Abstract
Percutaneous coronary intervention (PCI) with stent deployment has become a mainstay in the treatment of patients with coronary artery disease. These metallic endoprostheses prevent abrupt artery closure, the major drawback of PCI in the era of transluminal balloon angioplasty. However, the recurrent arterial narrowing at the site of intervention (restenosis) resulting from excessive smooth muscle cell proliferation is a major shortcoming in 15–30% of patients receiving a bare metal stent. The recent introduction of drug-eluting stents that locally deliver high doses of antiproliferative drugs, such as sirolimus and paclitaxel, has revolutionized the field of revascularization owing to a dramatic reduction in the incidence of in-stent restenosis, target lesion revascularization, and major adverse cardiac events. They are, however, limited by an increased risk of late stent thrombosis that forces a long-term oral dual antiplatelet therapy. A key factor contributing to late stent thrombosis after implantation of drug-eluting stents seems to be the apparent incomplete reendothelialization due to the cytostatic and cytotoxic effects that the active drugs exert on the underlying and neighboring endothelial cells. Here we review therapeutic strategies to limit neointimal cell proliferation in animal models of vascular injury; summarize some of the drug-eluting stents that are currently in clinical use; and discuss new approaches under investigation to optimize the efficacy and safety of this technology to improve PCI outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Percutaneous Coronary Intervention
- Major Adverse Cardiac Event
- Bare Metal Stents
- Target Lesion Revascularization
- Neointima Formation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
A Brief Overview of Stenting
Percutaneous coronary intervention (PCI) has become the most widely used strategy for the treatment of patients with coronary artery disease since its introduction by Grüntzig et al. in 1977 [1]. Angioplasty was plagued with multiple problems in the balloon catheter era, including acute collapse and dissection of the treated artery and recurrent luminal obstruction (restenosis), a pathological process that forced target vessel revascularization in 25–50% of cases, typically within 2–12 months post-PCI. A second revolution in the field of interventional cardiology materialized with the introduction of balloon-mounted stents, which consist of a self-expandable stainless-steel mesh that acts as a scaffold that maintains radial support to neutralize elastic recoil. Palmaz and colleagues introduced in 1985 the use of bare metal stents in peripheral arteries of dogs [2]. Schatz et al. then developed the first commercially successful stent, the Palmaz-Schatz stent [3]. In 1987, Sigwart et al. provided the first evidence that implantation of bare metal stents in patients with iliac, femoral, and coronary artery disease may offer a safe and useful way to prevent subacute occlusion and dissections and limit the occurrence of restenosis [4]. Following these pilot studies, the first Palmaz-Schatz stent was approved for use in the USA, and different bare metal stents platforms developed over the next decade confirmed the benefits of stenting compared with conventional transluminal balloon angioplasty, leading to the era of elective stenting. However, restenosis after base metal stents implantation still affected about 15–30% of patients, causing in the Western world an estimated annual cost exceeding $1 billion USD. After the identification of excessive proliferation of vascular smooth muscle cells (VSMCs) as a key feature of experimental and clinical neointimal thickening postangioplasty and the results of numerous animal studies demonstrating the utility of antiproliferative strategies to prevent this pathological process (reviewed in [5, 6]), a new era in interventional cardiology began nearly a decade ago with the advent of drug-eluting stents (also referred to as “coated” or “medicated” stents) that locally deliver high doses of antiproliferative drugs. Pilot studies using the sirolimus-eluting Bx Velocity™ stent demonstrated negligible neointimal thickening at follow-up [7, 8]. The superior performance of several drug-eluting stents platforms versus bare metal stents has been irrefutably confirmed in large multicenter clinical trials demonstrating dramatic reductions in restenosis rates, in target lesion revascularization, and in major adverse cardiac events [9]. Although the initial clinical trials with drug-eluting stents did not report significant adverse effects, recent case reports in real life patients have recognized an increased risk of late stent thrombosis potentially due to a mismatch between the vessels and the stent (late stent malapposition), hypersensitivity, or incomplete reendothelialization consider changing to “due to” to the cytostatic and cytotoxic effects that the active drugs exert on the underlying and neighboring ECs or the proinflammatory effects of the biostable polymeric coatings (Table 8.1) [9, 14]. The current clinical guidelines therefore recommend prolonged potent antiplatelet and antithrombotic adjunctive therapies in patients receiving drug-eluting stents. Because of these shortcomings, further research is essential in order to improve the long-term safety and efficacy of drug-eluting stents.
Etiopathogenesis of In-Stent Restenosis and Cell Cycle Control in Mammalian Cells
Stenting can result in acute damage to the endothelial cell (EC) monolayer, triggering a chronic inflammatory response that may promote exuberant neointimal hyperplasia (Fig. 8.1) [5, 15]. Localized platelet activation and thrombosis accompanied by recruitment of circulating monocytes, neutrophils, and lymphocytes into the intimal area characterize the acute early phase of restenosis. Numerous chemotactic and mitogenic factors produced by neointimal cells provoke a first hyperplastic response of medial VSMCs, which migrate toward the growing neointimal lesion where they maintain high proliferative activity. Compared with VSMCs in normal adult arteries, which are fusiform and display a differentiated so-called contractile phenotype characterized by reduced proliferative activity and motility, activated VSMCs within the injured vessel wall exhibit an undifferentiated “synthetic” phenotype characterized by broader and flatter shape, expression of embryonic isoforms of contractile proteins, high responsiveness to growth and chemotactic stimuli, and abundant synthesis of extracellular matrix components. Accumulating evidence indicates that recruitment of bone marrow-derived and adventitial VSMC progenitors and adventitial myofibroblasts also plays a role in neointimal lesion development, but the relative contribution of this phenomenon to restenosis remains unclear [15]. At later stages, resolution of inflammation is associated with restoration of the “contractile” phenotype of neointimal VSMCs and normalization of the composition of the extracellular matrix, which more closely resembles the undamaged vessel wall. Consistent with the complexity of restenosis, numerous animal and human studies have identified a plethora of candidate regulators of neointimal hyperplasia, including signal transduction pathways (e.g., MEK/ERK and PI3K/Akt signaling cascades), transcription factors (e.g., AP-1, YY1, Gax, NF-κB, E2F, c-myb, c-myc), growth factors (e.g., PDGF, FGF, TGFβ, VEGF, IGF, EGF), inflammatory cytokines (e.g., TNFα), chemotactic factors (e.g., MCP-1, CCR2), thrombogenic factors (e.g., thrombin receptor, tissue factor), cell adhesion molecules (e.g., VCAM, ICAM, Mac-1, LFA-1), metalloproteases (e.g., MMP-2, MMP-9), and cell cycle regulatory proteins (e.g., CDK2, CDC2, cyclin B1, PCNA, pRb, p27, p21).
