Abstract
Many RD-causing mutations lead to a dysregulation of cyclic guanosine monophosphate (cGMP), making cGMP signalling a prime target for the development of new treatment approaches. We showed previously that an analogue of cGMP, which inhibited cGMP signalling targets, increased photoreceptor viability in three rodent RD models carrying different genetic defects, in different RD genes. This raises the question of the possible generality of this approach as a treatment for RD. Here, we review RD genes that can be associated with high cGMP and discuss which RD genes might be amenable to a treatment aimed at inhibiting excessive cGMP signalling.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
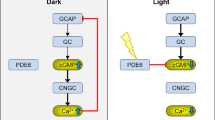
The degeneration and loss of photoreceptors in retinal degeneration (RD)-type diseases are a major unmet medical problem. Development of treatments for RD is hampered by the vast genetic heterogeneity of this group of diseases, with disease-causing mutations known in over 260 genes (https://sph.uth.edu/retnet; information retrieved October 2018). Mutations causing RD often affect genes relating to the photoreceptor phototransduction cascade. Within this cascade, the signalling molecule cyclic guanosine monophosphate (cGMP) occupies a key position. cGMP is synthesized by retinal guanylyl cyclase (retGC) in a highly regulated way, allowing cGMP to activate and open its prototypic photoreceptor target, the cyclic nucleotide gated (CNG) ion channel. This raises intracellular Na+ and Ca2+ levels and promotes the conversion of light to an electrochemical signal (Kulkarni et al. 2016). Light-dependent sequential activation of the photopigment rhodopsin and the G-protein transducin activate the enzyme phosphodiesterase 6 (PDE6), which hydrolyses cGMP, leading to the closure of CNG channels. Seminal research performed already in the 1970s established that high levels of cGMP were associated to and likely causal for photoreceptor degeneration (Farber and Lolley 1974; Lolley et al. 1977). Because of this pathologic aspect of cGMP in photoreceptors, we hypothesized several years ago that interventions in cGMP signalling might constitute a viable therapeutic avenue applicable to many different RD-causing mutations.
2 Inhibition of cGMP Signalling Protects Photoreceptors
In an initial study, we highlighted the possibility to use inhibitory analogues of cGMP to reduce photoreceptor cell death in vitro, in organotypic retinal explant cultures derived from the Pde6b-mutant rd1 mouse and the Prph2-mutant rd2 mouse (Paquet-Durand et al. 2009). In a comparative study, using ten different RD animal models, we subsequently found also that mutations in RD genes different from PDE6 could cause an excessive accumulation of cGMP in photoreceptors (Arango-Gonzalez et al. 2014), underlining the possibility that cGMP could represent a relative mutation independent target in RD. We then set out to develop a combination of inhibitory cGMP analogues with a drug delivery system (Maussang et al. 2016) that would enable these compounds not only to reach the photoreceptors in vivo, across the blood retinal barrier, but also to achieve favourable pharmacokinetics and improved bioavailability. Notably, the lead compound formulation protected rod photoreceptors in vivo in the rd1 , rd2 and rd10 animal models for the RD-type disease retinitis pigmentosa (RP) (Vighi et al. 2018). Furthermore, the rod photoreceptor protection observed with this treatment resulted in marked preservation of cone photoreceptor functionality.
We thus found that animals suffering from mutations in very different genes (rd1: Pde6b – functioning in phototransduction; rd2: Prph2 – important for outer segment structure) showed high levels of photoreceptor cGMP and benefitted from this treatment. Together with the fact that high cGMP indeed has been seen in yet other models (Arango-Gonzalez et al. 2014), this raises the question which else of the vast array of RD genes can be, in one way or the other, linked to cGMP dysregulation and which thus may be identified as additional targets for such treatment (Fig. 40.1).
RD genes associated with high levels of photoreceptor cGMP. The different circles show RD genes related to cGMP signalling in various ways: the innermost circle displays genes directly involved in cGMP synthesis and hydrolysis. The second circle relates to genes that regulate the synthesis/hydrolysis, while the third level indicates genes known to affect intracellular cGMP via genes shown in the second circle. The grey box shows genes found or reasoned to be associated with high cGMP but for which no clear causal relationship has been established so far
To give us a better perspective of this, we here reviewed RD genes that can be surmised to associate with high levels of cGMP in photoreceptors. This in turn could help to produce future estimates as to how many RD patients might be amenable to a treatment targeting cGMP signalling.
