Abstract
Recent experiments using electromagnetic levitation in reduced gravity have confirmed prior observations of anomalous nucleation of the solid in undercooled melts under specific conditions. All indications are that this effect is dynamical, not chemical: the same sample undercools over 300 °C before and after the anomalous event, but maintains the liquid state for only a few seconds when held at a more modest undercooling in the range of 0–50 °C. The new experimental results and related modeling will be examined in comparison to the hypothesis that the solidification is triggered by cavitation in the melt. This platform may provide data relevant to a better quantitative understanding of the effect of ultrasonic processing on grain refinement of terrestrial castings.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Nucleation of crystals in a liquid, both heterogeneous and homogeneous, has been well-studied (e.g. Kelton and Greer [1]). In undercooled liquids, particularly in pure liquid metals, the rate of nucleation increases from approximately zero to very large over a narrow range of temperature. The temperatures involved are characteristic of the properties of the liquid and the crystal, for homogeneous nucleation , or of the heterogeneous site for heterogeneous nucleation .
In electromagnetic levitation (EML) in vacuum, heterogeneous nucleation is minimized. No contact is needed with a container, nor with gas. Accordingly, large undercoolings are commonly achieved, of the order of hundreds of degrees Celsius. Usually the undercooling is limited by chemical contamination , either on the surface of the sample or within its bulk.
Hofmeister et al . [2] showed that for two high-purity zirconium samples processed in EML in vacuum in reduced gravity, the undercooling was very reproducible, at 334 ± 4 °C for 110 free-cooling cycles. This is consistent with the expected behavior of pure metals, for which only the very flat top of the TTT (time-temperature-transformation) curve is accessible with macroscopic samples. It should be possible to hold samples at even slightly lower undercoolings for a very long time without the onset of solidification .
However, for 6 cycles where the sample was held at smaller undercoolings, solidification did occur within seconds or minutes. The anomalous nucleation reported was not consistent with chemical contamination of the sample; upon remelting and free cooling, the sample again achieved deep undercooling . Furthermore, the time to nucleation under constant-temperature conditions was of the order 10s of seconds. This time is very different than the time for heterogeneous nucleation in undercooled liquid metals, which is sub-microsecond.
A theory was offered, suggesting that cavitation of the liquid under the influence of local negative hydrostatic pressure in the sample was the cause of the observed nucleation . Models of the magnetohydrodynamic convection showed that the stirring under the holding conditions may have been sufficient to produce a locally negative hydrostatic pressure in the centers of the recirculating loops in the flow. These local low-pressure zones would then excite any void in the fluid that was convected into the low-pressure zone. On exiting the low-pressure zone, the void could collapse. If the collapse were symmetrical, then the resulting impact would cause a very small region of very high pressure, as described by the Rayleigh–Plesset equation. A local pressure of the order GPa would shift the local melting point, and thus the local undercooling, enough to activate nucleation of the crystal. As the rest of the liquid would still be undercooled, even though not undercooled enough to cause nucleation , the nucleus created by the collapse of the void would then grow beyond the high pressure region, into the rest of the drop.
Similar anomalous nucleation was observed in high-purity Zr samples in a different experimental facility, the MSL-EML (Materials Science Laboratory Electromagnetic Levitator) on the International Space Station, in June 2016 and again in July 2018. These new results are on a different sample, decades later, in a different apparatus. Again, the sample undercooled deeply under free cooling, but solidified reliably within a few seconds to a few hundred seconds on isothermal hold. Analysis of these new data is ongoing and will be discussed in the presentation; this paper will be limited to discussion of the results of Hofmeister et al . [2].
Cavitation and Nucleation in Metals
The nucleation process is believed to be affected by fluid flow in some circumstances [3, 4], usually related to the perturbation of thermal and solutal gradients; however, the direct observation of nucleation with fluid flow is difficult for current techniques. For the conditions of the experiments described here, the effect of fluid flow on nucleation by these mechanisms is negligible.
The effects of cavitation in engineering are caused by the collapse of cavitation bubbles, which results in extreme high local pressure instantly. This effect is used industrially with ultrasound to promote grain refining and degassing .
