Abstract
An integrated biotechprocess has been developed for fungal biomass protein production and wastewater reclamation from starch processing wastewater. The process resulted in producing 9.0 g/L fungal biomass, and removing total suspended solids, 95% BOD and 75% nitrogen. The biomass products contained 45% protein and appreciable quantities of amino acids, and they would be nutritive and edible for animal consumption. The reclaimed wastewater could be used for farm irrigation. This technology appeared to be technically feasible and economically beneficial for food and agricultural industries.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
20.1 Introduction
Each year, billions of tons of organic substances are processed through the food and other processing industries. A significant portion of the organic substances become organic pollutants through wastewater streams. This is a major environmental concern in most parts of the world. Yet, much of these organic substances can potentially be utilised as valuable resources. Sustainability requires not only the treatment of the wastewater streams but also the recovery and utilization of resources, both the water itself and the substances in it. Bioconversion of wastes is the natural way to recover the useful resources. In industries, biotechnology can be used to facilitate the natural recycling processes. Biotechnological treatment of wastewater streams can produce valuable end-products, such as microbial biomass protein (MBP), utilizing the organic substances in wastewater as substrates.
In order to achieve the dual objectives of water reclamation and MBP production, it is necessary to have (a) suitable organism species for any given wastewater streams, which can utilise the organic pollutants in wastewater as the substrates with no or minimum pre-treatment and nutrient supplementation, and (b) suitable bioreactor system that can be operated under non-aseptic conditions and facilitate both the MBP production and water reclamation. The cultivation of suitable fungal species with starch processing wastewater (SPW) in external air lift bioreactors (EALB) can achieve these objectives. Pilot plant studies have demonstrated that the system is technically feasible and economically beneficial for the simultaneous production of fungal biomass proteins (FBP) and wastewater reclamation (1, 2). These types of processes can potentially be extended to the treatment and utilization of organic wastewater streams from other industries.
20.2 Fungal Biomass Protein Production
20.2.1 Fungal Biomass Protein
Microfungi play an important role in food industries. They have a number of properties, which make them important both scientifically and industrially. They have a wide range of enzymes and are capable of metabolizing complex mixtures of organic compounds occurring in most wastes (3–5). The production of biomass proteins from microfungi is particularly attractive for a number of reasons. These include the following: (a) the cells of most species of microfungi contain reasonably high levels of proteins, (b) microfungi contain low levels of nucleic acids when compared to yeasts and bacteria, (c) FBP products are relatively stable and can be easily separated from the cultivation media and (d) food produced from fungi is traditionally eaten in many parts of the world (6). In addition, fresh fungal biomass has a pleasant odour, which further facilitates the utilization of the biomass. For example, fresh dewatered fungal biomass products could be directly supplied to an animal farm as stock feeds (7). In this case, the processing costs of drying are avoided.
Another distinctive advantage of microfungus cultivation is the easiest way of separating fungal biomass from the culture media. The cost of separating the biomass from the spent cultivated broth is a significant part of the capital and operating costs for biomass protein production. In the case of microfungus cultivation, the filamentous or pellet form of the microfungi leads to an easy and cheap harvesting of mycelial biomass.
The nutrient contents of the FBP produced by Aspergillus oryzae DAR 3,699 and Rhizopus arrhizus DAR 2,062 from SPW have been analysed in detail (7–9). The biomass of both Aspergillus and Rhizopus contain more than 45% (w/w) crude proteins. The analytical results for crude proteins, metabolizable energy, fat, fibre and other nutrient quality parameters are listed in Table 20.1; and the amino acid compositions of the FBP are listed in Table 20.2. Tables 20.1 and 20.2 indicate that the nutrient quality of the FBP is relatively high. For example, Table 20.2 shows that the fungal biomass contained appreciable quantities of essential amino acids that are clearly superior to those of the FAO reference protein of UN WHO (10), except for amino acids tryptophan and tyrosine, which appear to have slightly lower contents than the FAO references.
20.2.2 Fungal Biomass Protein Production
The production of FBP from raw materials is a field with the largest volume capacity in modern industrial biotechnology. It is one of the most investigated topics in biotechnology. Microfungi have been used extensively in the fermentation industries as a traditional beverage and for fermented foods in the Orient for more than 2,000 years (3, 11, 12).
Because of the properties of easy harvesting, low nucleic acid content and acceptability as traditional food, filamentous fungi have become more and more attractive in MBP production and biotechnological waste treatment processes. A considerable amount of research is being devoted to the growth of cellulose fungi such as Trichodermasp. on cheap cellulosic materials or waste products. Two microfungi, Trichoderma viride and Geotrichum candidum, are grown in a submerged culture for 60 h, giving rise to a product containing 20% crude protein and 23% fibre with an in vitro digestibility of about 65% (13, 14). Attempts have also been made to grow filamentous fungi on plant cell biomass. Fungi, including Botritis cinerea and Trichoderma viride, grow well on waste plant cell biomass as the sole nutrient source (15). Botritis cinerea, a plant pathogen, which has a recognised ability to degrade plant cells rapidly, is a particularly suitable fungus for MBP production when grown on waste plant cells. The starch using fungi, such as Aspergillus niger or R. arrhizus, is hydrolysed to glucose and the protein content increases as the fungus grows (16, 17).
