Abstract
Oxidative stress plays a critical role in the development of cardiovascular diseases. Catechins are major components of green tea with many biological functions, including antioxidative, anti-inflammatory, and anticarcinogenic effects. Antioxidative effects of tea catechins are characterized by the ability to inhibit free radical generation and to scavenge free radicals, among other effects. They also influence activation of transcription factors such as nuclear factor kappa B, a multipotential promoter of inducible nitric oxide synthase and adhesion molecules. Although these characteristics of catechins have been well documented, antioxidative effects of catechins on cardiovascular diseases have not been well investigated. In this chapter, we review recent clinical and experimental papers to reveal the antioxidative effects of catechins in cardiovascular diseases. We performed oral administration of catechins in murine and rat models of cardiac transplantation, myocarditis, and myocardial ischemia to reveal the effects of catechins on the oxidative stress–induced ventricular and arterial remodeling. From our results and those of other investigations, we conclude that catechins are potent agents for the treatment and prevention of oxidative stress–related cardiovascular diseases because they are critically involved in the suppression of the stress. In this chapter, we review these reports and other investigations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
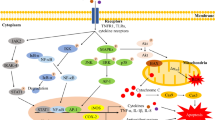
Green tea has favorable effects to prevent cardiovascular diseases; tea consumption is known to be associated with lower mortality of clinical myocardial infarction [1, 2]. Recently, it has been reported that green tea consumption reduced cardiovascular disease mortality in the Japanese population [3–5]. Catechins are key components of green tea with many biological functions, including antioxidative, anti-inflammatory, and anticarcinogenic effects [6–9]. Antioxidative effects of tea catechins are characterized by the ability to inhibit free radical generation and to scavenge free radicals, among other effects [6]. They also influence activation of transcription factors such as nuclear factor kappa B (NF-κB), a multipotential promoter of inducible nitric oxide synthase and adhesion molecules [10]. The major tea catechins are epigallocatechin-3 gallate (EGCG), epigallocatechin (EGC), epicatechin-3 gallate (ECG), and epicatechin (EC). Among them, EGCG is the most active polyphenol [11] and primarily responsible for the green tea effect [12]. In addition, EGCG demonstrated potent antioxidant properties [13]. EGCG possesses two triphenolic groups in its structure, which are reported to be important for its strong activity [14]. Luczay et al. arranged their antioxidative properties as follows: EGCG = EGC >> ECG = EC [15–17]. Antioxidative properties of catechins are manifested by their abilities to inhibit free radical generation, to scavenge free radicals, and to chelate transition metal ions, which are catalysts of free radical reactions [16]. Based on the standard one-electron reduction potential values, catechins can scavenge free radicals generated in an organism [18–21]. It is also noteworthy that EGCG is thought to act as an antioxidant in biological systems. Yin et al. revealed that EGCG increased cell viability, decreased reactive oxygen species (ROS) formation, and improved mitochondrial membrane potential in hippocampal neurons that had been exposed to lead. They concluded that EGCG is a potential agent in the treatment of chronic lead intoxication through its antioxidative character [22].
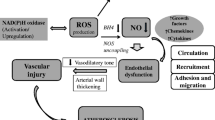
Oxidative stress is an important factor in organ and tissue injury [23]. Oxidative stress is defined as damage to cells, tissues, and organs caused by ROS. ROS are generated exogenously and intracellularly and include superoxide anion, hydrogen peroxide, hydroxyl radicals, and peroxynitrite. The principal intracellular sources of ROS include the mitochondrial electron transport system, peroxisomes, cytochrome P-450, and NADPH oxidase enzymes [24]. It is also known that exogenous factors involved in the generation of ROS are inflammatory cytokines, chemotherapeutic drugs, and toxins. Antioxidants constitute the defense mechanism against oxidative stress injury and include both enzymes and nonenzymatic factors. Copper–zinc and manganese superoxide dismutase (CuZn-SOD and Mn-SOD), catalase, and glutathione peroxidase (GPX) are key antioxidant enzymes. In contrast, glutathione and vitamins A, C, and E are also known to be the major nonenzymatic antioxidant molecules. The balance between ROS production and antioxidant defenses defines the degree of oxidative stress [25]. Whereas ROS play an important role in cell proliferation, differentiation, and apoptosis [26–28], oxidant signals can alter and denature nucleic acids, carbohydrates, lipids, and proteins, resulting in cell toxicity. The deleterious effects of oxidative stress have been reported in the pathophysiology of aging [24, 25] and neoplastic [29], hypertensive [30, 31], cardiovascular [32–34], and chronic kidney diseases [35–38].
