Abstract
Chemical communication is an important aspect of courtship and reproduction in salamanders ( Urodela ), where males secrete several protein pheromones from various sexually dimorphic glands . The most widely used sex pheromone system consists of proteins of the Sodefrin Precursor-like Factor (SPF) family . This protein family already started diversifying through gene duplications in the Late Palaeozoic and continued to do so in various urodelan lineages. As a result, males of multiple extant salamander species secrete different sets or ratios of SPF protein pheromones which, as shown in behavioral tests, can evoke various female responses during the courtship process, such as following behavior , cloacal gaping , or an acceleration of courtship in general. Still, all observable effects essentially are a consequence of female receptivity enhancement, indicating a preserved role for SPF pheromones. Salamandridae is a large family with currently 119 described species that show a considerable variety in courtship behaviour . In this family , different lineages evolved various modes of pheromone transfer, ranging from direct application during amplexus on land, to an indirect transfer in which males abandoned physical contact and instead tail-fan pheromones underwater toward a nearby female. In one genus, an additional decapeptide pheromone, sodefrin , originated by cleavage from a precursor of the SPF family and was co-opted alongside uncleaved SPF protein pheromones. Here we discuss our current understanding and knowledge gaps on the use of the SPF pheromone system in salamandrids, and we define some testable hypotheses on its evolution in relation to changes in mating habitat and courtship modes.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
1 Introduction
Throughout the animal kingdom, sex pheromones play an essential role in eliciting a variety of responses during reproductive interactions between males and females. In amphibians, the role of chemical signals during courtship is especially well documented in salamanders ( Urodela ). Most salamanders have internal fertilization, meaning that eggs and sperm merge inside the female body. Since salamanders do not have a copulatory organ, females of species in the internally fertilizing clade (Fig. 1) have to pick up the male spermatophore with their cloaca from the environment to complete courtship and reproduction successfully. The use of pheromones is an essential component in coordinating the courtship process that precedes fertilization. To this aim, males often possess sexually dimorphic glands , which hypertrophy during the breeding season and have been demonstrated or postulated to produce courtship pheromones (Sever 1991; Sever 2003; Van Bocxlaer et al. 2015). Some well-known examples are the mental glands on the chin in plethodontids (Houck et al. 2008) and the highly specialized dorsal glands in the cloaca and abdominal cavity of newts (Kikuyama et al. 1995; Van Bocxlaer et al. 2015). The chemicals secreted from these glands are transmitted from male to female either by direct contact (e.g., transfer of courtship pheromones from mental glands to nares or skin -abrasions in terrestrially mating plethodontids: Arnold 1977; Houck et al. 1998; Rollmann et al. 2003; Houck et al. 2008) or, provided that both sexes are in close proximity, by transmission through water (e.g., transfer of pheromones from the cloaca by tail-fanning in aquatically mating newts ; Halliday 1974, 1977, 1990; Arntzen and Sparreboom 1989; Wells 2007; Treer et al. 2013; Van Bocxlaer et al. 2015).
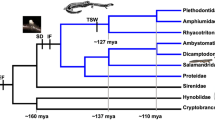
Known SPF pheromone use in salamanders . The phylogeny and timescale are based on TimeTree (www.timetree.org). With the exception of terrestrial representatives of the families Plethodontidae, Ambystomatidae, and Salamandridae , the remaining salamanders mate in water. The urodelan ancestor had external fertilization (EF), while internal fertilization (IF) originated in the ancestor of all Salamandroidea. The confirmed use of SPF pheromones in three extant families suggests the potential of pheromone use in all internally fertilizing salamanders , while the use of both alpha and beta SPF (products of a gene duplication that happened before the diversification of extant salamanders ) in Salamandridae and Ambystomatidae suggests extension of the potential use of SPF pheromones to all (i.e., including externally fertilizing) salamanders
Salamandridae forms a diverse group of 119 species in three subfamilies, Pleurodelinae (newts, 99 species), Salamandrinae (true salamanders , 18 species) and Salamandrininae (2 species) (AmphibiaWeb. 2018. https://amphibiaweb.org University of California, Berkeley, CA, USA. Accessed 17 January 2018). This family evolved an interesting diversity in mating strategies, with different courtship modes both on land and in water, the presence of various forms of contact during courtship , and different modes of sperm transfer (Halliday 1977, 1990; Houck and Arnold 2003; Sparreboom 2014). Since salamandrids only started diversifying in the Late Cretaceous (Zhang et al. 2008; Kieren et al. 2018), they form an interesting group to study pheromone evolution in relation to different courtship strategies over a relatively short evolutionary time.
One of the best-known modes of underwater pheromone transfer in salamandrids is tail-fanning behaviour in Pleurodelinae , in which males use their tail to create a water current that carries courtship pheromones from the cloaca toward the female’s snout. This behavior has already been described and illustrated in the eighteenth (Spallanzani 1780) and early nineteenth centuries (Rusconi 1821), and detailed courtship sequences have been outlined in multiple species since then (e.g., Tinbergen and ter Pelkwijk 1938; Nelson 1960; Halliday 1977, 1990; Duellman and Trueb 1994). The female responds by essentially following the male path and taking up his spermatophore. In search for molecules secreted by tail-fanning males, the decapeptide sodefrin , which was isolated from the male dorsal glands of the Japanese fire belly newt Cynops pyrrhogaster , was the first salamander pheromone to be identified (Kikuyama et al. 1995). Sodefrin was shown to function as a close-range attractant, and variants were detected in different populations of this species, as well as in its sister species C. ensicauda (Yamamoto et al. 2000; Iwata et al. 2004, 2005; Nakada et al. 2007). Subsequent studies have shown that proteins of about 20 kDa act as courtship pheromones in several salamander families (Houck et al. 2008; Van Bocxlaer et al. 2015; Maex et al. 2016). Although these pheromones do not share any similarity with sodefrin at amino acid level, their transcripts and precursors are phylogenetically related with those of sodefrin , and they were therefore termed Sodefrin Precursor-like Factors (SPF ).