In the Pekilo process, mycelia of the filamentous fungus Paecilomyces variothi are continuously cultivated in a medium, which contains dissolved carbohydrates. The yield of biomass approached 55% of the reducing substrate consumed a value exceeding that originally anticipated. The dried Pekilo protein is sold to feed compounding mills and has a crude protein content of 52–57% (18). Several fungal processes in a submerged culture for the treatment of starch wastes have been described. Balagopal and Maini (19) grew fungi in a suspension culture containing 25 g/L of cassava starch wastes and found Aspergillussp. NRRL 330 and Rhizopussp. to be superior in terms of mycelial weight and protein production. Trichoderma harzianum can be grown in suspensions of cassava meal (4%), and an enriched product with 38% protein from the original cassava containing 2.4% protein can be obtained. Aspergillus niger mutants have been applied for increasing the protein content of cassava starch wastes up to 20%. Penicillium notatum and P. digitatum also grew well on potato processing wastes, resulting in a biomass of 9–24 g/L. Solid-state cultures are of minor importance today, although a number of enzymes are still produced from Aspergillus, Mucor, or Rhizopus species. Fungi can be grown with almost any waste products that contain carbohydrates, such as confectionery and distillery waste, vegetable waste, and wood processing effluents (4, 20–22).
20.2.3 Fungal Biomass Protein Production from Starch Processing Wastewater
The manufacturing of starch products from wheat, corn and potato uses large quantities of water. The high level of water usage results in the generation of vast quantities of SPW. The wastewater streams contain high levels of chemical oxygen demand (COD) and other pollutants, as shown in Table 20.3 for a typical SPW stream (1, 2), which contained an organic loading of 16–22 g/L COD and 2.1–3.1 g/L starch. Therefore, they are highly polluting and can impose heavy loads on the environment or could be expensive in terms of sewer disposal. On the other hand, SPW represents an important energy-rich resource, with a relatively high percentage of carbohydrates, cellulose, protein and plant nutrients (Table 20.3). This resource has been shown to be a suitable substrate for biological conversion to FBP (1, 2, 20).
In addition, starch waste materials also offer the advantages of availability and consistent quality, being a readily convertible material at competitively low costs from which a wide variety of products can be produced. The pH range of SPW (5.2–6.0) and the temperature (around 38∘C) of SPW are also highly suitable for fungal cultivation. The low pH range of the SPW is particularly important as this inhibits the growth of contaminating bacteria that may be present in a non-aseptic culture environment.
A system of FBP production and SPW reclamation has been extensively studied (1, 2, 7–9, 15) and is used as a model system here. In this system, SPW with characteristics as listed in Table 20.3 were used as the culture media, and two enzyme producing fungal species of A. oryzae DAR 3,699 and R. arrhizus DAR 2,062 were used for FBP production. The two fungal species were selected from a group of potential fungal species in a comprehensive screening and selection study and were found to be particularly suitable for FBP production and wastewater reclamation (1). Laboratory testing indicated that they have fast growth kinetics and the biomass produced is readily separated from the liquid phase. The optimal temperature for the cultivation was also determined to be around 35∘C.
The seed cultures used in this system were prepared in a three-stage process as spore, suspensions and pre-cultures. The strains of the fungal species were maintained on potato dextrose agar (PDA) slants at 4∘C and recultured bimonthly. Phialospore suspensions were prepared from PDA slants on Petri dishes. The slants were incubated at 28∘C for 4 days. Spores were harvested from the surface of each slant into 10 mL of sterile water. This suspension containing \(1 \times 1{0}^{7}\mbox{ \textendash }1 \times 1{0}^{8}\) spores per mL, determined by haemocytometer counts, was used as inoculum.
The fungal biomass growth kinetics of the system is illustrated in Fig. 20.1, in which the amount of biomass produced (biomass yield) are plotted against the batch cultivation time (7, 8), together with corresponding COD reaction during the cultivation process. The biomass growth follows the typical characteristics of microbial growth kinetics. The kinetics profile contains four growth phases. The first phase is the lag phase. In this phase of the first 2 h of cultivation, there is little fungal growth, as the fungal cells grow under an incubation process in the culture medium. After this particular incubation period, the growth rate of the fungal cells increases dramatically, as they enter the exponential growth phase. In Fig. 20.1, the exponential growth phase occurs between 6 and 10 h, and 8 and 12 h for A. oryzae 3,699 and R. arrhizus 2,062, respectively.
During the exponential growth, the growth kinetics of the biomass can be described by the Monad kinetics as follows:
where μ is specific growth rate of the biomass, X is the biomass concentration, t is time, C s is the concentration of the limiting substrate, and μmax and K m are constants. The specific growth rates of the system in Fig. 20.1 can be estimated to be 0. 18 h− 1 for A. oryzae 3,699 and 0. 14 h− 1 for R. arrhizus 2,062.
