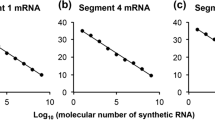
Abstract
Influenza viruses use an RNA-dependent RNA polymerase (RdRp) to transcribe and replicate their segmented negative-stranded RNA genomes. The influenza A virus RdRp consists of a heterotrimeric complex of the proteins PB1, PB2, and PA. The RdRp is associated with the incoming influenza A viral RNA (vRNA) genome bound by the viral nucleoprotein (NP), in complexes called viral ribonucleoproteins, vRNPs. During the viral replication cycle, the RdRp snatches capped primers from nascent host mRNAs to carry out primary viral transcription. Viral mRNA translation produces new copies of the RdRp subunits and NP, which are required to stabilize and encapsidate complementary copies of the genome (cRNAs), forming cRNPs. These cRNPs then use the cRNAs to make new vRNAs, which are encapsidated into new vRNPs. Secondary transcription by new vRNPs results in further viral mRNAs and an increase of the viral protein load in the cell. The activities of the RdRp (mRNA, cRNA, and vRNA synthesis) in the influenza virus replication cycle can be measured on several levels, ranging from assessment of the accumulation of RNA products in virus-infected cells, through in situ reconstitution of the RdRp from cloned cDNAs, to in vitro biochemical assays that allow the dissection of individual functions of the RdRp enzyme. Here we describe these assays and point out the advantages and drawbacks of each.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
te Velthuis AJ, Fodor E (2016) Influenza virus RNA polymerase: insights into the mechanisms of viral RNA synthesis. Nat Rev Microbiol 18:473–493
Krug RM, Fodor E (2013) The virus genome and its replication. In: Textbook of influenza, 2nd edn. John Wiley and Sons, Oxford, pp 57–66
Hengrung N, El Omari K, Serna Martin I, Vreede FT, Cusack S et al (2015) Crystal structure of the RNA-dependent RNA polymerase from influenza C virus. Nature 527:114–117
Pflug A, Guilligay D, Reich S, Cusack S (2014) Structure of influenza a polymerase bound to the viral RNA promoter. Nature 516:355–360
Reich S, Guilligay D, Pflug A, Malet H, Berger I et al (2014) Structural insight into cap-snatching and RNA synthesis by influenza polymerase. Nature 516:361–366
Arranz R, Coloma R, Chichón FJ, Conesa JJ, Carrascosa JL et al (2012) The structure of native influenza virion ribonucleoproteins. Science 338:1634–1637
Moeller A, Kirchdoerfer RN, Potter CS, Carragher B, Wilson IA (2012) Organization of the influenza virus replication machinery. Science 338:1631–1634
Eisfeld AJ, Neumann G, Kawaoka Y (2015) At the Centre: influenza a virus ribonucleoproteins. Nat Rev Microbiol 13:28–41
Gabriel G, Fodor E (2014) Molecular determinants of pathogenicity in the polymerase complex. Curr Top Microbiol Immunol 385:35–60
York A, Hutchinson EC, Fodor E (2014) Interactome analysis of the influenza a virus transcription/replication machinery identifies protein phosphatase 6 as a cellular factor required for efficient virus replication. J Virol 88:13284–13299
Robb NC, Smith M, Vreede FT, Fodor E (2009) NS2/NEP protein regulates transcription and replication of the influenza virus RNA genome. J Gen Virol 90:1398–1407
Kawakami E, Watanabe T, Fujii K, Goto H, Watanabe S et al (2011) Strand-specific real-time RT-PCR for distinguishing influenza vRNA, cRNA, and mRNA. J Virol Methods 173:1–6
Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, García-Sastre A (1999) Rescue of influenza a virus from recombinant DNA. J Virol 73:9679–9682
Neumann G, Zobel A, Hobom G (1994) RNA polymerase I-mediated expression of influenza viral RNA molecules. Virology 202:477–479
Fodor E, Crow M, Mingay LJ, Deng T, Sharps J et al (2002) A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase inhibits endonucleolytic cleavage of capped RNAs. J Virol 76:8989–9001
