Abstract
The radiological diagnosis of breast carcinoma is based on three different techniques today: mammography, magnetic resonance imaging (MRI), and breast ultrasound (echography). A major disadvantage of mammography is the lack of precise anatomical references. In fact, the radiologist using this technique makes a global analysis of the connective and fatty tissues containing the fibro-glandular block but the lobes of the breast are not outlined on the normal mammogram. MR mammography is based on contrast enhancement dependent on angiogenesis and as such gives no opportunity to relate the lesions to lobar anatomy of the breast. Conventional breast echography with orthogonal vertical and horizontal scanning is only a transcript of the mammographic findings from the radiologist’s point of view. It does not allow viewing anatomical structures, and only a limited part of the breast volume can be studied. Description of lobes, lobules, and ducts, or the localization of specific terminal ductal-lobular unit (TDLUs) groups never appears in an echography report.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
8.1 Introduction
The radiological diagnosis of breast carcinoma is based on three different techniques today: mammography, magnetic resonance imaging (MRI), and breast ultrasound (echography). A major disadvantage of mammography is the lack of precise anatomical references. In fact, the radiologist using this technique makes a global analysis of the connective and fatty tissues containing the fibro-glandular block but the lobes of the breast are not outlined on the normal mammogram. MR mammography is based on contrast enhancement dependent on angiogenesis and as such gives no opportunity to relate the lesions to lobar anatomy of the breast. Conventional breast echography with orthogonal vertical and horizontal scanning is only a transcript of the mammographic findings from the radiologist’s point of view. It does not allow viewing anatomical structures, and only a limited part of the breast volume can be studied. Description of lobes, lobules, and ducts, or the localization of specific terminal ductal-lobular unit (TDLUs) groups never appears in an echography report.
As the first consequence of these observations, one could ask whether the mammary gland is the only organ of the human body which radiological images should not be interpreted relying on the knowledge of its normal anatomy. The second question is how to detect a “millimetric” (a few millimeters in size) lesion if one does not know how to search for it and where and how it develops. For these purposes, we aimed to introduce a new concept taking large-format histology sections of the breast as a model (Fig. 8.1). This concept was termed ductal echography. The accuracy and the reproducibility of the results using this approach give us a new alternative for breast imaging, improve our diagnostic skills, and allow better understanding of the lobar breast morphology and its physiologic variations.
First of all, we will underline the complexity and variability of the lobes regarding their origin, their size, and shape, raised and discussed previously in this book. The possibility of detecting “millimetric” lesions increases parallel to improved understanding of the modifications of lobar morphology at the earliest phases of breast cancer natural history. It all becomes simple if we understand where exactly the cancer could appear, how it would evolve, and which method should be used for detecting it.
After many years of utilizing ductal echography in diagnostic routine and thousands of analyzed cases, we can postulate that we have been able to enhance our diagnostic accuracy, particularly in detecting multifocal, multicentric, and diffusely growing cancers. The detection rates for these tumors are superior to those of mammography itself or mammography combined with conventional echography. Tumors can also be detected earlier this way as the stromal reaction, which facilitates the detection with mammography, is initially absent. In the meantime, it is important to recall that ductal echography is not an anatomo-pathology technique and that it does not allow detecting all the “millimetric” lesions, a few of them will still be missed.
The multimodality radiological approach combining mammography with ductal echography (and doppler sonography, elastography, 3D ultrasound images) is the best possible approach today in routine diagnosis of early breast carcinomas. MR mammography is a valuable complement to these techniques in some precisely defined indications.
