Abstract
In the recent years, the biosynthesis and application of noble nanoparticles have been emerged as escalating field with a great impact on biology, medicine and electronics. Diverse strategies including high-energy physical to toxic chemical procedures have been used for the synthesis of nanoparticles. Moreover, higher production cost with raising environmental risk becomes the major issue. To overcome these, green synthesis of nanoparticles is considered as the potential alternative. Green synthesis involves exploitation of biological entities like algae including microalgae, plants, and microorganisms. Microorganisms have innate potential for the synthesis of nanoparticles and could be regarded as potential biofactories for nanoparticles synthesis. So far, the wealth of microbial resources such as bacteria, algae, fungi, actinomycetes and viruses has been exploited for the development of different metallic nanoparticles. Microbial-nanoparticle syntheses have attracted a great attention due to their rich diversity and wider application with simple, cost-effective, non-toxic, and eco-friendly methods for production of technologically important materials. Hence, exploitation of organisms of microbial origin for the synthesis of nanoparticles is considered a valuable approach in green nanotechnology. In this chapter, we provide an overview of green synthesized nanoparticles using various microbes as biotemplates, which highlights from their substantial mechanism to incredible applications for the purpose of minimizing the negative impacts of synthetic procedures, their accompanying chemicals, and derivative compounds.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Nanotechnology is an emerging field blending the flavor of the scientific basis of physics, chemistry, and biology to exploit distinct technological advances for manipulating and creating tools or structure of individual molecules or their organized aggregates referred as nanoparticles (NPs). These nanoparticles (NPs) are the functional products of nanotechnology and possess the catalytic, optical, electronic, magnetic, and antimicrobial properties compared to the conventional polycrystalline materials. These NPs can be produced in various shapes such as spheres, triangles, hexagons, rods, wires, and tubes (Mandal et al. 2006) usually having dimensions of 100 nm or less (Huber 2005). Traditionally, they can be synthesized by a wide variety of physical and chemical methods such as UV irradiation, pyrolysis, lithography, laser ablation, ultrasonic fields, chemical vapor deposition, photoinduced reduction, aerosol technologies, and microemulsion synthesis techniques which are employed for the production of various metal NPs such as gold, silver, platinum, cadmium, iron, and palladium (Dhillon et al. 2012). These approaches end up with several limitations in terms of high energy, temperature, pH, and pressure requirement with huge monetary expenses. Moreover, the use of hazardous substances such as organic solvents, reducing agents, and stabilizers and the release of toxic by-products enhance the toxicity problems. Hence, the contamination of toxic agents with the synthesized NPs has prevented their clinical, agricultural, and other biological and environmental application (Prasad et al. 2014). In order to address these limitations, green synthesis came into existence recently to restrain the ecosystem through available naturally biodegradable resources for its biosynthesis (Gibney 2015; Malik et al. 2014).
Green synthesis of NPs has gained the momentum over the last few years due to their distinctive properties, wider applicability, and environmental sustainability. The development of NPs through green chemistry is an eco-friendly technique (Rai et al. 2012; Prasad 2014; Prasad et al. 2018a). The superiority of green NPs over their chemical counterpart lies on their organic origin and non-toxic property. Besides this, green synthesis involves a wide range of environmentally acceptable methodology with low-cost production in lesser time (Prasad et al. 2017). These green NPs are synthesized using diverse biological resources available in nature such as plants (Pantidos and Horsfall 2014), algae (Castro et al. 2013; Aziz et al. 2014, 2015), actinomycetes (Singh et al. 2016), bacteria (Li et al. 2011; Vijayaraghavan and Kamala Nalini 2010; Prasad et al. 2016), yeast (Ingale and Chaudhari 2013; Menon et al. 2017), fungi (Dhillon et al. 2012; Moghaddam et al. 2015; Prasad 2016, 2017; Prasad et al. 2018b), and viruses (Merzlyak and Lee 2006; Shah et al. 2015), or their secondary metabolites and by-products. The natural ability of these organisms and possibility to utilize them for synthesizing inorganic materials on a nano- and microscale served as driving fuel for the development of a relatively new and unexplored area of research. Feasibility of biosynthesis of “green” NPs primarily lies on the choice of appropriate medium (Li et al. 2011). Among many organisms, plant extracts are extensively exploited as the good media and are suitable for large-scale biosynthesis. But stability of plant-originated NPs differs significantly with the variation in biochemical composition of plant extracts of the same species (Duran et al. 2011). To overcome this problem, synthesizing NPs using microbial resource is relatively advantageous as microbe-secreted proteins confer better stabilization of NPs (Selvakumar et al. 2014). Furthermore, huge structural diversity and easy culturability of microbes ensure their innate property for synthesis of green nanoparticle (NP) which could be regarded as potential biofactories for NP synthesis.
Exploitation of microbial resource for green synthesis of NPs is considered an excellent strategy. So far, wealth of information is available on microbial nanotechnology, but very little information highlights their substantial mechanism of biosynthesis and stabilization along with their potential applications. In this article, we try to incorporate all possible aspects of NP synthesis procedures with aim of long-term stabilization and wider application in biomedical sciences, biosensors, chemical sensing, antimicrobial, drug delivery, mechanics, magnetic, energy science, agricultural, environmental, and other industrial sectors (Prasad et al. 2016). This will not only provide an overview of green synthesis of NPs using various microbes as a biotemplates but also would focus to find out possible approaches for minimizing the negative impacts of synthetic procedures and their accompanying chemicals and derivative compounds and ultimately to harness their sustainability in long run.
3.2 Nanoparticles and Their Green Sources
The term “nano” has been derived from its Greek origin “nanos” meaning dwarf. Usually the particles or molecules of any shape having a dimension in the range of 1 × 10−9 to 1 × 10−7 m are called nanoparticles (Banik and Sharma 2011). A NP is a collection of about tens to thousands of atoms measuring about 1–100 nanometers in diameter, created by atom aggregation to form amorphous, crystalline, or semi-crystalline, zero-dimensional (0Do) nano structure (with no dimensions longer than 100 nm). The essential criteria to be a NP is its size in nanoscale and structural arrangement, ability to work at the atomic or molecular level, and its novel, significantly changed, and useful physical, chemical, and biological properties not previously created or observed (Cao 2004). These may be available in the form of nanopowder or nanocluster or nanocrystal. These nanomolecule or nanoparticles are utilized for the creation of materials having dimension beyond the nanometer scale (>100 nm) and fundamentally new molecular organizations and functional characteristics. Other than changes in physical, chemical, and biological properties, two principal factors, i.e., the ratio of surface area to volume and the size of the particle, become the main determinant (Mazhar et al. 2017). Usually, NPs with lower particle size have a much greater surface area as compared to larger particles; and as the particle size decreases, dominance of the surface atoms increases over the interior of the particle. This results in the enhancement in catalytic activity of NPs, both in isolation and its interaction with other materials that helps to form nanocomposites, having special properties such as increased strength and chemical resistance (Mohanpuria et al. 2008; Prasad et al. 2017). Thus, size and shape of the nanomaterials can be controlled experimentally to generate one-dimensional, two-dimensional, or three-dimensional nanostructures like nanotubes, nanorods, nanowires, nanofilms, nanolayers, nanocoatings, etc.
NPs are generally available in organic or inorganic form. Of them, metallic NPs are the most common. They can be synthesized physiochemically, biologically, or through a hybrid of these techniques (Mazhar et al. 2017). Various physical methods, such as vapor deposition, lithography, mechanical attrition, laser ablation, laser pyrolysis, and ion sputtering, have been utilized, but these are not very popular, and the yield is very less (Li et al. 1999; Narayanan and Sakthivel 2010; Panigrahi et al. 2004). However, NP synthesis by chemical process is the traditionally most exploited approach. Their chemical synthesis involves the conversion of metallic ions to NPs with the help of various reducing agents, such as sodium borohydride, potassium bitartrate, methoxypolyethylene glycol, hydrazine, etc., via several methods like hydrothermal, conventional heating, anodization, deposition precipitation, wet oxidation, electrodeposition, and sonication (Kim et al. 2007; Li et al. 1999; Mallick et al. 2004; Panigrahi et al. 2004; Tan et al. 2003). However, these methods are usually highly expensive and very labor-intensive. Moreover, the use of some toxic chemicals and generation of hazardous by-products raise the environmental safety issue and health concerns to living organisms. Therefore, their application is restricted in biological, medical, and agricultural sectors (Prasad et al. 2017; Tarafdar et al. 2013).
