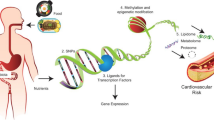
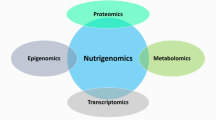
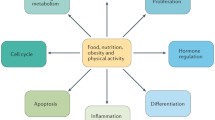
Abstract
Nutrigenomics refers to the interaction between one’s diet and his/her genes. These interactions can markedly influence digestion, absorption, and the elimination of bioactive food components, as well as influence their site of actions/molecular targets. Nutrigenomics comprises nutrigenetics, epigenetics, and transcriptomics, coupled with other “omic,” such as proteomics and metabolomics, that apparently account for the wide variability in cancer risk among individuals with similar dietary habits. Multiple food components including essential nutrients, phytochemical, zoochemicals, fungochemical, and bacterochemicals have been implicated in cancer risk and tumor behavior, admittedly with mixed results. Such findings suggest that not all individuals respond identically to a diet. This chapter highlights the influence of single-nucleotide polymorphism, copy number, epigenetic events, and transcriptomic homeostasis as factors influencing the response to food components and ultimately health, including cancer risk. Both breast and colorectal cancers are reviewed as examples about how nutrigenomics may influence the response to dietary intakes. As the concept that “one size fits all” comes to an end and personalized approaches surface, additional research data will be required to identify those who will benefit most from dietary change and any who might be placed at risk because of an adjustment.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Büchner, F.L., Bueno-de-Mesquita, H.B., Ros, M.M. et al. (2009) Consumption of vegetables and fruit and the risk of bladder cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 125, 2643–2651.
Migianno, G.A. and De Sanctis, R. (2006) Nutritional Genomics :toward a personalized diet. Clin Ter 157, 355–361.
Milner, J.A. (2004) Molecular targets for bioactive food components. J Nutr 134, 2492S–2498S.
Tan, A.C., Konczak, I, Sze, D.M., et al. (2011) Molecular pathways for cancer chemoprevention by dietary phytochemicals. Nutr Cancer 63, 495–505.
Newman, D.J. and Cragg, G.M. (2009) Microbial antitumor drugs: natural products of microbial origin as anticancer agents. Curr Opin Investig Drugs 10, 1280–1296.
Fenech, M., El-Sohemy, A., Cahill, L., et al. (2011) Nutrigenetics and Nutrigenomics: Viewpoints on the Current Status and Applications in Nutrition Research and Practice. J Nutrigenet Nutrigenomics 28, 69–89.
Ross, S.A. (2010) Evidence for the relationship between diet and cancer. Exp Oncol 32, 137–142.
Trujillo, E., Davis, C., and Milner, J. (2010) Nutrigenomics, proteomics, metabolomics, and the practice of dietetics. J Am Diet Assoc 106, 403–443.
Simopoulos, A.P. (2010) Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp Biol Med 235,785–795.
Inoue, M., Robien, K., Wang, R., et al. (2008) Green tea intake, MTHFR/TYMS genotype and breast cancer risk: the Singapore Chinese Health Study. Carcinogenesis 1967–1972.
World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. 2007. American Institute for Cancer Research, Washington, DC. Retrieved February 20, 2009, from: http://www.dietandcancerreport.org.
Milner, J.A., Romagnolo, D.F., et al. (2010) Bioactive compounds and Cancer 45–46, 393, 469–496, 580.
Martin, K.R. (2007) Using nutrigenomics to evaluate apoptosis as a preemptive target in cancer prevention. Curr Cancer Drug Targets 7, 438–446.
Davis, C.D., Emenaker, N.J., and Milner, J.A. (2010) Cellular proliferation, apoptosis and angiogenesis: molecular targets for nutritional preemption of cancer. Semin Oncol 37, 243–257.
Teng, X. and Xiao, H. (2009) Perspectives of DNA microarray and next-generation DNA sequencing technologies. Sci China C Life Sci 52, 7–16.
Masotti, A., Da Sacco, L, Bottazzo, G.F., et al. (2010) Microarray technology: a promising tool in nutrigenomics. Crit Rev Food Sci Nutr 50, 693–698.