At the end of the exponential growth phase, the maximum fungal biomass concentration is reached. Beyond that, the biomass growth moves into a stationary phase. During the stationary phase, the microfungi are stable in the growth medium for more than 10 h (Fig. 20.1). The last phase is the decline phase or death phase. In this phase, a number of viable fungus cells and autolysis of cells occur. Usually, contamination with other species also occurs, especially due to an increasing pH (higher than 6.5) during the incubation period, which is conducive to bacterium growth competing for the limited nutrients. For the cultivation of the fungal species, as shown in Fig. 20.1, the decline phase starts at around 22 h.
The COD reduction profiles in Fig. 20.1 show that COD reductions of around 90–95% are achieved after the exponential growth phase and maintained during the stationary phase. The COD reductions start to deteriorate at the start of the decline phase, corresponding to the autolysis of the fungal cells, which releases COD into the water phase. It is also noted that, although biomass yield and the COD reduction remained relatively stable, and the variations are relatively small, during the stationary phase, the maximum biomass yields and the maximum COD reduction may not occur at the same time. Therefore, optimal operating conditions for maximum biomass production and those for maximum COD reduction may be slightly different.
Table 20.4 shows the typical water quality parameters of the reclaimed wastewater for the fungal cultivation with SPW (2, 23). Table 20.4 demonstrates a high efficiency of biodegradation and the removal of starch materials with high bioconversion rates. Associated with valuable FBP production, removal of more than 95% organic loading and insoluble solids, and approximately 75% N and P from the SPW are achieved. The reclaimed wastewater contains low organic compounds and very low minerals, and may be used for a number of applications such as farm irrigation.
Another key parameter for microbial cultivation is the conversion yield. For the fungal cultivation with SPW, the COD can be used as the measure of substrate concentration and the conversion yield from COD to biomass can be defined as
Example 1.
From the growth kinetics in Fig. 20.1, estimate (a) the specific growth rate of A. oryzae and (b) the COD to biomass yield.
Answer
(a) From Fig. 20.1, at the cultivation time of 8 h, the biomass growth rate can be estimated from the slope of the growth phase to be about 1.25 g/L/h and the biomass concentration is about 7.0 g/L. Therefore, the specific growth rate is 1.25/7.0 = 0.18 L/h. (b) From Fig. 20.1, the maximum biomass concentration is about 8.3 g/L and the corresponding COD reduction is about 96%. The initial COD value of the SPW is \(20,670\,\mathrm{mg}/\mathrm{L} = 20.67\,\mathrm{g}/\mathrm{L}\). Therefore, the change in COD is \(-0.96 \times 20.67 = -19.8\,\mathrm{g}/\mathrm{L}\), and the conversion yield is \(-8.3/(-19.8) = 0.42.\)
20.3 Reactor Configuration and Process Flow Diagram
20.3.1 Reactor Configuration
The central processing unit in an FBP production plant is the external air-lifted bioreactor (EALB), which is schematically illustrated in Fig. 20.2. The bioreactor has three major sections: a riser (main column in the diagram), a downcomer (side column) and a gas separator (top column), as well as the controlling and monitoring accessaries. At the bottom of the riser, an air sparger is fitted to provide for aeration. The liquid flows upwards in the riser and downwards in the downcomer. The gas flows as bubbles together with the liquid. The gas flow is actually responsible for inducing the circulation flows, which is necessary to provide the mixing and to facilitate the oxygen transfer processes. To further facilitate the oxygen transfer, a second air sparger may also be fitted at the bottom of the downcomer. In this case, the air flow in the downcomer is also upwards, counter-current to the liquid flow. It has been shown that the use of a second air diffuser can significantly increase the oxygen transfer efficiencies (24, 25). The riser provides the main reaction volume and may also be fitted with a water jacket to control the reaction temperature. The gas separator at the top of the reactor is needed to provide the space for gas liquid separation and the mechanism for the removal of products. A micro screen may also be fitted in the gas separator for the separation and recycling of the fungal biomass during continuous cultivations.
In designing the dimensions of an EALB, the ratio between the cross section areas of the downcomer and the riser is a key parameter, which is in the range of 0.3–0.6. Another important ratio is that of length to diameter of the riser and the downcomer (the lengths of the two are about the same), which can be within the range of 6–12. A higher ratio increases the retention times of the air bubbles and hence the oxygen transfer rate, while a lower ratio leads to better mixing characteristics and a lower pressure drops. The diameter of the gas separated is usually 1.5–2 times of that of the riser to ensure effective gas liquid separation. The height of the gas separator can be in the range of 1–1.5 times of the diameter to provide sufficient space for gas liquid separation and product removal, as well as the accommodation of accessaries.
Example 2.