Although the characteristics of tea catechins have been well documented [39], their effects on oxidative stress in cardiovascular diseases have not been well investigated. Recently, we have reported the effects of tea catechins on oxidative stress–related cardiovascular diseases, such as myocardial ischemia [40], acute myocarditis [41], and rejection after heart transplantation [42]. In this chapter, we review these reports and other investigations.
2 Catechins Suppress Oxidative Stress in Myocardial Ischemia
Myocardial ischemia and ventricular remodeling causes significant damage leading to cardiac death. It is well known that NF-κB–related inflammation is enhanced by ischemia and reperfusion of myocardium. Oxidative stress is known to cause adverse effects by increasing the infarct size or altering ventricular remodeling [43]. Results from Askari et al. [44] revealed the relationship between inflammation and modulation of left ventricular remodeling. Several leukocyte-derived enzymes are responsible for the increase of oxidative stress in the myocardium after acute myocardial infarction [45]. In ischemic conditions, superoxide is generated by nicotinamide adenine dinucleotide phosphate oxidase [46], hypochlorous acid by myeloperoxidase (MPO) [47], and peroxynitrite from inducible nitric oxide synthase [48]. The systems lead to the oxidation of proteins and lipids in the infarct zone. By identifying the oxidation products present after inflammation, the responsible oxidant systems should be elucidated. It is noteworthy that statins can block inflammation in several of these systems by limiting the generation of superoxide [49, 50]. Results from previous studies suggest that altered protease activation may contribute to remodeling [51]. Oxidative stress directly affects protease activation, and the phenotype observed in MPO-knockout mice was similar to that observed in urokinase and plasminogen-knockout mice [52]. Protease activation follows a well-described cascade of events. Urokinase cleaves plasminogen to plasmin, whereas plasmin can cleave pro–matrix metalloproteinase-9 to its active form, matrix metalloproteinase-9 (MMP-9). Urokinase is inhibited by binding to the plasminogen activator inhibitor-1 (PAI-1), leading to the generation of an irreversibly inactivated molecule. Each of these proteases has been shown to significantly affect the inflammatory response after acute myocardial infarction [53].
Activation of NF-κB induces adhesion molecules, cytokines, and MMPs involved in myocardial ischemia. Thus, decoy against NF-κB reduces myocardial inflammation induced by ischemia/reperfusion injury [54]. As proinflammatory factors are key components in the positive feedback loop of inflammation, the inhibition is an effective therapy for myocardial reperfusion injury by preventing inflammation [55–58]. The effects of catechins are induced by the suppression of several inflammatory factors including ROS induced by NF-κB [10]. To clarify the role of catechins in the ischemic heart, we produced a rat myocardial ischemia model by ligation of the left anterior descending coronary artery, and this was continued for 28 days. After ischemic injury, the nontreated ischemia group showed significant decline of blood pressure compared with the nontreated sham-operated group. However, catechin administration suppressed the decline of the blood pressure compared with that of the nontreated ischemia group. Echocardiography revealed that the nontreated ischemia group showed significantly impaired left ventricular contraction compared with that of the nontreated sham-operated group. However, the catechin treatment significantly improved left ventricular wall motion compared with that of the nontreated ischemia group. Pathologically, the anterior wall of each heart of the nontreated ischemia group was completely fibrotic, and the remaining area showed interstitial fibrosis and cell infiltration. However, catechin-treated hearts showed significantly less infarct size, infarct length, left ventricular circumference, and left ventricular inner diameter than those of the nontreated ischemia group. Immunohistochemically, increased numbers of CD4, CD8, CD11b, intercellular adhesion molecule-1 (ICAM-1), and ED-1 positive infiltrating cells were observed in the nontreated ischemia group, whereas catechin administration suppressed the numbers significantly. To prove the effect of catechins on NF-κB, we performed a sensitive multiwell colorimetric assay. It revealed that increased NF-κB activity was observed in hearts in the nontreated ischemia group. However, this enhanced NF-κB activity was abolished by the catechin treatment. Finally, to reveal the role of MMPs, the infarct region and myocardium were separated under a dissecting microscope and used with zymography as previously reported. This showed that increased gelatinase (MMP-2 and MMP-9) activity was observed in hearts in the nontreated ischemia group. However, this enhanced gelatinase activity was decreased by catechin administration [40]. We clearly revealed that catechins prevented chronic ventricular remodeling after ischemic injury because of the suppression of proinflammatory factors. Oxidation and inflammation have a significant role in left ventricular remodeling after acute myocardial infarction. Administration of catechin, a compound that downregulates the oxidative stress–induced modulation of the biological function of components of the MMP cascade, could inhibit the negative remodeling that occurs after myocardial infarction and improve patient outcomes.
