Abstract
Natural killer (NK) cells can be expanded upon activation by proliferative cytokines (such as IL-2 and IL-15). The NK cell expansion can be greatly enhanced by proteins from feeder cells such as tumor cell lines or PBMCs. Therefore, coculture systems of irradiated feeder cells and NK cells in media containing IL-2 and IL-15 have been developed to generate large numbers of NK cells, although NK cell expansion protocol using anti-CD3 antibody (OKT-3) without feeder cells has also been developed. Commonly used feeder cell lines are RPMI8866, Epstein-Barr lymphoblastoid cell line (EBV-LCL), and K562. Stimulation with NK-sensitive K562 cells is known to augment NK cell proliferation to IL-2, IL-15, and IL-21 in combination.
Recently, remarkable NK cell-expansion rates are achieved when genetically engineered (GE) feeder cells are used. Dr. Dario Campana’s group found that membrane-bound IL-15 and 4-1BBL, coexpressed by K562 cells, acted synergistically to augment K562-specific NK stimulatory capacity, resulting in vigorous expansion of peripheral blood CD56+ CD3− NK cells without concomitant growth of T lymphocytes. Here, we describe an in vitro expansion method of human NK cells among PBMCs by coculturing with GE_K562 cells.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
NK cells are defined phenotypically as CD56+ CD3− lymphocytes , comprising 5–20 % of peripheral blood mononuclear cells (PBMCs) in humans. Based on their phenotype and function, two NK cell subsets have been characterized: CD56dim CD16+ NK cells which represent approximately 90 % of circulating NK cells and have high cytotoxic activity, and CD56bright CD16neg/dim NK cells which constitute approximately 10 % and have the capacity to produce abundant cytokines and chemokines [1, 2].
NK cells can directly kill target cells via the perforin–granzyme pathway, antibody-dependent cellular cytotoxicity (ADCC ) , and death receptor-ligand induced apoptosis . The killing of target tumors and virus infected cells by NK cells is regulated by the balance between activating and inhibitory receptors, including killer cell Ig-like receptors (KIRs), NKG2A, NKG2D; and natural cytotoxic receptors NKp30, NKp44, NKp46 [3, 4]. In addition to direct cytotoxicity , NK cells produce an array of cytokines and chemokines in response to target cells to modulate immune responses. On the basis of their antitumor killing activity and cytokine production, NK cells are one of the attractive tools for targeting cancer in immunotherapy [3, 5]. However, the selective expansion of NK cells to yield relevant amounts of these lymphocytes has been a major hurdle in the development of methods for clinical therapeutic use. Recently expansion and activation of NK cells has been achieved using cytokines (IL-2 , IL-15) and feeder cells (tumor cell lines or PBMCs) that selectively activate NK cells in the cell-cell contact dependent manner. Among widely used feeder cells, GE_K562 (i.e., K562-mb15-41BBL) cells are shown to be the best candidate for remarkable NK cell expansion [6–8]. The NK cell expansion protocol using the GE_K562 cells was originally developed by Dr. Dario Campana group and then modified by others [6, 8–11]. Here, we describe an in vitro expansion method of human NK cells among PBMCs by coculturing with irradiated GE_K562 cells. Notably, this method with slight modification can also be used for the expansion of canine NK cells [12].
2 Materials
-
1.
Vacutainer blood collection tubes with sodium heparin (BD)
-
2.
Whole blood remaining in the leukoreduction system (LRS) chambers of Trima Accel (Gambro BCT) after platelet pheresis from healthy donors.
-
3.
1× phosphate buffered saline (PBS).
-
4.
Fetal bovine serum (FBS, Gibco), with or without heat inactivation (30 min, 56 °C).
-
5.
Lymphoprep 1.077 g/ml (Axis-Shield PoC AS, Oslo, Norway).
-
6.
NK cell media: RPMI 1640 medium (Gibco) supplemented with 10 % heat-inactivated FBS, 4 mM l-glutamine (Gibco), and 1 % antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin, Lonza).
-
7.
Complete RPMI 1640 media: RPMI 1640 medium supplemented with 10 % heat-inactivated FBS, and 1 % antibiotics.
-
8.
0.2 μm disposable filter systems (Corning).
-
9.
Interleukin-2 (Peprotech Inc.).
