (Muñoz-Clares et al. 2014).
The first plant BADH crystal structure obtained was from spinach, which showed that there are four aromatic residues Tyr160, Trp167, Trp285, and Trp456 at the active site (Díaz-Sanchez et al. 2012). By using in silico model building, kinetic studies, and site-directed mutagenesis of SoBADH, it was found that the aromatic ring of Tyr160 is of great importance for BA binding, followed by Trp285 and Trp167 (Díaz-Sanchez et al. 2012). The position that occupies in the active site pocket Trp456 is determined by the conformation adopted by the side chain of the amino acid residue in position 441 [Ile, Ala or Cys], to allow or not the proper positioning of the trimethylammonium group of BA, so Ile size would push Trp456 to such a position that there would be no adequate space for the binding of trimethylammonium group (Díaz-Sanchez et al. 2012; Muñoz-Clares et al. 2014). Interestingly, a great number of BADH from GB accumulators’ plants possess an Ala or Cys in position 441 (Muñoz-Clares et al. 2014).
ALDH10 isoenzymes evolved from the gene coding to an Ile in position 441 as a consequence of environmental pressure; however, all plants conserved isoenzymes with one of three amino acids in the 441 position (Ile, Ala, and/or Cys), which allow isoenzymes to perform other metabolic functions in plants (Muñoz-Clares et al. 2014).
Different studies have demonstrated that BADH in plants is a homodimer of ≈ 120 kDa, except in wild amaranth and pea which are heterodimeric and homotetrameric, respectively (Weretilnyk and Hanson 1989; Valenzuela-Soto and Muñoz-Clares 1994; Figueroa-Soto and Valenzuela-Soto 2001; Ŝebela et al. 2000; Livingstone et al. 2002; Oishi and Ebina 2005; Fujiwara et al. 2008). Plant BADHs show an acidic pI, an optimum pH ranging from 8.0 to 8.5, and they exhibit preference to use NAD+ as coenzyme (Weretilnyk and Hanson 1989; Trossat et al. 1997; Valenzuela-Soto and Muñoz-Clares 1994; Incharoensakdi et al. 2000; Hibino et al. 2001; Fujiwara et al. 2008).
Similar to cis-acting regulatory elements described before for CMO, the BADH is also regulated at the genetic level. Analysis of the BADHs’ gene promoter sequence from Suaeda liaotungensis revealed regulatory elements, such as a TATA-box, a CAAT-box, a GC-motif, EIRE, MRE, WUNmotif, a heat shock element, ABRE, methyl jasmonate-responsive element, and ethylene-responsive element [ERE] (Zhang et al. 2008; Xu et al. 2018).
2.2 Glycine Betaine Degradation Routes
In animals and some bacteria, GB is catabolized to methionine and glycine by betaine homocysteine methyl transferase [BHMT], it removes a methyl group from GB to produce dimethylglycine, and the methyl group is transferred to homocysteine for methionine synthesis (Fig. 1c) (Pajares and Perez-Salas 2006). A glycine betaine transmethylase was proposed in Rhizobium meliloti as the enzyme to convert GB to dimethylglycine (Smith et al. 1988), whereas in the cyanobacteria Aphanothece halophytica, GB was catabolized by BHMT under hyperosmotic conditions (Incharoensakdi and Waditee 2000; Waditee and Incharoensakdi 2001). After a deep search about BHMT in plants, no information was found. This being the reason why is not possible to relate methionine synthesis with GB degradation. However, it is possible to speculate that BHMT has not been searched and therefore identified.
2.3 Cellular Compartment of Glycine Betaine Synthesis
GB synthesis has been localized in chloroplasts, peroxisomes, and cytoplasm. It has been suggested that in dicotyledons GB synthesis takes place in the chloroplast, while in monocotyledons it occurs in the peroxisome (Nakamura et al. 1997; Mitsuya et al. 2011). BADH isoenzyme localization differs between plants, e.g., in spinach, one of them is targeted to chloroplast and the other to cytosol; in barley, one isoenzyme is directed to the peroxisome and the other to the cytosol; and in rice, both isoenzymes are targeted to the peroxisome, whereas in Avicennia marina, one of them is delivered to the chloroplast and the other to peroxisome (Weigel et al. 1986; Nakamura et al. 1997; Hibino et al. 2001; Nakamura et al. 2001; Shirasawa et al. 2006). BADH isoenzymes targeted to chloroplast or to peroxisome possess a short signal peptide [seven or three residues, respectively]; in barley, the signal peptide is located in the C-terminus, whereas in spinach it is in the N-terminus (Weretilnyk and Hanson 1990; Nakamura et al. 2001). To this date, it is known the BADHs with high BA affinity are located in the chloroplast (Weigel et al. 1986; Hibino et al. 2001).
2.4 GB Synthesis in Plant Tissue
In plants capable of synthesizing and accumulating GB, it has been found that GB is distributed throughout the whole plant under stress conditions (Yamada et al. 2009). The leaf is the tissue with the highest content of GB, but it is influenced by the leaf age; in barley and sugar beet, it was found that GB is synthesized mainly in old leaves where CMO activity was detected (Nakamura et al. 1996; Hattori et al. 2009; Yamada et al. 2009). The root has the ability to synthesize GB; however, the expression of CMO and BADH is lower compared to the leaf (Bhuiyan et al. 2007; Yamada et al. 2009). On the other hand, BADH was detected in old and young leaves and roots of sugar beet, so it is concluded that the synthesis of GB is limited by the availability of CMO (Bhuiyan et al. 2007; Fujiwara et al. 2008; Yamada et al. 2009).