Mechanisms of in-stent restenosis. The left and right images show cross sections through a stented artery immediately after intervention and at a late time point showing excessive neointimal lesion development, respectively. The scheme between both images represents a longitudinal section through the vessel wall (for simplicity, neither the native atherosclerotic plaque that compromised blood flow before performing angioplasty nor the stent struts are depicted). Platelets are recruited into the damaged vessel wall and provoke thrombi formation. Blood-borne leukocytes adhere to thrombi via selectins and integrins and, driven by locally produced chemokines, they migrate across the fibrin-platelet layer toward the intimal area. Medial VSMCs exhibiting a differentiated so-called contractile phenotype revert to a “synthetic” less-differentiated phenotype characterized by abundant extracellular matrix protein synthesis and high responsiveness to mitogenic and migratory stimuli. Activated VSMCs migrate toward the growing neointimal lesion and proliferate very actively, thus contributing to neointimal thickening
Neointimal hyperplasia following PCI can thus be viewed as the arterial wall’s healing response to acute mechanical injury, which encompasses excessive hyperplastic growth of VSMCs. The proliferation of mammalian cells requires a series of sequential events that constitute the mitotic cell cycle (Fig. 8.2). Under normal conditions, most differentiated cells are maintained in a nonproliferative state (G0 phase). After stimulation with growth factors, cells enter the first gap phase (G1), during which proteins necessary for DNA replication are synthesized and/or activated. In the subsequent synthesis phase (S) the DNA is replicated, then cells enter a second gap phase (G2) that allows the synthesis and activation of proteins required for mitosis (M phase). Cell cycle progression is orchestrated by the activation of various holoenzymes composed of the regulatory subunit cyclin and a catalytic cyclin-dependent protein kinase (CDK). Several mechanisms sequentially activate distinct CDK/cyclin complexes during different phases of the cell cycle, including the periodic synthesis and degradation of cyclins and the phosphorylation/dephosphorylation of CDKs and cyclins. Another important level of cell cycle regulation is the inhibition of CDK/cyclin holoenzymes through their interaction with CDK inhibitory proteins (CKIs) of two families: Cip/Kip (CDK interacting protein/kinase inhibitory protein: p21Cip1, p27Kip1, p57Kip2) and Ink4 (inhibitor of CDK4: p16Ink4a, p15Ink4b, p18Ink4c, p19Ink4d) [16]. Cip/Kip proteins bind to and inhibit many CDK/cyclin complexes, while Ink4 proteins specifically interact with and inhibit cyclin D-associated CDKs (Fig. 8.2). The rates of synthesis and degradation of CKIs, as well as their redistribution among different CDK/cyclin heterodimers are modulated by mitogenic and antimitogenic stimuli. The tumor suppressor p53 and other proteins modulate CKI expression and function to ensure that cell cycle progression is halted if environmental conditions are not appropriate and/or cells accumulate genetic damage. CDK/cyclin activity regulates E2F/DP- and retinoblastoma protein (pRb)-dependent transcription of target genes involved in cell cycle control and DNA biosynthesis (Fig. 8.2). In nonproliferating cells, low CDK/cyclin activity keeps pRb in its hypophosphorylated form, which binds to and inactivates the dimeric transcription factor E2F/DP. In contrast, high CDK/cyclin activity in proliferating cells causes the accumulation of hyperphosphorylated pRb during late G1-phase, thus leading to the release of E2F/DP and transactivation of various target genes necessary for cell cycle progression.
Cell cycle regulation in mammalian cells. Activation of specific CDK/cyclin complexes drives progression through the different phases of the mammalian cell cycle (G1 Gap 1, S synthesis of DNA, G2 Gap 2, M mitosis). Advance through G1/S is orchestrated by CDK/cyclin-dependent hyperphosphorylation of pRb, which allows the transcriptional activation of E2F/DP target genes that are required for cell proliferation. CDK inhibitory proteins (CKIs) of the Cip/Kip and Ink4 families interact with and inhibit the activity of CDK/cyclin holoenzymes. Cip/Kip proteins bind to and inhibit a wide spectrum of CDK/cyclins, while Ink4 proteins are specific for cyclinD-associated CDKs. CDK1 is also known as CDC2
MicroRNAs (miRNAs) represent an additional layer of the complex regulatory network that controls cell cycle progression, and evidence is accumulating that they may be of particular therapeutic interest in the context of pathological vascular remodeling [17]. For instance, miRNA-221 and miRNA-222 have been shown to limit VSMC proliferation by targeting the CKIs p27Kip1 and p57Kip2, and the growth-factor receptor c-Kit [18, 19]. Importantly, knockdown of these microRNAs inhibits arterial cell proliferation and neointimal formation in the rat carotid artery injury model [19].
Pharmacological Antiproliferative Strategies to Limit Neointimal Thickening After Mechanical Injury of the Vessel Wall
The recognition that excessive VSMC proliferation is a hallmark of restenosis postangioplasty in animal models and humans has fueled extensive research into the molecular mechanisms that control the cell cycle in these cells in vitro and in vivo. Moreover, numerous preclinical studies have been conducted to assess whether antiproliferative strategies are efficient at limiting neointimal lesion development, including gene therapy and drug-based approaches. Although gene therapy targeting cell cycle regulatory factors (e.g., inhibition of positive cell cycle regulators and overexpression of growth suppressors) has shown undisputable therapeutic efficacy in animal models of restenosis [5, 6], its clinical use awaits the overcoming of current limitations of gene therapy in humans. We have therefore focused our discussion on drug-based strategies that limited neointimal lesion development in animal models of angioplasty, some of which have demonstrated clinical benefits when administered in drug-eluting stent platforms.
Animal models are critical to provide mechanistic insight into neointimal thickening associated with balloon angioplasty and stenting, and to establish safety margins, efficacy, and toxicity [20–22]. The rat carotid model of balloon angioplasty has been extensively used to gain insight into the molecular mechanisms that provoke neointimal thickening induced by mechanical injury; however, the porcine and the rabbit models are considered standard for the evaluation of drug-eluting stents prior to human use [20–22]. Nevertheless, there are shortcomings associated with animal models that limit their biological significance. Ideally, drug-eluting stents should be tested in atherosclerotic arteries to more closely resemble the clinical situation; however, preclinical studies are generally performed in atherosclerosis -free vessels. Moreover, neointimal responses associated with stent deployment are exaggerated in pigs and rabbits, and the time course of healing is reduced compared to humans (about 4–6 weeks in swine and rabbits compared to roughly 6–9 months in humans). It is also noteworthy that the rabbit model does not offer the possibility of coronary stenting due to its anatomical size; thus, the aorta or the iliac arteries are used for stent placement in rabbits. Albeit the site of stenting can be considered as a critical limitation of the rabbit model, it resembles more closely than the pig the healing process observed in humans and is therefore widely used to examine inflammatory, proliferative, and thrombotic processes subsequent to vascular injury and stenting [20].
Inhibitors of Mammalian Target of Rapamycin
The mammalian target of rapamycin (mTOR) protein is a member of the phosphoinositide 3-kinase (PI3K)-related proteins kinases (PIKK) family that forms the catalytic subunit of two different complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) [23, 24]. Signaling through the mTOR pathway links cell cycle activity with energy and nutrient availability and therefore plays a key role in maintaining homeostasis. A potent inhibitor of mTOR is rapamycin (also known as rapamune, sirolimus), a fungal macrolide produced by Streptomyces hygroscopicus that impairs mTOR complex assembly via sequestration of the intracellular receptor FKBP12 [23, 24]. Treatment of VSMCs with sirolimus upregulates p27kip1, inhibits pRb phosphorylation, and limits cell proliferation and migration in vitro [25–29]. These findings are in agreement with the observation that the p27kip1/CDK/pRb pathway regulates VSMC proliferation and migration in a coordinated manner [30, 31].