To overcome such issues, green synthesis approach opens up new window to develop low-cost, non-toxic, biocompatible, and environmentally safe metallic NPs that have wider application in various sectors. A vast natural source of biological entities including plants, plant extracts, algae, fungi, yeast, diatoms, bacteria, actinomycetes, and viruses have been exploited in the development of metallic NPs (Li et al. 2011; Prasad et al. 2016; Thakkar et al. 2010). Due to their rich diversity and the innate potential for synthesizing different kinds of NPs, they could be regarded as potential biotemplate for NP synthesis. This green approach gains importance due to non-requirement of high pressure, energy, pH, temperature, and toxic compounds that ensure added advantage over chemical and physical methods. In addition, the size and shape of the NPs can be controlled by manipulating the growth and other cellular activities of the template organism (Gericke and Pinches 2006; Mazhar et al. 2017). Furthermore, their secondary metabolites like amino acids, peptides and organic acids are also very efficient for biological formulation of various nanostructures.
Comparison of different biological entities is very crucial to determine their potential and utility for efficient synthesis of NPs. So far, plants are most commonly utilized as a cost-effective resource for the metal NP production with the advantage of faster rate of biosynthesis than other biological entities. Besides, lack of complexity in preparation and maintenance of bioculture ensure its suitability for large-scale production (Makarov et al. 2014; Shah et al. 2015). However, variation in biochemical composition of the plant extract creates problem in stabilization, structural variation, and catalytic activity of metal NPs.
Thus focus is now shifted toward the utilization of microbial resource for synthesis of NPs. Horizon of this spread from simple prokaryotes to complex eukaryotic organisms like bacteria, fungi, yeasts, actinomycetes, algae, and viruses, as they can be easily harvested for the fabrication of NPs due to their natural abundance and ease in culturing (Prasad et al. 2016). Their metal biodegradation potential can also be exploited for this purpose. These microorganisms have the ability to detoxify heavy metals that are present in their environment and turn them into their elemental form via oxidation and reduction reactions (Labrenz et al. 2000; Mehra and Winge 1991; Stephen and Maenaughton 1999). This process is performed either intra- or extracellularly through enzymatic action. The extracellular enzymes of microbial origin are capable of reducing heavy metals present in the surrounding of cell, whereas intracellular enzymes target only the metals (ions) that are transported inside the microbial cell (Li et al. 2011). The particles generated in these ways have higher catalytic activity with greater surface area like their microbial origin; these particles can sustain various environmental conditions of varying temperature, pH, and pressure (Shah et al. 2015). Thus, microbe-originated NPs can be very efficient for diverse fields of application (Zhang et al. 2011). Furthermore, these NPs can be designed in various shapes and size in comparison with other sources. Due to their non-hazardous nature and an improved efficiency, microbial resource is considered as one of the best resource for synthesis of metal nanoparticles.
3.3 Microbes as Biotemplates for Nanoparticle Synthesis
Biosynthesis of various NPs using microbial template is already well established. This microbial approach is evolved as an efficient system for synthesis of NPs due to their distinctive properties. Most importantly, these microbiological systems have huge structural variation from micro- to macroscale length that may be exploited as suitable biotemplate (Table 3.1, Fig. 3.1). Besides various microbial structures, their cellular components sized in nanoscale are also preferred. The biochemical constituents of the whole cell and its parts or cell extracts like exo-polysaccharides, proteins, and enzymes are involved in the catalysis of NP synthesis pathway, and hence, understanding their significance and mechanism in NP synthesis is crucial.
3.3.1 Bacteria as Biotemplate
3.3.1.1 Bacterial Whole-Cell Template
Various bacterial cells, possessing different morphological features and surface structures can be used as a biotemplate for nanoparticle synthesis. The composition of cell surface structure including cell wall with exo-polysaccharides or surface layer proteins is the key determinant. Basically sugars, proteins, and enzymes present in the microbial cell wall have different functional groups like carboxyl, phosphate, and amide groups that favor the metal binding and nucleation process for the aggregation and formation of NPs or nanostructures (Selvakumar et al. 2014; Iravani 2014). One such component is bacterial exo-polysaccharide that is used as a potential biotemplate for the metal NP synthesis. The polyanionic functional groups (i.e., hydroxyl, carboxyl, and amino groups) of the exo-polysaccharide layer form an interface with metal cations and speed up their reduction process. This exo-polysaccharide is also used as the efficient capping agents for stabilizing the primary structures of metal NPs through chelation (Gomaa 2017; Sanyasi et al. 2016; Sathiyanarayanan et al. 2017), thus controlling the particle size and shape. The mucoadhesion property of exo-polysaccharides leads to a neutral, low surface energy in NPs, thus preventing their agglomeration and ensuring uniform particle dispersion after long-term storage.
Similar to exo-polysaccharides, the bacteria cell wall also contains surface layer proteins (S layer) that form self-assembled nanostructures on its surface to maintain the cell shape. This S layer possesses large surface area with uniform pore size and interacts weakly with metal ions. This induces specific binding of molecules on S-layer lattices via covalent and non-covalent bond which can be used for the synthesis of metal NPs like gold (Au), cadmium (Cd), etc. (Selvakumar et al. 2014). After isolation, S layer protein is re-crystallized into defined symmetries on the solid support matrices like electron microscope grids, silica, glass, mica, liposome, carbon, or polymers. Usually modification in self-assembling of S layer can be possible depending upon the metal NPs to be synthesized. S layer-coated grids act as the biotemplate and are dipped in a solution containing metal salt for coating and synthesis of NPs of defined shape and size (Iravani 2014).
3.3.1.2 Bacterial Cell Appendages
Other than the whole bacterial cell, appendages like flagella and pili are also used as the biotemplate for the synthesis of NPs. These flagella and pili are self-assembled filamentous nanostructure made of proteins, like flagellin and pilin, respectively. Their self-assembling property is explored and exploited for the fabrication of nanostructured materials with precise shape and size (Sleytr et al. 1993).
Bacterial flagella have unique mechanical properties like stiffness and elasticity that ensure durability and stability to withstand very high temperature and extreme pH and adaptability to the changing environment through helical rearrangement of flagellin. This property of flagellin protein can be used for de-polymerization into monomers followed by re-polymerization into modulated flagellar filaments of desirable dimensions and structures (Kowshik et al. 2002a, b). During re-polymerization, reshuffling of amino acids leads to different sequence and nature of polymers. These polymerized flagella can be used as the biotemplate for the synthesis of NPs with superior electrical conductivity (Gopinathan et al. 2013; Velusamy et al. 2016). Further, the flagellin protein subunits can be modified genetically to enhance their affinity for metal ions and fabrication of hybridized metal nanotubes.
Bacterial pili are a small hair-like structure with diverse functional category. One such functional category of pili promotes interaction with inorganic electron acceptors and is involved in extracellular electron transfer. Such types of electrically conductive pili (e-pili) act as microbial electrode, called “biological nanowires,” and are found in Geobacter sp. and Shewanella sp. This property of pili can be exploited for the biosynthesis of different nanostructures. Deposition of NPs like titanium dioxide (TiO2) , zinc oxide (ZnO), and aluminum oxide (Al2O3) on these pili forms nanofibers that enhance the extracellular electron transfer. These oxide-coated nanostructures are also applicable in bioelectric production (Selvakumar et al. 2014).
3.3.2 Fungi as Biotemplate
Like bacterial cell, fungi are also a potential candidate for the synthesis of metal NPs. The enzymes and proteins synthesized by fungi help in reducing the metal salts to NPs; therefore, they are invariably used for the synthesis of metal NPs. Usually fabrication of metal NPs of varying size and shape occurs either extracellularly or intracellularly (Sastry et al. 2003; Abdel-Aziz et al. 2018). Extracellular synthesis occurs through biosorption of metal ions on the cell wall of fungal filament and reduction by secretion of extracellular enzymes bacterial cell and metabolites, whereas intracellular synthesis involves influx of metal salts within the fungal cell followed by bioreduction into metal ions (Dhillon et al. 2014; Pantidos and Horsfall 2014). Usually, fungi are preferred over bacteria for large-scale production of NPs. Their versatility in shapes (from unicellular structures to microfilaments), wider adaptability in distinct ecology, and tolerance to environmental stress favor them for the purpose (Velusamy et al. 2016). Furthermore, fungal mycelia have large surface area for binding with metal salts and grow more biomass which results into rapid synthesis of metal NPs. Hence, exploration and exploitation of fungi for the fabrication of NPs become more advantageous (Prasad 2016, 2017; Aziz et al. 2016, 2019).