That high local pressure was theorized to trigger the nucleation of metals during undercooling process by Hofmeister et al . [2]. Based on the Clausius-Clapeyron Equation [5], the melting point temperature is elevated under the condition of high pressure , which means even at the same temperature, the tiny amount of material surrounding the high pressure point is subjected to a much deeper undercooling . Equation 1 gives the relation between the vapor pressure and temperature as Clausius-Clapeyron Equation, where P1 and P2 are the pressure at temperatures of T1 and T2. R is gas constant and ∆Hvap is enthalpy of vaporization.
Hofmeister et al . [2] tested a sample of pure zirconium in 7 mm diameter to study nucleation process on Spacelab mission. The experiment completed over 115 melt cycles on two samples, using a containerless technique (TEMPUS) to reduce the contamination from facility. Each sample was melted, cooled to solidify and then melted again to re- peat certain numbers of melt cycles during levitation. There was fluid flow occurred in the liquid sample and driven by electromagnetic force. The fluid flow rate was kept in controlled by modulating the heating and positioning voltage.
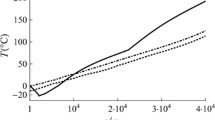
In the velocity range between 5 and 43 cm/s, when the heating was turned off and the convection in the liquid sample was driven by positioning field, the effect of fluid flow on nucleation was not found. Figure 1 gives the maximum flow rates and corresponding undercooling levels. It can be noticed that there is no obvious difference between undercooling temperatures within the flow rate range.
Undercooling as a function of holding conditions for Zr processed in EML in vacuum in reduced gravity [2]
When the interior fluid flow at rates above 50 cm/s, the effect of convection on nucleation was observed. To accelerate the fluid flow, the positioning voltage was held constant and the heating field was applied. A limitation of the undercooling level was found that the nucleation occurred with less undercooling under the condition of convection greater than 50 cm/s. Two samples were tested in undercooling and their performances were given as a function of applied heater voltage. The pressure difference within the liquid calculated by magnetohydrodynamic (MHD) models was shown in Fig. 1. The convection in the liquid sample was assumed to be laminar in these simulations, although contemporary work showed that this sample was surely turbulent [6]. It can be noticed that when the pressure difference increased rapidly, the undercooling level was limited to a small range. The dynamic pressure of interior fluid flow was believed to exceed the static pressure of the liquid sample so that cavitation was favored; for details see Hofmeister et al. [2]. Then the collapse of the cavitation bubbles generated sufficient high local pressure to trigger the nucleation start.
Homogeneous bubble nucleation means the nucleation starts in a liquid without pre-existing bubble nuclei. The classical theory of homogeneous bubble nucleation provides a definition of critical radius of bubble, rc [7]. If the radius of this bubble nucleus is larger than the critical size rc, the bubble nucleus will grow freely with decreasing free energy . If the radius of a bubble nucleus is less than rc, this nucleus needs more energy for growth. These sub-critical bubbles are on average shrinking.
Fisher [8] believed that cavitation is the fracture of liquids and determined the negative pressure in liquid when the first cavitation bubble appears. Blander and Katz [9] provided an estimation of critical cavitation bubble size for certain liquid pressure, with radius rc, (Eq. 2).
where σ is surface tension ; P∗ is the saturation pressure at given temperature; P is the bulk phase pressure; and δ is the correction factor which is expressed as
where ρV is the density of the vapor and ρL is the density of the liquid. Note that rc in Eq. 2 is for the radius of bubbles in local equilibrium with the surrounding liquid, in the absence of the transient dynamic effects during excitation or collapse. These dynamic effects enable the large pressure rise on collapse of the void, and as mentioned above, are governed by the Rayleigh-Plesset equation.
Instead of water as considered by Fisher, the present work calculated the negative pressure required to form the first cavitation bubble in zirconium. The negative pressure needed for homogeneous nucleation of a critical bubble is negative 4.5 GPa.
Based on the MHD/CFD simulation, the pressure in the droplet is around 200 Pa. By using the Eq. 2, the size of the critical bubble nucleus is estimated and to be 3 cm, which is 5 times the droplet size. In this estimation, the value of surface tension adopted 1.58 N/m which from the experimental measurements by our group, and the correction factor is 1. It means, any bubble nuclei appear in the droplet must be smaller than the critical size and, therefore, according to the classical cavitation homogeneous nucleation , they usually disappear or collapse instead of growing bigger to cavitation .