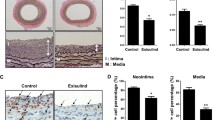
Preclinical studies in different animal models have demonstrated the utility of sirolimus to limit neointimal thickening induced by arterial injury. Oral or intramuscular application of sirolimus reduces neointimal thickening in porcine and rat balloon injury models [32–34]. As in cultured VSMCs [28, 29], the reduction in neointimal proliferation observed in the porcine coronary model is associated with increased p27kip expression and reduced pRB phosphorylation [33]. Sirolimus eluting-stents have also demonstrated protection against neointimal thickening in porcine [35–37], rabbit [38], and rat [39] models. Likewise, Pires and colleagues reported that sirolimus-eluting cuffs placed around the femoral artery significantly reduce intimal thickening in both normocholesterolemic wild-type mice [40] and atherosclerotic hypercholesterolemic apoE*3-Leiden transgenic mice [41] with no systemic adverse effects or effect on cuffed contralateral femoral arteries. However, evidence has been presented demonstrating that sirolimus has unfavorable in vitro and in vivo effects on ECs. Barilli et al. demonstrated that prolonged treatment of human ECs with sirolimus impairs cell viability (increased apoptosis and necrosis) and function (reduced proliferation and mobility and increased actin stress fiber formation), possibly through mTORC2 inhibition [42]. Suppression of reendothelialization and revascularization by sirolimus also correlates with increased EC mortality via apoptosis and autophagy [43], a process activated in response to cellular damage and nutrient deprivation that mediates the degradation of cellular components in lysosomes [44]. Using a porcine model of epicardial coronary artery stenting, Frey and colleagues noted delayed vascular healing (endothelialization) with slow-release sirolimus-eluting stents compared with bare metal stents and extended-release sirolimus-eluting stents [45]. Sirolimus treatment might also delay reendothelization through induction of endothelial progenitor cell senescence, possibly due to increased expression of p27kip and inactivation of telomerase [46]. Moreover, sirolimus suppresses the coordinated proadhesive and proinflammatory gene expression that normally occurs in renal artery segments subjected to mechanical injury, which in turn may reduce the recruitment of leukocytes and hematopoetic progenitor cells that participate in vascular healing [47]. These adverse effects of sirolimus on mature ECs and endothelial progenitors might contribute to increased risk of late stent thrombosis in patients receiving sirolimus-eluting stents.
Several sirolimus derivatives have been developed with the goal of optimizing mTOR inhibitory therapies. Everolimus exhibits a shorter half-life and reduced unwanted side-effects compared with sirolimus if delivered systemically, yet both drugs elicit similar protection against neointimal formation in a porcine coronary artery model [48, 49]. Zotarolimus exhibits increased retention in the arterial wall and reduces neointima development after stent deployment in a porcine coronary artery model [50]. Finally, compared with sirolimus-eluting stents, a polymer-free stent coated with the sirolimus analog biolimus A9 has demonstrated equivalent early and superior late reduction of intimal proliferation in a porcine model [51]. Remarkably, delayed arterial healing with biolimus A9 was minimal, and there was no increased inflammation at 180 days compared with implantation of sirolimus-eluting stents.
Taxanes
Taxol (paclitaxel) is a microtubule stabilizing drug that impairs centrosomal function, induces abnormal mitotic spindles, and suppresses spindle microtubule dynamics during mitosis causing G2/M-phase arrest [52]. In vitro treatment of VSMCs with paclitaxel upregulates both p53 and its downstream target p21Cip1, and also disorganizes cytoskeleton structures and increases apoptosis. These effects are associated with a significant inhibition of processes that promote restenosis, including cell proliferation and migration and extracellular matrix production [27, 53–55]. Accordingly, oral paclitaxel treatment markedly reduces neointimal lesion formation after rat balloon angioplasty without causing significant toxicity [53, 56], and local delivery of paclitaxel to the balloon angioplasty site in rabbit carotid artery disorganizes microtubules and inhibits neointimal thickening [57]. Likewise, studies in the porcine coronary artery model have demonstrated long-term effects of paclitaxel-eluting stents [58, 59]. However, the cytotoxic effects of paclitaxel may partly explain its reduced long-term efficacy and safety compared with sirolimus [60]. Wessely and colleagues demonstrated that both drugs efficiently block VSMC proliferation, but paclitaxel has more deleterious effects on ECs, such as more potent antiproliferative and proapoptotic activities [27]. Moreover, paclitaxel-eluting cuffs placed around the femoral artery effectively prevent neointimal thickening on the atherosclerotic plaques of hypercholesterolemic apoE*3-Leiden transgenic mice, but high concentration demonstrated adverse vascular pathology and transcriptional responses (e.g., increased mRNA level of the proapoptotic factors FAS, BAX, and caspase 3), suggesting a narrower therapeutic range of this drug [41]. Given the high cytotoxicity of paclitaxel, major efforts are underway to improve the safety of this drug while maintaining therapeutic benefits. Such strategies include programmable drug release [61], addition of paclitaxel to contrast media [62], or drug coating of the angioplasty balloon rather than the stent [63].
Estradiol
Estradiol, the most abundant sex hormone in humans, has numerous effects in vascular cells, including modulation of cell proliferation and migration, which are for the most part mediated by its binding to the estrogen receptors α and β [64, 65]. Upon binding of estradiol, these intracellular receptors act as transcription factors that modulate the expression of a large number of genes [64]. In cultured cells, estradiol inhibits VSMC proliferation and migration and, conversely, promotes EC proliferation [66]. Mechanistically, the effects of estradiol appear to be mediated by changes in the activity of various signaling proteins, including the mitogen-activated protein kinases p38 and ERK1/2 [66–68] or the GTPase Rac1 [69]. Therefore, estradiol may be effective at preventing vascular restenosis after arterial injury with low risk of late stent thrombosis, as it would be predicted to enhance reendothelialization. Supporting this notion, a number of preclinical studies have demonstrated the protective function of this hormone against vascular injury. For example, systemic delivery of estradiol in rodents and rabbits accelerates reendothelization after vessel denudation [70–73], reduces neointimal thickening in the injured carotid artery [74–81] and aorta [82], and inhibits VSMC proliferation in vivo [75, 76, 82, 83]. Similarly, cathether-mediated local delivery of estradiol in balloon-injured porcine coronary artery reduces VSMC proliferation and neointimal thickening [84], and estrogen-coated stent implantation reduces neointimal formation in a similar porcine model [85]. These preclinical studies demonstrate that both systemic and local delivery of estradiol prevent adverse vascular remodeling after arterial injury and provide rationale for the assessment of the therapeutic potential of estradiol-eluting stents in humans.
Other Drugs
Based on reported capacity to inhibit VSMC proliferation and neointima formation in different animal models of vascular injury, other drugs might prove effective at inhibiting clinical restenosis. For example, the 3-hydroxi-3-methilylglutaryl-CoA (HMG CoA) reductase inhibitor cerivastatin is one of the most promising compounds owing to its pleiotropic effects, which include inhibition of cell proliferation and improvement of EC function [86]. Preclinical assessment in a rat carotid model has revealed that cerivastatin-eluting stent deployment limits neointima formation [87]. Treatment of VSMC cultures with cerivastatin increases p21Cip1 and p27Kip1 levels, downregulates cyclin A and D1, and decreases CDK2 activity and pRb phosphorylation, leading to reduced cell proliferation, and all these effects of cerivastatin are less pronounced in ECs [87]. Therefore, local application of statins might limit restenosis while decreasing the risk of late stent thrombosis associated with defective reendothelialization.
Another compound of potential therapeutic interest in the setting of restenosis is flavopiridol, a synthetic CDK inhibitor that induces VSMC growth arrest in parallel with increased levels of the growth suppressors p21Cip1, p27Kip1, and p53 and decreased accumulation of hyperphosphorylated pRb [88, 89]. Accordingly, both oral and stent-mediated administration of flavopiridol significantly reduce neointima formation after rat carotid injury [88, 89].