3 Catechins Suppress Oxidative Stress in Myocarditis
Myocarditis is a serious disease in humans. Patients with myocarditis may present with rapidly progressive heart failure, shock, or arrhythmia in its severe form. Although acute myocardial inflammation is an essential etiology for the progression, no effective treatment has been elucidated [59–63]. Experimental autoimmune myocarditis (EAM) is a rat model that is characterized by severe myocardial damage and multinucleated giant cell infiltration. This has been used as a disease model of human acute myocarditis [64–68]. Liu et al. investigated the therapeutic role of thioredoxin-1 (TRX-1), a redox-regulatory protein with anti-oxidant and anti-inflammatory effects, in a murine myocarditis model [69]. They revealed that TRX-1 attenuates myocarditis by suppressing chemokine expressions and leukocyte chemotaxis in the murine model. TRX-1 is well known as a scavenger of ROS. The study using 8-OHdG, a marker for tissue oxidative damage, showed that 8-OHdG was strongly expressed in the heart with myocarditis, whereas the area immunopositive for 8-OHdG was much smaller in the heart with TRX-1 treatment. It also has been reported that, in the initial stage of EAM, cardiac dendritic cells and infiltrating macrophages and neutrophils attack the cardiomyocytes, forming rosette figures as a sign of active cardiomyocytolysis. Subsequently, the infiltration by macrophages and T lymphocytes plays a crucial role in the generation of myocarditis [70]. A recent study showed that ROS are involved in the antigen-presenting function of dendritic cells and that antioxidants suppress the activation of dendritic cells [71]. TRX-1 has radical scavenging functions; thus, administration of TRX-1 may also attenuate the activation of dendritic cells in this model. Shioji et al. [72] also reported that the suppression of dendritic cell functions by immunoglobulin therapy in the early phase attenuated giant cell autoimmune myocarditis. The evidence strongly suggests that the reducing activity of TRX-1 plays an important therapeutic role in EAM. In addition, chemokines, such as macrophage inflammatory protein-1 (MIP-1) and MIP-2, have recently been reported to be involved in the recruitment of inflammatory cells in EAM [73–76]. In the study, the cardiac expression of chemokines was markedly suppressed by TRX-1. Thus, the authors concluded that the attenuation of EAM by TRX-1 is due to its suppression of chemokine-induced chemotaxis of inflammatory cells in the initial phase of EAM.
To clarify the role of catechins in myocarditis, we produced a rat EAM model. The rats were supplemented with diet containing catechins or diet with saline without catechins for controls. After the induction of EAM, the catechins significantly reduced the heart weight/body weight ratio compared with that of nontreated EAM controls. Echocardiogram revealed the catechins improved cardiac function compared with that of the controls. Pathologically, nontreated control EAM animals showed severe myocardial cell infiltration and fibrotic lesions. However, the catechin treatment showed significantly less myocardial cell infiltration and fibrosis areas compared with those in controls. Immunohistochemistry revealed that enhanced expression of CD4, CD8, CD11b, ICAM-1, and NF-κB in infiltrating and arterial endothelial cells was observed in nontreated EAM hearts, whereas the catechins suppressed the expression. To examine expression of cytokine mRNA in EAM hearts, RNase protection assay was used. Tumor necrosis factor (TNF)-α mRNA level was markedly decreased in the catechin-treated group compared with that of the control group. On the other hand, mRNA levels of Th2 cytokines such as interleukin (IL)-4 and IL-10 in the catechin-treated group were markedly enhanced compared with those of the control group. We revealed that myocardial cell infiltration, fibrosis, and proinflammatory cytokines were enhanced in the EAM progression, and the catechins suppressed the development of these changes with suppressed cytokine expression and oxidative stress [41].