Another hypothesis for the formation of bubbles in the zirconium droplet is that the formation is driven by turbulent eddies. At the center of an eddy, the pressure is further reduced, beyond the time-average pressure. The additional pressure drop may contribute to the excitation of bubbles, but is still of the order Pascals, not Gigapascals. If voids are present in the liquid, they are not nucleated homogeneously due to the negative hydrostatic pressure.
Alternative Mechanisms
If voids in the liquid zirconium cannot be nucleated homogeneously under the conditions present in the experiments, then either voids are nucleated heterogeneously, or some other mechanism than cavitation must be responsible for the nucleation of the solid. Given the high temperature and high reactivity of the zirconium sample and its very high equilibrium solubility for oxygen, it is not clear what kind of heterogeneous nucleation site for voids or bubbles would be possible.
Contact with solid particles of dust, aerosol, or other contamination could cause the undercooled sample to solidify. However, there are several arguments against this explanation. First, all of the cycles with free cooling experienced significant time in the undercooled range, but none solidified except near the maximum undercooling . If there were solid contaminants in the vacuum chamber, some of these cycles should also have shown nucleation due to collision with a solid particle. Furthermore, solid particles are not produced in small numbers; typically in EML experiments, either no particles are observed, or else a dense “smoke” of thousands or millions of particles is produced. There was no evidence of such particles in the video record, nor in the 110 free-cooling cycles.
Conclusions
Prior work [2] reported experimental observations that a sample which undercooled deeply and repeatable nonetheless solidified after a hold of seconds at a modest undercooling . This experimental observation was confirmed by new experiments on the MSL-EML in 2016 and 2018. All three sets of experiments provide strong evidence that the nucleation is anomalous; that is, not consistent with either heterogeneous or homogenous nucleation according to classical nucleation theory.
The prior work reported a theory that the cause of this anomalous nucleation was cavitation of the liquid metal under the influence of magnetohydrodynamic stirring . A closer look at the conditions needed to nucleate voids in liquid metals indicates that such voids must be nucleated heterogeneously, as homogeneous nucleation of voids is impossible under the specified experimental conditions. Given the high temperature and high reactivity of the zirconium sample and its very high equilibrium solubility for oxygen, it is not clear what kind of heterogeneous nucleation site for voids or bubbles would be possible.
References
K.F. Kelton and A.L Greer, Nucleation in Condensed Matter: Applications in Materials and Biology, Boston: Elsevier, 2010.
Hofmeister, WH, Bayuzick, RJ, Hyers, R, and Trapaga, G. Cavitation-induced nucleation of zirconium in low earth orbit. Applied physics letters 74, 18 (1999), 2711–2713.
Garabedian, H, and Strickland-Constable, RF. Collision breeding of crystal nuclei: Sodium chlorate. i. Journal of Crystal Growth 13 (1972), 506–509.
Frawley, JJ, and Childs, WJ. Dynamic nucleation of supercooled metals. TRANS MET SOC AIME 242, 2 (1968).
Hunt, JD, and Jackson, KA. Nucleation of solid in an undercooled liquid by cavitation. Journal of Applied Physics 37, 1 (1966), 254–257.
Hyers, Robert W, Trapaga, G, and Abedian, B. Laminar-turbulent transition in an electromagnetically levitated droplet. Metallurgical and materials transactions B 34, 1 (2003), 29–36.
Landau, LD, and Lifshitz, EM. Statistical physics, vol. 5. Course of theoretical physics 30 (1980).
Fisher, J C, The fracture of liquids. Journal of Applied Physics 19, 11 (1948), 1062–1067.
Blander, Milton, and Katz, Joseph L. Bubble nucleation in liquids. AIChE Journal 21, 5 (1975), 833–848.
Acknowledgements
The authors would like to thank Prof. Dieter Herlach for fruitful discussions about the nature of nucleation of cavities, and Prof. Dirk Holland-Moritz and Priv. Doz. Dr. Juergen Brillo for discussions about alternative explanations for the experimental observations. The UMass team acknowledges support from NASA under grant NNX16AB40G.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Hyers, R.W., Zhao, J., Bracker, G.P., Wunderlich, R., Fecht, H. (2019). Anomalous Nucleation in Undercooled Melts Processed by Electromagnetic Levitation. In: Chesonis, C. (eds) Light Metals 2019. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-05864-7_198
Download citation
DOI: https://doi.org/10.1007/978-3-030-05864-7_198
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05863-0
Online ISBN: 978-3-030-05864-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)