3.3.3 Algae as Biotemplate
Synthesis of NPs using algal biotemplate is another unique approach. The use of algal species in fabrication of metal NPs is totally subjected to their structural/physical features and secreted biomolecules (Davis et al. 2003; Siddiqi and Husen 2016). Diverse biomolecules like sugars, proteins and secondary metabolites secreted by various algae play crucial role in the biogenesis of NPs. Since algal membrane proteins are important for templating metals ions, extracellular polysaccharides help in the reduction of different metal ions and stabilization of metal NPs. Secondary metabolites (flavonoids and terpenoids) are effective for capping and stabilizing the metal nanoparticles; thus, altogether the design and size and shape of metal NPs (Anwar 2018; Kannan et al. 2013; Sharma et al. 2015; Vasquez et al. 2016). Furthermore, existence of skeletal and morphological variability within algal species supports the production of nanostructures. NPs of different dimensions and morphology (spherical, elongated or irregular) are obtained depending on algal species (Patel et al. 2015). Algae are supposed to be advantageous for nanoparticle synthesis because it favors cost-effective and large-scale synthesis of highly stable, safe, and non-toxic nanoparticles with better biological properties (Aziz et al. 2014, 2015).
3.3.4 Virus as Biotemplate
Virus is a nucleoprotein particle containing genomic nucleic acid surrounded by proteinaceous capsid. Due to their unique sizes in nanoscale and shapes, they are considered natural nanoarchitectures. The three-dimensional (3Do) structure of viral particles is densely covered by protein subunits, which in turn is made up of amino acid with carboxylate, amino, and thiol group side chains that represent a highly reactive layer and have high affinity for interacting with metal ions, and thus helps in their nucleation and metallization at the external surface (Flenniken et al. 2009; Shah et al. 2015; Selvakumar et al. 2014). Further, virus particles have a hollow inner cavity inside the viral capsid, where some metals can diffuse and interact with internally projecting amino acid side chains (Pokorski and Steinmetz 2011; Wen et al. 2012). Thus, inner cavity of viral particles can serve as a nanoreactor for fabrication of metal nanomaterials of desired sizes and shapes. Hence, external surface of coat protein and its interior cavity can be exploited for the designing of various metal nanowares, nanorods and nanotubes.
3.4 Approaches for Microbes-Derived Nanoparticle Synthesis
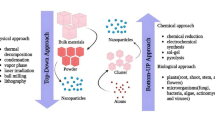
Synthesis of NPs can be achieved via two basic approaches, viz., top-down approach and bottom-up approach (Iqbal et al. 2012). In the top-down approach, a large bulk material is fragmented into fine particles by size reduction using lithographic techniques (grinding, ball milling, chemical etching, sputtering, thermal/laser ablation, etc.). This leads to the severe plastic deformation into nanoscale. It is a costly, very slow, and time-consuming approach and, thus, not suitable for large-scale production. However, in the bottom-up approach, NPs are synthesized through self-assembling of atoms or molecules into new nuclei that build up to NPs or nanostructures (Mazhar et al. 2017). Fabrication of NPs through this approach is more advantageous because of more homogeneous and less defective production of nanostructures with minimum cost. The aggregation of atoms or ions to produce NPs can be induced using chemical and biological methods. Among chemical methods, chemical precipitation, vapor deposition, sol-gel process, and pyrolysis are mostly applicable. These methods involve the chemical reduction of ions using different organic and inorganic reducing agents, such as ascorbic acid, citrate, glucose, dextrose, ethylene glycol, hydrazine, paraffin, sodium borohydride (NaBH4), etc. (Ahmed et al. 2016; Iravani et al. 2014). This is followed by the use of capping agents for the stabilization of synthesized NPs. Although chemical synthesis of NPs is well established and very common, this approach is ecologically unfriendly and a highly expensive one. Thus, biological synthesis of “green” NPs is favored using diverse biological entities like microorganisms, plant extract, plant biomass, etc. (Bhattacharya and Gupta 2005; Dhillon et al. 2012). This green synthesis of NPs follows the bottom-up strategy where the reduction of atoms or ions occurs by biological reducing agents like polysaccharides, proteins, enzymes or metabolites of microbial origin. This is followed by nucleation of reduced metal atoms for subsequent growth and formation of nanostructures using microbial structures and other biological substances as the biotemplate. The advantage of green synthesis includes feasibility of designing various nanostructures like nanorods, nanosheets, etc. with more homogeneous composition and stability due to reduction of Gibb’s free energy (Thanh et al. 2014; Shah et al. 2015). Thus green synthesis of NPs is progressing as a key approach for the production of NPs and could be preferred over chemical and physical methods due to its inexpensive, energy-saving, and eco-friendly nature (Reddy et al. 2012).
Biosynthesis of microbial NPs can be done using intracellular and extracellular synthetic methods. For intracellular synthesis, the microbial cell culture is employed with optimized metal salt concentration and incubated at desirable temperature and pH for production of NPs, whereas for extracellular synthesis, the crude extract of microbial cell culture (free from microbial cells) is mixed with metal salt solution and incubated for the reduction and stabilization of NPs (Fig. 3.2) (Baker et al. 2013; Velusamy et al. 2016). To synthesize NPs of precise size and shape, various physiological parameters such as microbial source, nutrient composition, pH, temperature, metal salts concentration, etc. have to be optimized for specific incubation period and condition (static or shaking) (Baker et al. 2013). To get desirable NPs, selection of microbial agents is done based on their growth rate and ability to produce diverse biomolecules like enzymes, proteins, and metabolites that are essential for reducing metal ions. For optimization of bioreduction process, harvesting time of microbial cells or their biomolecules is also an important factor as the protein function and enzyme kinetics are variable at different courses of time for incubation (Khandel and Shahi 2016; Prasad et al. 2017). Thus optimization of all the physiological parameters is necessary for production of NPs with precise size, shape, chemical composition, etc.
3.5 Application of Microbial Nanoparticle
Nanoparticles of microbial origin have numerous potential applications in different fields such as cosmetics, coatings, packaging, electronics, agriculture, food and beverages, bioremediation, diagnostics, and biomedicines. Some of these applications are discussed in this section.
3.5.1 Cosmeceutical Industry
Novel nanocarriers like liposomes, nanoemulsions, solid lipid NPs, nanostructured lipid carrier, nanocapsules, etc. are used as cosmeceuticals to condition the skin, hair, and nail and for lip care, aging, and hyperpigmentation due to their biocompatible, self-cleansing, skin-compatible, antimicrobial, and dermatological behavior (Singh et al. 2016). Generally, NPs possess a wavelength below the critical wavelength of light which renders them transparent. This property makes them suitable for application in cosmeceutical industry (Raj et al. 2012). Metal NPs, viz., zinc (Zn) and titanium (Ti) oxide NPs, have been used in sunscreens, as they are transparent to visible light as well as absorb and reflect UV rays encountered in their way to be effective for skin conditioning (Pierfrancesco 2010). These properties of nanoscale materials in cosmetics impart a widespread use in personal care industries.
3.5.2 Electronics and Catalysis
Day by day, increasing importance of less power-consuming, large-sized, highly bright display technology encourages the use of nanoparticles. Nanocrystalline lead telluride, cadmium sulfide, titanium dioxide, zinc selenide, and zinc sulfide are mostly used in the light-emitting diodes (LED)-based modern displays system in computer monitors and television (Ealias and Saravanakumar 2017). Further, development of portable electronic gadgets such as mobile phones, laptops, and computers led to the extensive demand for compact, lightweight, and high-capacity batteries. In such batteries, considerably high energy can be stored compared to traditional batteries due to their foam-like (aerogel) structure. NPs are the ideal choice for separator plates in batteries made from nanocrystalline nickel, palladium, carbon-coated silicon nanowires, and metal hydrides, due to their large surface area requiring increased energy densities and thus decreased recharge times and long-lasting storage density (Lu et al. 2010).
