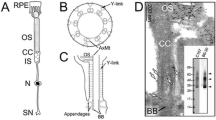
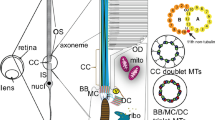
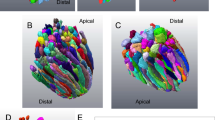
Abstract
The connecting cilium of the rod photoreceptor is a tubular structure that bridges two adjacent cellular compartments, the inner segment, the major site of biosynthesis and energy metabolism, and the outer segment, a highly specialized ciliary structure responsible for phototransduction. The connecting cilium allows for active processes of protein sorting and transport to occur between them. Mutations affecting the cargo, their transporters, and the structural components of the primary cilium and basal body lead to aberrant trafficking and photoreceptor cell death. Understanding the overall design of the cilium, its architectural organization, and the function of varied protein complexes within the structural hierarchy of the cilium requires techniques for visualizing their native three-dimensional structures at high magnification. Here we describe methods for isolating retinas from mice, purifying fragments of rod cells that include much of the inner segment and the rod photoreceptor cilia, vitrifying the cell fragments, and determining their structures by cryo-electron tomography.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Wang J, Deretic D (2014) Molecular complexes that direct rhodopsin transport to primary cilia. Prog Retin Eye Res 38:1–19
Young RW (1967) The renewal of photoreceptor cell outer segments. J Cell Biol 33:61–72
Wensel TG (2012) Molecular biology of vision. In: Brady ST, Albers RW, Price D, Siegel JG (eds) Basic neurochemistry: principles of molecular, cellular, and medical neurobiology, 8th edn. Elsevier, London, pp 889–903
Besharse JC, Horst CJ (1990) The photoreceptor connecting cilium. A model for the transition zone. In: Bloodgood RA (ed) Ciliary and flagellar membranes. Springer, New York, pp 389–417
Kozminski KG, Johnson KA, Forscher P et al (1993) A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A 90:5519–5523
Rosenbaum JL, Cole DG, Diener DR (1999) Intraflagellar transport: the eyes have it. J Cell Biol 144:385–388
Nair KS, Hanson SM, Mendez A et al (2005) Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron 46:555–567
Gilliam JC, Chang JT, Sandoval IM et al (2012) Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell 151:1029–1041
Yahav T, Maimon T, Grossman E et al (2011) Cryo-electron tomography: gaining insight into cellular processes by structural approaches. Curr Opin Struct Biol 21:670–677
Dubochet J, Adrian M, Chang JJ et al (1988) Cryo-electron microscopy of vitrified specimens. Q Rev Biophys 21:129–228
Dierksen K, Typke D, Hegerl R et al (1992) Towards automatic electron tomography. Ultramicroscopy 40:71–87
Dierksen K, Typke D, Hegerl R, Baumeister W (1993) Towards automatic electron tomography. II. Implementation of autofocus and low-dose procedures. Ultramicroscopy 49:109–120
Frank J (1992) Electron tomography: three-dimensional imaging with the transmission electron microscope. Plenum, New York
Kremer JR, Mastronarde DN, McIntosh JR (1996) Computer visualization of three-dimensional image data using IMOD. J Struct Biol 116:71–76
Mastronarde DN (1997) Dual-axis tomography: an approach with alignment methods that preserve resolution. J Struct Biol 120:343–352
Grimm R, Singh H, Rachel R et al (1998) Electron tomography of ice-embedded prokaryotic cells. Biophys J 74:1031–1042
Grimm R, Typke D, Barmann M et al (1996) Determination of the inelastic mean free path in ice by examination of tilted vesicles and automated most probable loss imaging. Ultramicroscopy 63:169–179
Leis A, Rockel B, Andrees L et al (2009) Visualizing cells at the nanoscale. Trends Biochem Sci 34:60–70
Nickell S, Park PS, Baumeister W et al (2007) Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol 177:917–925
Nicastro D (2009) Cryo-electron microscope tomography to study axonemal organization. Methods Cell Biol 91:1–39
Mastronarde DN (2005) Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol 152:36–51
Sandberg K, Mastronarde DN, Beylkin G (2003) A fast reconstruction algorithm for electron microscope tomography. J Struct Biol 144:61–72
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this protocol
Cite this protocol
Wensel, T.G., Gilliam, J.C. (2015). Three-Dimensional Architecture of Murine Rod Cilium Revealed by Cryo-EM. In: Jastrzebska, B. (eds) Rhodopsin. Methods in Molecular Biology, vol 1271. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-2330-4_18
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2330-4_18
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-2329-8
Online ISBN: 978-1-4939-2330-4
eBook Packages: Springer Protocols