3 RD Genes Connected to Excessive Photoreceptor cGMP Production
In photoreceptors, cGMP is produced by retGC, a protein encoded by the GUCY2D gene in human rods, and in which gain-of-function mutations cause rapid cGMP-dependent photoreceptor loss (Sato et al. 2018). The activity of retGC is regulated by a guanylyl cyclase activating protein (GCAP), encoded by the GUCA1A and GUCA1B genes in rods and cones. When Ca2+ levels are low, GCAP activates retGC to produce cGMP, while in high Ca2+ concentrations, GCAP inhibits retGC (Tucker et al. 1999).
This dependence of cGMP synthesis on Ca2+ levels provides for a regulatory feedback loop in which cGMP-dependent activation of CNG channels leads to Ca2+ influx, which in turn inhibits further cGMP synthesis and limits photoreceptor cGMP to physiological levels of 1–5 μM (Olshevskaya et al. 2002). This also means that when CNG channels are dysfunctional, the negative feedback control of retGC is missing, allowing cGMP levels to overshoot to extremely high concentrations. Such dysfunction may come from the known RD connected mutations in the CNG subunit genes CNGA1, CNGA3, CNGB1 and CNGB3 (Reuter et al. 2008; Biel and Michalakis 2009; Paquet-Durand et al. 2011). retGC is additionally inhibited by the RD3 protein, so that loss-of-function mutations in the RD3 gene will also cause high cGMP and photoreceptor death (Peshenko et al. 2016)
Furthermore, retGC activity critically depends on the availability of its substrate GTP. The rate-limiting step in the synthesis of the required guanine nucleotides is mediated by inosine monophosphate dehydrogenase 1 (IMPDH1). Remarkably, RD-causing mutations in the IMPDH1 gene preserve its enzymatic activity; however, the mutant enzyme is no longer regulated by nucleotide binding (Xu et al. 2008), possibly causing an overproduction of retGC substrate.
4 RD Genes Involved in cGMP Hydrolysis
The enzyme hydrolysing cGMP in photoreceptors is PDE6, a heterodimer consisting of one alpha (PDE6A) and one beta (PDE6B) subunits in rods (McLaughlin et al. 1993; Dryja et al. 1995), which in turn form a complex with the small inhibitory gamma (PDE6G) subunit (Tsang et al. 1996). Cone PDE6 is a homodimer encoded for by the PDE6C gene (Thiadens et al. 2009), with the specific gamma subunit encoded by the PDE6H gene (Brennenstuhl et al. 2015). In both rods and cones, the functional assembly of the subunits is mediated by the AIPL1 protein. Hence, loss-of-function mutations in the AIPL1 gene lead to PDE6 dysfunction, cGMP accumulation and rapid photoreceptor loss (Ramamurthy et al. 2004).
PDE6 is activated by transducin, a protein encoded by the GNAT1 gene in rods and GNAT2 in cones. Transducin in turn is activated by rhodopsin (RHO) and cone opsins, respectively, once their photopigment retinal has been activated by a photon of light. Thus, PDE6 activity depends on the function of both rhodopsin and transducin, explaining why certain mutations in these genes lead to insufficient cGMP hydrolysis (Kohl et al. 2002; Arango-Gonzalez et al. 2014; Mejecase et al. 2016).
5 Other RD Genes that May Be Associated with High cGMP in Photoreceptors
The gene PRPH2 encodes for the peripherin-2 protein, which together with the rod outer segment membrane protein 1 (ROM1) is essential for the formation of photoreceptor outer segments (Goldberg et al. 2016). Mutations in PRPH2 lead to an absence of outer segments, which likely leads to an ectopic and dysregulated expression of outer segment enzymes. Interestingly, Prph2 mutations in the mouse cause high levels of photoreceptor cGMP (Paquet-Durand et al. 2009), suggesting that Rom1 mutations would have similar effect.
We may expect that also photoreceptor-specific transcription factors such as CRX, NRL and NR2E3, which regulate the expression of genes linked to phototransduction (Pittler et al. 2004; Xu et al. 2013), when mutated may eventually lead to lack of phototransduction activity and consequently increased cGMP. Similarly, mutations in genes involved in the correct trafficking of phototransduction proteins could result in aberrant cGMP production. For instance, the REEP6 protein appears to be important for the trafficking of retGC to the photoreceptor outer segment (Agrawal et al. 2017). Whether REEP6 gene mutations cause RD via aberrant cGMP ectopic production in the photoreceptor cytoplasm is currently not known.