Since GB has been found in tissues that do not contain CMO activity, the mobilization of GB has been investigated. Two transporters have been found: one in sugar beet and another in barley called BvBet/ProT1 and HvProT2, respectively; they transport proline and GB with the highest affinity detected for GB (Yamada et al. 2009; Fujiwara et al. 2010). BvBet/Pro1 and HvProT2 were localized in plasma membrane: BvBet/Pro1 was more abundant in old than in young leaves, while HvProT2 is distributed in old leaves and roots (Yamada et al. 2009; Fujiwara et al. 2010).
3 GB Synthesis and Control of Plant Growth and Development
GB synthesis requires choline in any cellular compartment where it is carried out; at the date, the important aspects that limit the synthesis of GB are the availability of choline and the structural characteristics to carry out the union of the substrates and the catalysis of the CMO and BADH isoenzymes (Nuccio et al. 1998; Díaz-Sanchez et al. 2012; Muñoz-Clares et al. 2014; Carrillo-Campos et al. 2018). Synthesis of P-choline is strongly favored in the cytosol; however, it depends on the dephosporylation of choline because only choline can be transported to chloroplast or vacuole (Bligny et al. 1989; McNeil et al. 2000a). On the other hand, choline produced is distributed between vacuole, chloroplast, and cytosol which limit the availability of choline to GB synthesis (Nuccio et al. 1998; McNeil et al. 2000a; Sahu and Shaw 2009).
Considering that the concentration of choline is not limiting, the synthesis of GB would require a high concentration of SAM which would immediately cause a decrease in the synthesis of ethylene and polyamines (Ravanel et al. 1998; Sahu and Shaw 2009; Wang et al. 2010; Khan et al. 2014). In addition, SAM is required for chlorophyll synthesis, DNA replication, cell wall synthesis, etc.; therefore, GB synthesis cannot be very high, which corresponds with GB concentrations found in plants (Huang et al. 2000; Holstrom et al. 2000; Sakamoto and Murata 2001; Quan et al. 2004; Tabuchi et al. 2005; Wei et al. 2017). Tabuchi et al. (2005) suggested a co-regulation between the levels of S-adenosyl-L-methionine synthetase [SAMS] transcript with those of CMO and PEAMT; this would allow sustain active GB production without significantly diminishing the synthesis of other metabolites dependent on SAM.
Plants capable of synthesizing and accumulating GB show tolerance to stress mainly to drought, salinity, and extreme temperature [cold and heat] stress; the growth and development of those plants are affected depending on the stage of development of the plant, as well as the type and species of the plant. It has been demonstrated that drought, salinity, and low and high temperature decrease root and shoot growth and development, but GB’s synthesizing plants manage to reduce the effect of stress on both parameters. The degree or level of protection varies between species and even between varieties of the same species.
Under stress conditions, GB participates in maintaining of fundamental processes for growth and development such as (a) photosynthesis, energy production [ATP], and carbon skeletal, (b) conservation of the cell-reducing environment, and (c) enzyme functionality. Since GB can be present in all parts of the plant [either by synthesis or transport], its effect can occur in the entire plant. With all the information generated, it can be said that GB effects on plants under stress conditions are related to its ability to stabilize protein structure and regulate gene transcription and enzyme activities; those functions are the ways on how GB can play a role in plant growth and development.
Photosynthesis is inhibited by heat, chilling, salinity, and drought stress; however, GB contributes to maintain the photosynthesis activity through the PSII damaged reparation increasing the expression of D1 protein and increasing its degradation when it is damaged (Fig. 3) (Onishi and Murata 2006; Murata et al. 2007; Yang et al. 2008; Fan et al. 2012). In addition, PSII oxygen-evolving complex structure, Mn cluster, and PSII association with extrinsic polypeptides 18, 23, and 33 kDa are strongly stabilized by GB in plants under stress (Murata et al. 1992; Papageorgiou and Murata 1995; Allakhverdiev et al. 1996; Allakhverdiev et al. 1999). An adequate electron transport in the thylakoid maintains adequate levels of photosynthetic parameters as photosynthetic rate [A], intercellular CO2 [Ci], transpiration rate [E], stomatal conductance [gs], and maximal efficiency of PSII [Fv/Fm] (Fig. 3) (Zhao et al. 2007; Yang et al. 2008; Guha et al. 2010; Wei et al. 2017).
The other face of photosynthesis is the CO2 fixation by the RUBISCO and the flux of carbon skeletal through the Calvin cycle enzymes. RUBISCO, Rubisco activase, fructose biphosphatase [FBPase], fructose biphosphatase aldolase [FBPaldolase], and phosphoribulose kinase [PRKase] are activated by GB by stabilizing their structure under stress conditions (Fig. 3) (Makela et al. 2000; Yang et al. 2005; Murata et al. 2007; Konrad and Zvi 2008; Fan et al. 2012). Interestingly, Yang et al. (2005) found that under heat stress conditions, Rubisco activase is associated with thylakoid membrane, which is avoided by GB. A suitable CO2 fixation has been proposed by Murata et al. (2007) as an important factor to decrease the PSII damage, because suppression of CO2 fixation drives to oxidative stress which inhibits D1 protein synthesis and the repair of PSII.