Design the key dimensions of an EALB with a reaction volume of 1,000 l (1. 0 m3), if the area ratio and the length (L) to diameter ratio (d) are to be 0.48 and 8, respectively.
Answer
As the cross sectional area is proportional to the square of the diameter, the diameter ratio between the downcomer and the riser is 0. 480. 5 = 0. 69. If the diameter of the riser is d, then the total working volume of the reactor is
20.3.2 Process Flow Diagram
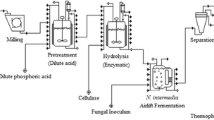
A process flow diagram of a pilot plant for the FBP production and wastewater reclamation is illustrated in Fig. 20.3 (7). The pilot plant consists of the cultivation, separation and drying stages. Drum rotary filter with 200 μm stainless steel mesh is used for separating the fungal biomass from cultivated broth. The separated wet biomass is dewatered by belt-pressure filter, and then followed by a flash air drying process to dry the final products. The filtered effluent is collected as the reclaimed water.
In practical operations, there may be two processing options. After a simple filtration, the wet FBP products could be directly supplied to an animal farm due to their appetizing flavours. In this case, the drying process is unnecessary. Another option is to transfer the dewatered product for stockfeed production, which not only reduces the costs of capital and operation but may also produce a new stockfeed with high protein content as well.
20.4 Oxygen Transfer and Hydrodynamics
20.4.1 Oxygen Transfer
The cultivation of microfungi in EALB is an aerobic process and sufficient oxygen supply in the liquid phase is needed to ensure that the cultivation process is viable. Oxygen supply is usually provided through aeration with air. Therefore, the rate of oxygen transfer from the gas phase to the liquid phase is critical. In fungal cultivation processes, it is usually required that the dissolved oxygen (DO) level in the liquid phase to be at least 50% of the saturation value (24, 25).
The oxygen transfer rate is calculated by the following equation,
where \({N}_{{\mathrm{O}}_{\mathrm{2}}}\) is the oxygen transfer rate per unit reactor volume, K L a is the mass transfer coefficient for oxygen transfer, DO is the dissolved oxygen concentration in the liquid phase and DO* is the saturation oxygen concentration in the liquid phase. The value of DO∗ can be obtained from Henry’s law and the partial pressure of oxygen in the gas phase.
Example 3.
Calculate the unit volume oxygen transfer rate if the oxygen transfer coefficient is 600 L/h. Assume that the DO level in the liquid phase is 0.1 mmol/L and the saturation value is 0.25 mmol/L.
Answer
\({N}_{{\mathrm{O}}_{2}} = 600 \times (0.25 - 0.1) = 90\,\mathrm{mmol}/\mathrm{h}\).
From Eq. (20.3), the oxygen transfer rate can be increased by the value of (\(D{O}^{{_\ast}}\mbox{ \textendash }\mathrm{DO}\)) (driving force). As a minimum DO level must be kept in the liquid phase in order to maintain a viable cultivation process, the driving force for oxygen transfer may be increased by increasing the partial pressure of the oxygen in the gas phase. For example, instead of atmospheric air supply, pressurized air or pure oxygen can be used as the gas phase.
Example 4.
Calculate the oxygen transfer rate if the air pressure is doubled in the previous example. Assume that the oxygen transfer coefficient, the DO level in the liquid phase, remains the same.
Answer
The new oxygen transfer rate is \(600 \times (0.25 \times 2 - 0.1) = 240\,\mathrm{mmol}/\mathrm{h}\).
In practice, the improvement in oxygen transfer rate by increasing the air pressure will be much less that in the previous example, in the range of 20–40%, as the DO level in the liquid phase will also be higher.
The oxygen mass transfer coefficient is affected by a range of parameters, including the diffusion coefficient of oxygen in the liquid phase, the quantity and size distribution of the air bubbles, which in turm are determined by the rheological properties of the liquid phase, aerator design and the hydrodynamic characteristics (mixing, velocity and gas hold-up) of the reactor system. For a given system, when the reactor design is completed, these effects will be determined by the operational parameters, principally the aeration rate. Therefore, the determination of a proper aeration rate is critical to the operation of an EALB system.
The oxygen transfer coefficient is usually determined through experimentation and correlation, in which the following equation can be used for the cultivation of filamentous fungi (25).
where G is molar air flow rate (mol/h), y 1 and y 2 are the oxygen content of inlet and exit air (mol %), V is the liquid phase volume in reactor (l), P T is the total pressure in system (atm), DO is the dissolved oxygen level in liquid phase (mol/L) measured at top of the riser, and H is Henry’s constant (8. 345 ×102 l atm ∕ mol).