4 Catechins Suppress Oxidative Stress in Transplant Rejection
Transplantation has been established in humans; however, acute rejection and graft arterial diseases (GADs) are still problems [77–80]. Systemic biomarkers of oxidative stress are increased in kidney transplant recipients [81–86]. Fuentes et al. demonstrated the effects of N-acetylcysteine (NAC) on oxidative stress, lipids, and renal function in 25 patients with renal transplantation. They revealed that NAC treatment increased high-density lipoprotein cholesterol and antioxidant molecules in relation to glutathione peroxidase, with a positive relationship on renal function [87]. Moratalla et al. showed a possible causal influence of subclinical glucose metabolism impairment on the presentation of left ventricular diastolic dysfunction via the impaired oxidative stress status in kidney transplantation [88]. In cardiac transplantation, Nilakantan et al. examined whether continuous treatment of recipients with the superoxide dismutase (SOD) mimetic manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride (MnTmPyP) provided protection using rat acute cardiac transplant models. They revealed that the beneficial effect of MnTmPyP on graft function was related to oxidative stress and by decreasing apoptotic signaling, rather than by an effect on inflammatory cytokine gene expression [89]. Murata et al. also showed that a single injection of the macrocyclic SOD mimetic M40401 before revascularization decreased the extent of development of coronary artery disease in rat cardiac allografts [90]. The study suggests that ROS play a critical role in reperfusion injury in cardiac allografts. To confirm the antioxidative effects of catechins on rejection, we have used a class II allo-mismatch combination of mice to perform cardiac transplantation [91]. Some recipient mice were orally supplemented with tea catechins; the other transplanted mice were supplemented with normal water without the catechins for control. Although severe myocardial cell infiltration and fibrosis was observed in nontreated allografts at day 60, tea catechins markedly attenuated myocardial cell infiltration and fibrosis. Immunohistochemically, enhancement of CD4, CD8, CD11b, ICAM-1, and vascular cell adhesion molecule (VCAM)-1 expression was observed in nontreated allograft myocardium and coronary arteries. However, catechin markedly attenuated expression of all these factors. RNase protection assay was used to examine expression of cytokine mRNA in hearts. Levels of Th2 cytokine IL-10 was significantly elevated in the catechin-treated group compared with that of the nontreated group [42]. In the study, we have demonstrated that tea catechins reduced both myocardial remodeling and GAD formation with suppression of cell adhesion molecules. Because blockade of cell adhesion molecule resulted in suppression of rejection and induction of immunologic tolerance [92–95], adhesion molecule–related oxidative stress is critical in transplantation. Therefore, catechins may be clinically effective for suppression of transplant rejection because they effectively suppress oxidative stress.
5 Summary and Future Direction
In this review, we summarize the beneficial effects of catechins in cardiovascular diseases focusing on oxidative stress. Catechins exert cardiovascular protective effects through multiple mechanisms, including not only antioxidative but also anti-inflammatory, antihypertensive, antiproliferative, antithrombogenic, and lipid-lowering effects [96–100]. Although epidemiologic studies have shown a positive correlation between green tea consumption and cardiovascular health, detailed pathophysiology of catechin effects against oxidative stress has not yet been clarified. Therefore, further investigation is needed to develop a new antioxidative strategy to suppress clinical diseases using catechins or their related compounds.
References
Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA. Tea consumption and mortality after acute myocardial infarction. Circulation. 2002;105:2476–2481.
Arts ICW, Peter Hollman PCH, Feskens EJM, de Mesquita HBB, Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am J Clin Nutr. 2001;74:227–232.
Kuriyama S, Shimazu T, Ohmori K, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006;296:1255–1265.
Shimazu T, Kuriyama S, Hozawa A, et al. Dietary patterns and cardiovascular disease mortality in Japan: a prospective cohort study. Int J Epidemiol. 2007;36:600–609.