Li OT, Chan MC, Leung CS, Chan RW, Guan Y et al (2009) Full factorial analysis of mammalian and avian influenza polymerase subunits suggests a role of an efficient polymerase for virus adaptation. PLoS One 4:e5658
Brownlee GG, Fodor E, Pritlove DC, Gould KG, Dalluge JJ (1995) Solid phase synthesis of 5′-diphosphorylated oligoribonucleotides and their conversion to capped m7Gppp-oligoribonucleotides for use as primers for influenza A virus RNA polymerase in vitro. Nucleic Acids Res 23:2641–2647
Chung TD, Cianci C, Hagen M, Terry B, Matthews JT et al (1994) Biochemical studies on capped RNA primers identify a class of oligonucleotide inhibitors of the influenza virus RNA polymerase. Proc Natl Acad Sci U S A 91:2372–2376
Deng T, Vreede FT, Brownlee GG (2006) Different de novo initiation strategies are used by influenza virus RNA polymerase on its cRNA and viral RNA promoters during viral RNA replication. J Virol 80:2337–2348
Plotch SJ, Krug RM (1977) Influenza virion transcriptase: synthesis in vitro of large, polyadenylic acid-containing complementary RNA. J Virol 21:24–34
Mänz B, Brunotte L, Reuther P, Schwemmle M (2012) Adaptive mutations in NEP compensate for defective H5N1 RNA replication in cultured human cells. Nat Commun 3:802
Deng T, Sharps J, Fodor E, Brownlee GG (2005) In vitro assembly of PB2 with a PB1-PA dimer supports a new model of assembly of influenza a virus polymerase subunits into a functional trimeric complex. J Virol 79:8669–8674
Ponchel F, Toomes C, Bransfield K, Leong FT, Douglas SH et al (2003) Real-time PCR based on SYBR-green I fluorescence: an alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC Biotechnol 3:18
te Velthuis A, Robb NC, Kapanidis AF, Fodor F (2016) The role of the priming loop in influenza A virus RNA synthesis. Nat Microbiol 1:16029
Plotch SJ, Bouloy M, Ulmanen I, Krug RM (1981) A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell 23:847–858
Koppstein D, Ashour J, Bartel DP (2015) Sequencing the cap-snatching repertoire of H1N1 influenza provides insight into the mechanism of viral transcription initiation. Nucleic Acids Res 43:5052–5064
Reich S, Guilligay D, Cusack S (2017) An in vitro fluorescence based study of initiation of RNA synthesis by influenza B polymerase. Nucleic Acids Res 45:3353–3368
Bouloy M, Plotch SJ, Krug RM (1978) Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc Natl Acad Sci U S A 75:4886–4890
Robertson HD, Dickson E, Plotch SJ, Krug RM (1980) Identification of the RNA region transferred from a representative primer, beta-globin mRNA, to influenza mRNA during in vitro transcription. Nucleic Acids Res 8:925–942
te Velthuis AJ, Turrell L, Vreede FT, Fodor E (2013) Uncoupling of influenza A virus transcription and replication through mutation of the unpaired adenosine in the viral RNA promoter. J Virol 87:10381–10384
Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM et al (2012) An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science 337:199–204
Acknowledgments
AJWtV is supported by Wellcome Trust grants 098721/Z/12/Z and 206579/Z/17/Z. JSL is supported by BBSRC sLoLa grant BB/K002465/1. WSB is supported by Wellcome Trust grants 200187/Z/15/Z and 205100/Z/16/Z.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
te Velthuis, A.J.W., Long, J.S., Barclay, W.S. (2018). Assays to Measure the Activity of Influenza Virus Polymerase. In: Yamauchi, Y. (eds) Influenza Virus. Methods in Molecular Biology, vol 1836. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-8678-1_17
Download citation
DOI: https://doi.org/10.1007/978-1-4939-8678-1_17
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-8677-4
Online ISBN: 978-1-4939-8678-1
eBook Packages: Springer Protocols