Some antioxidants, such as carvedilol and probucol, have also demonstrated strong antiproliferative properties in the arterial wall. Oral treatment with carvedilol inhibits VSMC proliferation and blunts neointima formation in the rat carotid injury model [90, 91], and carvedilol-coated stents inhibit neointima hyperplasia in pigs [92]. In contrast, the results with the related antioxidant probucol are conflicting. On one hand, oral administration of probucol in rabbits decreases neointima formation and the number of lesional proliferating VSMCs in balloon-injured carotid artery [93] or abdominal aorta [94], and some studies suggest that probucol also promotes reendothelization [94, 95]. However, other studies do not find any protective effect of probucol against neointimal thickening following balloon angioplasty in the rat carotid artery [96] or stent deployment in porcine coronary artery [97]. Similarly, probucol-coated stents fail to demonstrate beneficial effects in lumen area, neointimal area, or arterial cell proliferation in a porcine coronary injury model [92].
Cilostazol is a novel and potent inhibitor of phosphodiesterase in platelets and VSMCs that exerts both antithrombotic and antiproliferative properties, and is therefore a promising therapeutic candidate in the setting of restenosis. Cilostazol inhibits mitogen-induced VSMC proliferation by increasing the concentration of cyclic adenosine monophosphate [98], resulting in activation of the p53-p21Cip1 axis [99]. Notably, oral cilostazol treatment inhibits neointima formation in the rat carotid balloon angioplasty model [100], and cilostazol-coated stents reduce neointimal thickening in porcine coronary arteries [101].
Some antidiabetic drugs have also demonstrated their effectiveness at reducing adverse vascular remodeling. Thiazolidinediones (e.g., rosiglitazone, pioglitazone) are peroxisome proliferator-activated receptor γ [PPAR-γ] agonists originally developed as insulin sensitizers, but also exhibit vascular protective properties. For example, among other beneficial effects in the arterial wall, thiazolidinediones inhibit VSMC proliferation via ERK inactivation and induction of GSK-3β-dependent signaling [102]. Studies in rodents have demonstrated that rosiglitazone treatment prevents neointimal thickening after mechanical injury of the carotid artery [102–104]. Similar beneficial effects of thiazolidinediones have been observed in balloon injury [105] or stent implantation [106] rabbit models, and stenting in porcine coronary arteries [107].
Tranilast is an inhibitor of TGF-β-dependent signaling that attenuates VSMC proliferation in vitro [108–111] by a complex mechanism that involves inhibition of ERK1/2 [110], downregulation of the transcription factor c-myc [109], and upregulation of p21Cip1 [112]. Studies in rabbits and rodents have demonstrated that oral administration of tranilast reduces neointimal growth after photochemical or balloon injury of the arterial wall [113–116], and similar results have been obtained in pigs after coronary artery stenting [117, 118].
Antiproliferative Strategies for the Treatment of Clinical Restenosis Using Drug-Eluting Stents
PCI is the preferred therapeutic option to treat symptomatic coronary artery disease in the majority of cases. Interventional cardiology, as well as special areas of interventional angiography such as stent- or balloon-based treatment of complex lesions of the superficial femoral artery or below-the-knee arteries that inevitably carry a high risk of restenosis, have greatly benefited from the introduction of drug-eluting interventional devices. The use of drug-eluting stents has now even paved the road to safely and reliably treat complex coronary artery disease even in cases that had been previously considered to be a domain of bypass surgery, such as left main coronary artery and multivessel disease, including in diabetic patients [119]. To date, numerous lesion and patient characteristics have been identified to benefit from the usage of drug-eluting stents (Table 8.2). Predictors of restenosis include stent length and the number of stents per lesion, lesion length and complexity, small vessel diameter (≤2.75 mm), residual diameter stenosis, and certain clinical scenarios (e.g., previous restenosis and diabetes mellitus), while premature antiplatelet therapy discontinuation, renal failure, bifurcation lesions, diabetes, and low ejection fraction have been identified as predictors of thrombotic events associated with drug-eluting stents deployment [14]. The diagnostic gold standard for restenosis is coronary angiography, but noninvasive diagnostic tools are being developed (e.g., computerized tomography, cardiac magnetic resonance tomography).
To date, the two major classes of pharmacological compounds used in clinical interventional cardiology are the mTOR inhibitors (referred to as “limus drugs”) and paclitaxel, which inhibit VSMC proliferation and migration, two key processes that contribute to neointimal thickening during in-stent restenosis (Fig. 8.3). The term “limus drugs” is confusing since pimecrolimus and tacrolimus are calcineurin inhibitors that only exhibit immunosuppressive activities and have yielded unsatisfactory results when used in drug-eluting stent platforms to prevent restenosis [129, 130]. By contrast, pivotal studies a decade ago using sirolimus- and paclitaxel-coated stents have shown a dramatic decrease of late lumen loss, the pathoanatomical correlate of angiographic and clinical restenosis, compared to uncoated bare metal stents [131]. Meta-analysis and recent clinical head-to-head trials have implicated superior performance of mTOR-inhibitor-eluting stents [132]. Interestingly, the first clinically available drug-eluting stents, Cordis’ sirolimus-eluting stent, has been unsurpassed in terms of clinical safety and efficacy as is becoming evident in recent randomized comparisons presented at large international meetings as well as peer-reviewed publications [133]. However, due to potential improvements in stent design, Abbott’s everolimus-eluting Xience V stent is the most frequently used stent in contemporary interventional cardiology.
Overview of drugs currently used on the vast majority of drug-eluting stents approved for human use. The name of the stent platform is provided in parenthesis. A large reduction in restenosis and need for target vessel revascularization has been conclusively demonstrated with drug-eluting stents that deliver mTOR inhibitors and paclitaxel, two unrelated families of drugs which cause cell cycle arrest in G0/G1-phase and M-phase, respectively. Tacrolimus and pimecrolimus are calcineurin inhibitors which only exhibit immunosuppressive properties and have yielded unsatisfactory clinical results in drug-eluting stents platforms
Several studies investigated the use of dual drug-eluting stents to inhibit the rate of restenosis. Most of the combinatorial approaches revealed no beneficial effect. Examples include the combination of paclitaxel and pimecrolimus [130] or sirolimus and estradiol [134]. Interestingly, a combination of sirolimus and probucol on the ISAR platform revealed a positive effect [135]. However, replication of clinical results by independent groups is not yet available.
Current Limitations of Drug-Eluting Stents and Optimization
As in many instances, medical devices such as drug-eluting stents do not exclusively alleviate clinical problems such as restenosis but are associated with limitations that merit further optimization. The major shortcomings associated with current FDA-approved drug-eluting stent platforms that can be associated with the development of late (between 1 and 12 months after stent placement) and very late (12 months and later) stent thrombosis are listed in Table 8.1. Since cell cycle inhibitors do not selectively inhibit proliferation of their main target cells, namely VSMCs, but also inhibit proliferation of other cells, most importantly ECs, cell cycle inhibitors can delay healing processes and thus precipitate acute and subacute, life-threatening events, in particular stent thrombosis. Whereas early stent thrombosis that occurs during the first 30 days after stent placement is generally associated with problems linked with PCI itself or shortcomings attributable to concomitant antithrombotic pharmacotherapy such as drug resistance or patient incompliance, late/very late stent thrombosis is often related to risks associated with ongoing local inflammatory processes and delayed arterial healing, thus leading to a prothrombotic milieu (Table 8.1). Since stent thrombosis is associated with considerable mortality, it has been the focus of many clinical investigations. Thus, current guidelines recommend prolonged dual antithrombotic therapy of at least 12 months after drug-eluting stent implantation, exceeding the 4-week recommendation for bare metal stents [136].