20.4.2 Rheological Properties and DO levels
The fluids of mycelial culture contain suspended mycelial particles and exhibits non-Newtonian flow behaviours. This is further complicated by the changes in the concentration of fungal cells during the course of the cultivation. A set of typical properties of the fluids at an operating temperature of 25∘C for the model fungal cultivation system are given in Table 20.5, and the profiles of mycelial biomass concentration (broth density), viscosity and DO level in the culture broth during a batch cultivation are shown in Fig. 20.4. The variation of mycelial biomass concentration follows a typical logarithmic growth phase. The rheological characteristics of the cultivated broth become increasingly viscous and non-Newtonian as the biomass concentration increased. It was observed that turbulence in the riser subsides considerably and the bubble size distribution also changes. Even at a relatively low biomass concentration of 2 g/L, large spherical-capped bubbles (4 mm in diameter) become predominant in the riser. As the broth became highly viscous, large bubbles are present in the riser along with some very small bubbles. The large bubbles rise rapidly through the riser and disengage at the top gas separator, while the smaller bubbles remain trapped inside the reactor (26).
In Fig. 20.4, the variations in DO concentrations within the broth have four phases during mycelial biomass growth: a high lag phase, a decrease phase, and increase phase and a low lag phase. During the high lag phase within the first few hours of cultivation, the DO remains at a relatively constantly high level, approaching the saturation, due to no or little oxygen consumption. A rapid decrease phase in DO level is followed during the exponential growth phase of mycelial biomass, as the O2 uptake by the mycelium is higher than the O2 transfer into the medium. The DO level increases slowly as the biomass growth shifts to the biomass stationary phase. This DO increase phase, however, extends over a relatively short time. After that, it remains at a constant level. This behaviour reflects the dependence of mycelial biomass growth on sufficient oxygen supply, as mycelium growth limited the O2 transfer into the cultivated broth.
20.4.3 Hydrodynamic Characteristics and Oxygen Transfer Coefficient
The gas velocity and the gas hold up are important parameters which affect the oxygen mass transfer coefficient. The measurement of the gas hold-up can be expressed as an average or overall hold-up, and the volume expansion method can be used. It is estimated as the percentage increase in volume of the liquid phase compared with that when there is no aeration in the liquid medium. Figure 20.5 shows the profiles of gas superficial velocity, gas hold-up, and the corresponding oxygen transfer coefficient in a cultivation process of the model system. The gas hold-up decreases significantly with the increase of broth viscosity, but slightly during the saturation phase. This phenomenon has been observed in other non-Newtonian solutions (27, 28). The variations of superficial gas velocity and oxygen transfer coefficient have a similar trend during the cultivation of mycelial biomass. Both decrease with biomass growth, and then remain at a constant value when mycelial biomass is at a stationary growth phase.
20.4.4 Aeration Rate and Oxygen Transfer Coefficient
As mentioned earlier, the aeration rate is a critical parameter for the hydrodynamic characteristics and oxygen mass transfer coefficient. Therefore, fundamental relationships between the air flow rate and superficial velocity, gas hold-up, oxygen transfer coefficient and DO level in the reactor are needed, and these are usually determined experimentally under different levels of biomass concentration. A set of typical relationships are shown in Figs. 20.6 and 20.7 for the model system. Here, the aeration rate is measured as the relative volume of air to the volume of the liquid phase per unit time.
From the relationships in Fig. 20.6, it can be seen that the superficial gas velocity increases linearly with the air flow rate in the range of \(0\mbox{ \textendash }1.00\,\mathrm{v}/\mathrm{v}/\mathrm{m}\), while a further increase in the air flow rate leads to a slower increase in gas velocity. The riser superficial gas velocity is usually represented by the aeration flow rate. However, Fig. 20.5 here indicates that the relationship between gas velocity and air flow rate varies with the mycelial biomass density.
From Figs. 20.6 and 20.7, the gas hold-up and the oxygen transfer rate also increases with air flow rate. Similar to the case of gas velocity, in the lower range of air flow rate, the relationships is close to linear, and then the influence of the flow rate becomes less pronounced in an increasing range of \(1.25\mbox{ \textendash }2.00\,\mathrm{v}/\mathrm{v}/\mathrm{m}\), when the mycelial biomass is highly concentrated in the bioreactor.
It can also be seen that the DO level can be improved dramatically by increasing the air flow rate, but the increasing rate of DO level decreases as the air flow rate exceeds a critical value (1.250 v/v/m) in the EALB (Fig. 20.7). At this level, the DO saturation rate is approximately 50%. Moreover, the enhancement of the DO level by increasing air flow rate in the cultivated broth with high biomass concentration is limited because of the highly viscous culture broth. For example, in Fig. 20.7, an increase in air flow rate from 1.25 to 2.00 v/v/m increases the DO by approximately 12% of saturation at biomass concentration of 2.0 g/L, by only approximately 8% increase in DO at Cb 4.0 g/L, and a negligible increase in DO at Cb 8.0 g/L. Clearly, an increase in air flow rate at a high concentration of biomass would not achieve a desired DO level to meet sufficient oxygen consumption for mycelial biomass growth.
The fundamental relationships, as shown in Figs. 20.6 and 20.7, can be used to design the aeration requirements for an EALB system. For most systems, the aeration rate can be set at a value at or higher than the critical value for the systems.
Example 5.