On the contrary, microorganisms secrete large amounts of enzymes, involved in the enzymatic reduction of metals ions to produce metal NPs, which have characteristic features similar to chemically synthesized nanoparticles (Palomo and Filice 2016). Extremely large surface area-to-volume ratio of these nanoparticles functions as efficient catalyst for the production of chemicals. So, they have been widely used to improve various reactions as catalysts (Crooks et al. 2001). It is exemplified in AuNPs which are biosynthesized by using cell-free extract of Trichoderma sp. (at 30 °C) for producing nanoparticles of size 20–30 nm (Li et al. 2004; Mishra et al. 2014) and unique, anisotropic planar shapes that are applied in photonics and optoelectronics. The reduction of AuNPs through different solvents enhances their utility as future chemical sensors for reducing cost and improving performance significantly.
3.5.3 Medicine and Health
Deployment of nanotechnology in the medical field is enriched by their application as nanomedicine in healthcare (Li et al. 2011). This application of nanobiomedicine is expanded in diverse aspects like biosensors (Mohanpuria et al. 2008), detoxification of biological fluids (Singh et al. 2016), drug and gene delivery agents (Li et al. 2011), detection and diagnosis of pathogens (Nath and Banerjee 2013), and treatment of human diseases via tissue designing (Gurunathan et al. 2009), DNA analysis (Razavi et al. 2015), tumor destruction via heating/hyperthermia (Shinkai et al. 1999), MRI contrast enhancement (Weissleder et al. 1990), and phagokinetic examinations (Parak et al. 2002). Of them, microbe-originated metallic nanoparticles have promising usage in drug delivery, formulation of antimicrobial agents, and development of biosensors.
3.5.3.1 Drug Delivery
Accurate positioning of drug in desired dosage to their target sites (in cells or tissue of choice) at right time is the main concern in designing and developing of novel drug delivery systems. Therefore, total drug consumption and its side effects can be reduced significantly to make it precise, safe drug delivery to achieve the maximum therapeutic impact (Dhillon et al. 2012). Since the last decade, NPs have been widely investigated as a carrier for drug delivery (Gref et al. 1994). Silver (Ag) nanoparticles have gained importance as drug conveyors which can easily reach at targeted place due to their tiny size through the blood-brain cellular barrier and the narrow epithelial joints. It also enhances pharmacokinetics and biodistribution of therapeutic factors and therefore reduces poison via their privileged gathering at the targeted position owing to their higher surface area-to-volume ratio (Moghaddam et al. 2015). Similarly, magnetic nanoparticles Fe3O4 (magnetite) and Fe2O3 (maghemite) have been dynamically investigated as they are known to be biocompatible for several purposes such as target cancer therapy (magnetic hyperthermia), stem cell sorting, gene therapy, trained drug delivery, DNA analysis, and MRI (Xiang et al. 2007). Additionally, metal NPs have been used in the spatial analysis of various biomolecules, including several metabolites, peptides, nucleic acids, lipids, fatty acids, glycosphingolipids, and drug molecules with higher sensitivity and spatial resolution (Li et al. 2011). The reproduction and repair of damaged tissue (tissue engineering) can be carried out with the help of metallic NPs. Among various metal NPs, AgNPs have been widely considered for surgical gloves and covers, antibacterial injury dressings, bed lines, and so forth; likewise, they have got various applications in the fields of indicative therapeutics.
3.5.3.2 Antimicrobial Agent
Nanoparticle synthesis using microbial template has been emphasized in recent years due to the huge abundance of microorganisms contributing resistance to multiple antibiotics. These biosynthesized nanoparticles can be utilized for many antimicrobial purposes, viz., antibacterial, antifungal, antiviral, and anti-inflammatory factors (Fayaz et al. 2010). Some inherent properties of nanoparticles such as a very high aspect ratio in the case of AgNPs allow them to easily interact with other particles and increase their antimicrobial efficiency manyfold (Thakkar et al. 2010). Fungi-mediated AgNPs showed powerful bactericidal potential against both Gram-positive and Gram-negative bacteria by anchoring and penetrating the bacterial cell wall and modulating the cellular signaling pathways through dephosphorylating putative key peptide substrates on tyrosine residues (Sadhasivam et al. 2010; Singh et al. 2008). Extracellularly synthesized silver nanoparticle or AuNPs using Fusarium oxysporum and Trichoderma sp. can be incorporated in several kinds of materials such as textile fabrics. These fabrics embedded with silver NPs possess antibacterial properties, are safe, and can be used in hospitals to prevent/minimize the infection of pathogenic bacteria such as Staphylococcus aureus (Duran et al. 2007). Owing to their important biomedicine properties , silver NPs produced intra- or extracellularly using living organisms could be of great value.
3.5.3.3 Biosensors
Nanoparticles retain electronic and optical properties which can be used in biosensor applications. Selenium (Se) nanomaterial crystals exhibit high surface-to-volume ratio, biocompatibility, and good adhering and electro-catalytic activity toward the reduction of H2O2. Therefore, they are particularly used for enhancing the production of settled materials for building HRP (horseradish peroxidase) biosensor. Thus, the Se nanomaterials-modified electrode will probably be promising for a wide range of applications related to the detection of H2O2 in food, clinical, pharmaceutical, industrial, and environmental analyses (Li et al. 2011). Similarly, AuNPs are utilized for various purposes, e.g., as labels for biosensors, for cure of hyperthermia, determination of glucose level in commercial glucose injections, and staining of biological tissues, and are being equipped for conveying vast estimation of biomolecules (Moghaddam et al. 2015). Therefore, NPs have been acted as a novel biosensor with high sensitivity providing non-hazardous intends to environmental quality and medication liberation.
3.5.4 Food Industry
At present, huge improvement in production, processing, packaging, and protection of food is achieved by incorporation of nanotechnology via increasing the shelf-life of different kinds of food materials and is also helpful in reduction of food wastage due to microbial infestation (Pradhan et al. 2015). Nowadays, nanocarriers are being utilized as delivery systems to hold food additives in food products without troubling their basic morphology. For example, a nanocomposite coating employed in a food packaging can directly introduce the antimicrobial substances and also provide a barrier from extreme thermal and mechanical shock on the coated film surface (Pinto et al. 2013). For example, an additive called nanodrops, is being used in the canola oil production industry, in order to transfer vitamins and minerals in the food (Sekhon 2014). Additionally, nanofiltration is a recent membrane filtration system for water purification widely used in food and dairy industries to remove solids, including bacteria and other parasites.
3.5.5 Construction
Nanotechnological application has also improvised the properties of cement-based materials for construction. Use of nanoscale materials in the preparation of cementing agents makes them quicker, inexpensive, and safer (Sanchez and Sobolev 2010). Nanosilica (SiO2) or hematite (Fe2O3) nanoparticle is mixed with the normal concrete to improve its mechanical properties, strength, and durability (Shah et al. 2009). Similarly, the properties of steel, the most widely used material in the construction industry, can also be improved by using nanotechnological application. Use of nano-sized steel offers stronger steel cables for the construction of bridge. Another important construction material is glass, which can be developed to have self-cleaning, sterilizing, and antifouling properties using (TiO2) nanoparticles for glass coat glazing that provides better blocking of light and heat penetrating through the windows (Sobolev et al. 2009). Hence, nano-SiO2, Al2O3, TiO2, and quartz can be used in the re-engineering of materials to improve their functionality and wider use in construction industry.