The major components of a typical drug-eluting stent platform that can be optimized to increase efficacy and safety of drug-eluting stents include the polymer, the delivery system, stent design, and the drug itself (Fig. 8.4) [137]. All current FDA-approved drug-eluting stents carry a nonerodible polymer to avoid boost release and retard drug delivery to the vascular wall, since prolonged drug release of several weeks is considered to be of pivotal importance for effective inhibition of restenosis. Thus, the issue of polymeric coating is of integral importance for the development of novel drug-eluting stent platforms. Yet, virtually all polymers are able to precipitate proinflammatory processes in the vascular wall and are therefore considered to be a major cause for late and very late stent thrombosis. To circumvent this important clinical dilemma, several possible solutions have been proposed and are currently under investigation. The major focus is now on biodegradable polymers such as a polylactic acid polymer that biodegrades into carbon dioxide and water over time, as it is used on the biolimus A9-eluting Biomatrix and Nobori drug-eluting stents platforms. A fairly large clinical trial has shown noninferiority of this stent platform compared to the current gold standard, the sirolimus-eluting stent [138]. Other approaches to limit or abstain from surface polymer coating are microporous stents [139], reservoir-based drug delivery [137], and bioactive surface technology [140].
Major components of a typical drug-eluting stent platform. Research endeavors to improve the efficacy and safety of drug-eluting stents include the identification of new drugs, new stent design, optimization of delivery systems (e.g., use of bioabsorbable stent platforms), and improved biodegradable polymers
Major attention has been recently drawn to bioabsorbable stent platforms. The rationale behind this intriguing approach is the limited presence of a vascular scaffold in the coronary artery. However, first-in-man clinical trials using these approaches revealed rather disappointing results, eventually leading, for example, to the cessation of the magnesium bioabsorbable stent program from Biotronik [141]. However, a polymer-based, fully erodible coronary stent that delivers everolimus has recently shown encouraging results in a limited number of patients [142]. Albeit widespread clinical use of this particular stent platform is not currently foreseeable, the interest and expectations regarding this technology remain high in the interventional cardiology community.
Conclusions
In the last two decades, numerous studies in animal models have conclusively demonstrated that inhibiting cell proliferation within the damaged vessel wall is a suitable strategy to limit neointimal thickening after angioplasty. Nowadays, the majority of coronary interventions utilize drug-eluting stents that deliver locally high doses of antiproliferative drugs, such as sirolimus (and derivatives) and paclitaxel. These medical devices have revolutionized the field of revascularization owing to a dramatic reduction in the incidence of restenosis, target lesion revascularization, and major adverse cardiac events. However, both efficacy and safety of drug-eluting stent platforms need to be improved to reduce the need for repeated revascularization and the development of late stent thrombosis due to delayed reendothelialization, which forces prolonged oral dual antiplatelet therapy. Major areas of drug-eluting stent research include the development of new drugs, approaches to limit or even avoid the presence of polymers (e.g., biodegradable polymers, microporous stents, reservoir-based drug delivery), use of antithrombotic coatings, bioactive surface technology to promote vascular healing (e.g., antibody-, peptide-, and nucleotide-coated stents), and development of bioabsorbable stent platforms. By combining different strategies, next-generation drug-eluting stent platforms may consist of polyvalent devices that embrace the three foundations of stent-based lesion therapy: antirestenotic, prohealing, and antithrombotic. Another goal will be to develop drug-eluting stents tailored to some patient or lesion subgroups (e.g., diabetics, patients presenting with acute myocardial infarction) and lesion characteristics. Achieving these ambitious objectives will certainly require the close interaction of specialists in different biomedical and medical disciplines.
References
Grüntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty. N Engl J Med. 1979;301(2):61–8.
Palmaz JC, Sibbitt RR, Reuter SR, et al. Expandable intraluminal graft: a preliminary study. Radiology. 1985;156(1):73–7.
Schatz RA, Palmaz JC, Tio FO, et al. Balloon-expandable intracoronary stents in the adult dog. Circulation. 1987;76(2):450–7.
Sigwart U, Puel J, Mirkovitch V, et al. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316(12):701–6.
Andrés V. Control of vascular cell proliferation and migration by cyclin-dependent kinase signalling: new perspectives and therapeutic potential. Cardiovasc Res. 2004;63(1):11–21.
Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8(11):1249–56.
Sousa JE, Costa MA, Abizaid A, et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001;103(2):192–5.
Rensing BJ, Vos J, Smits PC, et al. Coronary restenosis elimination with a sirolimus eluting stent: first European human experience with 6-month angiographic and intravascular ultrasonic follow-up. Eur Heart J. 2001;22(22):2125–30.
Serruys PW, Kutryk MJ, Ong AT. Coronary-artery stents. N Engl J Med. 2006;354(5):483–95.
Togni M, Windecker S, Cocchia R, et al. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J Am Coll Cardiol. 2005;46(2):231–6.
Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the research on adverse drug events and reports (RADAR) project. J Am Coll Cardiol. 2006;47(1):175–81.
Gonzalo N, Barlis P, Serruys PW, et al. Incomplete stent apposition and delayed tissue coverage are more frequent in drug-eluting stents implanted during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction than in drug-eluting stents implanted for stable/unstable angina: insights from optical coherence tomography. JACC Cardiovasc Interv. 2009;2(5):445–52.
Bavry AA, Bhatt DL. Appropriate use of drug-eluting stents: balancing the reduction in restenosis with the concern of late thrombosis. Lancet. 2008;371(9630):2134–43.
Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126–30.
Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111(17):2257–73.
Vidal A, Koff A. Cell-cycle inhibitors: three families united by a common cause. Gene. 2000;247(1–2):1–15.
Bonauer A, Boon RA, Dimmeler S. Vascular microRNAs. Curr Drug Targets. 2010;11:943–9.
Davis BN, Hilyard AC, Nguyen PH, et al. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284(6):3728–38.
Liu X, Cheng Y, Zhang S, et al. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104(4):476–87.
Schwartz RS, Chronos NA, Virmani R. Preclinical restenosis models and drug-eluting stents: Still important, still much to learn. J Am Coll Cardiol. 2004;44(7):1373–85.
Schwartz RS, Edelman ER, Carter A, et al. Drug-eluting stents in preclinical studies: recommended evaluation from a consensus group. Circulation. 2002;106(14):1867–73.
Virmani R, Kolodgie FD, Farb A, et al. Drug eluting stents: are human and animal studies comparable? Heart. 2003;89(2):133–8.
Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem. 2010;285(19):14071–7.
Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35.
Marx SO, Jayaraman T, Go LO, et al. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76(3):412–7.
Poon M, Marx SO, Gallo R, et al. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 1996;98(10):2277–83.
Wessely R, Blaich B, Belaiba RS, et al. Comparative characterization of cellular and molecular anti-restenotic profiles of paclitaxel and sirolimus. Implications for local drug delivery. Thromb Haemost. 2007;97(6):1003–12.
Luo Y, Marx SO, Kiyokawa H, et al. Rapamycin resistance tied to defective regulation of p27Kip1. Mol Cell Biol. 1996;16(12):6744–51.
Sun J, Marx SO, Chen HJ, et al. Role for p27(Kip1) in vascular smooth muscle cell migration. Circulation. 2001;103(24):2967–72.
Diez-Juan A, Andres V. Coordinate control of proliferation and migration by the p27Kip1/cyclin-dependent kinase/retinoblastoma pathway in vascular smooth muscle cells and fibroblasts. Circ Res. 2003;92(4):402–10.
Castro C, Diez-Juan A, Cortes MJ, et al. Distinct regulation of mitogen-activated protein kinases and p27Kip1 in smooth muscle cells from different vascular beds. A potential role in establishing regional phenotypic variance. J Biol Chem. 2003;278(7):4482–90.
