Abstract
Immune checkpoint inhibitors (ICIs) have shown significant benefit in cancer patients, but are associated with immune-related adverse events (irAEs), that can affect the gastrointestinal tract resulting in diarrhea and colitis. IrAEs range from mild self-limiting to severe life-threatening disease, which potentially limit the use of these medications. Diagnosis of ICI-induced colitis is based on clinical symptoms, physical examination, stool tests, endoscopic evaluation, and/or imaging. Current management strategy is mainly anti-diarrheal agents for mild symptoms, and immunosuppressants (e.g., corticosteroids, and infliximab or vedolizumab) for more severe cases.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
The Incidence of ICI-Induced Colitis
ICI-induced colitis, which shares some similarities with inflammatory bowel diseases (IBDs), is observed in 25–30% of patients receiving anti CTLA-4 agents [1,2,3]. Anti-PD-1 antibodies are associated with lower rate of gastrointestinal (GI) adverse events, approximately 10% [4]. However, combination therapy with both CTLA-4 and PD-1 blockers raised the risk of GI toxicities to about 45% which is much higher than monotherapy [5]. Grade 3 or 4 diarrhea was reported to be among the most commonly reported serious adverse events and occurs in 10% of cases receiving ICIs [3, 6].
Clinical Presentation of ICI-Induced Colitis
Among all the clinical symptoms of ICI-induced GI toxicities, the most common presentation is watery diarrhea followed by abdominal pain, hematochezia, nausea/vomiting, and fever [1, 2, 7]. Weight loss has also been found in patients with ICI-induced colitis [1]. Many patients often have only non-bloody self-limiting diarrhea without other associated symptoms [8, 9], whereas severe colitis may result in colonic perforation and death [10,11,12]. The severity of diarrhea and colitis is graded based on the Common Terminology Criteria for Adverse Events (version 4.03). Details of CTCAE criteria for diarrhea and colitis are shown in Table 7.1 [13].
Diarrhea adverse event generally occurs around 6–7 weeks following commencement of ICI treatment [11, 14]. However, the onset can range from immediately after the first dose to more than 4 months after the last dose [7, 15, 16].
Diagnostic Tools for the Evaluation of ICI-Induced Colitis
Patients on ICI treatment who develop acute onset of diarrhea should be evaluated for infectious etiology first [12]. Stool tests for bacterial infection, C. difficile, viral, parasitic, or fungus should be performed to rule out infectious causes before conferring a diagnosis of ICI-induced diarrhea or colitis [17, 18]. It was noted that in some cases, ICI-induced colitis and GI infection can coexist [19].
Currently, there are no available specific serologic or fecal markers for ICI-induced colitis [20]. Fecal calprotectin is a stool inflammatory marker that has been widely used in the clinical practice for patients with inflammatory bowel disease. It has also been reported as a diagnostic or predictive tool for ICI-induced colitis [2]. However, the association between the increased fecal calprotectin level and ICI-induced colitis is not well established [20].
For patients who have ≥grade 2 diarrhea and colitis symptoms, endoscopy with biopsies is highly recommended to further evaluate the severity of ICI-induced GI toxicity [21, 22]. Endoscopic manifestations often reveal erythema, edema, erosions, ulcers, exudates, granularity, loss of vascular pattern, and bleeding [23]. About 43% colonic inflammation is distributed throughout the ileum and colon, while 34% is limited to left colon alone. The rest is normal colon exam [24]. The inflammation pattern can vary from diffuse circumferential, patchy, segmental, to isolated and focal type. For patients who had normal appearing colon on the exam, routine biopsy is required to rule out a subtype of colitis, which mimics microscopic colitis [7, 25]. Although colitis presented with significant endoscopic inflammation accounts for 79% and normal endoscopic exam in 21% [24].
Microscopic findings from inflamed colon are presented with three categories: acute, chronic, and microscopic inflammation [22, 25]. Acute inflammation features include neutrophil and/or eosinophil infiltration, epithelium apoptosis, cryptitis, and crypt microabscesses, which account for 23% of colitis; chronic inflammation features include crypt architectural distortion, basal lymphoplasmocytosis, granuloma, and Paneth cell metaplasia, that account for 60% of colitis; and microscopic colitis can present with features of lymphocytic infiltration in the epithelium and/or sub-epithelial collagen band deposition which is 8% [24]. Chronic histologic features share fair similarity with both Crohn’s disease and ulcerative colitis. In addition, the absence of cytomegalovirus infection on histopathological examination of the colon tissue should be confirmed [2].