3.5.6 Environmental Remediation
The unique physicochemical properties of NPs have made them an ideal choice to decontaminate air, water, and soil under environmental remediation since the second decade of the twentieth century (Dhillon et al. 2014). These NPs are reported to clean up the environment via removal of heavy metals, pesticides, herbicides, fertilizers, oil spills, toxic gases, industrial effluents, sewage, and organic compounds (Liu 2006). Importantly, the ability of microorganisms to utilize its inherent biochemical processes to transform inorganic metallic ions into metal NPs with their surrounding environment has led to a relatively new and largely unexplored area of research. Songara et al. (2018) tested the rate of transformation of photodegraded products of benzyl butyl phthalate (BBP), an environmental pollutant, by Pseudomonas putida and the photocatalytic ZnO nanoparticles and concluded that the photocatalytic activity of ZnO nanoparticles was dose- and time dependent in transformation of BBP. Studies have also shown that extracellularly and intracellularly synthesized green metallic NPs (Ag, Fe, Pt, Pt-Co, Pt-Ni, Fe-Co) are involved in detoxification of contaminants via redox reaction (Liu 2006; Shah et al. 2015). Furthermore, zero-valent iron (granular form of iron) has reducing and absorption property and is used in remediation of chlorinated compounds (Oh et al. 2016). Hence, it is a relatively recent development that material scientists have been keeping interest in such microorganisms that can become possible eco-friendly input in a number of environmental remediation technologies.
3.5.7 Agricultural Applications
It is noticeable globally that the agricultural sector is continuously hampered by different pests and pathogens which are adversely affecting plant growth and agricultural production, which results in high economic losses and poses a risk to the global food security (Ingale and Chaudhari 2013). To control these plant pests and diseases, farmers use various agrochemicals indiscriminately which results in deterioration of soil and water quality. The excessive and repeated use of these chemicals causes ecotoxicological effects and occasionally results in development of resistance against agrochemicals (Prasad et al. 2017). In some cases, these chemicals also enter in the food chain and get accumulated in the human body. More efforts should be devoted toward developing safe management methods that can replace synthetic pesticides with higher efficiency and also pose less danger to humans and animal health (Benelli and Lukehart 2017). Thus, green synthesis of metal nanoparticles using microbial template not only offers a novel, easy, environmentally safe, and cost-effective approach but also ensures sustainable pest management strategy. These nanopesticides play an important role in the recent development of non-toxic and promising pesticide delivery systems for increasing global food production in sustainable agriculture by reducing rate of application at least 10–15 times smaller than the applied classical formulation (Kah and Hofmann 2014). Alternatively, due to special properties like sensitivity and performance, these nanoparticles could be employed as biosensors in global positioning systems with satellite imaging of fields for distantly detection of crop pests, soil analysis and physiological stress such as drought (Fraceto et al. 2016). NPs exhibit very good transduction properties which are being explored for analytical purpose of agricultural products. In this regard, AuNPs have intrinsic properties and may be used as transducers for several improvements in agricultural products (Kandasamy and Prema 2015). Hence, nanoscale carrier can be utilized for the efficient delivery of fertilizers, pesticides, herbicides, plant growth regulators, etc. in smaller amount and have much better and prolonged management in the agriculture sector.
3.6 Advantages and Future Trends
Green synthesis of microbial nanoparticles has scaffolding advantages, including biocompatible, ecofriendly, and cost-effective production methodologies. Apart from that, there is no requirement of further stabilizing agents as microbial cell constituents itself act as capping and stabilizing agents (Kalishwaralal et al. 2009). However, the surfaces of microbial NPs gradually and selectively adsorb biomolecules when they come into contact with complex biological fluids, forming a corona that interacts with biological systems which provides additional efficacy over bare biological nanoparticles. This makes it a less time-consuming, high yielding, and valuable one-step process by reducing the number of steps required in physiochemical synthesis and by including the attachment of some functional groups to the nanoparticle surface to make them biologically active (Khandel and Shahi 2016). Some of the other advantages like synthesized nanoparticle size can also be controlled easily by various parameters like pH and temperature (Gurunathan et al. 2009). Nanoparticles with smaller curvature have greater catalytic activity; hence, angular shapes are preferable over spherical particles due to their smaller radii of curvature in same volume (Li et al. 2011). Sometimes, nanoparticles are coated with a lipid layer that confers physiological solubility and stability, which is critical for biomedical applications and is the bottleneck of other synthetic methods (Razavi et al. 2015).
There have been tremendous developments in the exploration of microbial biotemplates for green synthesis of nanoparticles and their applications in various sectors over the last decade. However, the research work is still in the early stage, and yet further exploration is needed to improve their synthesis, efficiency, and stability, keeping in view the variegated parameters like type of microorganisms, growth phase of microbial cells, growth mediums, synthesis conditions, pH, substrate concentrations, target nanoparticles source compound, temperature, reaction time, and addition of non-target ions, which might result into obtaining sufficient control of particle size and monodispersity. Moreover, research efforts should be carried out in manipulating cells at the genomic and proteomic levels with better understanding of the synthesis mechanism on a cellular and molecular level, including isolation and identification of the compounds responsible for the reduction of nanoparticles. Further breakthroughs are desirable in order to revolve the impression of nanoparticle technology into a rational practical approach.
3.7 Conclusion
Since origin of life on earth, microbial entities have developed and evolved in environment containing various inorganic materials and these microorganisms play important role in transformation of minerals from one form to another in nature. Moreover, sustenance of life on the earth require a large number of minerals. Plethora of literatures have been found on multidisciplinary approaches of inorganic nanoparticles synthesized by microorganisms through either intracellular or extracellular routes. Presently, these nanoparticles have become a prominent platform for a diverse range of biological applications in a reliable and greener way instead of chemical and physical methods involving toxic chemicals and high temperatures that are not only hazardous to the environment but are costly too. Moreover, the shift from physico-chemical methods to greener synthesis of nanoparticles is preferred because they are environmentally salubrious, sustainable, safe and ecofriendly. Numerous microbial groups have focused on alternative ways of synthesizing nanoparticles as described in this chapter from its approaches to applications. Still, the field of microbial biosynthesis of metallic nanoparticles is relatively new and underexplored; however, it shows great potential in development of newer technology as it provides a single-step process for biosynthesis of nanoparticles which attracts more researchers to go for future developments in the area of electrochemical sensor, biosensors, healthcare, pharmaceutical, environmental technology, and agriculture. Development of improved technology will surely open up several new and exciting possibilities in the use of bioprocessed nanoparticles in every sphere of life which can become a boon to the society.