Design the aeration requirements for a 1,000 L EALB from the fundamental relationships in Figs. 20.6 and 20.7.
Answer
From Figs. 20.6 and 20.7, the critical value of aeration rate is about 1.25 v/v/m. Therefore, the design aeration rate can be set at slight higher at 1.5 v/v/m, and the aeration requirement is determined to be \(1.5 \times 1,000 = 1,500\ \mathrm{L}/\mathrm{m}\).
Example 6.
Estimate the oxygen transfer coefficient and the oxygen transfer rate at an air flow rate of 1.5 v/v/m. Assume that the saturation DO is 0.25 mmol/L.
Answer
From Fig. 20.6, the oxygen transfer coefficient ranges from 360 to 540 L/h at various biomass concentrations, and the corresponding saturation DO ranges from 45 to 70%. Therefore, the oxygen transfer rate ranges are as follows:
20.5 Process Design and Operation
20.5.1 Batch Process
The EALB can be operated in batch, semi-continuous, and continuous modes of operations. In the batch process, the SPW production medium is inoculated with a small amount of pre-culture, usually in the range of 5–8% (v/v), and the reactor system is operated without influent and effluent water streams. The operating temperature is usually controlled at the optimal cultivation temperature (e.g. 35∘C), and the pH of the cultivation medium can be adjusted into the optimal pH range of the culture species (e.g. 5.5–7.0). As the oxygen transfer rates usually decreases during the course of batch operations, the aeration rate is also regulated, typically starting with a lower aeration rate (e.g. 0.6 v/v/m) to a higher aeration rate (e.g. 1.2 v/v/m) to maintain a DO level above 50% of saturation.
In practice, batch cultivation finishes after the stationary phase is reached, either when the maximum biomass production or the maximum COD reduction is achieved. The reactor can then be prepared for the next batch process. Therefore, the operating time of a batch operation consists of both the cultivation time and the preparation time needed for the next batch of cultivation, both of which should be considered in the design of the reactors. The total volume (V t) of the bioreactors are designed as the product of the volumetric processing rate (F) and the total operating time (t):
Example 7.
Design the bioreactor volume required to batch process 1 l/d of SPW for the model system kinetics as given in Fig. 20.1.
Answer
From Fig. 20.1, the stationary phases for the two microfungi are reached around 10–12 h. If the operation time is set as 12 h, the reactor volume required will be
20.5.2 Semi-continuous Process
The semi-continuous mode of operation is also known as repeated fed batch process. It is conducted in a similar fashion as in the batch mode of operation. In addition, at the end of the initial cultivation cycle, when the maximum growth of the biomass has been reached, the cultivated broth is withdrawn from the bioreactor at a fixed V out ∕ V t value (ratio of the volume drawn out to the total culture volume). Afterwards, SPW medium is added into the bioreactor and the cultivated broth remained in the bioreactor serves as the inoculum for the next cycle. A set of typical results obtained from the semi-continuous cultivation of A. oryzae 3,699 and R. arrhizus 2,062 in SPW are shown in Figs. 20.8–20.10 for three different values of V out ∕ V t (8).
In the semi-continuous process, the value of the V out ∕ V t affects the productivity of the bioreactor. A lower V out ∕ V t value leaves more inoculum in the bioreactor and reduces the
shock to the microorganisms. The bioreactor shortens the lag phase needed, which in turn shortens the operation time needed and increases the overall productivity of the bioreactor. On the other hand, a higher V out ∕ V t value produces more products per cycle and reduces the preparation times needed between the cycles. Typically, a value of 0.5–0.9 is used for EALB.
The design equation for semi-continuous mode is similar to that of the batch operation, except a correction factor of V out ∕ V t is needed.
where t is the operation time needed for one cycle of cultivation.
Example 8.
Design the bioreactor volume required to process 1 l/d of SPW for the system in Fig. 20.9 with Microfungus A. oryzae and a V out ∕ V t value of 0.7.
Answer
From Fig. 20.9, the operation time needed for one cycle of cultivation is 8 h. Therefore,
20.5.3 Continuous Process
For a large scale process, the continuous operation mode is usually more efficient. However, this mode of cultivation is much more difficult to operate. For example, the biomass may accumulate on the walls and probes inside the culture vessel. Air supply also becomes a problem because the mycelium clogs up the air sparger, which leads to a low level of oxygenation and eventually unstable operations.
A continuous operation is initiated towards the completion of a batch cultivation, starting at the beginning of a stationary phase and then run at fixed liquid and air flow rates. The SPW medium is continuously fed into the bioreactor and the cultivated broth is continuously withdrawn. A recycle stream from the effluent can also be used to increase the stability of the continuous operation. When a steady state is established, the following design equation can be obtained:
where X is the biomass concentration and r X is the biomass growth rate. Equation (20.7) can be rewritten as
or
where D is the dilution rate.
