Abstract
Natural killer (NK) cells are an attractive cell population for immunotherapy. Adoptive transfer of NK cells has been tested in multiple clinical trials including acute myeloid leukemia (AML) and ovarian cancer, although limitations do exist especially for treatment of solid tumors. In order to overcome these limitations, mouse xenograft models are needed for evaluation of various NK cell populations, as well as routes of NK cell administration. Here, we describe the methods used for the establishment of an intraperitoneal (ip) ovarian cancer mouse xenograft model with ip delivery of NK cells. This model has been successfully employed with multiple ovarian cell lines and could be applied to other tumor models where the tumor’s primary location is in the peritoneal cavity. It is also compatible with multiple routes of NK cell administration. Bioluminescent imaging for monitoring tumor formation and response provides for easy visualization of NK cell tumor inhibition. This xenograft model is superior to other models because the tumor is implanted into the same physiological space where ovarian cancer is found, which allows for improved mimicking of actual disease.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Natural killer (NK) cells are cytotoxic lymphocytes known to play an important role in the control of viral infections and various malignancies [1]. The adoptive transfer of haploidentical NK cells isolated from peripheral blood (PB-NK) can mediate dramatic anti-tumor effects against hematological malignancies, especially in the case of acute myeloid leukemia (AML) [2]. Furthermore, intravenous (iv) delivery of PB-NK cells has also been evaluated for the treatment of ovarian cancer and other solid tumors [3]. Additionally, the NK cell line, NK-92, has been used in clinical trials for advanced renal cell carcinoma and AML [4, 5]. These studies support the use of adoptively transferred NK cells for cancer immunotherapy.
Currently, NK cells used for immunotherapy are isolated from an apheresis product followed by CD3 and CD19 depletion for the removal of T cells and B cells, respectively [2]. This procedure results in a heterogeneous cell population that typically consists of only approximately 30 % NK cells [6]. Recently, new techniques have been defined for the ex vivo expansion and activation of PB-NK cells [7, 8]. Furthermore, methods for the generation of NK cells from either human embryonic stem cells (hESCs) or induced pluripotent stem cells (iPSCs) have also been developed [9–12]. These methods allow for the production of a >97 % pure NK cell population that have the potential to become a standardized, off-the-shelf NK cell-based immunotherapy product.
In order to compare the various NK cell sources and routes of administration, we have developed a mouse xenograft model using bioluminescent imaging to monitor tumor growth (Fig. 1) [13]. Given that ovarian cancer is restricted to the peritoneal cavity, we have used this model to study the intraperitoneal (ip) delivery of NK cells rather than iv dosing [13]. This chapter will describe the methods used for the establishment of this mouse model for the study of ovarian cancer, as well as methods used for monitoring NK cell persistence.
Schematic diagram of the intraperitoneal xenograft mouse model. (a) Overview of the experimental outline including injection of luciferase (luc+) positive tumor cells, radiation of tumor-bearing mice with 225 cGy, injection of NK cells, and cytokine administration. (b) Timeline of injection schedule for cells and cytokines as well as imaging schedule
2 Materials
2.1 Cell Lines
-
1.
MA148/GFP:Luc Cells. MA-148 cells were kindly provided by Sundaram Ramakrishnan (University of Minnesota). The insertion of the GFP:Luc construct has been previously reported [13]. These cells are maintained in RPMI-1640 plus l-glutamine, 10 % FBS, and 1 % Penicillin/Streptomycin.
-
2.
PB-NK Cells. Cells were maintained in RPMI-1640, 10 % FBS, 2 mM l-glutamine, 1 % P/S, and 50 U/mL IL-2 . The cells were stimulated with lethally irradiated membrane bound (mb) IL-21-expressing artificial antigen presenting cells (aAPCs) weekly, as has been previously reported [8, 9].
2.2 Mice
-
1.
NOD.Cg-Prkdc scid Il2rg tm1wjl/SzJ mice, commonly known as NOD scid gamma-c or NSG mice, were obtained from The Jackson Laboratory. These mice lack mature T cells and B cells, have no functional NK cells, and have defective function of myeloid immune cells [14].