To contend with oxidative stress, transgenic plants or wild-type plants able to synthesize GB increase the expression of enzymes of antioxidant system; an increase in mRNA of the enzymes superoxide dismutase [SOD], catalase [CAT], ascorbate peroxidase [APX], glutathione reductase [GR], glutathione peroxidase [GPX], and dehydroascorbate reductase [DHAR] has been found in different plant species (Fig. 3) (Hoque et al. 2008; Islam et al. 2009; Fan et al. 2012; Hasanuzzaman et al. 2014; Zhang et al. 2016; Yao et al. 2018). Increases in antioxidant enzyme activity decrease the lipid peroxidation and protein carbonylation protecting cell survival (Hoque et al. 2008; Islam et al. 2009; Karabudak et al. 2014). Likewise, increases in the concentration of metabolites with antioxidant activity have been found, e.g., increases in glutathione reduced [GSH], ascorbate reduced [ASA], phenolic compounds, and flavonoids (Hoque et al. 2008; Islam et al. 2009; Ahmed et al. 2013; Wang et al. 2019).
Changes in the activity of enzymes involved in Calvin cycle, antioxidant system (enzymatic and nonenzymatic), or proline synthesis are consequence of changes in their gene expression or changes in the enzyme activity induced by GB. In animals, GB induces changes in DNA methylation status and interacts with transcription factors to modify gene expression (Song et al. 2007; Zhang et al. 2013; Deminice et al. 2015; Idriss et al. 2017); in plants, there is no information about it. However, it is tempting to propose that something similar to what happens in animals may be happening in plants.
Synthesis of ATP under stress conditions is less studied; Jin et al. (2015) found a high ATP/ADP ratio induced by GB in loquat fruit submitted to a low-temperature conditioning. It has been proposed that GB improves the lipid composition of cell membranes, that is, thylakoid membranes of wheat were protected by GB application, which provoked changes in the fat acid composition (Zhao et al. 2007; Tiang et al. 2017). Changes in lipid composition of thylakoid membranes increased the membrane fluidity, improving their function (Zhao et al. 2007; Tiang et al. 2017). Therefore, if GB maintains the functionality of thylakoid membranes and positively modulates photosynthesis, then there is a good proton gradient to carry out the ATP synthesis (Yang et al. 2008; Zhao et al. 2007; Guha et al. 2010; Ogbaba et al. 2014; Wei et al. 2017; Tiang et al. 2017).
Drought, salinity, heat, and cold stress increase GB synthesis both in GB natural synthesizing and in transgenic plants; this GB increase has a strong impact in the plant growth. Several works have been demonstrated that GB increases the growth of root, shoot, hypocotyl, and plant measured as high, biomass [fresh weight or dry weight], or leaf area under stress conditions and relative of the control plant (Kishitani et al. 2000; Quan et al. 2004; Yang et al. 2005; Park et al. 2007; Yang et al. 2008; Guha et al. 2010; Goel et al. 2011; Fan et al. 2012; Karabudak et al. 2014; Ke et al. 2016; Manaf 2016). The spiking time in transgenic maize plants under stress was less affected compared with wild-type plants (Quan et al. 2004). GB increased the number of anthers, pistils, and petals in transgenic Arabidopsis plants (Sulpice et al. 2003).
In transgenic maize, the reproductive development is promoted by GB under drought stress (Quan et al. 2004). The percentage and time of germination of seeds of transgenic rice, tomato, and tobacco plants are promoted by GB under salt and drought stress (Park et al. 2007; Kathuria et al. 2009; Goel et al. 2011; Li et al. 2011). Plant productivity of plants under stress conditions is also promoted in those plants capable of synthesizing GB. Based on maintaining the growth and development of the plants synthesizing GB, the productivity of them is also positively affected. Sulpice et al. (2003) found in transgenic Arabidopsis plants a greater number of flowers and seeds per plant, whereas in transgenic maize, Quan et al. (2004) reported a greater number of seeds per plant and a greater weight per grain.
Despite all the positive effects of GB on growth and development of plants, there is no evidence that GB is directly promoting growth, so it has been proposed that GB could be interacting with plant hormones like auxins and ABA, since they are involved in the control of growth (Kurepin et al. 2015). To date it has been found that ABA and methyl jasmonate increase the synthesis of GB (Fig. 3) (Ishitani et al. 1995; Jagendorf and Takabe 2001; Xing and Rajashekar 2001; Xu et al. 2018). Salicylic acid seems to be playing a role of increasing the methionine content to support the SAM production used to GB synthesis and decreasing the ethylene production (Khan et al. 2014). Barley and poplar transgenic plants overexpressing CodA gene showed increased expression levels of auxin-responsive IAA genes (Li et al. 2014; Ke et al. 2016).
4 Conclusion and Future Perspectives
The growth of plants primarily requires sugars, protein synthesis, ATP, reducing power, and a reducing cellular environment, all of which are influenced directly or indirectly by GB. All those aspects are influenced by GB synthesis, transport, and accumulation, by which it has a positive influence in plant growth and development under stress conditions. The mechanism by which GB influences the expression of genes is much less known and is an important aspect to study. The capacity of GB to stabilize proteins and to induce their synthesis explains in part changes in the activity of enzymes studied up to now; however, it remains to be defined if GB interacts directly as activator or inhibitor of enzymes. A great advance has been reached in the knowledge of the impact that GB synthesis has on the growth and development of the plants, as well as in the structural and evolutionary characteristics of the enzymes that catalyze its synthesis. The role of plant hormones in the induction of GB synthesis also begins to be clearer, as well as the impact that the synthesis of GB has on the ethylene and polyamine synthesis pathways.
There are still important aspects of the GB synthesis that need to be defined to increase agricultural productivity through plants with the ability to synthesize GB. Photosynthesis requires all proteins involved in H2O hydrolysis and in the transport of electrons and protons to remain functional, just as the enzymes that participate in ATP synthesis and carbon skeletal synthesis, as well as the chloroplast and thylakoid membranes. However, GB synthesis requires the availability of choline whose synthesis demands a high content of SAM, methionine, and ethanolamine. All these points must be taken into account for the improvement or genetic engineering of plants that synthesize and accumulate GB.