Burke SE, Lubbers NL, Chen YW, et al. Neointimal formation after balloon-induced vascular injury in Yucatan minipigs is reduced by oral rapamycin. J Cardiovasc Pharmacol. 1999;33(6):829–35.
Gallo R, Padurean A, Jayaraman T, et al. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999;99(16):2164–70.
Jahnke T, Schafer FK, Bolte H, et al. Short-term rapamycin for inhibition of neointima formation after balloon-mediated aortic injury in rats: is there a window of opportunity for systemic prophylaxis of restenosis? J Endovasc Ther. 2005;12(3):332–42.
Carter AJ, Aggarwal M, Kopia GA, et al. Long-term effects of polymer-based, slow-release, sirolimus-eluting stents in a porcine coronary model. Cardiovasc Res. 2004;63(4):617–24.
Carter AJ, Wei W, Gibson L, et al. Segmental vessel wall shear stress and neointimal formation after sirolimus-eluting stent implantation: physiological insights in a porcine coronary model. Cardiovasc Revasc Med. 2005;6(2):58–64.
Tepe G, Muschick P, Laule M, et al. Prevention of carotid artery restenosis after sirolimus-coated stent implantation in pigs. Stroke. 2006;37(2):492–4.
Klugherz BD, Llanos G, Lieuallen W, et al. Twenty-eight-day efficacy and phamacokinetics of the sirolimus-eluting stent. Coron Artery Dis. 2002;13(3):183–8.
Langeveld B, Roks AJ, Tio RA, et al. Rat abdominal aorta stenting: a new and reliable small animal model for in-stent restenosis. J Vasc Res. 2004;41(5):377–86.
Pires NM, van der Hoeven BL, de Vries MR, et al. Local perivascular delivery of anti-restenotic agents from a drug-eluting poly(epsilon-caprolactone) stent cuff. Biomaterials. 2005;26(26):5386–94.
Pires NM, Eefting D, de Vries MR, et al. Sirolimus and paclitaxel provoke different vascular pathological responses after local delivery in a murine model for restenosis on underlying atherosclerotic arteries. Heart. 2007;93(8):922–7.
Barilli A, Visigalli R, Sala R, et al. In human endothelial cells rapamycin causes mTORC2 inhibition and impairs cell viability and function. Cardiovasc Res. 2008;78(3):563–71.
Hayashi S, Yamamoto A, You F, et al. The stent-eluting drugs sirolimus and paclitaxel suppress healing of the endothelium by induction of autophagy. Am J Pathol. 2009;175(5):2226–34.
Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13(5):495–504.
Frey D, Billinger M, Meier P, et al. Endothelialization of sirolimus-eluting stents with slow and extended drug release in the porcine overstretch model. J Invasive Cardiol. 2008;20(12):631–4.
Imanishi T, Kobayashi K, Kuki S, et al. Sirolimus accelerates senescence of endothelial progenitor cells through telomerase inactivation. Atherosclerosis. 2006;189(2):288–96.
Nuhrenberg TG, Voisard R, Fahlisch F, et al. Rapamycin attenuates vascular wall inflammation and progenitor cell promoters after angioplasty. FASEB J. 2005;19(2):246–8.
Andrés V, Castro C, Campistol JM. Potential role of proliferation signal inhibitors on atherosclerosis in renal transplant patients. Nephrol Dial Transplant. 2006;21 Suppl 3:iii14–7.
Carter AJ, Brodeur A, Collingwood R, et al. Experimental efficacy of an everolimus eluting cobalt chromium stent. Catheter Cardiovasc Interv. 2006;68(1):97–103.
Garcia-Touchard A, Burke SE, Toner JL, et al. Zotarolimus-eluting stents reduce experimental coronary artery neointimal hyperplasia after 4 weeks. Eur Heart J. 2006;27(8):988–93.
Tada N, Virmani R, Grant G, et al. Polymer-free biolimus a9-coated stent demonstrates more sustained intimal inhibition, improved healing, and reduced inflammation compared with a polymer-coated sirolimus-eluting cypher stent in a porcine model. Circ Cardiovasc Interv. 2010;3(2):174–83.
Abal M, Andreu JM, Barasoain I. Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Cancer Drug Targets. 2003;3(3):193–203.
Sollott SJ, Cheng L, Pauly RR, et al. Taxol inhibits neointimal smooth muscle cell accumulation after angioplasty in the rat. J Clin Invest. 1995;95(4):1869–76.
Axel DI, Kunert W, Goggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96(2):636–45.
Wiskirchen J, Schober W, Schart N, et al. The effects of paclitaxel on the three phases of restenosis: smooth muscle cell proliferation, migration, and matrix formation: an in vitro study. Invest Radiol. 2004;39(9):565–71.
Kim DW, Kwon JS, Kim YG, et al. Novel oral formulation of paclitaxel inhibits neointimal hyperplasia in a rat carotid artery injury model. Circulation. 2004;109(12):1558–63.
Herdeg C, Oberhoff M, Baumbach A, et al. Local paclitaxel delivery for the prevention of restenosis: biological effects and efficacy in vivo. J Am Coll Cardiol. 2000;35(7):1969–76.
Wilson GJ, Polovick JE, Huibregtse BA, et al. Overlapping paclitaxel-eluting stents: long-term effects in a porcine coronary artery model. Cardiovasc Res. 2007;76(2):361–72.
Heldman AW, Cheng L, Jenkins GM, et al. Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation. 2001;103(18):2289–95.
Wessely R, Schömig A, Kastrati A. Sirolimus and paclitaxel on polymer-based drug-eluting stents: similar but different. J Am Coll Cardiol. 2006;47(4):708–14.
Finkelstein A, McClean D, Kar S, et al. Local drug delivery via a coronary stent with programmable release pharmacokinetics. Circulation. 2003;107(5):777–84.
Scheller B, Speck U, Schmitt A, et al. Addition of paclitaxel to contrast media prevents restenosis after coronary stent implantation. J Am Coll Cardiol. 2003;42(8):1415–20.
Scheller B, Speck U, Abramjuk C, et al. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation. 2004;110(7):810–4.
Epstein FH, Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801–11.
Xing D, Nozell S, Chen Y-F, et al. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29(3):289–95.
Geraldes P, Sirois MG, Bernatchez PN, et al. Estrogen regulation of endothelial and smooth muscle cell migration and proliferation: Role of p38 and p42/44 mitogen-activated protein kinase. Arterioscler Thromb Vasc Biol. 2002;22(10):1585–90.
Geraldes P, Sirois MG, Tanguay J-F. Specific contribution of estrogen receptors on mitogen-activated protein kinase pathways and vascular cell activation. Circ Res. 2003;93(5):399–405.
Cheng B, Song J, Zou Y, et al. Responses of vascular smooth muscle cells to estrogen are dependent on balance between ERK and p38 MAPK pathway activities. Int J Cardiol. 2009;134(3):356–65.
Kappert K, Caglayan E, Huntgeburth M, et al. 17Beta-estradiol attenuates PDGF signaling in vascular smooth muscle cells at the postreceptor level. Am J Physiol Heart Circ Physiol. 2006;290(2):H538–46.
Krasinski K, Spyridopoulos I, Asahara T, et al. Estradiol accelerates functional endothelial recovery after arterial injury. Circulation. 1997;95(7):1768–72.
Toutain CE, Filipe C, Billon A, et al. Estrogen receptor alpha expression in both endothelium and hematopoietic cells is required for the accelerative effect of estradiol on reendothelialization. Arterioscler Thromb Vasc Biol. 2009;29(10):1543–50.
Filipe C, Lam Shang Leen L, Brouchet L, et al. Estradiol accelerates endothelial healing through the retrograde commitment of uninjured endothelium. Am J Physiol Heart Circ Physiol. 2008;294(6):H2822–30.