Kuriyama S. The relation between green tea consumption and cardiovascular disease as evidenced by epidemiological studies. J Nutr. 2008;138:1548S–1553S.
Ishikawa T, Suzukawa M, Ito T, et al. Effect of tea flavonoid supplementation on the susceptibility of low-density lipoprotein to oxidative modification. Am J Clin Nutr. 1997;66:261–266.
Junkun J, Selman SH, Swiercz R, Skrzypczak-Jankun E. Why drinking tea could prevent cancer. Nature. 1997;387:561.
Beecher GR, Warden BA, Merken H. Analysis of tea polyphenols. Proc Soc Exp Biol Med. 1999;220:267–270.
Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406.
Lin YL, Lin JK. (–)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-kappaB. Mol Pharmacol. 1997;52:465–472.
Campbell EL, Chebib M, Johnston GAR. The dietary avonoids apigenin and (−)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABAA receptors. Biochem. Pharmacol. 2004;68:1631–1638.
Stewart AJ, Mullen W, Crozier A. On-line high-performance liquid chromatography analysis of the antioxidant activity of phenolic compounds in green and black tea. Mol. Nutr. Food Res. 2005;49:52–60.
Qiong G, Baolu Z, Meifen L, Shengrong S, Wenjuan X. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim. Biophys. Acta. 1996;1304:210–222.
Matsuo N, Yamada K, Shoji K, Mori M, Sugano M. Effect of tea polyphenols on histamine release from rat basophilic leukemia (RBL-2H3) cells: the structure-inhibitory activity relationship. Allergy. 1997;52:58–64.
Dreosti IE. Bioactive ingredients: antioxidants and polyphenols in tea. Nutr Rev. 1996;54:51–58.
Rice-Evans CA, Miller NJ, Paganga G. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Med. 1996;20:933–956.
Ahmad N, Gupta S, Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor-kB in cancer cells versus nor mal cells. Arch Biochem Biophys. 2000;376:338–346.
Khan SG, Katiyar SK, Agarwal R, Makhtar H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: possible role in cancer chemoprevention. Cancer Res. 1992;52:4050–4052.
Guo Q, Zhao B, Li M, Shen S, Xin W. Studies on protective mechanism of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim Biophys Acta. 1996;1304:210–222.
Yoshida T, Mori K, Hatano T, Okumura T. Studies on inhibition mechanism of autooxidation by tannins and flavonoids. V. Radical scavenging effects of tannins and related polyphenols on 1,1-diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull. 1989;37:1919–1921.
Jovanavic SV, Hara Y, Steenken S, Simic MG. Antioxidant potential of gallocatechins. A pulse radiolysis and laser photolysis study. J Am Chem Soc. 1997;119:5337–5343.
Yin ST, Tang ML, Su L, Chen L, Hu P, Wang HL, Wang M, Ruan DY. Effects of Epigallocatechin-3-gallate on lead-induced oxidative damage. Toxicology. 2008;249:45–54.
Djamali A. Oxidative stress as a common pathway to chronic tubulointerstitial injury in kidney allografts. Am J Physiol Renal Physiol. 2007;293:F445–F455.
Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581.
Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247.
Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res. 2006;99:924–932.
Hildeman DA, Mitchell T, Kappler J, Marrack P. T cell apoptosis and reactive oxygen species. J Clin Invest. 2003;111:575–581.
Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxid Redox Signal. 2002;4:405–414.
Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267.
Minuz P, Patrignani P, Gaino S, Degan M, Menapace L, Tommasoli R, Seta F, Capone ML, Tacconelli S, Palatresi S, Bencini C, Del VC, Mansueto G, Arosio E, Santonastaso CL, Lechi A, Morganti A, Patrono C. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease. Circulation. 2002;106:2800–2805.
Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–593.
Haidara MA, Yassin HZ, Rateb M, Ammar H, Zorkani MA. Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr Vasc Pharmacol. 2006;4:215–227.
Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–192.
Nickenig G, Harrison DG. The AT1-type angiotensin receptor in oxidative stress and atherogenesis. Part I: oxidative stress and atherogenesis. Circulation. 2002;105:393–396.
Himmelfarb J, McMonagle E. Albumin is the major plasma protein target of oxidant stress in uremia. Kidney Int. 2001;60:358–363.