6 Conclusion
The data available to date indicates that mutations in at least 20 different RD genes may lead to excessive accumulation of cGMP in photoreceptors. Further studies on RD models, with mutations in genes mediating other functions in photoreceptors, are needed to comprehensively assess the effects of mutations on photoreceptor cGMP levels. Given the antecedents, one may assume that such studies will in the future connect even more RD genes to high photoreceptor cGMP. Furthermore, we need to consider that in each RD gene, gain-of-function and loss-of-function mutations can have opposite effects on cGMP (Power et al. 2019). For instance, most RD-causing mutations in retGC appear to be gain-of-function mutations (Sato et al. 2018), while a retGC gene knockout in the mouse, with expected no photoreceptor cGMP production, also causes photoreceptor loss (Yang et al. 1999). Irrespective of this, it is likely that therapeutic approaches aimed at lowering cGMP signalling will be applicable to several RD genes and gene mutations causing high photoreceptor cGMP.
References
Agrawal SA, Burgoyne T, Eblimit A et al (2017) REEP6 deficiency leads to retinal degeneration through disruption of ER homeostasis and protein trafficking. Hum Mol Genet 26:2667–2677
Arango-Gonzalez B, Trifunovic D, Sahaboglu A et al (2014) Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS One 9:e112142
Biel M, Michalakis S (2009) Cyclic nucleotide-gated channels. Handb Exp Pharmacol:111–136
Brennenstuhl C, Tanimoto N, Burkard M et al (2015) Targeted ablation of the Pde6h gene in mice reveals cross-species differences in cone and rod phototransduction protein isoform inventory. J Biol Chem 290:10242–10255
Dryja TP, Finn JT, Peng YW et al (1995) Mutations in the gene encoding the alpha subunit of the rod cGMP-gated channel in autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci U S A 92:10177–10181
Farber DB, Lolley RN (1974) Cyclic guanosine monophosphate: elevation in degenerating photoreceptor cells of the C3H mouse retina. Science 186:449–451
Goldberg AF, Moritz OL, Williams DS (2016) Molecular basis for photoreceptor outer segment architecture. Prog Retin Eye Res 55:52–81
Kohl S, Baumann B, Rosenberg T et al (2002) Mutations in the cone photoreceptor G-protein alpha-subunit gene GNAT2 in patients with achromatopsia. Am J Hum Genet 71:422–425
Kulkarni M, Trifunovic D, Schubert T et al (2016) Calcium dynamics change in degenerating cone photoreceptors. Hum Mol Genet 25:3729
Lolley RN, Farber DB, Rayborn ME et al (1977) Cyclic GMP accumulation causes degeneration of photoreceptor cells: simulation of an inherited disease. Science 196:664–666
Maussang D, Rip J, van Kregten J et al (2016) Glutathione conjugation dose-dependently increases brain-specific liposomal drug delivery in vitro and in vivo. Drug Discov Today Technol 20:59–69
McLaughlin ME, Sandberg MA, Berson EL et al (1993) Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet 4:130–134
Mejecase C, Laurent-Coriat C, Mayer C et al (2016) Identification of a novel homozygous nonsense mutation confirms the implication of GNAT1 in rod-cone dystrophy. PLoS One 11:e0168271
Olshevskaya EV, Ermilov AN, Dizhoor AM (2002) Factors that affect regulation of cGMP synthesis in vertebrate photoreceptors and their genetic link to human retinal degeneration. Mol Cell Biochem 230:139–147
Paquet-Durand F, Hauck SM, van Veen T et al (2009) PKG activity causes photoreceptor cell death in two retinitis pigmentosa models. J Neurochem 108:796–810
Paquet-Durand F, Beck S, Michalakis S et al (2011) A key role for cyclic nucleotide gated (CNG) channels in cGMP-related retinitis pigmentosa. Hum Mol Genet 20:941–947
Peshenko IV, Olshevskaya EV, Dizhoor AM (2016) Functional study and mapping sites for interaction with the target enzyme in retinal degeneration 3 (RD3) protein. J Biol Chem 291:19713–19723
Power M, Das S, Schütze K, Marigo V, Ekström P, Paquet-Durand F (2019) Prog Retin Eye Res. 30:100772. doi: 10.1016/j.preteyeres.2019.07.005. Cellular mechanisms of hereditary photoreceptor degeneration - Focus on cGMP.