Other pheromones have been described in salamanders (Rollmann et al. 1999; Houck et al. 2007; Nakada et al. 2017; Maex et al. 2018), but SPF proteins are the most widely distributed male courtship pheromones among urodeles. Here we review our current understanding on the use of pheromones of the SPF family (i.e., sodefrin and SPF ) in relation to the wide variety of courtship interactions in salamandrids (Kikuyama et al. 1995; Yamamoto et al. 2000; Janssenswillen et al. 2014, 2015; Van Bocxlaer et al. 2015, 2016).
2 Female Courtship Responses and the Biological Roles of SPF
Several behavioral experiments have been developed to demonstrate the presence of chemical communication between sexes in salamanders . For example, linear as well as Y-shaped olfactometers were used to perform choice tests with different species (e.g., Dawley 1984; Malacarne and Vellano 1987; Secondi et al. 2005; Poschadel et al. 2007). In other setups, the time that a newt spent in the vicinity of sponges containing potential pheromones was compared to that of sponges containing only water (e.g., Toyoda et al. 1994; Kikuyama et al. 1995; Yamamoto et al. 2000). These experiments all compared time that a female spent in olfactometer compartments with different stimuli and were able to indicate the presence of chemical communication . However, because such experiments are less suited for understanding the biological roles of these chemical stimuli , new tests were developed that allowed females to show more natural courtship responses in the presence of another individual. In plethodontids, the duration of courtship after application of putative pheromones on the female nares was measured to assay pheromone activity in a setup in which a female was coupled with a male whose pheromone gland (i.e., mental gland; Rollmann et al. 1999) was removed. In newts with internal pheromone glands that have courtship without contact, the problem of a pheromone-releasing male was circumvented by developing a two-female test, in which female responses were compared in water with and without male courtship pheromones (Treer et al. 2013). The latter test allows females to show the same courtship responses, including following behavior , as in the presence of a courting male. In newts with amplexus , i.e., where the male grasps the female, a two-female test was developed in which cloacal gaping in response to the presence of male pheromones was measured (Janssenswillen and Bossuyt 2016). Since opening the cloaca is a required response for insemination, this test also allows a more straightforward interpretation of the biological role of pheromones than general choice experiments . The development of all these tests enabled studying the effects of SPF pheromones in a more realistic biological context. Although SPF pheromones are able to attract females in olfactometers (Kikuyama et al. 1995), these proteins do not play a role in attracting females from a long distance, since males only start administering their pheromones when the female is already in close vicinity. Instead, an important role of SPF pheromones is to enhance female receptivity during close-range courtship , which at several instants during the courtship process results in observable changes, such as following behaviour , cloacal gaping , or an acceleration of the courtship process in general.
Despite the evident effects that male SPF courtship pheromones have on female receptivity in experimental setups, the exact impact of these chemical cues in the totality of all available cues during natural courtship in newts is not yet entirely understood. When breeding newts meet underwater, they can deliver short-range communication signals of different modalities at the same time, such as visual, chemical and tactile cues. On the one hand , courtship pheromones are able to induce courtship responses in female newts in the absence of a male, obviating the need for male visual and tactile cues to elicit female mating responses (Treer et al. 2013). On the other hand , while pheromones are beneficial for urodelan courtship , they do not always seem to be a necessary prerequisite for successful reproduction . For example, in the Spanish ribbed newt (Pleurodeles waltl), a successful mating without obvious delivery of male courtship pheromones to the female could be observed in about 5% of the cases (Janssenswillen and Bossuyt 2016). Similar behaviour was observed in plethodontid salamanders , which engaged in mating even when the male courtship pheromone -producing mental gland was ablated (Houck et al. 1998; Rollmann et al. 1999), although it must be noted that the availability of other pheromone -producing glands could not be excluded in these animals (Sever 1994, 2003; Rollins and Staub 2017). Altogether, behavioural experiments indicate a key role for SPF pheromones in influencing female courtship responses (Van Bocxlaer et al. 2015).
3 The SPF pheromone System
Phylogenetic analyses have shown that all transcripts of the SPF family are derived from genes that belong to the three-finger protein (TFP ) superfamily (Janssenswillen et al. 2014), which is present in most vertebrates and has a wide variety of biological functions (e.g., toxic, cholinergic signalling , and immune regulation; Tsetlin 1999; Loughner et al. 2016). Three-finger proteins are characterized by a distinct tertiary structure of three beta-strand loop fingers, shaped by a conserved cysteine pattern (Fleming et al. 1993; Casey et al. 1994; Kieffer et al. 1994; Palmer et al. 2007; Kini and Doley 2010). SPFs are two-domain three-finger proteins (2D-TFPs), often consisting of either an anterior 8-cysteine pattern and a posterior 6-cysteine pattern (resulting in the loss of one ‘finger’), or an anterior 10-cysteine pattern and a posterior 8-cysteine pattern (Fig. 2), within mature proteins of around 20 kDa (Janssenswillen et al. 2014). Little is known about the functions of 2D-TFPs in vertebrates . The Anolis carolinensis genome contains 19 2D-TFP genes, and several of these are highly expressed in male testes and dewlap, and in female ovaries (NCBI Protein Database. 2004. https://www.ncbi.nlm.nih.gov/protein/, accessed on 21 January 2018), suggesting a function in reproduction (Baeckens et al. 2016). In snakes, 2D-TFPs have been postulated to function as a self-protecting agent against phospholipase A2 from their own venom, which could enter the blood circulation (Dunn and Broady 2001; Fortes-Dias et al. 2017), but nonvenomous snakes secrete them as well (Okomura et al. 1999). In the genome of the frog Silurana tropicalis, 26 2D-TFPs were identified, but the function of most of these is unknown. A reconstruction of vertebrate 2D-TFP evolution suggests that amphibian 2D-TFPs (including SPF pheromones ) form a monophyletic group , suggesting that the diversification of these genes in Amphibia started after their divergence from Amniota (Janssenswillen et al. 2014). Other than SPF genes that are mainly expressed in male sexually dimorphic glands , genes of this clade are found to be expressed in the gastrula embryo stage in frogs (Amaya et al. 2006; Clark et al. 2016), and in the eyes and regenerative tissues of salamanders (Maki et al. 2010; Campbell et al. 2011), but the exact biological function in these tissues remains unknown.