-
10.
Interleukin-15 (Peprotech Inc.).
-
11.
24-well tissue culture plate.
-
12.
T75 tissue culture flask.
-
13.
Sterile autoclaved micropipette tips (10, 100, and 1000 μL).
-
14.
15 and 50 ml sterile centrifuge tubes.
-
15.
Sterile microcentrifuge tubes, 1.5 ml.
-
16.
Cesium-137 Gammacell-3000 Elan irradiator (Best Theratronics).
-
17.
GE_K562 (K562-mb15-41BBL cells, a gift of Dr. D. Campana, National University of Singapore).
-
18.
FACS buffer: 1 % FBS in 1× PBS.
-
19.
Fixation buffer: 1 % formaldehyde in 1× PBS.
-
20.
Mouse anti-human CD3 conjugated with FITC (BD Pharmingen).
-
21.
Mouse anti-human CD56 conjugated with PE-Cy5 (BD Pharmingen).
-
22.
Mouse anti-human CD16 conjugated with PE (BD Pharmingen).
-
23.
Mouse anti-human CD69 conjugated with PE (BD Pharmingen).
-
24.
Mouse anti-human NKG2D conjugated with PE (BD Pharmingen).
-
25.
Mouse anti-human NKp30 conjugated with PE (BD Pharmingen).
-
26.
Mouse anti-human NKp44 conjugated with PE (BD Pharmingen).
-
27.
Mouse anti-human NKp46 conjugated with PE (BD Pharmingen).
-
28.
Mouse anti-human CD158b conjugated with PE (BD Pharmingen).
-
29.
FACS Calibur cytometer and BD CellQuest software (BD Immunocytometry System).
-
30.
Freezing solution: 10 % dimethyl sulfoxide (DMSO) and 90 % heat-inactivated FBS.
3 Methods
3.1 Preparation of Genetically Engineered K562 Feeder Cells
-
1.
GE_K562 (i.e., K562-mb15-41BBL, a gift of Dr. D. Campana, National University of Singapore) cells are cultured in T-75 flasks containing 25 ml of complete RPMI 1640 media at 37 °C in a humidified incubator containing 5 % CO2.
-
2.
Harvest feeder cells, centrifuge at 400 × g for 3 min. Resuspend the cell pellet in 5 ml complete RPMI 1640 media.
-
3.
Irradiate feeder cells at 100 Gy with a Gammacell 3000 Elan irradiator (see Notes 1 and 2).
3.2 Isolation of Peripheral Blood Mononuclear Cells
-
1.
Whole blood from healthy donors are diluted with PBS at 1:2 ratio (10 ml blood: 20 ml PBS), overlay onto 15 ml Lymphoprep and centrifuge at 1200 × g for 25 min at room temperature with no brake (acceleration 1; deceleration 0).
-
2.
Harvest cells from the interface (buffy coat layer) and wash 3 times with PBS at 400 × g for 7 min.
-
3.
Resuspend the cell pellet by tapping and then add NK culture media, count the cells with trypan blue exclusion method.
3.3 Culture of NK Cells from Isolated PBMCs
-
1.
On Day 0: Seed 3 × 106 freshly prepared PBMCs and 0.5 × 106 100 Gy-irradiated GE_K562 in 1 ml NK cell media into 24 well plate (see Note 3) (An overview is presented in Fig. 1).
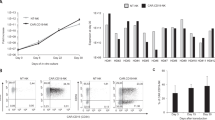
Fig. 1 Isolated PBMCs from healthy donors were cocultured in 24-well plate with cryopreserved-thawed irradiated GE_K562 feeder cells at a ratio of 6:1 (PBMCs:GE_K562) in NK cell media at 3 × 106 PBMCs/2 ml. NK phenotype, purity and fold expansion are checked every week. After a period of 2 or 3 weeks, sufficient numbers of expanded NK cells with high purity (>90 %) were obtained
-
2.
Add 1 ml NK cell media with 20 U/ml IL-2 to the well. Total media volume is 2 ml/well, final concentration of IL-2 is 10 U/ml. Mix well by gently pipetting.
-
3.
Incubate at 37 °C, 5 % CO2 incubator.
-
4.