Iwakura A, Luedemann C, Shastry S, et al. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108(25):3115–21.
Chen S-J, Li H, Durand J, et al. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation. 1996;93(3):577–84.
Iafrati MD, Karas RH, Aronovitz M, et al. Estrogen inhibits the vascular injury response in estrogen receptor-deficient mice. Nat Med. 1997;3(5):545–8.
Sullivan Jr TR, Karas RH, Aronovitz M, et al. Estrogen inhibits the response-to-injury in a mouse carotid artery model. J Clin Invest. 1995;96(5):2482.
Levine RL, Chen S-J, Durand J, et al. Medroxyprogesterone attenuates estrogen-mediated inhibition of neointima formation after balloon injury of the rat carotid artery. Circulation. 1996;94(9):2221–7.
Oparil S, Levine RL, Chen S-J, et al. Sexually dimorphic response of the balloon-injured rat carotid artery to hormone treatment. Circulation. 1997;95(5):1301–7.
Bakir S, Mori T, Durand J, et al. Estrogen-induced vasoprotection is estrogen receptor dependent: evidence from the balloon-injured rat carotid artery model. Circulation. 2000;101(20):2342–4.
Mori T, Durand J, Chen Y-F, et al. Effects of short-term estrogen treatment on the neointimal response to balloon injury of rat carotid artery. Am J Cardiol. 2000;85(10):1276–9.
Hanke H, Hanke S, Bruck B, et al. Inhibition of the protective effect of estrogen by progesterone in experimental atherosclerosis. Atherosclerosis. 1996;121(1):129–38.
Foegh ML, Asotra S, Howell MH, et al. Estradiol inhibition of arterial neointimal hyperplasia after balloon injury. J Vasc Surg. 1994;19(4):722–6.
White CR, Shelton J, Chen S-J, et al. Estrogen restores endothelial cell function in an experimental model of vascular injury. Circulation. 1997;96(5):1624–30.
Chandrasekar B, Tanguay J-F. Local delivery of 17-beta-estradiol decreases neointimal hyperplasia after coronary angioplasty in a porcine model. J Am Coll Cardiol. 2000;36(6):1972–8.
New G, Moses JW, Roubin GS, et al. Estrogen-eluting, phosphorylcholine-coated stent implantation is associated with reduced neointimal formation but no delay in vascular repair in a porcine coronary model. Catheter Cardiovasc Interv. 2002;57(2):266–71.
Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14(1):37–44.
Jaschke B, Michaelis C, Milz S, et al. Local statin therapy differentially interferes with smooth muscle and endothelial cell proliferation and reduces neointima on a drug-eluting stent platform. Cardiovasc Res. 2005;68(3):483–92.
Ruef J, Meshel AS, Hu Z, et al. Flavopiridol inhibits smooth muscle cell proliferation in vitro and neointimal formation in vivo after carotid injury in the rat. Circulation. 1999;100(6):659–65.
Jaschke B, Milz S, Vogeser M, et al. Local cyclin-dependent kinase inhibition by flavopiridol inhibits coronary artery smooth muscle cell proliferation and migration: implications for the applicability on drug-eluting stents to prevent neointima formation following vascular injury. FASEB J. 2004;11:1285–7.
Sung C-P, Arleth AJ, Ohlstein EH. Carvedilol inhibits vascular smooth muscle cell proliferation. J Cardiovasc Pharmacol. 1993;21(2):221–7.
Ohlstein EH, Douglas SA, Sung CP, et al. Carvedilol, a cardiovascular drug, prevents vascular smooth muscle cell proliferation, migration, and neointimal formation following vascular injury. Proc Natl Acad Sci U S A. 1993;90(13):6189–93.
Kim W, Jeong MH, Cha KS, et al. Effect of anti-oxidant (carvedilol and probucol) loaded stents in a porcine coronary restenosis model. Circ J. 2005;69(1):101–6.
Miyauchi K, Aikawa M, Tani T, et al. Effect of probucol on smooth muscle cell proliferation and dedifferentiation after vascular injury in rabbits: possible role of PDGF. Cardiovasc Drugs Ther. 1998;12(3):251–60.
Lau AK, Leichtweis SB, Hume P, et al. Probucol promotes functional reendothelialization in balloon-injured rabbit aortas. Circulation. 2003;107(15):2031–6.
Tanous D, Bräsen JH, Choy K, et al. Probucol inhibits in-stent thrombosis and neointimal hyperplasia by promoting re-endothelialization. Atherosclerosis. 2006;189(2):342–9.
Jackson CL, Pettersson KS. Effects of probucol on rat carotid artery responses to balloon catheter injury. Atherosclerosis. 2001;154(2):407–14.
Yokoyama T, Miyauchi K, Kurata T, et al. Effect of probucol on neointimal thickening in a stent porcine restenosis model. Jpn Heart J. 2004;45(2):305–13.
Takahashi S, Oida K, Fujiwara R, et al. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol. 1992;20(6):900–6.
Hayashi S, Morishita R, Matsushita H, et al. Cyclic AMP inhibited proliferation of human aortic vascular smooth muscle cells, accompanied by induction of p53 and p21. Hypertension. 2000;35(1):237–43.
Aoki M, Morishita R, Hayashi S, et al. Inhibition of neointimal formation after balloon injury by cilostazol, accompanied by improvement of endothelial dysfunction and induction of hepatocyte growth factor in rat diabetes model. Diabetologia. 2001;44(8):1034–42.
Tsuchikane E, Suzuki T, Katoh O. Examination of anti-intima hyperplastic effect on cilostazol-eluting stent in a porcine model. J Invasive Cardiol. 2007;19(3):109–12.
Lee C-S, Kwon Y-W, Yang H-M, et al. New mechanism of rosiglitazone to reduce neointimal hyperplasia: activation of glycogen synthase kinase-3beta followed by inhibition of MMP-9. Arterioscler Thromb Vasc Biol. 2009;29(4):472–9.
Phillips JW, Barringhaus KG, Sanders JM, et al. Rosiglitazone reduces the accelerated neointima formation after arterial injury in a mouse injury model of type 2 diabetes. Circulation. 2003;108(16):1994–9.
Desouza CV, Murthy SN, Diez J, et al. Differential effects of peroxisome proliferator activator receptor-alpha and gamma ligands on intimal hyperplasia after balloon catheter-induced vascular injury in Zucker rats. J Cardiovasc Pharmacol Ther. 2003;8(4):297–305.
Alessi A, FranÇa Neto O, Brofman P, et al. Use of rosiglitazone before and after vascular injury in hypercholesterolemic rabbits: assessment of neointimal formation. Thromb J. 2008;6(1):12.
Joner M, Farb A, Cheng Q, et al. Pioglitazone inhibits in-stent restenosis in atherosclerotic rabbits by targeting transforming growth factor-beta and MCP-1. Arterioscler Thromb Vasc Biol. 2007;27(1):182–9.
Kasai T, Miyauchi K, Yokoyama T, et al. Pioglitazone attenuates neointimal thickening via suppression of the early inflammatory response in a porcine coronary after stenting. Atherosclerosis. 2008;197(2):612–9.
Miyazawa K, Kikuchi S, Fukuyama J, et al. Inhibition of PDGF- and TGF-[beta]1-induced collagen synthesis, migration and proliferation by tranilast in vascular smooth muscle cells from spontaneously hypertensive rats. Atherosclerosis. 1995;118(2):213–21.
Miyazawa K, Hamano S, Ujiie A. Antiproliferative and c-myc mRNA suppressive effect of tranilast on newborn human vascular smooth muscle cells in culture. Br J Pharmacol. 1996;118(4):915.