8.2 Anatomical Background
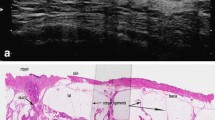
Detailed analysis of the large-format histology image in Fig. 8.2a–c gives us the basis for comparing anatomical structures with findings on ductal echography. Beginning from the surface of the skin and proceeding toward the chest wall, we will find the nipple, the areola, and the other structures of the skin (from left to right side of the image) with a thin layer of subcutaneous fatty tissue below. Next, we can see the linear connective structure of the superficial layer of the subcutaneous fascia (fascia superficialis). The typical fatty lobes are interrupted by the Cooper’s ligaments, which connect the fascia and the upper surface of the breast lobe. The lobe itself is a very well-defined structure being in connection with the nipple in the upper left part of the section, and ending up at the right margin of the image (partially represented in the image). The ductal axis and lobular groups are also visible within the lobe. The inferior surface of the lobe is bristled up by the inferior Cooper’s ligaments (giving a mirror pattern of the front lobe surface), which cross the fatty tissue on their way to the deep layer of the fascia (fascia inferior).
(a–c) Large-format histology section (a) showing a cross section of an entire breast lobe (Courtesy of Dr. Tibor Tot, Falun Sweden). Inverted (b) and original (c) ductal echography images produced with radial lobar scanning; note the perfect correlation of the echographic image and the histology image in Fig.8.2a
Our aim was to image breast anatomy in details as perfectly as this exceptional histology section. We asked whether it is possible to completely or at least partially reproduce this model with echography. Breast is an external organ particularly well suitable for ultrasound examination. However, the choice of the ultrasound technique proved to be crucial. Should we use orthogonal scanning of all the breast quadrants from the top to the bottom, from internal to external zones, or, alternatively, radial scanning around the areola with the objective of rediscovering the lobar and ductal architecture? Which ultrasound probe is best adapted to the analysis of the different lobes and to exploration of the axillary tissue?
8.3 Ductal Echography
As described for a long time ago, the breast has 15 to 20 lobes organized all around the nipple just like the daisy petals. The importance of this description remained for a long time purely theoretical as none of the imaging techniques could reveal such a disposition. The anatomical variations that the length of some of the lobes can be 14–16 cm indicated that only the use of the longest possible echography probe could image all the lobes. Since a dedicated probe of 9.5 cm has been designed, using 10 MHz frequency and an adapted water-bag, imaging of all the breast lobes has become a reality. Concentric scanning and/or a translation probe movements along the ductal axis and the axillary areas are required to image the largest lobes.
Using this approach, we can present all the lobes in equal echography projections on the same way, irrespective to the variations in their size and shape and to their localization within the breast. Indicating their position clockwise allows us to localize them very precisely (for example R.10 corresponds to the lobe at 10 o’clock in the right breast, L.6 indicates its position in the left breast at 6 o’clock).
This echographic presentation gives the opportunity to analyze breast tissue on the same way as using the large-format histology image in Fig. 8.2. Viewing from the skin toward the chest wall and from the left hand side to the right, the nipple (with some ducts within it) is seen at the upper left part of the images in Fig. 8.2b and c, the areola, and the skin right to it. The hyperechogenic fibrous tissue of the superior Cooper’s ligaments connects fascia superior and the front of the lobar surface, with a hyperechogenic small cone implantation. Likewise, the inferior Cooper’s ligaments connect the lower lobe surface, through the fatty lamina, to the inferior fascia, which itself parallels the surface of the pectoralis muscle. The lobe corresponds to a hyperechogenic area with different ductal axis visible within it, the largest one at the top of the lobe, the smallest in its depth. The lobules correspond to small hypoechogenic structures located along and mainly at the front part of the ducts.
The comparison of the histology and echography images resulted obviously in perfect matching proving that ductal echography is the method of choice among the noninvasive approaches for studying breast anatomy. No other imaging modality can generate comparable information. Getting more experienced, exploring the advantages of using appropriate equipment, and following Dr. Teboul’s teaching (Teboul 2004; Teboul and Halliwell 1995), we have become able to better understand the variations of the lobar breast morphology regarding the topographic position of the lobes, their age-related physiologic modifications, their changes under therapeutic and hormonal influences as well as under pathological conditions. We have also realized that our observations are concordant with the lobar character of breast cancer conceptualized by Dr. Tot (2007b).