References
Ahmed IM, Cao F, Zhang M, Chen X, Zhang G, Wu F (2013) Difference in yield and physiological features in response to drought and salinity combined stress during anthesis in tibetan wild and cultivated barleys. PLoS One 8(10):e77869
Allakhverdiev SI, Feyziev YM, Ahmed A, Hayashi H, Alie JA, Klimov VV, Murata N, Carpentier R (1996) Stabilization of oxygen evolution and primary electron transport reactions in photosystem II against heat stress with glycinebetaine and sucrose. Photochem Photobiol 34:149–157
Allakhverdiev YM, Mamedov MD, Ferimazova N, Papageorgiou GC, Gasanov RA (1999) Glycinebetaine stabilizes photosystem 1 and photosystem 2 electron transport in spinach thylakoid membranes against heat inactivation. Photosynthetica 37:423–432
Bhuiyan NH, Hamada A, Yamada N, Rai V, Hibino T, Takabe T (2007) Regulation of betaine synthesis by precursor supply and choline monooxygenase expression in Amaranthus tricolor. J Exp Bot 58(15):4203–4212
Bligny R, Foray M-F, Roby C, Douce R (1989) Transport and phosphorylation of choline in higher plant cells: phosphorus-31 nuclear magnetic resonance studies. J Biol Chem 264:4888–4895
Bradbury LMT, Gillies SA, Brushett DJ, Waters DLE, Henry RJ (2008) Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol Biol 68:439–449
Brauner F, Šebela M, Snégaroff J, Peč P, Meunier JC (2003) Pea seedling aminoaldehyde dehydrogenase: primary structure and active site residues. Plant Physiol Biochem 41(1):1–10
Carrillo-Campos J, Riveros-Rosas H, Rodríguez-Sotres R, Muñoz-Clares RA (2018) Bona fide choline monoxygenases evolved in Amaranthaceae plants from oxygenases of unknown function: evidence from phylogenetics, homology modeling and docking studies. PLoS One 13(9):e0204711
Chen TH, Murata N (2008) Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci 13(9):499–505
Chen TH, Murata N (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34(1):1–20
Craig SAS (2004) Betaine in human nutrition. Am J Clin Nutr 80:539–549
Datko AH, Mudd SH (1988) Phosphatidylcholine synthesis. Plant Physiol 88(3):854–861
Deminice R, da Silva RP, Lamarre SG, Kelly KB, Jacobs RL, Brosnan ME, Brosnan JT (2015) Betaine supplementation prevents fatty liver induced by a high-fat diet: effects on one-carbon metabolism. Amino Acids 47:839–846
Díaz-Sánchez AG, González-Segura L, Mújica-Jiménez C, Rudiño-Piñera E, Montiel C, Martínez-Castilla LP, Muñoz-Clares RA (2012) Amino acid residues critical for the specificity for betaine aldehyde of the plant ALDH10 isoenzyme involved in the synthesis of glycine betaine. Plant Physiol 158(4):1570–1582
Du Vigneaud V, Simmonds JP, Chandler, Cohn CM (1946) A further investigation of the role of betaine in transmethylation reactions in vivo. J Biol Chem 165(2):639–648
Fan W, Zhang M, Zhang H, Zhang P (2012) Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. PLoS One 7(5):e37344
Finkelstein JD, Martin JJ (1984) Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J Biol Chem 259(15):9508–9513
Fujiwara T, Hori K, Ozaki K, Yokota Y, Mitsuya S, Ichiyanagi T, Hattori T, Takabe T (2008) Enzymatic characterization of peroxisomal and cytosolic betaine aldehyde dehydrogenases in barley. Physiol Plant 134:22–30
Fujiwara T, Mitsuya S, Miyake H, Hattori T, Takabe T (2010) Characterization of a novel glycinebetaine/proline transporter gene expressed in the mestome sheath and lateral root cap cells in barley. Planta 232(1):133–143
Figueroa-Soto CG, Valenzuela-Soto EM (2001) Purification of a heterodimeric betaine aldehyde dehydrogenase from wild amaranth plants subjected to water deficit. Biochem Biophys Res Commun 285(4):1052–1058
Figueroa-Soto CG, Valenzuela-Soto EM (2018) Glycine betaine rather than acting only as an osmolyte also plays a role as regulator in cellular metabolism. Biochimie 147:89–97
Giri J (2011) Glycinebetaine and abiotic stress tolerance in plants. Plant Signal Behav 6(11):1746–1751
Goel D, Singh AK, Yadav V, Babbar SB, Murata N, Bansal KC (2011) Transformation of tomato with a bacterial CodA gene enhances tolerance to salt and water stresses. J Plant Physiol 168(11):1286–1294
Guha A, Sengupta D, Reddy AR (2010) Physiological optimality, allocation trade-offs and antioxidant protection linked to better leaf yield performance in drought exposed mulberry. J Sci Food Agric 90(15):2649–2659
Hanson AD, Rhodes D (1983) 14C-tracer evidence for synthesis of choline and betaine via phosphoryl base intermediates in salinized sugarbeet leaves. Plant Physiol 71:692–700
Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21(6):535–553
Hasanuzzaman M, Alam M, Rahman A, Hasanuzzaman M, Nahar K, Fujita M (2014) Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa) varieties. Biomed Res Int 2014:ID 757219
Hattori T, Mitsuya S, Fujiwara T, Jagendorf AT, Takabe T (2009) Tissue specificity of glycinebetaine synthesis in barley. Plant Sci 176:112–118