2.3 Injection of Tumor into Mice
-
1.
0.05 % Trypsin-EDTA for harvesting tumor cells (Invitrogen).
-
2.
Dulbecco’s phosphate-buffered saline (DPBS) as tumor cell carrier (HyClone).
-
3.
1 cc U-100 Insulin Syringe 28G1/2.
2.4 Animal Imaging
-
1.
d-Luciferin (Gold Biotechnology). Resuspend the 1 g of d-Luciferin in 40 mL DPBS and store at −80 °C in 0.5–1 mL aliquots. At this concentration, 100 μL is injected into each mouse.
-
2.
IVIS Spectrum (Perkin-Elmer).
-
3.
Living Image 4.0 for analysis of images (Perkin-Elmer).
-
4.
X-Rad 320 Biological Irradiator (Precision X-ray).
2.5 Cytokines
-
1.
Interleukin-2 (Proleukin (aldesleukin), Prometheus). Suspend 22 million units of IL-2 in 53 mL of DPBS. Store at −20 °C.
2.6 Analysis of NK Cell Survival
-
1.
Heparin (1000 U/mL).
-
2.
Ammonium Chloride (ACK) lysis buffer.
-
3.
Blocking serum: DPBS supplemented with 5 % human serum and 5 % FBS and sterile filtered.
-
4.
FACS buffer: 0.1 % sodium azide and 2 % FBS in DPBS.
3 Methods
3.1 Establishment of IP Tumor and Imaging
-
1.
Passage tumor cells so that they are no more than 70–80 % confluent on day of use. Typically we try and passage the cells 2 days prior to injection.
-
2.
Four days prior to NK cell administration, harvest 2 × 105 luciferase-expressing MA148 cells per mouse (see Note 1 ) using 0.05 % trypsin for 5 min at 37 °C.
-
3.
Centrifuge cells at 300 × g for 5 min and resuspend in DPBS at a concentration of 1 × 106 cells/mL in a capped tube.
-
4.
Inject 10–12-week-old female NSG mice (see Note 2 ) with 2 × 105 (see Note 1 ) luciferase-expressing tumor cells (200 μL). Injections should be given ip with a 1 cc U-100 insulin syringe 28G1/2 (see Note 3 ).
-
5.
72 h post-tumor injection (1 day prior to NK cell treatment). Image the mice using the IVIS Spectrum. Inject mice ip with 100 μL of d-Luciferin at a concentration of 25 mg/mL. Anesthetize the mice using Isoflurane (vaporizer setting at 2.5 % and a flow rate of 2.0 to anesthetize the mice and 0.5 L/min O2 while imaging). Place mice in the IVIS spectrum imaging platform and acquire image with presets of Medium Binning, 30 s exposure, and F Stop of 1 (Fig. 2) (Note 4 ). Collect the data in Radiance. Acquire images from 3 to 5 min after administering d-Luciferin.
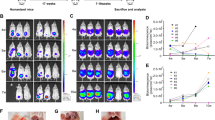
Fig. 2 Bioluminescence imaging of luciferase + tumor-bearing mice. Mice were injected ip with varying numbers of MA148/GFP:Luc+ ovarian cancer cells on day 4. Mice were imaged starting on day 1 and weekly thereafter to monitor tumor formation and growth in order to determine the optimal number of cells for tumor formation. Two mice for each group received irradiation (IRR) (225 cGy) on day 1 while two mice were left untreated
-
6.
Analyze the data using Living Image by drawing an identical region of interest (roughly 3.6 cm wide and 4 cm tall) for each mouse in order to measure radiance (see Note 5 ).
-
7.
Use the total flux (photons/s) to compare mice and assign into groups such that each group will have mice with similar total flux measurements (see Note 6 ).
-
8.
Immediately following imaging, mice are irradiated using an X-Rad 320 Biological Irradiator set to 12.5 mA and 320KV to deliver 2.25 cGy (225 rads) per animal at a height of 50 cm (see Note 7 ).
-
9.
Imaging is continued every 7 days to monitor tumor growth and effectiveness of the NK cell treatment.
3.2 Delivery of NK Cells and Cytokine Injections
-
1.