8.4 Morphologic Variations of the Lobes
The sonographic variations of lobar breast anatomy are numerous, and some of them may be difficult to analyze. Using a strict and reproducible protocol allows us to visualize all the lobes with the same accuracy irrespective to their dimensions or localization, to minimize the technical difficulties, and also to slightly reduce the examination time. The protocol makes the method less dependent of the skills of the examiner; larger number of cases can be analyzed this way but the results are dependent on thorough knowledge of the anatomic variations.
There are three main echographic types of the breast lobes:
-
The mainly fibrous lobe, which is hyperechogenic and contains only very few detectable epithelial structures
-
The mainly epithelial lobe, which is hypoechogenic, with much less connective tissue
-
The intermediate lobe with approximately equal amount of epithelial and fibrous tissue
The age-related variations of the lobar morphology are also numerous with two basic extremes:
-
The young women’s breast lobes rich in glandular tissue, with minimal amount of fatty tissue (illustrated in Fig. 8.3), and
-
The adult type breast lobe of women with a balance between the amount of parenchyma and fat (illustrated in Fig. 8.4)
Most of the lobes undergo involution, which is only partial in premenopausal women (Fig. 8.5) and more advanced in postmenopausal women. With progression of the involution, structures of the lobe may disappear leaving behind delicate residual connective structures, the lobar “skeleton” (illustrated in Fig. 8.6) or may be totally lost. As underlined above, the lobes also vary in their size and topographic localizations. The lobes in the medial quadrants of the breast and in the lower quadrants are smaller, the largest are the lobes in the upper outer quadrants; some of them may reach the axilla.
Variations also occur regarding the origin of the lobes. The largest lobes are first to develop during the adolescence in the upper outer quadrants, while the smaller ones in the medial inner quadrants appear later during the young women’s life. On the opposite, the smaller lobes are the first to disappear during and after the menopause, while those developing earlier located in the upper outer quadrants remain active for a longer time.
Additional variations can be observed regarding the orientation and the distribution of the periareolar lobes. Most of them are well orientated all around the nipple in radial fashion, but some of them have a circuitous way of approaching the nipple and they may overlap each other. This partial superposition of some of the lobes may give the false impression of existence of interlobar connections (anastomoses), and may lead to over- or underestimation of the real dimensions of the observed lobe. It is also difficult to separate the lobes within the retro-areolar area because of their short connection to the nipple. However, studying the mammary gland in adolescents, the partially involuted lobes in postmenopausal women, or in male gynecomastia, demonstrates that the lobes are totally independent of and well separated from each other and represent individual units.
Ductal echography represents an ideal tool of visualizing lobar breast anatomy; however, the normal ducts and lobules are hardly visible because of their small size. Proliferation of the epithelium inside the ducts and lobules causes local or diffuse distension as well as distortion of these structures, and modifies their acoustic impedance. Then they become “echo-detectable”: The hypoechogenic structures correspond to the luminal content of ducts and lobules; the walls of these structures remain invisible. Pathological processes lead to echographic changes that replace the echographic signs of the epithelial proliferation.
8.5 Lobar Implications in Mammary Pathology
The major target in ducto-radial echography is the modification of the normal patterns inside the ductal and lobular structures as well as in the surrounding connective tissue elements of the lobe. Nakama (1991) described the migration of the malignant cells accompanied by lymphocytes, histiocytes, and fibroblasts toward the skin inside the Cooper’s ligaments and fascia superficialis. These connective structures are then involved in cancer development at an early stage. (We illustrated the concept with a drawing in Fig. 8.7) This publication confirms Dr. Gallager’s conclusions in his article published in December 1969, particularly his conclusion number 2 that “the supportive connective tissue of the breast is also affected by carcinogenic agent” (Gallager and Martin 1969). Teboul (2004); Teboul and Halliwell (1995), as well as Stavros (2006) have also described involvement of the connective tissue and ligaments in cancer development at early stages. They have also underlined the multifocal nature of breast cancer in certain cases. Thanks to the precise echographic anatomy background, Teboul (2004) identified the specific ducto-lobular terminal unit groups, their localization as well as their involvement in the initial steps of the pathological alterations. As he stressed out, the cross section of the ductal axis and the axis of the ligament is the key zone where these groups are located and where our attention should be focused if aiming to study the early pathological modifications of the epithelial structures and of the connective tissue.