Wheatley, K.E., Nogueira, L.M., Perkins, S.N., et. al (2011) Differential effects of calorie restriction and exercise on the adipose transcriptome in diet-induced obese mice. J Obes 2011, 265417.
Satih, S., Chalabi, N., Rabiau, N., et al. (2010) Gene expression profiling of breast cancer cell lines in response to soy isoflavones using a pangenomic microarray approach. OMICS 14, 231–238.
Thomas, P. and Fenech, M. (2011) Cytokinesis-block micronucleus cytome assay in lymphocytes. Methods Mol Biol 682, 217–234.
Bull, C.F., Beetstra-Hill, S., Benassi-Evans, B.J., et al. (2011) Application and adaptation of the in vitro micronucleus assay for the assessment of nutritional requirements of cells for DNA damage prevention. Mutagenesis 26, 193–197.
Davis, C.D. (2007) Nutrigenomics and the prevention of colon cancer. Pharmacogenomics 8, 121–124.
Jacob, R.A., Gretz, D.M., Taylor, P.C., et al. (1995) Moderate folate depletion increases plasma homocysteine and decreases lymphocyte DNA methylation in postmenopausal women. Cancer Res 55, 1894–901.
Pogribny, I.P., Basnakian., A.G., Miller, B.J, et al. (1995) Breaks in genomic DNA and within the p53 gene are associated with hypomethylation in livers of folate/methyl-deficient rats. Cancer Res 55, 1894–1901.
Wallwork, J.C. and Duerre, J.A. (1985) Effect of zinc deficiency on methionine metabolism, methylation reaction and protein synthesis in isolated perfused rat liver. J.Nutr 115, 252–262.
Davis, C.D., Uthus, E.O., and Finley, J.W. (2000) Dietary selenium and arsenic affect DNA methylation in vitro in Caco −2 cells and in vivo in rat liver and colon. J Nutr 130, 2903–2909.
Wolff,G.L., Kodell, R.L., Moore, S.R., et al. (1998) Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J 12, 949–957.
Cooney, C.A., Dave, A.A., and Wolff, G.L. (2002) Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr 132, 2393 S–2400 S.
Fraga, M.F., Ballestar, E., and Villar-Garea, A. (2005) Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 37, 391–400.
Ho, E, Clarke, J.D., and Dashwood, R.H. (2009) Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention. J Nutr 139, 2393–2396.
Nian, H., Delage, B., Ho, E., et al. (2009) Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: studies with sulforaphane and garlic organosulfur compounds. Environ Mol Mutagen 50, 213–221.
American Cancer Society. Cancer Facts and Figures 2010. (2010). Atlanta, Georgia, American Cancer Society.
http://www.cancer.gov/clinicaltrials/results/summary/2010/prophylactic-surgery0910
Ghadirian, P., Narod, S., and Fafard, E. (2009) Breast cancer risk in relation to the joint effect of BRCA mutations and diet diversity. Breast Cancer Res Treat 117, 417–422.
Vissac-Sabatier, C., Bignon, Y.J., and Bernard–Gallon, D.J. (2003) Effects on the phytoestrogens genistein and daidzein on BRCA2 tumor suppressor gene expression in breast cell lines. Nutr Cancer 45, 247–255.
Vissac-Sabatier, C., Coxam, V., Dechelotte, P., et al. (2003) Phytoestrogen-rich diets modulate expression of BRCA-1 and BRCA-2 tumor suppressor genes in mammary gland in female Wistar rats. Cancer Res 63, 6607–6612.
Cabanes, A., Wang, M., Olivo, S., et al. (2004) Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis 25, 741–748.
Fink, B.N., Gaudet, M.M., Britton, J.A., et al. (2006) Fruits, vegetables, and micronutrient intake in relation to breast cancer survival. Breast Cancer Res Treat 98, 199–208.
Chlebowski, R.T., Blackburn, G.L., Thomson, C.A., et al. (2006) Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst 98, 1767–1776.
Terry, P., Suzuki, R., and Hu, F.B. (2001) A prospective study of major dietary patterns and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev 10, 1281–1285.