Himmelfarb J, McMonagle E, McMenamin E. Plasma protein thioloxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000;58:2571–2578.
Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538.
Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderatetoseverechronickidneydisease. Kidney Int. 2004;65:1009–1016.
Benelli R, Vene R, Bisacchi D, Garbisa S, Albini A. Anti-invasive effects of green tea polyphenol epigallocatechin-3-gallate (EGCG), a natural inhibitor of metallo and serine proteases. Biol Chem. 2002;383:101–105.
Suzuki J, Ogawa M, Maejima Y, et al. Tea catechins attenuate chronic ventricular remodeling after myocardial ischemia in rats. J Mol Cell Cardiol. 2007;42:432–440.
Suzuki J, Ogawa M, Futamatsu H, Kosuge H, Sagesaka YM, Isobe M. Tea catechins improve left ventricular dysfunction, suppress myocardial inflammation, fibrosis, and alter cytokine expression in rat autoimmune myocarditis. Eur J Heart Fail. 2007;9:152–159.
Suzuki J, Ogawa M, Sagesaka YM, Isobe M. Tea catechins attenuate ventricular remodeling and graft arterial diseases in murine cardiac allografts. Cardiovasc Res. 2006;69:272–279.
Penn MS. The role of leukocyte-generated oxidants in left ventricular remodeling. Am J Cardiol. 2008;101:30D–33D.
Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112:2812–2820.
Shishehbor MH, Brennan ML, Aviles R, Fu X, Penn MS, Sprecher DL, Hazen SL. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426–431.
Hoffmeyer MR, Jones SP, Ross CR, Sharp B, Grisham MB, Laroux FS, Stalker TJ, Scalia R, Lefer DJ. Myocardial ischemia/reperfusion injury in NADPH oxidase-deficient mice. Circ Res. 2000;87:812–817.
Askari A, Brennan ML, Zhou X, Drinko J, Morehead A, Thomas JT, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase and plasminogen activator inhibitor-1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197:615–624.
Jones SP, Greer JJ, Ware PD, Yang J, Walsh K, Lefer DJ. Deficiency of iNOS does not attenuate severe congestive heart failure in mice. Am J Physiol Heart Circ Physiol. 2005;288:H365–H370.
Rueckschloss U, Galle J, Holtz J, Zerkowski HR, Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation. 2001;104:1767–1772.
Wassmann S, Laufs U, Baumer AT, Muller K, Ahlbory K, Linz W, Itter G, Rosen R, Bohm M, Nickenig G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–1457.
Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–1142.
Creemers E, Cleutjens J, Smits J, Heymans S, Moons L, Collen D, Daemen M, Carmeliet P. Disruption of the plasminogen gene in mice abolishes wound healing after myocardial infarction. Am J Pathol. 2000;156:1865–1873.
Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62.
Morishita R, Sugimoto T, Aoki M, et al. In vivo transfection of cis element “decoy” against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997;3:894–899.
Li C, Browder W, Kao RL. Early activation of transcription factor NF-κB during ischemia in perfused rat heart. Am J Physiol. 1999;45:H543–H552.
Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734.
Chandrasekar B, Freeman GL. Induction of nuclear factor B and activation protein 1 in postischemic myocardium. FEBS Lett. 1997;401:30–34.
Onai Y, Suzuki J, Kakuta T, et al. Inhibition of IkB phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;63:51–59.
Haas GJ. Etiology, evaluation, and management of acute myocarditis. Cardiol Rev. 2001;9:88–95.
Oakley CM. Myocarditis, pericarditis and other pericardial diseases. Heart. 2000;84:449–454.
Batra AS, Lewis AB. Acute myocarditis. Curr Opin Pediatr. 2001;13:234–239.
Zee-Cheng CS, Tsai CC, Palmer DC, Codd JE, Pennington DG, Williams GA. High incidence of myocarditis by endomyocardial biopsy in patients with idiopathic congestive cardiomyopathy. J Am Coll Cardiol. 1984;3:63–70.
Parrillo JE, Aretz HT, Palacios I, Fallon JT, Block PC. The results of transvenous endomyocardial biopsy can frequently be used to diagnose myocardial diseases in patients with idiopathic heart failure: endomyocardial biopsies in 100 consecutive patients revealed a substantial incidence of myocarditis. Circulation. 1984;69:93–101.