These observations are essential in the history of breast echography: radial echographic sections, lobar analysis, search for ductal axis, identification of ligamental ways, and analysis of fascia and skin element alterations. Understanding the origin of the lesion and their development is essential for this analysis. The concept of ductal echography has radically changed the way of examination of the breast. One should not focus on to discover the lesion(s) itself using systematic orthogonal echographic projections in different breast quadrants. Instead, the observer should carry out a lobar analysis (according to the well-established protocol above). The lesions are then usually detectable within the expected structures, within the expected areas, and their development will follow the expected pattern.
The abnormalities should be studied in comparison to the normal lobar anatomy. All the epithelial lesions (both anechogenic/liquid and hypoechogenic/solid) are linked either to a duct, or to a lobule: for example, to a duct in case of ductal ectasia/dilatation and papillomas and to lobules in supraductal alterations. Differentiation between ductal and lobular microcysts will become possible this way. Intralobular localization of fibroadenomas will also become evident. Overlapping of many small fibroadenomas developed in lobules close to each other explains the lobulated silhouette of larger fibroadenomas. Similarly, detection of ductal dilatation due to epithelial proliferation will be possible routinely as well as discovering multifocal lesions near the specific TDLUs. It is crucial to underline that the indirect ligamental signs, often associated with cancer, are absent in benign lesions. These delicate early echographic signs precede the radiological signs related to stromal reaction and, of course, they also precede the clinical signs.
A typical case is demonstrated in Figs. 8.8a–d. The initial diagnosis of breast cancer has been made with mammography regarding a palpable nodule. Echographic examination and MR mammography detected five additional foci of invasive carcinoma. The multifocal and multicentric character of the tumor indicated mastectomy, which was analyzed by Professor Di Marino (Anatomic Laboratory, Marseille Medical University). The specimen was also documented using a large-format histological section by Dr. Rojat-Habib on the Pathology Department of the same University. The pathohistological section corresponded to a 10 cm long radial echography sections. The comparison of the histology section and the echography sections reveals a perfect correlation between these two techniques. Analysis of the different ductal axis shows the signs of duct ectasia. At the distal part of the lobe, two cancer foci are seen (one centimetric and the other millimetric), localized in connection to the Cooper’s ligaments. Some ligaments are difficult to study at histology because they are partially represented in the very thin section. Additional tumor foci were detected in other cutting levels, not shown in Fig. 8.8. Like mammography, ductal echography allows a quick, precise, and cheap analysis of multifocal and multicentric lesions. The method allowed a real progress in diagnosing early breast cancer.
8.6 Multifocality, Multicentricity, and Diffuse Lesions
Breast radiology has imaged breast carcinoma for decades like a lesion being most often unifocal and sometimes multifocal. Multifocal and multicentric lesions were distinguished on the basis of the distance between two foci (4 cm). With introduction of ductal echography, a new definition of multiple lesions has become possible: Multifocal cancers should now be defined as those developing within the same lobe along the ductal axis, while multicentric cancers develop in different lobes (they can also be solitary or multifocal). This definition is in agreement with histologic studies.