Hibino T, Meng YL, Kawamitsu Y, Uehara N, Matsuda N, Tanaka Y, Ishikawa H, Baba S, Takabe T, Wada K, Ishii T, Takabe T (2001) Molecular cloning and functional characterization of two kinds of betaine-aldehyde dehydrogenase in betaine-accumulating mangrove Avicennia marina (Forsk,) Vierh. Plant Mol Biol 45(3):353–363
Hibino T, Waditee R, Araki E, Ishikawa H, Aoki K, Tanaka Y, Takabe T (2002) Functional characterization of choline monooxygenase, an enzyme for betaine synthesis in plants. J Biol Chem 277(44):41352–41360
Holmström KO, Somersalo S, Mandal A, Palva ET, Welin B (2000) Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J Exp Bot 51(543):177–185
Hoque MA, Banu MN, Nakamura Y, Shimoishi Y, Murata Y (2008) Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification system and reduce NaCl-induced damage in cultured tobacco cells. J Plant Physiol 165:813–824
Huang J, Hirji R, Adam L, Rozwadowski KL, Hammerlin JK, Keller WA, Selvaraj G (2000) Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: metabolic limitations. Plant Physiol 122(3):747–756
Idriss AA, Hu Y, Sun Q, Jia L, Jia Y, Omer NA, Abobaker H, Zhao R (2017) Prenatal betaine exposure modulates hypothalamic expression of cholesterol metabolic genes in cockerels through modifications of DNA methylation. Poult Sci 96:1715–1724
Incharoensakdi A, Matsuda N, Hibino T, Meng YL, Ishikawa H, Hara A, Funaguma T, Takabe T, Takabe T (2000) Overproduction of spinach betaine aldehyde dehydrogenase in Escherichia coli. Structural and functional properties of wild-type mutants and E. coli enzymes. Eur J Biochem 267(24):7015–7023
Incharoensakdi A, Waditee R (2000) Degradation of glycinebetaine by betaine-homocysteine methyltransferase in Aphanothece halophytica: effect of salt downshock and starvation. Curr Microbiol 41:227
Iordachescu M, Imai R (2008) Trehalose biosynthesis in response to abiotic stresses. J Integr Plant Biol 50(10):1223–1229
Ishitani M, Nakamura T, Han SY, Takabe T (1995) Expression of the betaine aldehyde dehydrogenase gene in barley in response to osmotic stress and abscisic acid. Plant Mol Biol 27:307–315
Islam MM, Hoque MA, Okuma E, Banu MN, Shimoishi Y, Nakamura Y, Murata Y (2009) Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol 166(15):1587–1597
Jagendorf AT, Takabe T (2001) Inducers of glycinebetaine synthesis in barley. Plant Physiol 127:1827–1835
Jin P, Zhang Y, Shan T, Huang Y, Xu J, Zheng Y (2015) Low-temperature conditioning alleviates chilling injury in loquat fruit and regulates glycine betaine content and energy status. J Agric Food Chem 63(14):3654–3659
Karabudak T, Bor M, Özdemir F, Türkan I (2014) Glycine betaine protects tomato (Solanum lycopersicum) plants at low temperature by inducing fatty acid desaturase7 and lipoxygenase gene expression. Mol Biol Rep 41(3):1401–1410
Kathuria H, Giri J, Nataraja KN, Murata N, Udayakumar M, Tyagi AK (2009) Glycinebetaine-induced water-stress tolerance in CodA-expressing transgenic indica rice is associated with upregulation of several stress responsive genes. Plant Biotechnol J 7:512–526
Ke Q, Wang Z, Ji CY, Jeong JC, Lee HS, Li H, Xu B, Deng X, Kwak SS (2016) Transgenic poplar expressing CodA exhibits enhanced growth and abiotic stress tolerance. Plant Physiol Biochem 100:75–84
Khan MS, Yu X, Kikuchi A, Asahina M, Watanabe KN (2009) Genetic engineering of glycine betaine biosynthesis to enhance abiotic stress tolerance in plants. Plant Biotechnol 26(1):125–134
Khan MI, Asgher M, Khan NA (2014) Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol Biochem 80:67–74
Kishitani S, Takanami T, Suzuki M, Oikawa M, Yokoi S, Ishitani M, Alvarez-Nakase AM, Takabe T, Takabe T (2000) Compatibility of glycinebetaine in rice plants: evaluation using transgenic rice plants with a gene for peroxisomal betaine aldehyde dehydrogenase from barley. Plant Cell Environ 23(1):107–114
Konrad Z, Bar-Zvi D (2008) Synergism between the chaperone-like activities of the stress regulated ASR1 protein and the osmolyte glycine-betaine. Planta 227(6):1213–1219
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63(4):1593–1608
Kurepin LV, Ivanov AG, Zaman M, Pharis RP, Allakhverdiev SI, Hurry V, Hüner NP (2015) Stress-related hormones and glycinebetaine interplay in protection of photosynthesis under abiotic stress conditions. Photosynth Res 126(2–3):221–235
Lawson-Yuen A, Levy HL (2006) The use of betaine in the treatment of elevated homocysteine. Mol Genet Metab 88(3):201–207
Lever M, Slow S (2010) The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem 43(9):732–744
Li S, Li F, Wang J, Zhang W, Meng Q, Chen TH, Murata N, Yang X (2011) Glycinebetaine enhances the tolerance of tomato plants to high temperature during germination of seeds and growth of seedlings. Plant Cell Environ 34(11):1931–1943
Li H, Wang Z, Ke Q, Ji CY, Jeong JC, Lee HS, Lim YP, Xu B, Deng XP, Kwak SS (2014) Overexpression of CodA gene confers enhanced tolerance to abiotic stresses in alfalfa. Plant Physiol Biochem 85:31–40