Kodama M, Matsumoto Y, Fujiwara M, Masani F, Izumi T, Shibata A. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol. 1990;57:250–262.
Kodama M, Izumi T. Experimental autoimmune myocarditis. Acta Med Bio. 1991;39:1–10.
Kitabayashi H, Isobe M, Watanabe N, Suzuki J, Yazaki Y, Sekiguchi MFTY. 720 prevents development of experimental autoimmune myocarditis through reduction of circulating lymphocytes. J Cardiovasc Pharmacol. 2000;35:410–416.
Yokoseki O, Suzuki J, Kitabayashi H, et al. cis element decoy against nuclear factor-κB attenuates development of experimental autoimmune myocarditis in rats. Circ Res. 2001;89:899–906.
Futamatsu H, Suzuki J, Kosuge H, et al. Attenuation of experimental autoimmune myocarditis by blocking activated T cells through inducible costimulatory molecule pathway. Cardiovasc Res. 2003;59:95–104.
Liu W, Nakamura H, Shioji K, et al. Thioredoxin-1 ameliorates myosin-induced autoimmune myocarditis by suppressing chemokine expressions and leukocyte chemotaxis in mice. Circulation 2004;110:1276–1283.
Izumi T, Suzuki K, Saeki M, et al. An ultrastructural study on experimental autoimmune myocarditis with special reference to effector cells. Eur Heart J. 1995;16(suppl O):75–77.
Matsue H, Edelbaum D, Shalhevet D, et al. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol. 2003;171:3010–3018.
Shioji K, Kishimoto C, Sasayama S. Fc receptor–mediated inhibitory effect of immunoglobulin therapy on autoimmune giant cell myocarditis: concomitant suppression of the expression of dendritic cells. Circ Res. 2001;89:540–546.
Kishimoto C, Kawamata H, Sakai S, et al. Role of MIP-2 in coxsackievirus B3 myocarditis. J Mol Cell Cardiol. 2000;32:631–638.
Kishimoto C, Kawamata H, Sakai S, et al. Enhanced production of macrophage inflammatory protein 2 (MIP-2) by in vitro and in vivo infections with encephalomyocarditis virus and modulation of myocarditis with an antibody against MIP-2. J Virol. 2001;75:1294–1300.
Toyozaki T, Saito T, Shiraishi H, et al. Macrophage inflammatory protein-1alpha relates to the recruitment of inflammatory cells in myosin-induced autoimmune myocarditis in rats. Lab Invest. 2001;81:929–936.
Song HK, Noorchashm H, Lin TH, et al. Specialized CC-chemokine secretion by Th1 cells in destructive autoimmune myocarditis. J Autoimmun. 2003;21:295–303.
Taylor DO, Edwards LB, Boucek MM, Trulock EP, Keck BM, Hertz MI. The registry of the international society for heart and lung transplantation: twenty-first official adult heart transplant report—2004. J Heart Lung Transplant. 2004;23:796–803.
Pinney SP, Mancini D. Cardiac allograft vasculopathy: advances in understanding its pathophysiology, prevention, and treatment. Curr Opin Cardiol. 2004;19:170–176.
Suzuki J, Isobe M, Aikawa M, et al. Nonmuscle and smooth muscle myosin heavy chain expression in rejected cardiac allograft. A study in rat and monkey models. Circulation. 1996;96:1118–1124.
Hosenpud JD. Immune mechanism of cardiac allograft vasculopathy: an update. Transplant Immunol. 1993;1:237–249.
Campise M, Bamonti F, Novembrino C, Ippolito S, Tarantino A, Cornelli U, Lonati S, Cesana BM, Ponticelli C. Oxidative stress in kidney transplant patients. Transplantation. 2003;76:1474–1478.
Cristol JP, Vela C, Maggi MF, Descomps B, Mourad G. Oxidative stress and lipid abnormalities in renal transplant recipients with or without chronic rejection. Transplantation. 1998;65:1322–1328.
Raj DS, Lim G, Levi M, Qualls C, Jain SK. Advanced glycation end products and oxidative stress are increased in chronic allograft nephropathy. Am J Kidney Dis. 2004;43:154–160.