References
Abdel-Aziz SM, Prasad R, Hamed AA, Abdelraof M (2018) Fungal nanoparticles: a novel tool for a green biotechnology? In: Prasad R, Kumar V, Kumar M, Wang S (eds) Fungal nanobionics: principles and applications. Springer, Singapore, pp 61–87
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, Sastry M (2003a) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Coll Surf B Biointerfaces 28:313–318
Ahmad A, Senapati S, Khan MI, Kumar R, Sastry M (2003b) Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir 19:3550–3553
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res 7(1):17–28
Anwar SH (2018) A brief review on nanoparticles: types of platforms, biological synthesis and applications. Res Rev J Mat Sci 6(2):109–116
Aziz N, Fatma T, Varma A, Prasad R (2014) Biogenic synthesis of silver nanoparticles using Scenedesmus abundans and evaluation of their antibacterial activity. J Nanopart:689419. https://doi.org/10.1155/2014/689419
Aziz N, Faraz M, Pandey R, Sakir M, Fatma T, Varma A, Barman I, Prasad R (2015) Facile algae-derived route to biogenic silver nanoparticles: synthesis, antibacterial and photocatalytic properties. Langmuir 31:11605−11612. https://doi.org/10.1021/acs.langmuir.5b03081
Aziz N, Pandey R, Barman I, Prasad R (2016) Leveraging the attributes of Mucor hiemalis-derived silver nanoparticles for a synergistic broad-spectrum antimicrobial platform. Front Microbiol 7:1984. https://doi.org/10.3389/fmicb.2016.01984
Aziz N, Faraz M, Sherwani MA, Fatma T, Prasad R (2019) Illuminating the anticancerous efficacy of a new fungal chassis for silver nanoparticle synthesis. Front Chem 7:65. https://doi.org/10.3389/fchem.2019.00065
Bai HJ, Zhang ZM (2009) Microbial synthesis of semiconductor lead sulfide nanoparticles using immobilized Rhodobacter sphaeroides. Mater Lett 63(9):764–766. https://doi.org/10.1016/j.matlet.2008.12.050
Baker S, Harini BP, Rakshith D, Satish S (2013) Marine microbes: invisible nanofactories. J Pharm Res 6:383–388
Banik S, Sharma P (2011) Plant pathology in the era of nanotechnology. Indian Phytopathol 64:120–127
Bansal V, Rautaray D, Ahmad A, Sastry M (2004) Biosynthesis of zirconia nanoparticles using the fungus Fusarium oxysporum. J Mater Chem 14:3303–3305
Bansal V, Rautaray D, Bharde A, Ahire K, Sanyal A, Ahmad A, Sastry M (2005) Fungus-mediated biosynthesis of silica and titania particles. J Mater Chem 15:2583–2589
Benelli G, Lukehart CM (2017) Applications of green-synthesized nanoparticles in pharmacology, parasitology and entomology. J Clust Sci 28(1):1–2. https://doi.org/10.1007/s10876-017-1165-5
Bhattacharya D, Gupta RK (2005) Nanotechnology and potential of microorganisms. Crit Rev Biotechnol 25(4):199–204
Cao G (2004) Nanostructures and nanomaterials: synthesis, properties and applications, vol 2. World Scientific Series in Nanoscience and Nanotechnology. Imperial College Press, London, pp 1–433
Castro L, Blázquez ML, Munoz JA, Gonzalez F, Ballester A (2013) Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol 7(3):109–116
Chakraborty N, Banerjee A, Lahiri S, Panda A, Ghosh AN, Pal R (2009) Biorecovery of gold using cyanobacteria and an eukaryotic alga with special reference to nanogold formation-a novel phenomenon. J Appl Phycol 21:145–152
Chen JC, Lin ZH, Ma XX (2003) Evidence of the production of silver nanoparticles via pretreatment of Phoma sp.3.2883 with silver nitrate. Lett Appl Microbiol 37(2):105–108
Crooks RM, Zhao M, Sun L, Chechik V, Yeung LK (2001) Dendrimer-encapsulated metal nanoparticles : synthesis, characterization, and applications to catalysis. Acc Chem Res 34(3):181–190
Dameron CT, Reese RN, Mehra RK, Kortan AR, Carroll PJ, Steigerwald ML, Brus LE, Winge DR (1989) Biosynthesis of cadmium sulphide quantum semiconductor crystallites. Nature 338:596–597
Das SK, Das AR, Guha AK (2009) Gold Nanoparticles: microbial synthesis and application in water hygiene management. Langmuir 25(14):8192–8199. https://doi.org/10.1021/la900585p
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330. https://doi.org/10.1016/S0043-1354(03)00293-8
Dhillon GS, Brar SK, Kaur S, Verma M (2012) Green approach for nanoparticle biosynthesis by fungi. Curr Trends Appl 32:49–73
Dhillon GS, Kaur S, Brar SK (2014) Facile fabrication and characterization of chitosan-based zinc oxide nanoparticles and evaluation of their antimicrobial and antibiofilm activity. Int Nano Lett 4:107. https://doi.org/10.1007/s40089-014-0107-6
Duran N, Marcato PD, De S, Gabriel IH, Alves OL, Esposito E (2007) Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J Biomed Nanotechnol 3:203–208
Duran N, Marcato PD, Duran M, Yadav A, Gade A, Rai M (2011) Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl Microbiol Biotechnol 90:1609–1624
Ealias AM, Saravanakumar MP (2017) A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf Series: Mater Sci Eng 263:032019. https://doi.org/10.1088/1757-899X/263/3/032019
Fayaz M, Balaji K, Kalaichelvan PT, Venkatesan R (2009) Fungal based synthesis of silver nanoparticles- an effect of temperature on the size of particles. Colloids Surf B: Biointerfaces 74(1):123–126
Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R (2010) Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against Gram-positive and Gram-negative bacteria. Nanomed: Nanotechnol Biol Med 6(1):103–109
Flenniken ML, Uchida M, Lipold L, Kang S, Young MJ, Douglas T (2009) A library of protein cage architectures as nanomaterials. Curr Top Microbiol Immunol 327:71–73
Fraceto LF, Grillo R, de Medeiros GA, Scognamiglio V, Rea G, Bartolucci C (2016) Nanotechnology in agriculture: which innovation potential does it have? Front Environ Sci 4:20. https://doi.org/10.3389/fenvs.2016.00020
Gade AK, Bonde P, Ingle AP, Marcato PD, Duran N, Rai MK (2008) Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biobased Mater Bioenergy 2(3):243–247
Gericke M, Pinches A (2006) Biological synthesis of metal nanoparticles. Hydrometallurgy 83:132–140
Gibney E (2015) Buckyballs in space solve 100-year-old riddle. Nat News. https://doi.org/10.1038/nature.2015.17987
Gomaa EZ (2017) Silver nanoparticles as an antimicrobial agent: a case study on Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram-negative bacteria. J Gen Appl Microbiol 63(1):36–43. https://doi.org/10.2323/jgam.2016.07.004
Gopinathan P, Ashok AM, Selvakumar R (2013) Bacterial flagella as biotemplate for the synthesis of silver nanoparticle impregnated bionanomaterial. Appl Surf Sci 276(1):717–722
Gref R, Minamitake Y, Perracchia MT, Trubeskoy V, Torchilin V, Langer R (1994) Biodegradable long-circulating polymeric nanospheres. Sci 263(5153):1600–1603
Gurunathan S, Lee KJ, Kalishwaralal K, Sheikpranbabu S, Vaidyanathan R, Eom SH (2009) Antiangiogenic properties of silver nanoparticles. Biomaterials 30:6341–6350
Haefeli C, Franklin C, Hardy K (1984) Plasmid-determined silver resistance in Pseudomonas stutzeri isolated from a silver mine. J Bacteriol 158(1):389–392
Huber DL (2005) Synthesis, properties, and applications of iron nanoparticles. Small 1(5):482–501
Husseiney MI, El-Aziz MA, Badr Y, Mahmoud MA (2007) Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim Acta A Mol Biomol Spectrosc 67(3–4):1003–1006
Ingale AG, Chaudhari AN (2013) Biogenic synthesis of nanoparticles and potential applications: an eco-friendly approach. J Nanomed Nanotechol 4:165. https://doi.org/10.4172/2157-7439.1000165
Ingle A, Rai M, Gade A, Bawaskar M (2009) Fusarium solani: a novel biological agent for the extracellular synthesis of silver nanoparticles. J Nanopart Res 11:2079–2085
Iqbal P, Preece JA, Mendes PM (2012) Nanotechnology: the “Top-Down” and “Bottom-Up” approaches. In: Gale PA, Steed JW (eds) Supramolecular chemistry: from molecules to nanomaterials. John Wiley & Sons Ltd, Chichester, pp 3589–3602. https://doi.org/10.1002/9780470661345.smc195
Iravani S (2014) Bacteria in nanoparticle synthesis: current status and future prospects. International Scholarly Research Notices, Article ID 359316, 18 pages. https://doi.org/10.1155/2014/359316
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9(6):385–406