Watanabe S, Matsuda A, Suzuki Y, et al. Inhibitory mechanism of tranilast in human coronary artery smooth muscle cells proliferation, due to blockade of PDGF-BB-receptors. Br J Pharmacol. 2000;130(2):307–14.
Tanaka K, Honda M, Kuramochi T, et al. Prominent inhibitory effects of tranilast on migration and proliferation of and collagen synthesis by vascular smooth muscle cells. Atherosclerosis. 1994;107(2):179–85.
Sata M, Takahashi A, Tanaka K, et al. Mouse genetic evidence that tranilast reduces smooth muscle cell hyperplasia via a p21(WAF1)-dependent pathway. Arterioscler Thromb Vasc Biol. 2002;22(8):1305–9.
Fukuyama J, Ichikawa K, Miyazawa K, et al. Tranilast suppresses intimal hyperplasia in the balloon injury model and cuff treatment model in rabbits. Jpn J Pharmacol. 1996;70(4):321.
Fukuyama J, Ichikawa K, Hamano S, et al. Tranilast suppresses the vascular intimal hyperplasia after balloon injury in rabbits fed on a high-cholesterol diet. Eur J Pharmacol. 1996;318(2–3):327–32.
Miyazawa N, Umemura K, Kondo K, et al. Effects of pemirolast and tranilast on intimal thickening after arterial injury in the rat. J Cardiovasc Pharmacol. 1997;30(2):157–62.
Kikuchi S, Umemura K, Kondo K, et al. Tranilast suppresses intimal hyperplasia after photochemically induced endothelial injury in the rat. Eur J Pharmacol. 1996;295(2–3):221–7.
Ward MR, Agrotis A, Kanellakis P, et al. Tranilast prevents activation of transforming growth factor-beta system, leukocyte accumulation, and neointimal growth in porcine coronary arteries after stenting. Arterioscler Thromb Vasc Biol. 2002;22(6):940–8.
Ishiwata S, Verheye S, Robinson KA, et al. Inhibition of neointima formation by tranilast in pig coronary arteries after balloon angioplasty and stent implantation. J Am Coll Cardiol. 2000;35(5):1331–7.
Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961–72.
Kim YH, Park SW, Lee CW, et al. Comparison of sirolimus-eluting stent, paclitaxel-eluting stent, and bare metal stent in the treatment of long coronary lesions. Catheter Cardiovasc Interv. 2006;67(2):181–7.
Colmenarez HJ, Escaned J, Fernandez C, et al. Efficacy and safety of drug-eluting stents in chronic total coronary occlusion recanalization: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(17):1854–66.
Spaulding C, Henry P, Teiger E, et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006;355(11):1093–104.
Ardissino D, Cavallini C, Bramucci E, et al. Sirolimus-eluting vs uncoated stents for prevention of restenosis in small coronary arteries: a randomized trial. JAMA. 2004;292(22):2727–34.
Holmes Jr DR, Teirstein PS, Satler L, et al. 3-Year follow-up of the SISR (sirolimus-eluting stents versus vascular brachytherapy for in-stent restenosis) trial. JACC Cardiovasc Interv. 2008;1(4):439–48.
Latib A, Ferri L, Ielasi A, et al. Comparison of the long-term safety and efficacy of drug-eluting and bare-metal stent implantation in saphenous vein grafts. Circ Cardiovasc Interv. 2010;3(3):249–56.
Stenestrand U, James SK, Lindback J, et al. Safety and efficacy of drug-eluting vs. bare metal stents in patients with diabetes mellitus: long-term follow-up in the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J. 2010;31(2):177–86.
Sukhija R, Aronow WS, Palaniswamy C, et al. Major adverse cardiac events in patients with moderate to severe renal insufficiency treated with first-generation drug-eluting stents. Am J Cardiol. 2010;105(3):293–6.
Lee MS, Kobashigawa J, Tobis J. Comparison of percutaneous coronary intervention with bare-metal and drug-eluting stents for cardiac allograft vasculopathy. JACC Cardiovasc Interv. 2008;1(6):710–5.
Morice MC, Bestehorn HP, Carrie D, et al. Direct stenting of de novo coronary stenoses with tacrolimus-eluting versus carbon-coated carbostents. The randomized JUPITER II trial. EuroIntervention. 2006;2(1):45–52.
Verheye S, Agostoni P, Dawkins KD, et al. The GENESIS (randomized, multicenter study of the pimecrolimus-eluting and pimecrolimus/paclitaxel-eluting coronary stent system in patients with de novo lesions of the native coronary arteries) trial. JACC Cardiovasc Interv. 2009;2(3):205–14.
Morice M-C, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346(23):1773–80.
Stettler C, Allemann S, Wandel S, et al. Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis. BMJ. 2008;337:a1331.
Cassese S, Piccolo R, Galasso G, et al. Twelve-month clinical outcomes of everolimus-eluting stent as compared to paclitaxel- and sirolimus-eluting stent in patients undergoing percutaneous coronary interventions. A meta-analysis of randomized clinical trials. Int J Cardiol. 2011;150:84–9.
Adriaenssens T, Mehilli J, Wessely R, et al. Does addition of estradiol improve the efficacy of a rapamycin-eluting stent? Results of the ISAR-PEACE randomized trial. J Am Coll Cardiol. 2007;49(12):1265–71.
Byrne RA, Mehilli J, Iijima R, et al. A polymer-free dual drug-eluting stent in patients with coronary artery disease: a randomized trial vs. polymer-based drug-eluting stents. Eur Heart J. 2009;30(8):923–31.
Grines CL, Bonow RO, Casey Jr DE, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulations. 2007;115(6):813–8.
Wessely R. New drug-eluting stent concepts. Nat Rev. 2010;7(4):194–203.
Windecker S, Serruys PW, Wandel S, et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008;372(9644):1163–73.
Wessely R, Hausleiter J, Michaelis C, et al. Inhibition of neointima formation by a novel drug-eluting stent system that allows for dose-adjustable, multiple, and on-site stent coating. Arterioscler Thromb Vasc Biol. 2005;25(4):748–53.
Clapper JD, Pearce ME, Guymon CA, et al. Biotinylated biodegradable nanotemplated hydrogel networks for cell interactive applications. Biomacromolecules. 2008;9(4):1188–94.
Erbel R, Di Mario C, Bartunek J, et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007;369(9576):1869–75.
Ormiston JA, Serruys PW, Regar E, et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371(9616):899–907.
Acknowledgments
We thank M.J. Andrés-Manzano for her help with figure preparation. Work in the author’s laboratory is supported by grants from the Spanish Ministry of Science and Innovation (MICINN) (grant SAF2010-16044), Instituto de Salud Carlos III (RECAVA, grant RD06/0014/0021), and the Dr. Léon Dumont Prize 2010 awarded to V.A. by the Belgian Society of Cardiology. C.S.R is the recipient of a predoctoral fellowship from Fundación Mario Losantos del Campo. The CNIC is supported by the MICINN and the Fundación Pro-CNIC.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media New York
About this chapter
Cite this chapter
Andrés, V., Fuster, J.J., Silvestre-Roig, C., Wessely, R. (2012). Modulating the Proliferative Response to Treat Restenosis After Vascular Injury. In: Homeister, J., Willis, M. (eds) Molecular and Translational Vascular Medicine. Molecular and Translational Medicine. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-61779-906-8_8
Download citation
DOI: https://doi.org/10.1007/978-1-61779-906-8_8
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-61779-905-1
Online ISBN: 978-1-61779-906-8
eBook Packages: MedicineMedicine (R0)