Ling MY, Wang YM, Zhang DA, Nii N (2001) Isolation of a choline monooxygenase cDNA clone from Amaranthus tricolor and its expressions under stress conditions. Cell Res 11(3):187–193
Livingstone JR, Yoshida I, Tarui Y, Hirooka K, Yamamoto Y, Tsutui N, Hirasawa E (2002) Purification and properties of aminoaldehyde dehydrogenase from Avena sativa. J Plant Res 115(5):393–400
Livingstone JR, Maruo T, Yoshida I, Tarui Y, Hirooka K, YamamotoY TN, Hirasawa E (2003) Purification and properties of betaine aldehyde dehydrogenase from Avena sativa. J Plant Res 116(2):133–140
Luo D, Niu X, Wang Y, Zheng W, Chang L, Wang Q, Wei X, Yu G, Lu BR, Liu Y (2007) Functional defect at the rice choline monooxygenase locus from an unusual post-transcriptional processing is associated with the sequence elements of short-direct repeats. New Phytol 175(3):439–447
Luo D, Niu X, Yu J, Yan J, Gou X, Lu BR, Liu Y (2012) Rice choline monooxygenase (OsCMO) protein functions in enhancing glycine betaine biosynthesis in transgenic tobacco but does not accumulate in rice (Oryza sativa L ssp. Japonica). Plant Cell Rep 31:1625–1635
Makela P, Karkkainen J, Somersalo S (2000) Effect of glycinebetaine on chloroplast ultrastructure, chlorophyll and protein content, and RuBPCO activities in tomato grown under drought or salinity. Biol Plant 43:471–475
Manaf HH (2016) Beneficial effects of exogenous selenium, glycine betaine and seaweed extract on salt stressed cowpea plant. Ann Agric Sci 61(1):41–48
McNeil SD, Nuccio ML, Rhodes D, Shachar-Hill Y, Hanson AD (2000a) Radiotracer and computer modeling evidence that phospho-base methylation is the main route of choline synthesis in tobacco. Plant Physiol 123(1):371–380
McNeil SD, Rhodes D, Russell BL, Nuccio ML, Shachar-Hill Y, Hanson AD (2000b) Metabolic modeling identifies key constraints on an engineered glycine betaine synthesis pathway in tobacco. Plant Physiol 124(1):153–162
Meng YL, Wang YM, Zhang DB, Nii N (2001) Isolation of a choline monooxygenase cDNA clone from Amaranthus tricolor and its expressions under stress conditions. Cell Res 11(3):187–193
Mitsuya S, Kuwahara J, Ozaki K, Saeki E, Fujiwara T, Takabe T (2011) Isolation and characterization of a novel peroxisomal choline monooxygenase in Barley. Planta 234(6):1215–1226
Mudd SH, Datko AH (1989a) Synthesis of methylated ethanolamine moieties. Plant Physiol 90(1):306–310
Mudd SH, Datko AH (1989b) Synthesis of methylated ethanolamine moieties. Regulation by choline in Lemna. Plant Physiol 90(1):296–305
Muñoz-Clares RA, Valenzuela-Soto EM (2008) Betaine aldehyde dehydrogenases: evolution, physiological functions, mechanism, kinetics, regulation, structure, and stability. In: Advances in protein physical chemistry. Research Signpost, Kerala, pp 279–302
Muñoz-Clares RA, Riveros-Rosas H, Garza-Ramos G, Gonzales-Segura L, Mújica-Jiménez C, Julián-Sanchez A (2014) Exploring the evolutionary route of the acquisition of betaine aldehyde dehydrogenase activity by plant ALD10 enzymes: implications for the synthesis of the osmoprotectant glycine betaine. BMC Plant Biol 14:149
Murata N, Mohanty PS, Hayashi H, Papageorgiou GC (1992) Glycinebetaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen-evolving complex. FEBS Lett 296:187–189
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochem Biophys Acta 1767(6):414–421
Nakamura T, Ishitani M, Harinasut P, Nomura M, Takabe T, Takabe T (1996) Distribution of glycinebetaine in old and young leaf blades of salt-stressed barley plants. Plant Cell Physiol 37(6):873–877
Nakamura T, Yokota S, Muramoto Y, Tsutsui K, Oguri Y, Fukui K, Takabe T (1997) Expression of a betaine aldehyde dehydrogenase gene in rice, a glycinebetaine nonaccumulator, and possible localization of its protein in peroxisomes. Plant J 11(5):1115–1120
Nakamura T, Nomura M, Mori H, Jagendorf AT, Ueda A, Takabe T (2001) An isozyme of betaine aldehyde dehydrogenase in barley. Plant Cell Physiol 42(10):1088–1092
Nyyssola A, Kerovuo J, Kaukinen P, von Weymarn N, Reinikainen T (2000) Extreme halophiles synthesize betaine from glycine by methylation. J Biol Chem 275:22196–22201
Nuccio ML, Russell BL, Nolte KD, Rathinasabapathi B, Gage DA, Hanson A (1998) The endogenous choline supply limits glycine betaine synthesis in transgenic tobacco expressing choline monooxygenase. Plant J 16:487–496
Nuccio ML, Ziemak MJ, Henry SA, Weretilnyk EA, Hanson AD (2000) cDNA cloning of phosphoethanolamine N-methyltransferase from spinach by complementation in Schizosaccharomyces pombe and characterization of the recombinant enzyme. J Biol Chem 275:14095–11410
Oishi H, Ebina M (2005) Isolation of cDNA and enzymatic properties of betaine aldehyde dehydrogenase from Zoysia tenuifolia. J Plant Physiol 162(10):1077–1086
Ohnishi N, Murata N (2006) Glycinebetaine counteracts the inhibitory effects of salt stress on the degradation and synthesis of D1 protein during photoinhibition in Synechococcus sp. PCC 7942. Plant Physiol 141(2):758–765