Figures 8.9a and b illustrate a unifocal breast cancer. Multifocal cancer correspond to hypoechogenic foci, taking place along the ductal axis (picture of a string of pearls), located in the specific TDLUs (Figs. 8.10 and 8.11). Their size depends on their age, and they are more or less associated with indirect anatomic (ligamentary) or echographic (posterior absorption shadow) signs. Although identifying many foci, ductal echography may still underestimate their real number because the smallest ones (most recently appeared) could possibly not be seen. Discovering multiple cancers has become possible through technology progress and practicing using fully digital machines with dedicated probes and through improvement in our way of examination followed by increase of our knowledge.
The rate of detecting multifocal and multicentric breast cancer cases started with very low values to reach 10–20% a few years ago and to get up to as impressive numbers as 45% nowadays (Tot 2007a). These numbers were surprising, as we were so far away from them in our classical views regarding breast carcinoma. However, the numbers are real as they have been confirmed by other ductal echography specialists and, most of all, by breast pathologists. It seems to us that the “sick lobe theory” (Tot 2007b) reflects the reality we daily observe “in vivo.”
Diffuse cancers represent a separate and special group of lesions (Tot 2003). They are difficult to detect and very difficult if not impossible to be differentiated from epithelial proliferation zones. Their characterization often represents the limits of this method and may result in failure of diagnosing (Fig. 8.12).
8.7 Surgical Aspects
The end-result of breast mammographic-echographic examination should be a report giving the maximum of information considering the presence of abnormalities, the biological nature of the abnormalities, their number, and their exact location. A precise mapping of the lesions (with an exact clockwise localization) will allow the therapists to choose the best treatment. Notably, eventual recurrences are not the surgeons’ responsibility if the preoperative diagnostic information was suboptimal. The role of the diagnostician is crucial and the information he or she generates may have serious consequences. Surgeons such as Professor Dolfin (Dolfin et al. 2008) or Professor Durante (2006) have been able to develop a specific surgical procedure of lobectomy technique thanks to this background of ducto-radial echography (with pre- and postoperative but also, if necessary, peroperative control examinations). This is an absolutely innovating way of breast surgery. Close collaboration of radiologists, surgeons, oncologists, therapeutics, and the other members of the breast team is mandatory to achieve the best results.
8.8 Conclusion
Ductal echography represents in our opinion an irreplaceable innovation and a major progress in breast carcinoma diagnosis. The concept is based on analyzing lobar anatomy, understanding the morphologic and physiologic variations of the lobes and understanding the appearance and natural history of benign and malignant pathological lesions within the breast structures. The perfect correlation between the echographic and histologic observations is the best proof of the many advantages of ductal echography. Highlighting each lobe individually allows appropriate analysis (in contrast to the conventional echography looking at single lesion inside a quadrant). In our experience, the number of the lobes in most of the breasts is 12 to 15 (less than the 15 to 20 originally described by Going and Mohun 2006). Particular attention should be paid to specific TDLUs and the zones of their localization as this is a very important phase of examination. Subtle alterations in connective tissue structures (seemingly accessories, like the Cooper’s ligament, the fascias, and the subcutaneous elements) allow early detection of breast carcinoma hardly ever seen with conventional methods (Amy 2005; Amoros et al. 2009).
The modern echographic approach still remains limited because of the limited probe resolution as well as the availability of special echographic machines. Nevertheless, all the thousands of the lobules within the breast can never ever be properly examined. But the fact that those of them showing the signs of epithelial proliferation or pathological alterations are currently detectable seems to us to be satisfactory. Histological confirmation we had in more or less half of the examined cancer cases, also multifocal or multicentric, is fundamental. Further development is needed for accurate differentiation of breast cancer subtypes, like ductal, lobular, and mixed ducto-lobular.
It is debatable whether we will ever be able to detect the earliest changes in cancer development using echography, but we are already able to observe alterations of the normal morphological constituents of the breast during the disease. Multifocality/multicentricity is often explained by migration of the malignant cells, a phenomenon that cannot be echographically followed; however, observing lesions of different size and age within the same lobe may be a consequence of local cell migration (Fig. 8.13). Finding early bifocal or multifocal cancer foci separated by a distance of several centimeter within a single lobe (Figs. 8.10 and 8.11.) as well as the presence of lesions of the same size within the same and/or in different lobes leads us to the conclusion that multiple malignant foci may develop independently from each other as a result of simultaneous and/or asynchronous malignant process.