Conserved cysteine patterns in alpha and beta SPF proteins . The shown sequences are from Cynops pyrrhogaster (GenBank-numbers KU213615 and KU213626). SPF proteins have two three-finger motifs (TFM1 and TFM2), containing 8 and 6 cysteines (alpha SPF ), or 10 and 8 cysteines (beta SPF ). Cysteines are indicated in gray. TFM = three-finger motif
The SPF gene repertoire has diversified substantially through gene duplications that started already during the early amphibian evolution . Phylogenetic analyses have shown that several extant species of salamandrids and ambystomatids secrete pheromone proteins , termed SPF alpha and beta, whose divergence can be traced back to a gene duplication that happened in the late Palaeozoic, before the diversification of extant salamanders (Van Bocxlaer et al. 2015; Maex et al. 2016). Each of these gene lineages further diversified through additional duplications during more recent urodelan evolution , and several extant salamander species now secrete highly divergent isoforms of both alpha and beta SPF proteins alongside each other during courtship . The genus Cynops has an additional set of transcripts, making up about 15% of the total SPF family blend (Van Bocxlaer et al. 2016), that leads to the secretion of another pheromone , the decapeptide sodefrin (Kikuyama et al. 1995).
4 The Origin of Sodefrin
Sodefrin and SPF proteins are products from the same gene family , and a large part of their precursors is homologous at the nucleotide level. However, sodefrin transcripts contain an insertion of 62 base pairs that causes a frameshift in protein translation. This frameshift results in a different amino acid sequence with new cleavage sites, which allows generating the decapeptide sodefrin (Fig. 3). SPF transcripts do not have this insert and are secreted as full-length proteins (Janssenswillen et al. 2014). Because of the frameshift, the C-terminus of the sodefrin and SPF amino acid precursor sequences are nonhomologous, and as a consequence sodefrin shows no resemblance to intact SPF proteins. Precursors containing the insert were only found in the genus Cynops (Janssenswillen et al. 2014), indicating that a sodefrin -like decapeptide originated and obtained a pheromone function in the shadow of uncleaved SPF proteins shortly after the divergence of Cynops from its sister clade (Fig. 4) (Janssenswillen et al. 2014; Van Bocxlaer et al. 2016). The unique combination of an ancient pheromone blend (SPF ) with a new pheromone (sodefrin ) sheds light on how a random molecule can become a pheromone, even though it shows no similarity to existing signals. Because most SPF genes continued to encode for uncleaved SPF pheromones, the insertion in one of the genes likely did not have a large effect on the overall pheromone function of the altered protein blend. However, because sodefrin was secreted alongside functional SPF proteins, females may have been able to associate the new scent with existing pheromones , making it easier for the new decapeptide to evolve a sex pheromone function (Janssenswillen et al. 2014).
Comparison of SPF and sodefrin precursors. SPF and sodefrin pheromones originate from the same gene family , and are largely homologs at the nucleotide level (RNA precursors are indicated in the same tint of gray). SPF protein precursors become mature pheromones after cleavage (indicated with scissors) of the signal peptide (SP) (shown on the left). Sodefrin RNA precursors differ from SPF RNA precursors by an insertion of 62 base pairs that, compared to SPF , causes a translational frameshift (shown on the right). This leads to the origin of a protein precursor that shows no amino acid sequence similarity with the SPF precursor at the C-terminal region (indicated in dark gray). The new amino acid sequence contains additional cleavage sites that allow cleavage of the decapeptide sodefrin (indicated in black)
The evolution of courtship in Salamandridae . The figure gives an overview of courtship and mating in salamandrids, with an indication of known use of SPF and sodefrin -like decapeptide pheromones . Squares with question marks indicate probably present (black) or probably absent (white), based on presence or absence in studied species and their phylogenetic relationships. The timescale follows Zhang et al. 2008 and is only given as indicative measure. Males are drawn in gray, females in white. The decapeptide pheromone sodefrin (and its variants) evolved relatively recently in the lineage to Cynops
Based on behavioural experiments in which responses of single females were assessed in the absence of other individuals (Toyoda et al. 1994; Kikuyama et al. 1995; Yamamoto et al. 2000; Nakada et al. 2007), sodefrin and its variants have been described as attractants. Different components of the Cynops pheromone blend thus would serve distinct but complementary functions, in which the decapeptide functions as an attractant, while full-length SPF proteins are used to enhance female receptivity . However, the decapeptides are produced together with uncleaved SPF pheromones in the male dorsal glands , and both are simultaneously secreted via the cloaca during male tail-fanning at close distance (Kikuyama and Toyoda 1999; Van Bocxlaer et al. 2016). Additionally, SPF protein pheromones from modern European newts were also shown to attract females from a short distance in a Y-maze setup that includes a visual newt stimulus (Treer and Bossuyt 2010). We, therefore, consider it possible that decapeptides and SPF proteins have highly similar functions, but this hypothesis remains to be tested. If proteins and decapeptides would show comparable effects in both behavioural setups (i.e., two-female test and attractant test), the observed short-range attraction for both pheromones could be recognized as a logical consequence of the enhanced female receptivity .