On Days 3 and 5: change media; remove half of the media and add 1 ml fresh media containing sufficient fresh IL-2 to make 10 U/ml for 2 ml final volume. (See Note 4)
-
5.
On Day 7: Count the number of cells in culture at the end of 1 week. If the cell number is more than 4 × 106 cells/ml, split the cell from one to four wells. If not, split the cell from one to two wells.
-
6.
The concentration of IL-2 is increased to 100 U/ml and an additional 5 ng/ml of IL-15 is added to the media. In this method, NK cells are stimulated with GE_K562 cells only once at Day 0 during the entire 21 day period (see Notes 5 and 6)
-
7.
Assess the purity of NK cells using a fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD3 and a PE-Cy5-conjugated mouse anti-human CD56 monoclonal antibodies for every week.
-
8.
Day 10 and 12: change media; remove half of the media and add 1 ml fresh media containing 200 U/ml IL-2 and 10 ng/ml IL-15.
-
9.
Day 14: Count the number of cells in culture at the end of 2 weeks. If the cell number is more than 4 × 106 cells/ml, split the cell from one to four wells. If not, split the cell from one to two wells. Check the purity of expanded NK cells after 2 weeks (as in step 7).
-
10.
Day 17 and 19: change media; remove half of the media and add 1 ml fresh media containing 200 U/ml IL-2 and 10 ng/ml IL-15.
-
11.
Day 21: Count the number of NK cells by trypan blue exclusion method, and check the purity of expanded NK cells using a FITC-anti-human CD3 and a PE-Cy5 anti-human CD56 monoclonal antibodies at the end of 3 weeks. Overall fold expansion and purity of NK cells that can be achieved by this protocol is shown in Fig. 2.
Fig. 2 The morphological and flow cytometric analysis of NK cells before (day 0) and after (day 14) expansion was performed by May-Grünwald-Giemsa staining and flow cytometry. PBMC (day 0) and expanded NK cells (day 14) were stained with CD3, CD56 monoclonal antibody and analyzed by FACS. These photomicrographs were prepared with May-Grünwald-Giemsa-stained cytocentrifuged slides. The large granular lymphocytes indicate the expanded NK cells
-
12.
Harvest all expanded NK cells, which may be used directly or cryopreserved for later use.
3.4 Receptor Expression on Cultured NK Cells
Surface expression of NK cell activating and inhibitory receptors on cultured NK cells are checked on days 0, 7, 14, and 21 by flow cytometry .
-
1.
Recover 2 × 105 cells per staining condition, and spin at 400 × g for 5 min.
-
2.
Remove the supernatant and resuspend cells in 100 μL FACS buffer per 2 × 105 cells.
-
3.
Transfer 100 μL FACS buffer containing cells to FACS tubes.
-
4.
Add 5 μL of fluorescence-conjugated target Abs or isotype control Ab, incubate on ice for 15 min. (Standard surface expressions of receptors assessed on expanded NK are CD16, CD69, NKG2D, NKp30, NKp44, NKp46, and CD158b as well as appropriate isotype controls).
-
5.
Wash the cells after staining with 1 ml FACS buffer and spin at 400 × g for 5 min.
-
6.
Repeat the washing step two more times.
-
7.
Remove supernatant and resuspend stained cells in fixation buffer and analyze them by flow cytometry (Fig. 3).
Fig. 3 Changes of expression of surface receptors on natural killer (NK) cells expanded using GE_K562 feeder cells. Phycoerythrin (PE)-conjugated antibody to human CD16, CD69, natural killer group-2, member D (NKG2D), NKp30, NKp44, NKp46, and CD158b (G) antibodies were used to analyze the NK cell receptors on day 0 (upper) and day 14 (lower). The peak at the extreme left of each figure shows the isotope control
4 Notes
-
1.
In order to prevent cancer feeder cell overgrowth, several methods such as fixing with 10 % formalin, treatment of methanol–acetic acid (3:1) mixture, heating, freezing–thawing, and irradiation have been used. Among them, irradiation (at dose from 30 Gy to 100 Gy) is a safe and effective method to prevent overgrowth in the coculture with NK cells [13, 14]. Our group uses 100 Gy for irradiation.
-
2.