On the day NK cells are to be given, count and determine the cell viability (see Note 8 ).
Centrifuge (300 × g for 5 min) enough NK cells for a dose of 20 × 106 cells/mouse and wash once with DPBS. Centrifuge again and resuspend cells at 66.6 × 106 cells/mL in DPBS and place in a capped tube.
-
2.
Inject 300 μL (20 × 106 cells) ip into each mouse using a 1 cc U-100 insulin syringe.
28G1/2. Be sure to use a pipette to mix the cells immediately prior to loading each syringe (see Note 3 ).
-
3.
The day following NK cell dosing, start the administration of cytokine injections (see Note 9 ). We give IL-2 at 5 μg/mouse/day (83,000 U/mouse/day) by injecting 200 μL of stock solution prepared as in the Subheading 2.
-
4.
Administer cytokine dosing as follows: every day for 7 days followed by three times a week (Monday, Wednesday, and Friday) for an additional 3 weeks (4 weeks total).
3.3 Monitoring NK Cell Engraftment and Survival in Blood
-
1.
Draw blood from mice on both 7 and 21 days after giving them NK cells (see Note 10 ). Collect ~100 μL of blood through facial vein bleeding into 1.5 mL Eppendorf tubes containing 50 μL of heparin to prevent clotting.
-
2.
Lyse red blood cells (RBCs) using ACK lysis buffer. Add 800 μL of ACK to the blood and incubate on ice for 5 min.
-
3.
Centrifuge at 300 × g for 4 min and remove supernatant.
-
4.
Repeat steps 2 and 3 one more time.
-
5.
Add 1 mL blocking serum and incubate on ice for 20 min.
-
6.
C entrifuge at 300 × g for 4 min and remove supernatant.
-
7.
Wash with FACS buffer once and stain with huCD45 and huCD56 for 20 min on ice. Other NK cell markers can be used if desired such as KIRs or NK cell markers NKp44 or NKp46.
3.4 Intraperitoneal Washes to Measure NK Cell Persistence
-
1.
NK cell persistence within the peritoneal cavity can also be evaluated (see Note 11 ). Euthanize the mouse and carefully separate the skin from the peritoneal wall.
-
2.
Once the skin is separated, make a small hole to allow access into the peritoneal cavity. This is best done by holding the peritoneum with tweezers while making a small cut with a scissors.
-
3.
Use a glass pipette with a pipette bulb and carefully flush out the cavity with DPBS. Be careful not to puncture the liver with the pipette to avoid getting excess RBCs in the wash.
-
4.
Repeat the washes with up to 10 mL of DPBS trying to rinse the entire cavity.
-
5.
Keep the cells on ice until all of the samples are collected and then centrifuge the cells (300 × g for 4 min).
-
6.
The washes should be clear unless there was ascites in the cavity. If there are any RBCs present, perform an RBC lysis as in Subheading 3.3.
-
7.
Stain cells with anti-huCD45 and anti-huCD56 to identify NK cells. Other antibodies can be used as desired, as described in Subheading 3.3.
4 Notes
-
1.
We have performed this with three different ovarian cell lines, MA148, A1847, and A2780 cells and in each case 2 × 105 cells resulted in consistent engraftment within 4 days (Fig. 2). If a different cell line is to be used, we recommend doing a dose response test by injecting different numbers of cells and imaging 3 days and 10 days later to ensure engraftment will occur. The ideal cell number is the fewest cells that will provide reliable engraftment. Too high of tumor burden at the beginning may lead to tumor growth faster than NK cell-mediated activity can be effective.
-
2.
It is best to use mice aged 10–12 weeks, but we have been successful using mice as early as 8 weeks and as late as 14 weeks. Additionally, we have found that it is prudent to inject 5 extra mice at start of the studies, as there are often times a few mice where the tumor will not engraft.
-
3.
Immediately prior to loading the syringe and injecting the mice, make sure to pipette the tumor cells gently to ensure they are well mixed. Each syringe can be used to inject 5 mice. Do not preload all of the syringes prior to injection, rather load one at a time and inject into mice immediately. We have found that a wider gauge needle can result in tract tumors subcutaneously and interfere with results.
