Abstract
Cranial electrical stimulation (CES) is a noninvasive brain stimulation technology that uses a low intensity alternating current (AC) applied to the head through one or more electrodes. CES variants using a range of frequencies have been used clinically and in research for approximately 100 years. From early human and animal studies of the so-called electrosleep and electro-analgesia, to later twentieth century and contemporary explorations of potential usefulness for mild to moderate depression, anxiety, insomnia, and pain, CES devices have persisted, mostly outside the mainstream of psychiatric and neurological treatment. Low-powered or poor quality studies, varied stimulation parameters and device names, and associations with alternative medicine all have been barriers to CES being scientifically studied and refined. This may soon change, for several reasons: CES is very affordable and easy to use; it appears to have a good safety profile; biomedical engineers are optimizing CES devices; new CES variants targeted to individual cranial nerve afferents show some success in treating specific neurological conditions; and recent modeling and human laboratory data suggest CES may be particularly well suited to modulating endogenous brain oscillations. This chapter gives an overview of CES history, of evidence for CES efficacy for diverse clinical conditions, discusses proposed mechanisms of action, safety and regulatory issues, and the future of CES.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Transcranial Magnetic Stimulation
- Deep Brain Stimulation
- Alternate Current
- Default Mode Network
- Vagus Nerve Stimulation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Cranial electrical stimulation (CES) is a noninvasive brain stimulation technology that uses a low intensity (0.1–16 mAmp) alternating current (AC) applied to the head through one or more electrodes. Preset, often patented stimulus frequency patterns vary across different CES devices. Treatment typically is given once or twice per day for 20–60 min, although some newer versions of CES devices designed to stimulate cranial nerves stimulate overnight for 8 h per day. CES frequencies range from 0.5 to 15,000 Hz, often with bursts of high-frequency stimulation separated by low frequency stimulation to produce recurring high-frequency pulse bursts. The higher frequencies are better able to overcome the high impedance of the skull. Some commercial devices offer several intensity settings for individual titration for efficacy and comfort, and for different clinical applications. Many CES devices use two electrodes on opposite sides of the head (e.g., at the temples, on the mastoid processes, on the earlobes using ear clips), and some include a third or fourth electrode as well (e.g., on the forehead). Newer devices designed to stimulate cranial nerves may use one or two electrodes, supraorbitally (centrally on the forehead above the eye sockets). Other devices used clinically or in research may use one electrode over a target area of cortex and a reference electrode on the top of the head (vertex) or on the neck, arm or another location.
Stimulation parameters vary greatly among CES instruments and experimental paradigms. Commercial devices often use special patented patterns of stimulation that combine a range of low to high frequencies, which the original inventors believed produced therapeutically potent stimulation. The alternating current can be sinusoidal or square waves. Alternating current stimulation used in laboratory experiments to probe brain function usually involves a single frequency sinusoidal alternating current applied to the head/scalp, or earlobes and usually is known as transcranial alternating current stimulation (tACS).
Use of diverse forms of low intensity electrical stimulation of the brain—both alternating and direct (galvanic) current—goes back to antiquity [1, 2]. Roman physicians Galen and Scribonius Largus prescribed application of Mediterranean electric fishes to the human head to alleviate melancholia, and to the feet for gout and headaches. More recently in the eighteenth and nineteenth centuries, Volta, Aldini, and others studied medical and physiological effects of direct current (DC). Aldini reported the successful galvanic treatment of patients with melancholia in 1804 [2]. In the twentieth century, various low intensity AC and DC current devices applied to the head have been investigated periodically. Electroconvulsive Therapy (ECT), a high intensity, high frequency AC therapy, introduced in the 1930s by Bini and Cerletti, had been the dominant psychiatric and neurological device until the development of Transcranial Magnetic Stimulation (TMS), Deep Brain Stimulation (DBS) Magnetic Seizure Therapy (MST), and Vagus Nerve Stimulation (VNS), along with revived interest in transcranial Direct Current Stimulation (tDCS), and in diverse forms of Cranial Electrical Stimulation (CES) in recent decades [2].