Pittler SJ, Zhang Y, Chen S et al (2004) Functional analysis of the rod photoreceptor cGMP phosphodiesterase alpha-subunit gene promoter: Nrl and Crx are required for full transcriptional activity. J Biol Chem 279:19800–19807
Ramamurthy V, Niemi GA, Reh TA et al (2004) Leber congenital amaurosis linked to AIPL1: a mouse model reveals destabilization of cGMP phosphodiesterase. Proc Natl Acad Sci U S A 101:13897–13902
Reuter P, Koeppen K, Ladewig T et al (2008) Mutations in CNGA3 impair trafficking or function of cone cyclic nucleotide-gated channels, resulting in achromatopsia. Hum Mutat 29:1228–1236
Sato S, Peshenko IV, Olshevskaya EV et al (2018) GUCY2D cone-rod dystrophy-6 is a “Phototransduction Disease” triggered by abnormal calcium feedback on retinal membrane guanylyl cyclase 1. J Neurosci 38:2990–3000
Thiadens AA, den Hollander AI, Roosing S et al (2009) Homozygosity mapping reveals PDE6C mutations in patients with early-onset cone photoreceptor disorders. Am J Hum Genet 85:240–247
Tsang SH, Gouras P, Yamashita CK et al (1996) Retinal degeneration in mice lacking the gamma subunit of the rod cGMP phosphodiesterase. Science 272:1026–1029
Tucker CL, Woodcock SC, Kelsell RE et al (1999) Biochemical analysis of a dimerization domain mutation in RetGC-1 associated with dominant cone-rod dystrophy. Proc Natl Acad Sci U S A 96:9039–9044
Vighi E, Trifunovic D, Veiga-Crespo P et al (2018) Combination of cGMP analogue and drug delivery system provides functional protection in hereditary retinal degeneration. Proc Natl Acad Sci U S A 115:E2997–E3006
Xu D, Cobb G, Spellicy CJ et al (2008) Retinal isoforms of inosine 5′-monophosphate dehydrogenase type 1 are poor nucleic acid binding proteins. Arch Biochem Biophys 472:100–104
Xu J, Morris L, Thapa A et al (2013) cGMP accumulation causes photoreceptor degeneration in CNG channel deficiency: evidence of cGMP cytotoxicity independently of enhanced CNG channel function. J Neurosci 33:14939–14948
Yang RB, Robinson SW, Xiong WH et al (1999) Disruption of a retinal guanylyl cyclase gene leads to cone-specific dystrophy and paradoxical rod behavior. J Neurosci 19:5889–5897
Acknowledgement and Funding
This work was supported by grants from the European Union (DRUGSFORD; HEALTH-F2-2012-304963, transMed; H2020-MSCA-ITN-765441) and Deutsche Forschungsgemeinschaft (PA1751/8-1).
Conflict of Interest Statement
François Paquet-Durand, Valeria Marigo, and Per Ekström have filed for three patents on the synthesis and use of cGMP analogues (PCTWO2016/146669A1, PCT/EP2017/066113, PCT/EP2017/071859) and have obtained a European Medicine Agency orphan drug designation for the use of the cGMP analogue DF003 for the treatment of retinitis pigmentosa (EU/3/15/1462). François Paquet-Durand, Valeria Marigo, and Per Ekström are shareholders of, or have other financial interest in, the company Mireca Medicines GmbH (www.mireca.eu), which intends to forward clinical testing of DF003.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this paper
Cite this paper
Paquet-Durand, F., Marigo, V., Ekström, P. (2019). RD Genes Associated with High Photoreceptor cGMP-Levels (Mini-Review). In: Bowes Rickman, C., Grimm, C., Anderson, R., Ash, J., LaVail, M., Hollyfield, J. (eds) Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology, vol 1185. Springer, Cham. https://doi.org/10.1007/978-3-030-27378-1_40
Download citation
DOI: https://doi.org/10.1007/978-3-030-27378-1_40
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27377-4
Online ISBN: 978-3-030-27378-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)