The productivity and the COD reduction rate of the bioreactor is strongly affected by the dilution rate. This can be illustrated as in Figs. 20.11 and 20.12 for the continuous cultivation of A. oryzae 3,699 and R. arrhizus 2,602 on SPW at dilution rates ranging from 0.06 to 0.20 L/h (7). The results clearly demonstrate that an optimal dilution rate based on either the biomass productivity or COD reduction rate can be obtained. For example, a maximum biomass productivity of 0.92 g/L/h for A. oryzae 3,699 and 0.87 g/L/h for R. arrhizus 2,062 are obtained at a dilution rate of 0.14 and 0.12 L/h, respectively. Similarly, from Fig. 20.12, the highest COD reduction rate of 1.91 g/L/h for A. oryzae 3,863 is obtained at a dilution rate of 0.14 L/h with 96.5% COD reduction and for R. arrhizus 2,062, a maximum COD reduction rate of 1.75 g/L/h with 95.8% COD reduction at a dilution rate of 0.12 L/h.
Example 9.
Design the bioreactor volume required to continuously process 1 L/d of SPW for the system in Figs. 20.11 and 20.12 with microfungus A. oryzae for maximum biomass production and COD reduction.
Answer
From Figs. 20.11 and 20.12, the optimal dilution rate for both biomass productivity and COD reduction for R. arrhizus 2,062 is 0.14 L/h. Therefore, from Eq. (20.8),
20.6 Summary and Conclusions
With the development of an EALB system, simultaneous production of fungal biomass protein and wastewater reclamation from starch processing wastewater can be effectively achieved. In particular, microfungi A. oryzae and R. arrhizus can be successfully cultivated using raw SPW, while treating the SPW. The processes have a number of advantages including (1) a high bioconversion efficiency of starch materials and a short cultivation time, (2) high protein contents of the biomass produced and high nutrient qualities of amino acids that may be safe for human and animal consumption and (3) high COD reduction rates from SPW. The temperature and pH conditions of the SPW are also close to the optimal operating conditions of the microfungal species, and thus the operational costs can be reduced.
In the cultivation of the microfungal species, the oxygen transfer rate and the resulting DO level is critically important. Sufficient aeration rate is needed to maintain the level of DO above 50% of the saturation value. A higher oxygen pressure can be used to increase the oxygen transfer rates in the bioreactor.
The EALB can be operated in batch, semi-continuous and continuous processes. While the continuous process has higher efficiency of operation, a smaller bioreactor can be used and the batch mode of processing offers flexibility and ease of operation. The performance of the semi-continuous process is between the other two modes of operation. The bioreactor volumes can be designed from the fungal growth kinetics and optimal operating conditions obtained from pilot studies, as illustrated by design examples. The fundamentals of fungal biomass can be found from the literature (29–31).
20.7 Nomenclature
-
C s = Substrate concentration
-
D = Dilution rate
-
DO = Dissolved oxygen concentration
-
DO∗ = Equilibrium dissolved oxygen concentration
-
F = Volumetric flow rate
-
G = Molar air flow rate
-
H = Henry’s constant
-
y 1 = Inlet oxygen mole fraction
-
y 2 = Exit oxygen mole fraction
-
\({N}_{{\mathrm{O}}_{2}}\) = Oxygen transfer rate
-
K L a = Oxygen mass transfer coefficient
-
K m = Monad kinetic constant
-
P t = Total pressure
-
r X = Biomass growth rate
-
t = Time
-
V = Liquid phase volume in reactor
-
V out ∕ V t = Ratio of volume withdrawn to total culture volume
-
V t = Total reactor volume
-
X = Biomass concentration
-
Y COD ∕ X = COD to biomass yield
-
μ = Biomass specific growth rate
-
μmax = Maximum biomass specific growth rate
References
Jin B (1998) Microbial biomass protein production by microfungi in starch processing wastewater treatment. Ph.D Thesis, The University of New England, Australia
Lopes A, Sabaini NM, Gomes-Da-Costa SM (2009) Biomass production of sun-mushroom and shiitake in liquid culture media with agro-industrial residues. Boletim Do Centro De Pesquisa De Processamento De Alimentos 27:183–190
Bergmann FW, Abe J, Hizukurii S (1988) Selection of microorganisms which produce raw-starch degrading amylases. Appl Microbiol Biotechnol 27:443–446
Carlsen M, Nielsen J, Villadsen J (1995) Growth and α-amylase production by Aspergillus oryzae during continuous cultivation. J Biotechnol 45:81–93
Fogarty WM, Kelly C (1980) Amylase, amyloglucosidase and related gluconases. In: Rose AH (ed) Microbial enzymes and bioconversions economic microbiology, vol 5. Academic Press, London, pp 115–170