Radiology especially CT scan is important to evaluate bowel perforation, obstruction, and toxic megacolon that are complications of severe ICI-induced colitis. Features of colonic inflammation on imaging include diffuse wall thickening, mesenteric vessel engorgement, peri-colic fat stranding, and mucosal enhancement in patients with ICI-induced colitis [2, 26]. Free intraperitoneal air indicates the presence of bowel perforation [27]. However, the sensitivity of detecting evidence of colitis on imaging is only 50% if endoscopy is used as the gold standard for inflammation [24]. For selected patients with high suspicion for toxic megacolon or perforation, abdominal imaging should be obtained to provide early guidance for further management.
Management and Clinical Outcomes of ICI-Induced Colitis
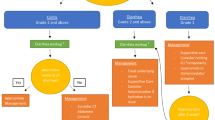
Current management of ICI-induced diarrhea and colitis depends on the severity of the symptoms [28]. For patients with grade 1 diarrhea, usually conservative managements with over the counter anti-diarrheal agents, adequate oral hydration, diet modification, and close follow-up monitoring are recommended. It has also been reported that 5-ASA may be effective in those with milder grade diarrhea [29]. Usually, ICI can be continued for grade 1 symptoms. If patients fail conservative management, or symptoms progress to higher grade level, more aggressive management strategy is required.
For grade 2 and above diarrhea and colitis, holding immunotherapy is highly recommended [30, 31]. The main treatment options for higher grade of ICI-induced diarrhea/colitis are immunosuppressants to reverse the effect of ICI, and hamper the inflammation. These include corticosteroids and other nonsteroidal immunosuppressants, e.g., infliximab and/or vedolizumab [3, 32, 33]. The forms of corticosteroid reported to be used for ICI-colitis include hydrocortisone enema, oral budesonide, and systematic use of corticosteroids (intravenous form of steroid and oral prednisone). Intravenous corticosteroid is indicated in patients who have severe symptoms that require hospitalization especially for grade 3 and above toxicities. Long steroid taper duration over 4–6 weeks is recommended to minimize the rebound symptoms. The standard dose of initial steroid treatment is 1 mg/kg/day, but can be increased to 2 mg/kg if symptoms are refractory within 2–3 days. The use of steroid enema and budesonide was reported in case studies only [14, 17, 29, 34]. For cases refractory to corticosteroid treatment, anti-TNF agents such as infliximab and adhesion molecule blocker, e.g., vedolizumab had been reported to be successful in case studies [3, 32, 35]. Indeed, early use of infliximab is associated with shorter duration of immunosuppressant treatment and improved clinical outcome [32, 33, 36]. The contraindications for biological agents include bowel perforation and infection, especially sepsis [11]. The response to infliximab therapy is usually within 1–3 days [7], while some patients may need more than one dose [29]. The reported response rate to infliximab was as high as 83–100% [2].
When symptoms resolve or improve to grade 1 or less after steroid treatment, resuming checkpoint inhibitor may be considered especially non-CTLA-4 agents [11]. Recurrent GI symptoms after the initial episode can occur months after successful treatment and may require complete evaluation for the same etiology [17].
Other immunosuppressive agents such as tacrolimus or mycophenolate mofetil have also been reported in case studies for the treatment of ICI-induced colitis [33]. It should be noted that, for patients with high suspicion of bowel perforation or toxic megacolon, steroids should be withheld and a surgical consultation should be obtained [11]. Surgery with colectomy is usually reserved for patients with serious GI complications, e.g., colonic perforation [33, 37, 38]. Avoidance of nonsteroidal anti-inflammatory drugs (NSAID) is usually recommended to prevent exacerbation of gastrointestinal symptoms based on case reports [1, 39].
Conclusion
The recognition of ICI-induced colitis is increasing with the wide use of ICIs in the past few years. It shares some characteristics with IBD; however, presents with much broader range of manifestations than IBD. The diagnosis and the severity measures of ICI-induced colitis are based on multiple evaluation modalities. Early use of immunosuppressants, e.g., corticosteroids, infliximab and/or vedolizumab can lead to quick symptom improvement in severe cases. The ultimate goal is to provide maintenance treatment to keep the colitis in remission while keeping patients on ICI treatment to maximize its benefit if they are deemed to be good responders. Further studies are still required to further improve the management strategy.
References
Marthey L, Mateus C, Mussini C, et al. Cancer immunotherapy with Anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10:395–401.
Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42:406–17.
Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48.
Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49.
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34.
Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6(1):37.
Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–9.
Johnston RL, Lutzky J, Chodhry A, Barkin JS. Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab. Dig Dis Sci. 2009;54:2538–40.
de Guillebon E, Roussille P, Frouin E, Tougeron D. Anti program death-1/anti program death-ligand 1 in digestive cancers. World J Gastrointest Oncol. 2015;7:95–101.
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28.
Howell M, Lee R, Bowyer S, Fusi A, Lorigan P. Optimal management of immune-related toxicities associated with checkpoint inhibitors in lung cancer. Lung Cancer. 2015;88:117–23.
Ledezma B, Binder S, Hamid O. Atypical clinical response patterns to ipilimumab. Clin J Oncol Nurs. 2011;15:393–403.
Common terminology criteria for adverse events (CTCAE) version 4.0. US Department of Health and Human Services.
Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–7.
Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864–72.
Lord JD, Hackman RC, Moklebust A, et al. Refractory colitis following anti-CTLA4 antibody therapy: analysis of mucosal FOXP3+ T cells. Dig Dis Sci. 2010;55:1396–405.
Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28:264–8.
Eggermont AM, Chiarion-Sileni V, Grob JJ. Correction to Lancet Oncol 2015; 16: 522-30. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:e262.
McCutcheon JL, McClain CM, Puzanov I, Smith TA. Infectious colitis associated with ipilimumab therapy. Gastroenterol Res. 2014;7:28–31.
Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11.
Abu-Sbeih H, Ali FS, Luo W, Qiao W, Raju GS, Wang Y. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2018;6(1):95.
Wang Y, Abu-Sbeih H, Mao E, Ali N, Qiao W, Trinh VA, et al. Endoscopic and histologic features of immune checkpoint inhibitor-related colitis. Inflamm Bowel Dis. 2018;24(8):1695–705.
Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol. 2010;37:499–507.
Wang Y, Abu-Sbeih H, Mao E, et al. Endoscopic and histologic features of immune checkpoint inhibitor-related colitis. Inflamm Bowel Dis. 2018;24(8):1695–705.
Choi K, Abu-Sbeih H, Samdani R, Nogueras Gonzalez G, Raju GS, Richards DM, et al. Can immune checkpoint inhibitors induce microscopic colitis or a brand new entity? Inflamm Bowel Dis. 2018.
Kim KW, Ramaiya NH, Krajewski KM, et al. Ipilimumab-associated colitis: CT findings. Am J Roentgenol. 2013;200:W468–74.
Mitchell KA, Kluger H, Sznol M, Hartman DJ. Ipilimumab-induced perforating colitis. J Clin Gastroenterol. 2013;47:781–5.
Singal AK, Jampana SC, Singal V, Kuo YF. Hepatocellular carcinoma predicts in-hospital mortality from acute variceal hemorrhage among patients with cirrhosis. J Clin Gastroenterol. 2012;46:613–9.
Tarhini A, Lo E, Minor DR. Releasing the brake on the immune system: ipilimumab in melanoma and other tumors. Cancer Biother Radiopharm. 2010;25:601–13.
Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95.
Hwang JH, Shergill AK, Acosta RD, et al. The role of endoscopy in the management of variceal hemorrhage. Gastrointest Endosc. 2014;80:221–7.
Dadu R, Zobniw C, Diab A. Managing adverse events with immune checkpoint agents. Cancer J. 2016;22:121–9.
Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60.
Collins M, Michot JM, Danlos FX, et al. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol. 2017;28:2860–5.
Minor DR, Chin K, Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24:321–5.
Johnson DH, Zobniw CM, Trinh VA, Ma J, Bassett RL Jr, Abdel-Wahab N, et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer. 2018;6(1):103.
Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–30.
Burdine L, Lai K, Laryea JA. Ipilimumab-induced colonic perforation. J Surg Case Rep. 2014;2014:rju010.
Ananthakrishnan AN, Higuchi LM, Huang ES, et al. Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann Intern Med. 2012;156:350–9.
Disclosure
The authors declared no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tian, Y., Abu-Sbeih, H., Wang, Y. (2018). Immune Checkpoint Inhibitors-Induced Colitis. In: Naing, A., Hajjar, J. (eds) Immunotherapy. Advances in Experimental Medicine and Biology, vol 995. Springer, Cham. https://doi.org/10.1007/978-3-030-02505-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-02505-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-02504-5
Online ISBN: 978-3-030-02505-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)