(a–d) Echography image (a) and inverted echography image (b) illustrating a sick breast lobe with a large initial cancer and several additional new foci on both sides of the ductal axis. Elastography score 4 (c) and Doppler positivity (d) in each of the new multiple millimetric foci within the distal part of the same lobe
The entire new concept presented in this chapter and in this book opens interesting perspectives of surgical and therapeutic character: multifocal, progressive, and extensive lobar involvement should not be treated the same way as a unifocal cancer. The therapeutic strategy should be different at the very early stage of breast cancer development and in the advanced stage, as well.
The diagnostic improvements achieved with introduction of ductal echography, with using large-format histology section, performing controlled breast lobectomy in surgery, introducing new molecular targets in oncology, or peroperative contact radiotherapy and cryotherapy will only be successful if the synergic effects between all these modalities continue. This interdisciplinary approach is more important than ever before. The progress of all this disciplines depends on improvements of the others.
Regarding echography, new equipment (digital machines, new generation of dedicated probes Doppler echography, elastography, three-dimensional reconstruction) have allowed the best of diagnostic performances. But it is also crucial to have dedicated and competent personnel continuously. Ductal echography has become a necessity in specialized breast centers. This is the way and the price of being able to give our patients the best chances for their recovery.
References
Amoros J, Dolfin G, Teboul M (2009) Atlas de Ecografia de la Mama. Ananke, Torino
Amy D (2005) Millimetric breast carcinoma ultrasonic detection. In: Leading Edge conference Pr. Goldberg B. USA
Dolfin G, Chebib A, Amy D, and Tagliabue P (2008) Carcinome mammaire et chirurgie conservatrice. 30e Seminaire Franco-Syrien d’Imagerie Médicale. Tartous, Syrie
Durante E (2006) Multimodality imaging and interventional techniques. IBUS Course Abstracts, Ferrara, Italy
Gallager HS, Martin JE (1969) Early phases in the development of breast cancer. Cancer 24:1170–1178
Going JJ, Mohun TJ (2006) Human breast duct anatomy, the ‘sick lobe’ hypothesis and intraductal approaches to breast cancer. Breast Cancer Res Treat 97:285–291
Nakama S (1991) Comparative studies on ultrasonogram with histological structure of breast cancer: an examination in the invasive process of breast cancer and the fixation to the skin. In: Kasumi F, Ueno E (eds) Topic in breast ultrasound. Shinohara, Tokyo
Stavros T (2006) Breast ultrasound. Lippincott, Philadelphia
Teboul M, Halliwell M (1995) Atlas of ultrasound and ductal echography of the breast. Blackwell Science, Oxford
Teboul M (2004) Practical ductal echography. Medgen, S.A. Madrid, Spain
Tot T (2003) The diffuse type of invasive lobular carcinoma of the breast: morphology and prognosis. Virchows Arch 443:718–724
Tot T (2007a) Clinical relevance of the distribution of the lesions in 500 consecutive breast cancer cases documented in large–format histologic sections. Cancer 110:2551–2560
Tot T (2007b) The theory of the sick breast lobe and the possible consequences. Int J Surg Pathol 1:68–71
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer London
About this chapter
Cite this chapter
Amy, D. (2010). Lobar Ultrasound of the Breast. In: Tot, T. (eds) Breast Cancer. Springer, London. https://doi.org/10.1007/978-1-84996-314-5_8
Download citation
DOI: https://doi.org/10.1007/978-1-84996-314-5_8
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-84996-313-8
Online ISBN: 978-1-84996-314-5
eBook Packages: MedicineMedicine (R0)