Ogbaga CC, Stepien P, Johnson GN (2014) Sorghum (Sorghum bicolor) varieties adopt strongly contrasting strategies in response to drought. Physiol Plant 152:389–401
Olthof MR, Verhoef P (2005) Effects of betaine intake on plasma homocysteine concentrations and consequences for health. Curr Drug Metab 6(1):15–22
Pajares MA, Pérez-Sala D (2006) Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism? Cell Mol Life Sci 63(23):2792–2803
Papageorgiou GC, Murata N (1995) The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving photosystem II complex. Photosynth Res 25:243–252
Park EJ, Jeknic Z, Chen TH, Murata N (2007) The codA transgene for glycinebetaine synthesis increases the size of flowers and fruits in tomato. Plant Biotechnol J 5(3):422–430
Paul MJ, Primavesi LF, Jhurreea D, Zhang YH (2008) Trehalose metabolism and signaling. Annu Rev Plant Biol 59:417–441
Peel GJ, Mickelbart MV, Rhodes D (2010) Choline metabolism in glycinebetaine accumulating and non-accumulating near-isogenic lines of Zea mays and Sorghum bicolor. Phytochemistry 71(4):404–414
Quan R, Shang M, Zhang H, Zhao Y, Zhang J (2004) Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol J 2:477–486
Rathinasabapathi B, Burnet M, Russell BL, Gage DA, Liao PC, Nye GJ, Scott P, Golbeck JH, Hanson AD (1997) Choline monooxygenase, an unusual iron-sulfur enzyme catalyzing the first step of glycine betaine synthesis in plants: prosthetic group characterization and cDNA cloning. Proc Natl Acad Sci U S A 94(7):3454–3458
Ravanel S, Gakière B, Job DR (1998) The specific features of methionine biosynthesis and metabolism in plants. Proc Natl Acad Sci U S A 95(13):7805–7812
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Ann Rev Plant Biol 44:357–384
Russell BL, Rathinasabapathi B, Hanson AD (1998) Osmotic stress induces expression of choline monooxygenase in sugar beet and amaranth. Plant Physiol 116(2):859–865
Sahu BB, Shaw BP (2009) Isolation, identification and expression analysis of salt-induced genes in Suaeda maritima, a natural halophyte, using PCR-based suppression subtractive hybridization. BMC Plant Biol 9(1):69
Sakamoto A, Murata N (2001) The use of bacterial choline oxidase, a glycine betaine-synthesizing enzyme, to create stress-resistant transgenic plants. Plant Physiol 125(1):180–188
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25(2):163–171
Scheibler C (1869) Ueber das betain, eine im Safte der Zuckerrüben (Beta vulgaris) vorkommende Pflanzenbase. Ber Dtsch Chem Ges 2:292–295
Šebela M, Brauner F, Radová A, Jacobsen S, Havliš J, Galuszka P, Peč P (2000) Characterisation of a homogeneous plant aminoaldehyde dehydrogenase. Biochem Biophys Acta 1480(1–2):329–341
Shirasawa K, Takabe T, Takabe T, Kishitani C (2006) Accumulation of glycinebetaine in rice plants that overexpress choline monooxygenase from spinach and evaluation on their tolerance to abiotic stress. Ann Bot 98(3):565–571
Singh TN, Aspinall D, Paleg LG (1972) Proline accumulation and varietal adaptability to drought in barley: potential metabolic measure of drought resistance. Nat New Biol 236:188–190
Smith LT, Pocard JA, Bernard T, Le Rudulier D (1988) Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol 170(7):3142–3149
Summers PS, Weretilnyk EA (1993) Choline synthesis in spinach in relation to salt stress. Plant Physiol 103:1269–1273
Song Z, Deaciuc I, Zhou Z, Song M, Chen T, Hill D, McClain CJ (2007) Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. Am J Physiol Gastrointest Liver Physiol 293(4):G894–G902
Sophos NA, Vasiliou V (2003) Aldehyde dehydrogenase gene superfamily: the 2002 update. Chem Biol Interact 143:5–22
Stewart GR, Lee JA (1974) Role of proline accumulation in halophytes. Planta 120(3):279–289
Sulpice R, Tsukaya H, Nonaka H, Mustardy L, Chen TH, Murata N (2003) Enhanced formation of flowers in salt-stressed Arabidopsis after genetic engineering of the synthesis of glycine betaine. Plant J 36(2):165–176
Tabuchi T, Kawaguchi Y, Azuma T, Nanmori T, Yasuda T (2005) Similar regulation patterns of choline monooxygenase, phosphoethanolamine N-methyltransferase and S-adenosyl-L-methionine synthetase in leaves of the halophyte Atriplex nummularia L. Plant Cell Physiol 46(3):505–513
Takabe T, Rai V, Hibino T (2006) Metabolic engineering of glycinebetaine. In: Abiotic stress tolerance in plants. Springer, Dordrecht, pp 137–151
Tiang F, Wang W, Liang C, Wang X, Wang G, Wang W (2017) Overaccumulation of glycine betaine makes the function of the thylakoid membrane better in wheat under salt stress. Crop J 5(1):73–82
Trossat C, Rathinasabapathi B, Hanson AD (1997) Transgenically expressed betaine aldehyde dehydrogenase efficiently catalyzes oxidation of dimethylsulfoniopropionaldehyde and ω-Aminoaldehydes. Plant Physiol 113(4):1457–1461
Ueland PM (2011) Choline and betaine in health and disease. J Inherit Metab Dis 34(1):3–15