5 Species-Specificity of the SPF Pheromone System
The expression of multiple, highly divergent SPF proteins in a single species prompts the question of how the underlying genes evolved to produce species-specific courtship signals throughout salamander evolution . Interspecific experiments have disclosed varying degrees of behavioural responses to courtship pheromones of the same versus other species. In both Cynops pyrrhogaster and Cynops ensicauda , females are attracted by the scent of their own species- and population-specific decapeptide pheromones (Kikuyama et al. 1995; Yamamoto et al. 2000; Iwata et al. 2004, 2005; Nakada et al. 2007). For species using SPF pheromones, females of one species ( Lissotriton helveticus ) showed responses to male courtship pheromones of a related species (L. vulgaris) in a two-female test, but they were much less pronounced than for proteins of their own species, indicating a preference for conspecific signals (Van Bocxlaer et al. 2015). Furthermore, the same species showed no responses to pheromones of a more distantly related newt ( Ichthyosaura alpestris ), suggesting that after speciation, cross-reaction to allospecific courtship pheromones gradually dilutes over evolutionary time as species diverge (Treer et al. 2013). One mechanism through which protein courtship pheromones can diverge to establish species-specificity was disclosed in an in-depth transcriptome study on the multicomponent SPF pheromone system of these species (Treer et al. 2017). Despite the vast diversity of available SPF gene copies, I. alpestris and L. helveticus predominantly expressed the same subset of orthologs in their pheromone blend. Neither gene loss nor co-option of distinct SPF genes has left a clear imprint on the courtship pheromones of both species, and the absence of major differences in SPF expression suggests that sequence divergence has been the predominant mode of pheromone diversification in these species. The observed species-specificity seems to be a consequence of speciation rather than the cause since cross-reaction between the two congeneric Lissotriton species still occurs. However, despite the fact that courtship pheromones do not necessarily prevent mating between closely related species or populations (Rollmann et al. 2003; Van Bocxlaer et al. 2015), they may still contribute to speciation by reducing gene flow through assortative mating (Kozak 2003).
6 Pheromone Glands in Salamandrids
Male salamandrids possess different sexually dimorphic glands and nuptial excrescences, which often are closely associated with specific courtship behavior . The most widely occurring pheromone glands are located in the male cloaca. In salamandrids, these glands are known as dorsal glands because they are situated in the dorsal region of the cloaca (Sever et al. 1990; Sever 1992). They are highly specialized skin glands that secrete via papillae situated at the posterior end of the cloaca and are homologous to vent glands of males of other salamander families (Sever 1991, 2003). In some newt genera, these dorsal glands are quite large and extend from the cloaca into the so-called abdominal cavity, and are therefore often called abdominal glands (Sacerdote 1956; Kikuyama et al. 1995). However, the term ‘abdominal glands ’ has been deemed inappropriate because of the lack of a true abdomen in salamanders (Sever 2003; Osikowski and Cierniak-Zuzia 2013). Pheromones produced in the dorsal glands are released into the environment via the cloaca. Amplecting species (see below) can, in addition to tail-fanning, use cloacal imposition to deliver these pheromones more directly to the female nares (Janssenswillen and Bossuyt 2016). For most other salamandrid male glands and excrescences, it is still unknown whether they are involved in pheromone communication. For example, some species have nuptial pads that assist in holding the female during amplexus (e.g., Pleurodeles and Notophthalmus), but also could play a role in pheromone application (Mitchell and Gibbons 2010; Sparreboom 2014; Gong et al. 2018). Additionally, males of several species independently evolved various head glands (Sever 2003) which are rubbed against the female nares while amplexing (e.g., Taricha; Davis and Twitty 1964; Arnold 1972), during tail-fanning (e.g., Cynops pyrrhogaster , Fig. 4a, Kawamura and Sawada 1959; and Lissotriton boscai, Rafinski and Pecio 1992), or in an amplexus -tail-fanning combination (e.g., Notophthalmus, Fig. 4e; Pool and Dent 1977; Verrell 1982; Janssenswillen et al. 2015). At this point, Notophthalmus is the only salamandrid species for which it was shown that non-cloacal glands—in this species the cheek glands —contain an evolutionary diverse set of SPF pheromones (Janssenswillen et al. 2015).
7 SPF and the Evolution of Courtship Behaviour
The salamandrid radiation holds terrestrially as well as aquatically courting species (Fig. 4), and is characterized by various modes and degrees of physical contact during courtship . The basal splits in the salamandrid phylogeny lead to a single evolutionary lineage with no contact (Salamandrina, Fig. 4m), and several lineages with amplexus , in which the male holds the female in various ways during courtship . In true salamanders (e.g., Lyciasalamandra, Fig. 4l) and primitive newts (e.g., Tylototriton and Pleurodeles, Fig. 4g, j), the male grabs the female from beneath, which results in a ventral amplexus (Arnold 1972, 1977; Sparreboom 2014). New World newts are largely characterized by some kind of dorsal amplexus (Fig. 4e, f), in which Notophthalmus differs from Taricha by the fact that the female is constrained by the male’s hind limbs around her neck (Fig. 4e; Gallien 1952; Verrell 1982; Petranka 2010). Two genera that independently colonized lotic water (Euproctus and Calotriton) have copulatory-like contact during courtship and use a form of restraint of the female with the tail or the mouth (Fig. 4d) (Ahrenfeldt 1955; Salthe 1967; Griffiths 1996; Guillaume 1999; Houck and Arnold 2003; Carranza and Amat 2005). Males of Eurasian modern newts largely rely on tail-fanning their pheromones to the female under water, with no or limited physical contact with the female (Fig. 4b, c). Given that the courtship strategies of males define their opportunities for pheromone application, an intriguing question is how evolutionary transitions between courtship habitats or modes on one hand , and pheromone use and the overall evolution of the SPF pheromone system on the other hand , have influenced each other in this family .