Simic-Ogrizovic S, Simic T, Reljic Z, Markovic S, Blagojevic R, Radivojevic D, Lezaic V, Djukanovic L, Mimic-Oka J. Markers of oxidative stress after renal transplantation. Transpl Int. 1998;11(Suppl1):S125–S129.
Simmons EM, Langone A, Sezer MT, Vella JP, Recupero P, Morrow JD, Ikizler TA, Himmelfarb J. Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end-stage renal disease patients. Transplantation. 2005;79:914–919.
Vos IH, Joles JA, Rabelink TJ. The role of nitric oxide in renal transplantation. Semin Nephrol. 2004;24:379–388, Biochem. 2002;86:376–393.
Ruiz Fuentes MC, Moreno Ayuso JM, Ruiz Fuentes N, Vargas Palomares JF, Asensio Peinado C, Osuna Ortega A. Treatment with N-acetylcysteine in stable renal transplantation. Transplant Proc. 2008;40:2897–2899.
Osorio Moratalla JM, Ferreyra Lanatta C, Baca Morilla Y, Romero Ramírez E, Moreno Ayuso JM, Galindo Sacristán P, Osuna Ortega A. Left ventricular structure and function in long-term kidney transplantation: the influence of glucose metabolism and oxidative stress. Transplant Proc. 2008;40:2912–2915.
Nilakantan V, Zhou X, Hilton G, Shi Y, Baker JE, Khanna AK, Pieper GM. Antagonizing reactive oxygen by treatment with a manganese (III) metalloporphyrin-based superoxide dismutase mimetic in cardiac transplants. J Thorac Cardiovasc Surg. 2006;131:898–906.
Murata S, Miniati DN, Kown MH, et al. Superoxide dismutase mimetic M40401 reduces ischemia-reperfusion injury and graft coronary artery disease in rodent cardiac allografts. Transplantation. 2004;78:1166–1171.
Suzuki J, Cole SE, Batirel S, et al. Tumor necrosis factor receptor-1 and -2 double deficiency reduces graft arterial disease in murine cardiac allografts. Am J Transplant. 2003;3:968–976.
Isobe M, Yagita H, Okumura K, Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992;255:1125–1127.
Kosuge H, Suzuki J, Gotoh R, et al. The induction of immunological tolerance to cardiac allograft by simultaneous blockade of inducible co-stimulator (ICOS) and CTLA4 pathway. Transplantation. 2003;75:1374–1379.
Kosuge H, Suzuki J, Kakuta T, et al. Attenuation of graft arterial disease by manipulation of the LIGHT pathway. Arterioscler Thromb Vasc Biol. 2004;24:1409–1415.
Koga N, Suzuki J, Kosuge H, et al. The blockade of the interaction between PD-1 and PD-L1 accelerates graft arterial disease in cardiac allografts. Arterioscler Thromb Vasc Biol. 2004;24:2057–2062.
Babu PV, Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 2008;15:1840–1850.
Wolfram S. Effects of green tea and EGCG on cardiovascular and metabolic health. J Am Coll Nutr. 2007;26:373S–388S.
Shenouda SM, Vita JA. Effects of flavonoid-containing beverages and EGCG on endothelial function. J Am Coll Nutr. 2007;26:366S–372S.
Basu A, Lucas EA. Mechanisms and effects of green tea on cardiovascular health. Nutr Rev. 2007;65:361–375.
Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea–a review. J Am Coll Nutr. 2006;25:79–99.
Acknowledgments
We would like to thank Ms. Noriko Tamura and Ms. Yasuko Matsuda for excellent technical assistance. All authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LCC
About this chapter
Cite this chapter
Suzuki, Ji., Isobe, M., Morishita, R., Nagai, R. (2010). Antioxidation in Prevention of Cardiovascular Diseases – An Effect of Polyphenols. In: Bondy, S., Maiese, K. (eds) Aging and Age-Related Disorders. Oxidative Stress in Applied Basic Research and Clinical Practice. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-60761-602-3_14
Download citation
DOI: https://doi.org/10.1007/978-1-60761-602-3_14
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-60761-601-6
Online ISBN: 978-1-60761-602-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)