Valenzuela-Soto EM, Muñoz-Clares RA (1994) Purification and properties of betaine aldehyde dehydrogenase extracted from detached leaves of Amaranthus hypochondriacus L. subjected to water deficit. J Plant Physiol 143(2):145–152
Vojtechová M, Rodríguez-Sotres R, Valenzuela-Soto EM, Muñoz-Clares RA (1997) Substrate inhibition by betaine dehydrogenase from leaves of Amaranthus hypochondriacus L. Biochim Biophys Acta 1341:49–5700
Waditee R, Incharoensakdi A (2001) Purification and kinetic properties of betaine-homocysteine methyltransferase from Aphanothece halophytica. Curr Microbiol 43(2):107–111
Waditee R, Bhuiyan MNH, Rai V, Aoki K, Tanaka Y, Hibino T, Suzukim S, Takano J, Jagendorf AT, Takabe T, Takabe T (2005) Genes for direct methylation of glycine provide high levels of glycinebetaine and abiotic-stress tolerance in Synechococcus and Arabidopsis. Proc Natl Acad Sci U S A 102(5):1318–1323
Wani SH, Singh NB, Haribhushan A, Mir JI (2013) Compatible solute engineering in plants for abiotic stress tolerance-role of glycine betaine. Curr Genomics 14(3):157–165
Wang LW, Showalter AM (2004) Cloning and salt-induced, ABA-independent expression of choline mono-oxygenase in Atriplex prostrata. Physiol Plant 120:405–412
Wang GP, Li F, Zhang J, Zhao MR, Hui Z, Wang W (2010) Overaccumulation of glycine betaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica 48:30–41
Wang L, Shan T, Xie B, Ling C, Shao S, Jin P, Zheng Y (2019) Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem 272:530–538
Wei D, Zhang W, Wang C, Meng Q, Li G, Chen THH, Yang X (2017) Genetic engineering of the biosynthesis of glycinebetaine leads to alleviate salt-induced potassium efflux and enhances salt tolerance in tomato plants. Plant Sci 257:74–83
Weigel P, Weretilnyk EA, Hanson AD (1986) Betaine aldehyde oxidation by spinach chloroplast. Plant Physiol 82(3):753–759
Weretilnyk EA, Hanson AD (1989) Betaine aldehyde dehydrogenase from spinach leaves: purification in vitro translation of the mRNA, and regulation by salinity. Arch Biochem Biophys 271(1):56–63
Weretilnyk A, Hanson AD (1990) Molecular cloning of a plant betaine-aldehyde dehydrogenase, an enzyme implicated in adaptation to salinity and drought. Proc Natl Acad Sci 87:2745–2749
Xing W, Rajashekar CB (2001) Glycine betaine involvement in freezing tolerance and water stress in Arabidopsis thaliana. Environ Exp Bot 46(1):21–28
Xu Z, Sun M, Jiang X, Sun H, Dang X, Cong H, Qiao F (2018) Glycinebetaine biosynthesis in response to osmotic stress depends on jasmonate signaling in watermelon suspension cells. Front Plant Sci 9:1469
Yamada N, Promden W, Yamane K, Tamagake H, Hibino T, Tanaka Y, Takabe T (2009) Preferential accumulation of betaine uncoupled to choline monooxygenase in young leaves of sugar beet – importance of long-distance translocation of betaine under normal and salt-stressed conditions. J Plant Physiol 166(18):2058–2070
Yancey PH, Clark ME, Hand C, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217:1214–1222
Yang X, Liang Z, Lu C (2005) Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol 138(4):2299–2309
Yang X, Liang Z, Wen X, Lu C (2008) Genetic engineering of the biosynthesis of glycinebetaine leads to increased tolerance of photosynthesis to salt stress in transgenic tobacco plants. Plant Mol Biol 66(1–2):73–86
Yao W, Xu T, Farooq SY, Jin P, Zheng Y (2018) Glycine betaine treatment alleviates chilling injury in zucchini fruit (Cucurbita pepo L.) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biol Technol 144:20–28
Zhao X-X, Ma Q-Q, Liang C, Fang Y, Wang YQ, Wang W (2007) Effect of glycine betaine on function of thylakoid membranes in wheat flag leaves under drought stress. Biol Plant 51:584–588
Zhang Y, Yin H, Li D, Zhu W, Li Q (2008) Functional analysis of BADH gene promoter from Suaeda liaotungensis K. Plant Cell Rep 27(3):585–592
Zhang W, Wang LW, Wang LK, Li X, Zhang H, Luo LP, Song JC, Gong ZJ (2013) Betaine protects against high-fat-diet-induced liver injury by inhibition of high-mobility group box 1 and Toll-like receptor 4 expression in rats. Dig Dis Sci 58(1):3198–31206
Zhang Y, Jin P, Huang Y, Shan T, Wang L, Li Y, Zheng Y (2016) Effect of hot water combined with glycine betaine alleviates chilling injury in cold-stored loquat fruit. Postharvest Biol Technol 118:141–147
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Valenzuela-Soto, E.M., Figueroa-Soto, C.G. (2019). Biosynthesis and Degradation of Glycine Betaine and Its Potential to Control Plant Growth and Development. In: Hossain, M., Kumar, V., Burritt, D., Fujita, M., Mäkelä, P. (eds) Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants. Springer, Cham. https://doi.org/10.1007/978-3-030-27423-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-27423-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27422-1
Online ISBN: 978-3-030-27423-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)