Phylogenetic reconstruction of the mating habitat shows that the early evolution of salamandrids is characterized by the split of terrestrially courting lineages (Salamandrina and the clade of “true” salamanders , with the exception of Mertensiella that has both aquatic and terrestrial mating) from otherwise aquatically courting newts (with the exception of Echinotriton) (Fig. 4). This indicates that salamandrid evolution is characterized by one transition from a terrestrial to an aquatic mating habitat (Fig. 4, in the ancestor of newts , between nodes 1 and 2), and one reversal (in Echinotriton). Use of SPF pheromones until now only has been demonstrated in aquatically courting species, but given the early origin of the SPF pheromone system (Fig. 1) and the presence of these pheromones in terrestrially courting plethodontids, we hypothesize that SPF proteins are also used by terrestrially mating salamandrids.
The conflicting phylogenetic relationships among major salamandrid lineages in different studies (for an overview, see Frost 2019) currently do not allow to reliably reconstruct transitions between different modes and levels of physical contact. Additionally, ancestral state reconstructions where different amplectic modes are coded as distinct states find absence of contact as the most probable character state for ancestors along the backbone of the salamandrid tree (Fig. 4, nodes 1 to 4) (Kieren et al. 2018), while coding amplexus as a present/absent state in a maximum parsimony framework shows presence of amplexus for these nodes, with loss in the ancestor of all modern newts (Fig. 4, between nodes 4 and 5). While reconstruction is inherently uncertain, the presence of facultative amplexus in multiple extant species (Notophthalmus, Pleurodeles, Tylototriton) is indicative for the plasticity of contact during courtship . For example, a study on the natural courtship behaviour of the Spanish ribbed newt (Pleurodeles waltl) showed that amplexus and associated pheromone use are context-dependent (Janssenswillen and Bossuyt 2016). Successful insemination in this species results from pinwheel behaviour , i.e., the couple going into a circular movement that ultimately leads to spermatophore deposition by the male and subsequent uptake through the cloaca by the female (Fig. 4k). Most often, this behaviour starts after the male has taken the female in a firm amplexus for an extended period of time, during which he releases one arm of his grip and regularly transfers pheromones by imposing his cloaca directly on the female nose (cloacal imposition). However, attentive observations on the natural courtship behaviour of these newts showed variations on this theme, in which males also use tail-fanning without contact to deliver their pheromones (Fig. 4i), or in which females engage in a pinwheel without a preceding amplexus , i.e., without a phase of male pheromone application (comparable to courtship in Tylototriton, Fig. 4h). Males that adapt their courtship behaviour in the function of the female’s responsiveness (facultative amplexus ) have also been found in the red-spotted newt (Notophthalmus viridescens) (Verrell 1982). Based on this plasticity in both primitive and New World newts, we hypothesize that the backbone of the salamandrid tree may have been equally characterized by ancestors in which amplexus varied across populations or was context-dependent. If true, complete loss of amplexus may have been achieved only in the ancestors of modern Eurasian newts (Fig. 4, between nodes 4 and 5) and could be correlated with the development of more pronounced dorsal glands (Sacerdote 1956; Osikowski and Cierniak-Zuzia 2013; Van Bocxlaer et al. 2015,2016; Treer et al. 2017; Kikuyama et al. 1995; Sever 1992) and increased tail-fanning behaviour , which in turn may have affected pheromone evolution in this clade.
8 Future Perspectives
Our current understanding of the SPF pheromone system will facilitate future studies on the evolution of chemical communication in relation to courtship behaviour in salamanders . To get better insights in pheromone use across evolutionary transitions (aquatic versus terrestrial mating, amplexus versus no amplexus ), integrative approaches that combine pheromone expression studies of the complete spectrum of secreted components with behavioural assays in a broader range of species with diverse courtship strategies would be very valuable. Examining both total pheromone blends and (combinations of) isolated or recombinantly obtained individual SPF proteins will provide insights in whether total quantity or rather genetic and posttranslational (e.g., glycosylation) diversity of specific SPF proteins determine efficiency of a pheromone blend. Furthermore, studies at the level of female chemoreceptors will be essential to understand how pheromones and their receptors have co-evolved and define the variation in behavioural courtship strategies we see today.
The use of SPF proteins by males to enhance female receptivity suggests selective advantages to both males and females in view of better coordination during courtship and shorter mating interval (Houck et al. 2008; Treer et al. 2013). Until now, behavioural tests were developed to investigate the ability of candidate pheromones to enhance female receptivity , but these tests did not examine to what extent females may prefer pheromone mixtures of some males above others (sexual selection). Still, it is plausible that females are choosy regarding the quantities (total SPF amount emitted) or diversity (SPF proteins originating from multiple gene copies) that a male produces. A recent study in the amplecting species Pleurodeles waltl (Janssenswillen and Bossuyt 2016) provided an interesting clue in this regard. The study showed that in about one on four natural courtships, the female avoided the stage of pinwheel behaviour (the stage necessary for successful reproduction in this species), either by thanatosis (i.e., feigning death) and/or by escaping from amplexus (Janssenswillen and Bossuyt 2016). This indicates that, even when amplected, females have a choice whether or not they want to engage in reproduction . Enhanced responsiveness to specific male pheromone mixtures could indicate an evolved female preference, for example, if pheromones would directly or indirectly convey information about male quality . Along the same line, the seasonal development of enlarged, conspicuous crests and tail fins, as well as visually conspicuous coloration patterns, suggest that elaborate sexually dimorphic male visual traits play a role in sexual selection as well. Especially in species that do not restrain a female (amplexus ) during courtship , males seem to use a combination of visual and chemical stimuli to persuade a female into courtship . Only an integration of all these aspects in overarching studies will allow to fully understand how sexual communication selection has shaped the salamandrid radiation.