Both cryopreserved and freshly prepared irradiated feed cells can be used for the NK cell expansion. We usually have good success in NK cell expansion by using cryopreserved irradiated GE_ K562 cells (K562-mb15-41BBL cells). Irradiated feeder cells can be cryopreserved with freezing medium for later use. The NK cell expansion rate, NK cytotoxicity , NK cell receptors, and level of interferon-γ secretion are similar in NK cell expanded by using freshly irradiated feeder cells and cryopreserved irradiated feeder cells [10].
-
3.
Direct cell contact between feeder cells and NK cells might be a substantial factor for NK cell expansion. In a 24-well plate, we start with 3 × 106 fresh PBMCs and 0.5 × 106 irradiated GE_K562 for optimal proliferation of NK cells. The optimal cell density of PBMCs: GE_K562 ratio is critical factor for successful NK cell expansion.
-
4.
To diminish effect of spontaneous evaporation during culture, we remove 950 μL and add 1000 μL of fresh media containing cytokines in 24-well plates when changing media half.
-
5.
The NK cell expansion protocol using GE_K562 (K562-mb15-41BBL cells) requires a low concentration of IL-2 (i.e., 10 U/ml of IL-2 ) during the first week, and 100 U/ml of IL-2 after the first week.
-
6.
It was reported that more than 95 % of GE_K562 (K562-mb15-41BBL) cells cannot survive at day 3 of NK cell expansion [10]. Therefore, the feeder cells might lose the function of genetically engineered molecules (i.e., membrane bound form of IL-15). However, in our protocol, we do not add GE_K562 cells every week, in contrast with other groups [7, 8]. Instead of adding GE_K562 cells every week, we add 5 ng/ml of IL-15 to the media after 7 days of culture and IL-15 every 2 days through the culture period. In the presence of 5 ng/ml of IL-15 as well as 100 U/ml of IL-2 , NK cells vigorously proliferated over a period of 3 weeks [9, 10].
-
7.
This protocol is a slight modification of the original one described in [6, 7].
References
Caligiuri MA (2008) Human natural killer cells. Blood 112:461–469
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22:633–640
Cho D, Kim SK, Carson WE (2011) NK cell-based immunotherapy for treating cancer: will it be promising? Korean J Hematol 46:3–5
Lanier LL (2008) Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 9:495–502
Ljunggren HG, Malmberg KJ (2007) Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 7:329–339
Imai C, Iwamoto S, Campana D (2005) Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 106:376–383
Fujisaki H, Kakuda H, Shimasaki N et al (2009) Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 69(9):4010–4017
Denman CJ, Senyukov VV, Somanchi SS et al (2012) Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One 7(1):e30264
Park YK, Shin DJ, Cho D et al (2012) Interleukin-21 increases direct cytotoxicity and IFN-γ production of ex vivo expanded NK cells towards breast cancer cells. Anticancer Res 32(3):839–846
Baek HJ, Kim JS, Yoon M et al (2013) Ex vivo expansion of natural killer cells using cryopreserved irradiated feeder cells. Anticancer Res 33(5):2011–2019
Lim DP, Jang YY, Kim S, et al (2014) Effect of exposure to interleukin-21 at various time points on human natural killer cell culture. Cytotherapy. Jun 17. pii: S1465-3249(14)00596-9
Shin DJ, Park JY, Jang YY et al (2013) Ex vivo expansion of canine cytotoxic large granular lymphocytes exhibiting characteristics of natural killer cells. Vet Immunol Immunopathol 153(3-4):249–259
Harada H, Saijo K, Watanabe S et al (2002) Selective expansion of human natural killer cells from peripheral blood mononuclear cells by the cell line, HFWT. Jpn J Cancer Res 93:313–319
Cho D, Campana D (2009) Expansion and activation of natural killer cells for cancer immunotherapy. Korean J Lab Med 29:89–96
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (NRF-2015R1D1A1A09058740) and by a grant from Korea Research Institute of Bioscience and Biotechnology (KGM4941612).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Phan, MT.T., Lee, SH., Kim, SK., Cho, D. (2016). Expansion of NK Cells Using Genetically Engineered K562 Feeder Cells. In: Somanchi, S. (eds) Natural Killer Cells. Methods in Molecular Biology, vol 1441. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3684-7_14
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3684-7_14
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3682-3
Online ISBN: 978-1-4939-3684-7
eBook Packages: Springer Protocols