Bissonauth,V., Shatenstein, B., and Ghadirian, P. (2008) Nutrition and breast cancer among sporadic cases and gene mutation carriers: An overview. Cancer Detect Prev 32, 52–64.
Pierce, J.P., Natarajan, L., and Caan, B.J. (2007) Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 298, 289–298.
Egeberg, R., Olsen, A., Autrup, H., et al. (2008) Meat consumption, N-acetyl transferase 1 and 2 polymorphism and risk of breast cancer in Danish postmenopausal women. Eur J Cancer Prev 17, 39–47.
Ericson, U., Sonestedt, E., and Ivarsson, M.I., (2009) Folate intake, methylenetetrahydrofolate reductase polymorphisms, and breast cancer risk in women from the Malmö Diet and Cancer cohort. Cancer Epidemiology Biomarkers Prev 18, 1101–1110.
Maruti, S.S., Ulrich, C.M., Jupe, E.R., et al. (2009) MTHFR C677T and postmenopausal breast cancer risk by intakes of one-carbon metabolism nutrients: a nested case–control study. Breast 11, R91
Sohn, K.J., Jang, H., Campan, M., et al. (2009) The methylenetetrahydrofolate reductase C677T mutation induces cell-specific changes in genomic DNA methylation and uracil misincorporation: a possible molecular basis for the site-specific cancer risk modification. Int J Cancer 124, 1999–2005.
Li,Y., Yuan, Y.Y., Meeran, S.M., et al. (2010) Synergistic epigenetic reactivation of estrogen receptor-α (ERα) by combined green tea polyphenol and histone deacetylase inhibitor in ERα-negative breast cancer cells. Mol Cancer. 14, 274.
Berletch JB, Liu C, Love WK, et al. (2008) Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem 103, 509–519.
Wu, A.H., Yu, M.C., and Tseng, C.C. (2003) Green tea and risk of breast cancer in Asian Americans. Int J Cancer 106, 574–579.
Wu, A.H., Tseng, C.C., Van Den Berg, D., et al. (2003) Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer 63, 7526–7529.
Lee, S.A., Fowke, J.H., Lu, W., et al. (2008) Cruciferous vegetables, the GSTP1 Ile105Val genetic polymorphism, and breast cancer risk. Am J Clin Nutr 87,753–760.
Antosiewicz, J., Ziolkowski,W., Kar,S., et al. (2008) Role of reactive oxygen intermediates in cellular responses to dietary cancer chemopreventive agents. Planta Med 74, 1570–1579.
Hail, N. Jr., Cortes, M., Drake, E.N., et al. (2008) Cancer chemoprevention: a radical perspective. Free Radic Biol Med 45, 97–110.
Syed Alwi, S.S., Cavell, B.E., Telang, U., et al. (2010) In vivo modulation of 4E binding protein 1 (4E-BP1) phosphorylation by watercress: a pilot study.Br J Nutr 104, 1288–1296.
Wang, J., John, E., Ingles, S.E., (2008) 5-Lipoxygenase and 5-Lipoxygenase-Activating Protein Gene Polymorphisms, Dietary Linoleic Acid, and Risk for Breast Cancer. Cancer Epidemiology Biomarkers Prev. 17, 2748–2754.
Dimri M., Bommi P.V., Sahasrabuddhe A.A., et al. (2010) Dietary omega-3 polyunsaturated fatty acids suppress expression of EZH2 in breast cancer cells. Carcinogenesis 31, 489–495.
Li, Y., Ambrosone, C.B. and McCullough, M.J. (2009) Oxidative stress-related genotypes, fruit and vegetable consumption and breast cancer risk. Carcinogenesis 777–784.
Iwasaki, M., Hamada, G.S., Nishimoto, I.N., et al. (2010) Dietary isoflavone intake, polymorphisms in the CYP17, CYP19, 17beta-HSD1, and SHBG genes, and risk of breast cancer in case–control studies in Japanese, Japanese Brazilians, and non-Japanese Brazilians. Nutr Cancer 62,466–475.