References
Ahrenfeldt RH (1955) Mating behaviour of Euproctus asper in captivity. Br J Herpetol 2:194–197
Amaya E, Ashurst JL, Bonfield JK, Croning MDR, Chen C-K, Davies RM, Francis MD, Garrett N, Gilchrist MJ, Grafham DV, McLaren SR, Papalopulu N, Rogers J, Smith JC, Taylor RG, Voigt J, Zorn AM (2006) Direct submission: nucleotide [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; [04 October 2006]—Accession No. CR761728.2, Xenopus tropicalis finished cDNA, clone TGas100l21 [cited 2018 January 14]. Available from: https://www.ncbi.nlm.nih.gov/nuccore/CR761728.2
Arnold SJ (1972) The evolution of courtship behaviour in salamanders (Doctoral dissertation). University of Michigan, Ann Arbor, Michigan, p 570
Arnold SJ (1977) The evolution of courtship behavior in new world salamanders with some comments on old world salamandrids. In: Taylor DH, Guttman SI (eds) The reproductive biology of amphibians. Plenum Press, New York, USA, pp 141–183
Arntzen JW, Sparreboom M (1989) A phylogeny for the old world newts, genus Triturus: biochemical and behavioural data. J Zool 219:645–664
Baeckens S, Driessens T, Van Damme R (2016) Intersexual chemo-sensation in a “visually-oriented” lizard. Anolis sagrei. PeerJ 4:e1874
Campbell LJ, Suárez-Castillo EC, Ortiz-Zuazaga H, Knapp D, Tanaka EM, Crews CM (2011) Gene expression profile of the regeneration epithelium during axolotl limb regeneration. Dev Dyn 240(7):1826–1840
Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW (2016) GenBank. Nucleic Acids Res 44(D1):D67–D72. https://doi.org/10.1093/nar/gkv1276
Carranza S, Amat F (2005) Taxonomy, biogeography and evolution of Euproctus (Amphibia: Salamandridae), with the resurrection of the genus Calotriton and the description of a new endemic species from the Iberian peninsula. Zool J Linn Soc 145(4):555–582
Casey JR, Petranka JG, Kottra J, Fleenor DE, Rosse W (1994) The structure of the urokinase-type plasminogen activator receptor gene. Blood 84:1151–1156
Davis WC, Twitty VC (1964) Courtship behavior and reproductive isolation in the species of Taricha (Amphibia, Caudata). Copeia 1964:601–610
Dawley EM (1984) Recognition of individual, sex and species odours by salamanders of the Plethodon glutinosus-P. jordani complex. Anim Behav 32(2):353–361
Duellman WE, Trueb L (1994) Biology of amphibians. Johns Hopkins University Press, USA, p 670
Dunn RD, Broady KW (2001) Snake inhibitors of phospholipase A2 enzymes. Biochim Biophy Acta (BBA)—Mol. Cell Biol. Lipids 1533(1):29–37
Fleming TJ, O’hUigin C, Malek TR (1993) Characterization of two novel Ly-6 genes. Protein sequence and potential structural similarity to alpha-bungarotoxin and other neurotoxins. J Immunol 150(12):5379–5390
Fortes-Dias CL, Campos PC, Fernandes CAH, Fontes MRM (2017) Phospholipase A2 inhibitors from snake blood (sbPLIs). In: Gopalakrishnakone P, Inagaki H, Mukherjee A, Rahmy T, Vogel CW (eds) Snake venoms. Toxinology. Springer, Dordrecht, pp 105–122
Frost DR (2019) Amphibian species of the world, an online reference. Version 6.0. Accessed on 10 January 2019. Electronic database accessible at http://research.amnh.org/herpetology/amphibia/index.html. American Museum of Natural History, New York, USA
Gallien L (1952) Elevage et comportement du Pleurodèle au laboratoire. Bulletin de Société Zoologique de France 77:456–461
Gong Y, Shu G, Huang F, He L, Li C, Xie F (2018) Courtship behaviour and male sexual competition of the Taliang crocodile newt, Liangshantriton taliangensis. Amphibia-Reptilia 39(3):275–288
Griffiths RA (1996) Newts and salamanders of Europe. Poyser Natural History, London, UK, p 188
Guillaume O (1999) Does the Pyrenean salamander Euproctus asper use chemical cues for sex identification and mating behaviour? Behav Proc 46(1):57–62
Halliday TR (1974) Sexual behaviour of the smooth newt Triturus vulgaris (Urodela, Salamandridae). J Herpetol 8(4):277–292
Halliday TR (1977) The courtship of European newts: an evolutionary perspective. In: Taylor DH, Guttman SI (eds) The reproductive biology of amphibians. Plenum Press, New York, USA, pp 185–232
Halliday TR (1990) The evolution of courtship behavior in newts and salamanders. Adv Study Behav 19:137–169
Houck LD, Arnold SJ (2003) Courtship and mating behaviour. In: Sever DM (ed) Phylogeny and reproductive biology of Urodela (Amphibia). Science Publishers, Enfield, New Hampshire, USA, pp 383–424
Houck LD, Bell AM, Reagan-Wallin NL, Feldhoff RC (1998) Effects of experimental delivery of male courtship pheromones on the timing of courtship in a terrestrial salamander, Plethodon jordani (Caudata: Plethodontidae). Copeia 1998:214–219
Houck LD, Palmer CA, Watts RA, Arnold SJ, Feldhoff PW, Feldhoff RC (2007) A new vertebrate courtship pheromone, PMF, affects female receptivity in a terrestrial salamander. Anim Behav 73(2):315–320
Houck LD, Watts RA, Mead LM, Palmer CA, Arnold SJ, Feldhoff PW, Feldhoff RC (2008) A candidate vertebrate pheromone, SPF, increases female receptivity in a salamander. In: Hurst JL, Beynon RJ, Roberts SC, Wyatt TD (eds) Chemical signals in vertebrates 11. Springer-Verlag Inc., New York, USA, pp 213–221