Shipman CH, Kao IC, Fan IT (1975) Single cell protein production by photosynthetic bacterium cultivation in agricultural by-products. Biotechnol Bioeng 17:1561–1570
Jin B, Yan XQ, Yu Q, van Leeuwen J (Hans) (2002) A comprehensive pilot plant system for fungal biomass protein production and wastewater reclamation. Adv Environ Res 6:179–189
Jin B, Yu Q, van Leeuwen J (Hans) (2001) A bioprocessing mode for fungal biomass protein production and wastewater treatment using external air-lift bioreactor. J Chem Technol Biotechnol 76:1041–1048
Jin B, van Leeuwen J (Hans), Patel B, Yu Q (1988) Utilization of starch processing wastewater for production of microbial biomass protein and fungal α-amylase by Aspergillus oryzae. Bioresour Technol 66:201–206
FAO (WHO) (1974) Protein advisory group guidelines no. 15 on the nutritional and safety aspects of novel sources of protein for animal feeding. United Nations, Rome
Barbesgard P, Heldt-Hansen HP, Diterichsen B (1992) On the safety of Aspergillus oryzae: a review. Appl Microbiol Biotechnol 9:569–572
Manjunath P, Shenoy BC, Rao MR (1983) Review: fungal glucoamylases. J Appl Biochem 5:235–260
Chen S, Wayman M (1992) Novel inducers derived from starch for cellulase production by Trichoderma reesei. Process Biochem 27:327–334
Ikenebomeh MJ (1981) Upgrading “garri” with single cell protein of Geotrichum candidum: preliminary investigation. J Canad Inst Food Sci Technol 14:168–173
Kositanont C, Charoensiri K, Bhumirtana M (1981) SCP Production from cellulosic material. In: Taguchi H (ed) Microbial utilisation of tenewable resources. II, Osaka University, Japan, 47–65
Tan KH, Ferguson LB, Carlton C (1984) Conversion of cassava starch to biomass, carbohydrates, and acids by Aspergillus niger. J Appl Biochem 6:80–90
Byrne GS, Ward OP (1989) Growth of Rhizopus in fermentation media. J Ind Microbiol 4:155–161
Romantschuk H, Lehtomaki M (1978) Operation experiments of first full scale Pekilo SCP-mill application. Process Biochem 13:16–29
Balagopal C, Maini SB (1977) Fungal protein from starch. J Root Crops 3:33–44
Collen SA, Kenneth FG (1987) Production of microbial biomass protein from potato process waste by Cephalosporim eichhoriae. Appl Environ Microbiol 53:824–291
Friendrich J, Cimerman A, Perdih A (1987) Mixed culture of Aspergillus awamori and Trichoderma reesei for bioconversion of apple distillery waste. Appl Microbiol Biotechnol 26:299–303
Moo-Young M, Chistri Y, Vilach D (1992) Fermentation conversion of cellulosic substrates to microbial protein by Neurospora sitophila. Biotechnol Lett 14(9):863–869
Jin B, van Leeuwen J (Hans), Yu Q, Patel B (1999) Screening and selection of microfungi for microbial biomass protein production and water reclamation from starch processing wastewater. J Chem Technol Biotechnol 74:106–110
Jin B, Yu Q, Yan XQ, van Leeuwen J (Hans) (2000) Characterization and improvement of oxygen transfer in pilot plant external air-lift bioreactor for mycelial biomass production from wastewater. World J Appl Microbiol Biotechnol 17:265–272
Malfait JL, Wilcox DJ, Mercer DG, Barker LD (1981) Cultivation of a filamentous mould in a glass pilot scale airlift fermentor. Biotechnol Bioeng 23:863–877
Jin B, van Leeuwen J (Hans), Doelle HW, Yu Q (1999) The influence of geometry on hydrodynamic and mass transfer characteristics in a new external airlift reactor for the cultivation of filamentous fungi. World J Microbiol Biotechnol 15:73–79
Yoshinori K, Moo-Young M (1986) Mixing and mass transfer in concentric-tube airlift fermentors: Newtonian and non-Newtonian media. J Chem Technol Biotechnol 36:527–538
Frohlich S, Lotz B, Larson B, Seekamp M (1990) Characterization of a pilot plant tower loop reactor: III. Evaluation of local properties of the dispersed gas phase during yeast cultivation and in model media. Biotechnol Bioeng 38:56–64
Wang LK, Pereira NC, Hung YT, Shammas NK (2009) Biological Treatment Processes. Humana Press, Totowa, NJ, 818 pp
Wang LK, Shammas NK, Hung YT (2009) Advanced Biological Treatment Proceses. Humana Press, Totowa, NJ, 737 pp
Wang LK, Ivanov V, Tay JH, Hung YT (2010) Environmental Biotechnology. Humana Press, Totowa, NJ, 975 pp
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Jin, B., Yu, Q., van Leeuwen, J.H., Hung, YT. (2010). An Integrated Biotechnological Process for Fungal Biomass Protein Production and Wastewater Reclamation. In: Wang, L., Tay, JH., Tay, S., Hung, YT. (eds) Environmental Bioengineering. Handbook of Environmental Engineering, vol 11. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-60327-031-1_20
Download citation
DOI: https://doi.org/10.1007/978-1-60327-031-1_20
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-58829-493-7
Online ISBN: 978-1-60327-031-1
eBook Packages: EngineeringEngineering (R0)