-
4.
These settings are recommended as a starting point. If the images become saturated, decrease the exposure time. We report our bioluminescence in total flux, which is independent of time. As the tumor grows, the images will remain saturated at even a 1 s exposure so increase the F stop on later days. Additionally, if imaging multiple mice at one time, place black dividing cards between the mice to avoid having the radiance from one mouse bleed over into nearby mice. This will not be immediately apparent, so don’t assume the cards aren’t necessary.
-
5.
Make sure to draw the box large enough the first time to measure the entire abdominal area so as the tumors grow the entire luciferase positive area is still measured.
-
6.
Negative controls typically are around 3–5 × 105 total flux. Mice with measured total flux less than 1 × 106 are typically discarded and removed from the experiment. Then, the remaining mice are ranked from highest to lowest total flux and each group is assigned mice in order.
-
7.
This low level of irradiation will not significantly affect tumor burden.
-
8.
If using an a APC expansion system, try to time the administration of NK cells so that all of the aAPCs have had time to be removed from the culture to avoid affecting the cell counts. Typically, we stimulate our NK cells on Fridays and dose the mice on Wednesday after changing the media on Tuesday.
-
9.
The administration of cytokines promotes NK cell engraftment and survival in vivo. We have found that IL-2 is equally effective as IL-15 in our model, but reports suggest IL-15 may be better in other models [15].
-
10.
Blood draws have been performed at day 7 and 14, but we have noticed that it tends to be fairly hard on the mice in combination with IL-2 administration, so we have moved to bleeding on day 7 and 21.
-
11.
NK cells can be found in the peritoneal cavity for up to 28 days.
References
Vivier E, Raulet DH, Moretta A et al (2011) Innate or adaptive immunity? The example of natural killer cells. Science 331:44–49
Miller JS, Soignier Y, Panoskaltsis-Mortari A et al (2005) Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105:3051–3057
Geller MA, Cooley S, Judson PL et al (2011) A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy 13:98–107
Cheng M, Chen Y, Xiao W, Sun R, Tian Z (2013) NK cell—based immunotherapy for malignant diseases. Cell Mol Immunol 10:230–252
Tonn T, Schwabe D, Klingemann HG et al (2013) Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 15:1563–1570
Koepsell SA, Miller JS, McKenna DH (2013) Natural killer cells: a review of manufacturing and clinical utility. Transfusion 53:404–410
Fujisaki H, Kakuda H, Shimasaki N et al (2009) Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 69:4010–4017
Denman CJ, Senyukov VV, Somanchi SS et al (2012) Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One 7, e30264
Knorr DA, Ni Z, Hermanson D et al (2013) Clinical—scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med 2:274–283
Ni Z, Knorr DA, Kaufman DS (2013) Hematopoietic and nature killer cell development from human pluripotent stem cells. Methods Mol Biol 1029:33–41
Woll PS, Martin CH, Miller JS, Kaufman DS (2005) Human embryonic stem cell—derived NK cells acquire functional receptors and cytolytic activity. J Immunol 175:5095–5103
Woll PS, Grzywacz B, Tian X et al (2009) Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood 113:6094–6101
Geller MA, Knorr DA, Hermanson DA et al (2013) Intraperitoneal delivery of human natural killer cells for treatment of ovarian cancer in a mouse xenograft model. Cytotherapy 15:1297–1306
Shultz LD, Lyons BL, Burzenski LM et al (2005) Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 174:6477–6489
Miller JS, Rooney CM, Curtsinger J et al (2014) Expansion and homing of adoptively transferred human natural killer cells in immunodeficient mice varies with product preparation and in vivo cytokine administration: implications for clinical therapy. Biol Blood Marrow Transplant 20:1252–1257
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Hermanson, D.L., Bendzick, L., Kaufman, D.S. (2016). Mouse Xenograft Model for Intraperitoneal Administration of NK Cell Immunotherapy for Ovarian Cancer. In: Somanchi, S. (eds) Natural Killer Cells. Methods in Molecular Biology, vol 1441. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3684-7_23
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3684-7_23
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3682-3
Online ISBN: 978-1-4939-3684-7
eBook Packages: Springer Protocols