Iwata T, Conlon JM, Nakada T, Toyoda F, Yamamoto K, Kikuyama S (2004) Processing of multiple forms of preprosodefrin in the abdominal gland of the red-bellied newt Cynops pyrrhogaster: regional and individual differences in preprosodefrin gene expression. Peptides 25(9):1537–1543
Iwata T, Ishizuka Y, Nakada T, Toyoda F, Yamamoto K, Conlon JM, Kikuyama S (2005) Regionally specific occurrence of an active sodefrin variant in the red-bellied newt. Ann NY Acad Sci 1040(1):351–353
Janssenswillen S, Bossuyt F (2016) Male courtship pheromones induce cloacal gaping in female newts (Salamandridae). PLoS ONE 11(1):e0144985
Janssenswillen S, Vandebergh W, Treer D, Willaert B, Maex M, Van Bocxlaer I, Bossuyt F (2014) Origin and diversification of a salamander sex pheromone system. Mol Biol Evol 32(2):472–480
Janssenswillen S, Willaert B, Treer D, Vandebergh W, Bossuyt F, Van Bocxlaer I (2015) High pheromone diversity in the male cheek gland of the red-spotted newt Notophthalmus viridescens (Salamandridae). BMC Evol Biol 15:54
Kawamura T, Sawada S (1959) On the sexual isolation among different species and local races of Japanese newts. J Sci Hiroshima Univ Ser B 18:17–31
Kieffer B, Driscoll PC, Campbell ID, Willis AC, van der Merwe PA, Davis SJ (1994) Three-dimensional solution structure of the extracellular region of the complement regulatory protein CD59, a new cell-surface protein domain related to snake venom neurotoxins. Biochemistry 33(15):4471–4482
Kieren S, Sparreboom M, Hochkirch A, Veith M (2018) A biogeographic and ecological perspective to the evolution of reproductive behaviour in the family Salamandridae. Mol Phylogenet Evol 121:98–109
Kikuyama S, Toyoda F, Ohmiya Y, Matsuda K, Tanaka S, Hayashi H (1995) Sodefrin: a female-attracting peptide pheromone in newt cloacal glands. Science 267:1643–1645
Kikuyama S, Toyoda F (1999) Sodefrin: a novel sex pheromone in a newt. Rev Reprod 4(1):1–4
Kini RM, Doley R (2010) Structure, function and evolution of three-finger toxins: mini proteins with multiple targets. Toxicon 56(6):855–867
Kozak KH (2003) Sexual isolation and courtship behavior in salamanders of the Eurycea bislineata species complex, with comments on the evolution of the mental gland and pheromone delivery behavior in the Plethodontidae. Southeast Nat 2(2):281–293
Loughner CL, Bruford EA, McAndrews MS, Delp EE, Swamynathan S, Swamynathan SK (2016) Organization, evolution and functions of the human and mouse Ly6/uPAR family genes. Hum Genomics 10(1):10
Maex M, Van Bocxlaer I, Mortier A, Proost P, Bossuyt F (2016) Courtship pheromone use in a model urodele, the Mexican axolotl (Ambystoma mexicanum). Sci Rep 6:20184
Maex M, Treer D, De Greve H, Proost P, Van Bocxlaer I, Bossuyt F (2018) Exaptation as a mechanism for functional reinforcement of an animal pheromone system. Curr Biol 28(18):2955–2960.e5
Maki N, Martinson J, Nishimura O, Tarui H, Meller J, Tsonis PA, Agata K (2010) Expression profiles during dedifferentiation in newt lens regeneration revealed by expressed sequence tags. Mol Vision 16:72–78
Malacarne G, Vellano C (1987) Behavioral evidence of a courtship pheromone in the crested newt, Triturus cristatus carnifex Laurenti. Copeia 1987(1):245–247
Mitchell J, Gibbons W (2010) Salamanders of the Southeast. University of Georgia Press, Georgia, USA, p 324
Nakada T, Toyoda F, Iwata T, Yamamoto K, Conlon JM, Kato T, Kikuyama S (2007) Isolation, characterization and bioactivity of a region-specific pheromone, [Val8] sodefrin from the newt Cynops pyrrhogaster. Peptides 28(4):774–780
Nakada T, Toyoda F, Matsuda K, Nakakura T, Hasunuma I, Yamamoto K, Onoue S, Yokosuka M, Kikuyama S (2017) Imorin: a sexual attractiveness pheromone in female red-bellied newts (Cynops pyrrhogaster). Sci Rep 7:41334
Nelson J (1960) Courtship display in the Italian crested newt (Triturus cristatus carnifex). Biol J 1:6–9
Okomura K, Masui K, Inoue S, Ikeda K, Hayashi K (1999) Purification, characterization and cDNA cloning of a phospholipase A2 inhibitor from the serum of the non-venomous snake Elaphe quadrivirgata. Biochem J 341(1):165–171
Osikowski A, Cierniak-Zuzia K (2013) Cloacal anatomy of the male Carpathian newt, Lissotriton montandoni (Amphibia, Salamandridae), in the breeding season. Zool Sci 30(9):748–753
Palmer CA, Watts RA, Houck LD, Picard AL, Arnold SJ (2007) Evolutionary replacement of components in a salamander pheromone signaling complex: more evidence for phenotypic-molecular decoupling. Evolution 61(1):202–215
Petranka J (2010) Salamanders od the United States and Canada. Smithsonian books, Washington, USA, p 592
Pool TB, Dent JN (1977) The ultrastructure and the hormonal control of product synthesis in the hedonic glands of the red-spotted newt Notopthalmus viridescens. J Exp Zool 201(2):177–201
Poschadel JR, Rudolph A, Plath M (2007) Nonvisual mate choice in the Pyrenean mountain newt (Euproctus asper): females prefer small males. Acta Ethologica 10(1):35–40
Protein [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004—[cited 2018 January 21]. Available from: https://www.ncbi.nlm.nih.gov/protein/
Rafinski J, Pecio A (1992) The courtship behaviour of the Bosca’s newt, Triturus boscai (Amphibia: Salamandridae). Folia Biol 40:155–165