Jha AK, Prasad K, Kulkarni AR (2009) Plantsystem: Nature’s nanofactory. Colloids Surf B: Biointerfaces 73:219–223
Kah M, Hofmann T (2014) Nanopesticides research: current trends and future priorities. Environ Int 63:224–235
Kalishwaralal K, Banumathi E, Pandian SRK, Deepak V, Muniyandi J, Eom SH, Gurunathan S (2009) Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf B: Biointerfaces 73:51–57
Kandasamy S, Prema RS (2015) Methods of synthesis of nanoparticles and its applications. J Chem Pharm Res 7:278–285
Kannan RRR, Stirk WA, Staden JV (2013) Synthesis of silver nanoparticles using the seaweed Codium capitatum P.C. Silva (Chlorophyceae). S Afr J Bot 86:1–4
Kathiresan K, Manivannan S, Nabeel M, Dhivya B (2009) Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf B Biointerfaces 71(1):133–137
Khandel P, Shahi SK (2016) Microbes mediated synthesis of metal nanoparticles: current status and future prospects. Int J Nanomater Biostruct 6(1):1–24
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ et al (2007) Antimicrobial effects of silver nanoparticles. Nanomed: Nanotechnol Biol Med 3:95–101
Kowshik M, Deshmukh N, Vogel W, Urban J, Kulkarni SK, Paknikar KM (2002a) Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol Bioeng 78:583–588
Kowshik M, Vogel W, Urban J, Kulkarni SK, Paknikar KM (2002b) Microbial synthesis of semiconductor PbS nanocrystallites. Adv Mater 14:815–818
Labrenz M, Druschel GK, Tomsen-Ebert T, Gilbert B, Welch SA, Kemner KM, Logan GA, Summons RE, Stasio GD, Bond PL, Lai B, Kelly SD, Banfeld JF (2000) Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science 290:1744–1747
Li Y, Duan X, Qian Y, Li Y, Liao H (1999) Nanocrystalline silver particles: synthesis. J Colloid Interface Sci 209:347–349
Li C, Cai W, Kan C, Fu G, Zhang L (2004) Ultrasonic solvent inducedmorphological change of Au colloids. Mat Lett 58:196–199
Li X, Xu H, Chen ZS, Chen G (2011) Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater 270974:16. https://doi.org/10.1155/2011/270974
Liu WT (2006) Nanoparticles and their biological and environmental applications. J Biosci Bioeng 102(1):1–7
Lu YC, Xu Z, Gasteiger HA, Chen S, Schifferli KH, Horn YS (2010) Platinum-Gold nanoparticles: a highly active bifunctional electrocatalyst for rechargeable Lithium-Air batteries. J Am Chem Soc 132(35):12170–12171. https://doi.org/10.1021/ja1036572
Luangpipat T, Beattie IR, Chisti Y, Haverkamp RG (2011) Gold nanoparticles produced in a microalga. J Nanopart Res 13(12):6439–6445
Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME, Kalinina NO (2014) Green nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae 6:35–44
Malik P, Shankar R, Malik V, Sharma N, Mukherjee TK (2014) Green chemistry based benign routes for nanoparticle synthesis. J Nanopart:302429. https://doi.org/10.1155/2014/302429
Mallick K, Witcomb MJ, Scurell MS (2004) Polymer stabilized silver nanoparticles: a photochemical synthesis route. J Matter Sci 39:4459–4463
Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P (2006) The use of microorganisms for the formation of metal nanoparticles and their application. Appl Microbiol Biotechnol 69:485–492
Mao C, Flynn CE, Hayhurst A, Sweeney R, Qi J, Georgiou G, Iverson B, Belcher AM (2003) Viral assembly of oriented quantum dot nanowires. Proc Natl Acad Sci USA 100(12):6946–6951
Mariekie G, Anthony P (2006) Microbial production of gold nanoparticles. Gold Bull 39:22–28
Mazhar T, Shrivastava V, Tomar RS (2017) Green synthesis of bimetallic nanoparticles and its applications: a review. J Pharm Sci Res 9(2):102–110
Mehra RK, Winge DR (1991) Metal ion resistance in fungi: molecular mechanisms and their regulated expression. J Cell Biochem 45:30–40
Menon S, Shanmugam RK, Venkat Kumar S (2017) A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resource-Efficient Technologies 3:516–527
Merzlyak A, Lee SW (2006) Phage as template for hybrid materials and mediators for nanomaterials synthesis. Curr Opin Chem Biol 10:246–252
Mishra A, Kumari M, Pandey S, Chaudhry V, Gupta KC, Nautiyal CS (2014) Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp. Bioresour Technol 166:235–242
Moghaddam BA, Namvar F, Moniri M, Md Tahir P, Azizi S, Mohamad R (2015) Nanoparticles biosynthesized by fungi and yeast: a review of their preparation, properties, and medical applications. Molecules 20(9):16540–16565
Mohanpuria P, Rana NK, Yadav SK (2008) Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res 10:507–517
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci 156:1–13
Nath D, Banerjee P (2013) Green nanotechnology–a new hope for medical biology. Environ Toxicol Pharmacol 36:997–1014
Oh SY, Seo YD, Kim B, Kim IY, Cha DK (2016) Microbial reduction of nitrate in the presence of zero-valent iron and biochar. Bioresour Technol 200:891–896
Palomo JM, Filice M (2016) Biosynthesis of metal nanoparticles: novel efficient heterogeneous nanocatalysts. Nanomaterials 6(5):84. https://doi.org/10.3390/nano6050084
Panigrahi S, Kundu S, Ghosh S, Nath S, Pal T (2004) General method of synthesis for metal nanoparticles. J Nanopart Res 6(4):411–414
Pantidos N, Horsfall LE (2014) Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. J Nanomed Nanotechnol 5:233. https://doi.org/10.4172/2157-7439.1000233
Parak WJ, Boudreau R, Le Gros M et al (2002) Cell motility and metastatic potential studies based on quantum dot imaging of phagokinetic tracks. Adv Mater 14(12):882–885
Patel V, Berthold D, Puranik P, Gantar M (2015) Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol Rep 5:112–119
Pierfrancesco M (2010) Use and potential of nanotechnology in cosmetic dermatology. Clin Cosmet Investig Dermatol 3:5–13
Pimprikar PS, Joshi SS, Kumar AR, Zinjarde SS, Kulkarni SK (2009) Influence of biomass and gold salt concentration on nanoparticle synthesis by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Colloids Surf B Biointerfaces 74(1):309–316. https://doi.org/10.1016/j.colsurfb.2009.07.040
Pinto RJB, Daina S, Sadocco P, Neto CP, Trindade T (2013) Antibacterial activity of nanocomposites of copper and cellulose. BioMed Res Int 6:280512. https://doi.org/10.1155/2013/280512
Pokorski JK, Steinmetz NF (2011) The art of engineering viral nanoparticles. Mol Pharm 8:29–43
Pradhan N, Singh S, Ojha N, Shrivastava A, Barla A, Rai V, Bose S (2015) Facets of nanotechnology as seen in food processing, packaging, and preservation industry. BioMed Res Int:365672. https://doi.org/10.1155/2015/365672
Prasad R (2014) Synthesis of silver nanoparticles in photosynthetic plants. J Nanopart:963961. https://doi.org/10.1155/2014/963961
Prasad R (2016) Advances and applications through fungal nanobiotechnology. Springer International Publishing, Switzerland. isbn:978-3-319-42989-2
Prasad R (2017) Fungal nanotechnology: applications in agriculture, industry, and medicine. Springer Nature, Singapore. isbn:978-3-319-68423-9
Prasad R, Kumar V, Prasad KS (2014) Nanotechnology in sustainable agriculture: present concerns and future aspects. Afr J Biotechnol 13:705–713. https://doi.org/10.5897/AJBX2013.13554
Prasad R, Pandey R, Barman I (2016) Engineering tailored nanoparticles with microbes: quo vadis. Wiley Interdiscip Rev Nanomed Nanobiotechnol 8:316–330. https://doi.org/10.1002/wnan.1363
Prasad R, Bhattacharyya A, Nguyen QD (2017) Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front Microbiol 8:1014. https://doi.org/10.3389/fmicb.2017.01014
Prasad R, Jha A, Prasad K (2018a) Exploring the realms of nature for nanosynthesis. Springer International Publishing. https://www.springer.com/978-3-319-99570-0. isbn:978-3-319-99570-0
Prasad R, Kumar V, Kumar M, Wang S (2018b) Fungal nanobionics: principles and applications. Springer Nature, Singapore. https://www.springer.com/gb/book/9789811086656. isbn:978-981-10-8666-3
Rai V, Acharya S, Dey N (2012) Implications of nanobiosensors in agriculture. J Biomater Nanobiotechnol 3:315–324. https://doi.org/10.4236/jbnb.2012.322039
Raj S, Jose S, Sumod US, Sabitha M (2012) Nanotechnology in cosmetics: opportunities and challenges. J Pharm Bioallied Sci 4(3):186–193. PMC3425166. https://doi.org/10.4103/0975-7406.99016