Qin, W., Zhu,W., Shi, H., et al. (2009) Soy isoflavones have an antiestrogenic effect and alter mammary promoter hypermethylation in healthy premenopausal women. Nutr Cancer 61, 238–244.
Jawaid, K., Crane, S.R., Nowers, J.L., et al. (2010) Long-term genistein treatment of MCF-7 cells decreases acetylated histone 3 expression and alters growth responses to mitogens and histone deacetylase inhibitors. J Steroid Biochem Mol Biol 120, 164–171.
Aiyer, H.S. and Gupta, R.C. (2010) Berries and ellagic acid prevent estrogen-induced mammary tumorigenesis by modulating enzymes of estrogen metabolism. Cancer Prev Res (Phila) 3, 727–737.
Lauber, S.N. and Gooderham, N.J. (2010) The cooked meat-derived mammary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine promotes invasive behaviour of breast cancer cells. Toxicology 279, 139–145.
Taioli, E., Garza, M.A., Ahn, Y.O., et al. (2009) Meta- and pooled analyses of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and colorectal cancer: a HuGE-GSEC review. Am J Epidemiol 170, 1207–1221.
Liu, J.J. and Ward, R.L. (2010) Folate and one-carbon metabolism and its impact on aberrant DNA methylation in cancer. Adv Genet 71, 79–121.
Cravo, M.L., Pinto, A.G., Chaves, P., et al. (1998) Effect of folate supplementation on DNA methylation of rectal mucosa in patients with colonic adenomas: correlation with nutrient intake. Clin Nutr 17, 45–49.
Figueiredo, J.C., Grau, M.V, Wallace, K., et al. (2009) Global DNA hypomethylation (LINE-1) in the normal colon and lifestyle characteristics and dietary and genetic factors. Cancer Epidemiol Biomarkers Prev 18, 1041–1049.
Economopoulos, K.P. and Sergentanis, T.N. (2010) GSTM1, GSTT1, GSTP1, GSTA1 and colorectal cancer risk: a comprehensive meta-analysis. Eur J Cancer 46, 1617–1631.
Ross, S.A. (2007) Nutritional genomic approaches to cancer prevention research. Exp Oncol 29, 250–256.
Novakovic, B., Sibson, M., Ng, H.K., et al. (2009) Placenta-specific methylation of the vitamin D 24-hydroxylase gene: implications for feedback autoregulation of active vitamin D levels at the fetomaternal interface. J Biol Chem 284, 14838–14848.
Dai, Q., Shrubsole, M.J., Ness, R.M., et al. (2007) The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am J Clin Nutr 86, 743–751.
Brevik, A., Joshi, A.D., Corral, R., et al. (2010) Polymorphisms in base excision repair genes as colorectal cancer risk factors and modifiers of the effect of diets high in red meat. Cancer Epidemiol Biomarkers Prev 19, 3167–3173.
Wong, H.L., Peters, U., Hayes, R.B., et al. (2010) Polymorphisms in the adenomatous polyposis coli (APC) gene and advanced colorectal adenoma risk. Eur J Cancer 46, 2457–2466.
Biswas, S.K., McClure, D., Jimenez, L.A., et al. (2005) Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal 7, 32–41.
Li, L., Aggarwal, B.B., Shishodia, S., et al. (2004) Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis.Cancer 101, 2351–2362.
Mudduluru, G., George-William, J.N., Muppala, S., et al. (2011) Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep 31,185–197.
Reuter, S., Gupta, S.C., Park, B., et al. (2011) Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr 6, 93–108.
Ströhle, A., Maike, W., and Hahn, A. (2007) Nutrition and colorectal cancer. Med Monatsschr Pharm 30, 25-32.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Riscuta, G., Dumitrescu, R.G. (2012). Nutrigenomics: Implications for Breast and Colon Cancer Prevention. In: Dumitrescu, R., Verma, M. (eds) Cancer Epigenetics. Methods in Molecular Biology, vol 863. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-61779-612-8_22
Download citation
DOI: https://doi.org/10.1007/978-1-61779-612-8_22
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-61779-611-1
Online ISBN: 978-1-61779-612-8
eBook Packages: Springer Protocols