Rollins RE, Staub NL (2017) The Presence of caudal courtship-like glands in male and female ouachita dusky salamanders (Desmognathus brimleyorum). Herpetologica 73(4):277–282
Rollmann SM, Houck LD, Feldhoff RC (1999) Proteinaceous pheromone affecting female receptivity in a terrestrial salamander. Science 285(5435):1907–1909
Rollmann SM, Houck LD, Feldhoff RC (2003) Conspecific and heterospecific pheromone effects on female receptivity. Anim Behav 66:857–861
Rusconi M (1821) Amours des salamandres aquatiques et developpement du tetard de ces salamandres depuis l’oeuf jusqu’a l’animal parfait. Paolo Emilio Giusti, Milano, Italy, p 91
Sacerdote M (1956) Per una migliore conoscenza della topografia delle ghiandole annesse alla cloaca nel tritone crestato. Bolletino di zoologia 23(1):33–50
Salthe SN (1967) Courtship patterns and the phylogeny of the urodeles. Copeia 1967:100–117
Secondi J, Haerty W, Lodé T (2005) Female attraction to conspecific chemical cues in the palmate newt Triturus helveticus. Ethology 111(8):726–735
Sever DM, Verrell PA, Halliday TR, Griffiths M, Waights V (1990) The cloaca and cloacal glands of the male smooth newt, Triturus vulgaris vulgaris (Linnaeus), with especial emphasis on the dorsal gland. Herpetologica 46(2):160–168
Sever DM (1991) Comparative anatomy and phylogeny of the cloacae of salamanders (Amphibia: Caudata). I. Evolution at the family level. Herpetologica 47(2):165–193
Sever DM (1992) Comparative anatomy and phylogeny of the cloacae of salamanders (Amphibia: Caudata) IV. Salamandridae. Anat Rec 233(2):229–244
Sever DM (1994) Comparative anatomy and phylogeny of the cloacae of salamanders (Amphibia: Caudata). VII. Plethodontidae. Herpetol Monogr 8:276–337
Sever DM (2003) Courtship and mating glands. In: Sever DM (ed) Phylogeny and reproductive biology of Urodela (Amphibia). Science Publishers, Enfield, New Hampshire, USA, pp 323–381
Spallanzani L (1780) Dissertazioni di fisica animale e vegetabile. Presso La Societa Tipografica, Modena, Italy, p 361
Sparreboom M (2014) Salamanders of the old world. KNNV Publishing, Zeist, The Netherlands, p 431
Tinbergen N, Ter Pelkwijk JJ (1938) De kleine watersalamander. De Levende Natuur 43(8):232–237
Toyoda F, Tanaka S, Matsuda K, Kikuyama S (1994) Hormonal control of response to and secretion of sex attractants in Japanese newts. Physiol Behav 55(3):569–576
Treer D, Maex M, Van Bocxlaer I, Proost P, Bossuyt F (2017) Divergence of species-specific protein sex pheromone blends in two related, nonhybridizing newts (Salamandridae). Mol Ecol 27(2):508–519
Treer D, Van Bocxlaer I, Matthijs S, Du Four D, Janssenswillen S, Willaert B, Bossuyt F (2013) Love is blind: indiscriminate female mating responses to male courtship pheromones in newts (Salamandridae). PLoS ONE 8(2):e56538
Treer D, Bossuyt F (2010) Chemical ‘versus’ visual cues in newts (Salamandridae): experiments in total darkness and in a Y-maze. In: 17th Benelux Congress of Zoology, Ghent, Belgium. Conference abstract, p 87
Tsetlin V (1999) Snake venom α-neurotoxins and other ‘three-finger’proteins. Eur J Biochem 264(2):281–286
Van Bocxlaer I, Maex M, Treer D, Janssenswillen S, Janssens R, Vandebergh W, Proost P, Bossuyt F (2016) Beyond sodefrin: evidence for a multi-component pheromone system in the model newt Cynops pyrrhogaster (Salamandridae). Sci Rep 6:21880
Van Bocxlaer I, Treer D, Maex M, Vandebergh W, Janssenswillen S, Stegen G, Kok P, Willaert B, Matthijs S, Martens E, Mortier A, de Greve H, Proost P, Bossuyt F (2015) Side-by-side secretion of late palaeozoic diverged courtship pheromones in an aquatic salamander. Proc R Soc Lond B Biol Sci 282:20142960
Verrell P (1982) The sexual behaviour of the red-spotted newt, Notophthalmus viridescens (Amphibia: Urodela: Salamandridae). Anim Behav 30(4):1224–1236
Wells KD (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago, USA, p 1148
Yamamoto K, Kawai Y, Hayashi T, Ohe Y, Hayashi H, Toyoda F, Kawahara G, Iwata T, Kikuyama S (2000) Silefrin, a sodefrin-like pheromone in the abdominal gland of the sword-tailed newt, Cynops ensicauda. FEBS Lett 472:267–270
Zhang P, Papenfuss TJ, Wake MH, Qu L, Wake DB (2008) Phylogeny and biogeography of the family Salamandridae (Amphibia: Caudata) inferred from complete mitochondrial genomes. Mol Phylogenet Evol 49(2):586–597
Acknowledgements
This work was supported by the European Research Council (ERC 204509 StG grant to FB), Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (FWO research project G026715 N), and the Strategic Research Program of the Vrije Universiteit Brussel (SRP30). We are very grateful to Kim Roelants for providing drawings.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this paper
Cite this paper
Bossuyt, F., Maex, M., Treer, D., Schulte, L.M., Van Bocxlaer, I., Janssenswillen, S. (2019). Chemistry Between Salamanders: Evolution of the SPF Courtship Pheromone System in Salamandridae. In: Buesching, C. (eds) Chemical Signals in Vertebrates 14. Springer, Cham. https://doi.org/10.1007/978-3-030-17616-7_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-17616-7_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-17615-0
Online ISBN: 978-3-030-17616-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)