Raliya R, Tarafdar JC (2013) ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agirc Res 2:48–57
Razavi M, Salahinejad E, Fahmy M, Yazdimamaghani M, Vashaee D, Tayebi L (2015) Green chemical and biological synthesis of nanoparticles and their biomedical applications. In: Basiuk VA, Basiuk EV (eds) Green processes for nanotechnology. Springer, Cham, pp 207–235
Reddy AS, Chen CY, Chen CC, Jean JS, Chen HR, Tseng MJ, Fan CW, Wang JC (2010) Biological synthesis of gold and silver nanoparticles mediated by the bacteria Bacillus subtilis. J Nanosci Nanotechnol 10(10):6567–6574
Reddy GAK, Joy JM, Mitra T, Shabnam S, Shilpa T (2012) Nano silver – a review. Int J Adv Pharm 2(1):09–15
Royston ES, Brown AD, Harris MT, Culver JN (2009) Preparation of silica stabilized tobacco mosaic virus templates for the production of metal and layered nanoparticles. J Colloid Interface Sci 332(2):402–407. https://doi.org/10.1016/j.jcis.2008.12.064
Sadhasivam S, Shanmugam P, Yun Y (2010) Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids and Surf B: Biointerfaces 81:358–362
Sanchez F, Sobolev K (2010) Nanotechnology in concrete-A review. Construct Build Mater 24:2060–2071
Sanghi R, Verma P (2009) Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour Technol 100(1):501–504. https://doi.org/10.1016/j.biortech.2008.05.048
Sanyasi S, Majhi RK, Kumar S, Mishra M, Ghosh A, Suar M, Satyam PV, Mohapatra H, Goswami C, Goswami L (2016) Polysaccharide-capped silver nanoparticles inhibit biofilm formation and eliminate multi-drug-resistant bacteria by disrupting bacterial cytoskeleton with reduced cytotoxicity towards mammalian cells. Sci Rep 6:24929. https://doi.org/10.1038/srep24929
Sarkar J, Ray S, Chattopadhyay D, Laskar A, Acharya K (2012) Mycogenesis of gold nanoparticles using a phytopathogen Alternaria alternata. Bioprocess Biosyst Eng 35(4):637–643
Sastry M, Ahmad A, Khan MI, Kumar R (2003) Biosynthesis of metal nanoparticles using fungi and actinomycetes. Curr Sci 85:162–170
Sathiyanarayanan G, Dineshkumar K, Yang YH (2017) Microbial exopolysaccharide-mediated synthesis and stabilization of metal nanoparticles. Crit Rev Microbiol 43(6):731–752. https://doi.org/10.1080/1040841X.2017.1306689
Sekhon BS (2014) Nanotechnology in agri-food production: an overview. Nanotechnol Sci Appl 7:31–53. https://doi.org/10.2147/NSA.S39406
Selvakumar R, Seethalakshmi N, Thavamani P, Naidu R, Megharaj M (2014) Recent advances in the synthesis of inorganic nano/microstructures using microbial biotemplates and their applications. RSC Adv 4:52156–52169. https://doi.org/10.1039/C4RA07903E
Shah SP, Konsta-Gdoutos MS, Metaxa ZS, Mondal P (2009) Nanoscale modification of cementitious materials. In: Bittnar Z, Bartos PJM, Nemecek J, Smilauer V, Zeman J (eds) Nanotechnology in construction 3. Springer, Berlin/Heidelberg, pp 125–130
Shah M, Fawcett D, Sharma S, Tripathy SK, Poinern GEJ (2015) Green synthesis of metallic nanoparticles via biological entities. Materials 8:7278–7308. https://doi.org/10.3390/ma8115377
Sharma D, Kanchi S, Bisetty K (2015) Biogenic synthesis of nanoparticles: a review. Arabian J Chem. https://doi.org/10.1016/j.arabjc.2015.11.002
Shenton W, Douglas T, Young M, Stubbs G, Mann S (1999) Inorganic-organic nanotube composites from template mineralization of tobacco mosaic virus. Adv Mater 11(3):253–256
Shinkai M, Yanase M, Suzuki M, Hiroyuki H, Wakabayashi T, Yoshida J, Kobayashi T (1999) Intracellular hyperthermia for cancer using magnetite cationic liposomes. J Magn Magn Mater 194(1):176–184
Siddiqi KS, Husen A (2016) Fabrication of metal and metal oxide nanoparticles by algae and their toxic effects. Nanoscale Res Lett 11:363. https://doi.org/10.1186/s11671-016-1580-9
Singaravelu G, Arockiamary JS, Kumar VG, Govindaraju K (2007) A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B Biointerfaces 57(1):97–101
Singh M, Singh S, Prasad S, Gambhir IS (2008) Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Digest J Nanomater Biostruct 3(3):115–122
Singh P, Kim YJ, Zhang D, Yang DC (2016) Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol 34(7):588–599
Sleytr UB, Messner P, Pum D, Sara M (1993) Crystalline bacterial cell surface layers. Mol Microbiol 10:911–916
Sobolev K, Flores I, Torres-Martinez LM, Valdez PL, Zarazua E, Cuellar EL (2009) Engineering of SiO2 nanoparticles for optimal performance in nano cement-based materials. In: 3rd international symposium on nanotechnology in construction, Prague, Czech Republic, pp 139–148
Songara J, Shanker R, Singh NK (2018) Transformation of benzyl butyl phthalate by Pseudomonas putida and photocatalytic ZnO nanoparticles. Int J Chem Stud 6(4):1334–1340
Stephen JR, Maenaughton S (1999) Developments in terrestrial bacterial remediation of metals. J Curr Opin Biotechnol 10:230–233
Sunkar S, Nachiyar CV (2012) Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac J Trop Biomed 2(12):953–959
Tan Y, Dai Y, Li Y, Zhua D (2003) Preparation of gold, platinum, palladium and silver nanoparticles by the reduction of their salts with a weak reductant–potassium bitartrate. J Mater Chem 13:1069–1075
Tarafdar JC, Raliya R, Rathore I (2012) Microbial synthesis of phosphorous nanoparticle from tri-calcium phosphate using Aspergillus tubingensis TFR-5. J Bionanosci 6(2):84–89
Tarafdar JC, Sharma S, Raliya R (2013) Nanotechnology: interdisciplinary science of application. Afr J Biotechnol 12:219–226
Thakkar KN, Mhatre SS, Rasesh Y, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanomed Nanotechnol Biol Med 6(2):257–262
Thanh NTK, Maclean N, Mahiddine S (2014) Mechanisms of nucleation and growth of nanoparticles in solution. Chem Rev 114(15):7610–7630
Vasquez RD, Apostol JG, de Leon JD, Mariano JD, Mirhan CMC, Pangan SS, Reyes AGM, Zamora ET (2016) Polysaccharide-mediated green synthesis of silver nanoparticles from Sargassum siliquosum J.G. Agardh: assessment of toxicity and hepatoprotective activity. OpenNano 1:16–24
Velusamy P, Venkat Kumar G, Jeyanthi V, Das J, Pachaiappan R (2016) Bio-inspired green nanoparticles: synthesis, mechanism, and antibacterial application. Toxicol Res 32(2):95–102
Vigneshwaran N, Kathe AA, Varadarajan PV, Nachane RP, Balasubramanya RH (2006) Biomimetics of silver nanoparticles by white rot fungus, Phaenerochaete chrysosporium. Colloids Surf B Biointerfaces 53(1):55–59
Vijayaraghavan K, Kamala Nalini SP (2010) Biotemplates in the green synthesis of silver nanoparticles. Biotechnol J 5:1098–1110
Weissleder R, Elizondo G, Wittenberg J, Rabito CA, Bengele HH, Josephson L (1990) Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology 175(2):489–493
Wen AM, Shukla S, Saxena P, Aljabali AA, Yildiz I et al (2012) Interior engineering of a viral nanoparticle and its tumor homing properties. Biomacromolecules 13:3990–4001
Xiang L, Wei J, Jianbo S, Guili W, Feng G, Ying L (2007) Purified and sterilized magnetosomes from Magnetospirillum gryphiswaldense MSR-1 were not toxic to mouse fibroblasts in vitro. Lett Appl Microbiol 45(1):75–81
Yan S, He W, Sun C, Zhang X, Zhao H, Li Z, Zhou W, Tian X, Sun X, Han X (2009) The biomimetic synthesis of zinc phosphate nanoparticles. Dye Pigment 80:254–258
Zhang X, Yan S, Tyagi RD, Surampalli RY (2011) Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 82(4):489–494. https://doi.org/10.1016/j.chemosphere.2010.10.023
Acknowledgments
The authors humbly acknowledge the assistance provided by the Honorable Vice Chancellor of S.D. Agricultural University, Sardarkrushinagar, Gujarat 385506 (India), for providing the facilities for preparation of this manuscript.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Purohit, J., Chattopadhyay, A., Singh, N.K. (2019). Green Synthesis of Microbial Nanoparticle: Approaches to Application. In: Prasad, R. (eds) Microbial Nanobionics. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-16534-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-16534-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16533-8
Online ISBN: 978-3-030-16534-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)