CES has been studied and used clinically for over 60 years in North America, Europe, Russia and the former Soviet Union, to treat insomnia, anxiety, depression, drug withdrawal, headache, other types of pain, and hypertension. Early Russian interest sprang from the work of Ivan Pavlov, who observed that his dogs frequently fell asleep during experiments using a similar electrical conditioning stimulus, hence the earlier term, electrosleep. This was hypothesized to happen because of spreading inhibition over the cortex, from a specific locus to generalized inhibition [3, 4]. Electrical stimulation to treat insomnia in humans was first reported by Robinovitch in 1914, [5]; he used rectangular pulses of 6–8 kHz, of approximately 4.0 mA (35 V) between forehead (−) and hand (+) electrodes.
Interest in electrosleep and other applications of CES in Russia has remained high throughout the twentieth century and continues to the present ([6–10] and many others). However, potentially useful information from a large body of work conducted in the former Soviet Union over many decades mostly has not been translated and therefore is little known in the West. The methodology of many Soviet era studies appears to predate modern clinical research standards. Klawansky et al. [11] considered some of this literature for their meta-analysis, and found that most of the published studies were uncontrolled and were therefore excluded from the meta-analysis. Some more recent Russian studies occasionally have been published in English (e.g., [8, 10, 12]).
From the early twentieth century to the present, many names have been used for low intensity alternating current devices applied to the head, including: cranial electrical stimulation (CES) [13], cranial electrotherapy stimulation (CES); transcranial alternating current stimulation (tACS) [14]; transcranial pulsed current stimulation (tPCS) [15]; transcutaneous cranial electrical stimulation (TCES), [16–18]; transcranial electrostimulation or transcranial electrical stimulation (TES) [8, 9]; cranial or cerebral electrotherapy (CET) [19, 20]; transcerebral electrotherapy (TCET); transcranial electric treatment (TET) [21]; neuroelectric therapy (NET); cranial transcutaneous electrical nerve stimulation (TENS); and descriptives such as electrosleep; brief high intensity pulsed stimulation [17, 22]; and auricular electrical stimulation [20].
In recent years, it has become customary to call this class of devices either CES (for example during the 2012 FDA hearings on possible re-classification of three of these devices; see below), or transcutaneous stimulation for devices intended to stimulate cranial nerves rather than directly stimulate the brain. However, based on stimulation parameters of transcutaneous devices, it is likely that these two categories of devices act through similar mechanisms. At this point, the relative importance of cranial nerve afferent stimulation vs. direct effects of current on brain tissue is unknown.
Compared to the other classes of brain stimulating devices, both FDA-approved for neuropsychiatric indications (Deep Brain Stimulation, Electroconvulsive Therapy, Vagus Nerve Stimulation, Transcranial Magnetic Stimulation) and still in development (Magnetic Seizure Therapy, transcranial Direct Current Stimulation), among commercial CES devices there is less standardization and less transparency regarding stimulation parameters. Among CES devices there is more variability in stimulus intensity (Amperage or Voltage), stimulus frequency (Hz), pattern and duration of stimulus delivery, size, number, and type of electrodes, and cranial placement of electrodes. CES stimulation is delivered at 16 mA or below, because intolerable scalp discomfort and pain are experienced at the electrode site as the intensity rises close to 16 mA. These doses are far below the seizure threshold.
Concerning the potential significance of the pattern of electrical stimulation, Datta et al. [15, 23] draw a distinction between clinical devices that use pulsed, varying frequencies of stimulation, as opposed to very low, constant frequency stimulation used in experimental laboratory studies to probe brain function, which is often called transcranial alternating current stimulation (tACS). They therefore advocate that CES be referred to instead as tPCS (transcranial pulsed current stimulation), a term Alon et al. also employ [24, 25] and the term tACS be used for stable frequencies used in experimental studies. To date, there has been little head-to-head comparison among stimulation parameters to determine differential biophysical or therapeutic effects [13–15, 23, 26, 27]. If differential therapeutic benefits related to the various parameters and methods of current delivery become clearer, this will likely lead to a more standardized device nomenclature.
CES types of devices have been in use for many years for a variety of clinical and subclinical symptoms, and a substantial and diverse body of information has accumulated. As of 2002, a bibliography by Kirsch listed 145 scientific studies of CES involving human subjects, reportedly encompassing over 8,800 people receiving active CES [28]. Nonetheless, poor understanding of CES efficacy and mechanism of action persists [29]. Potential mechanisms and brain areas affected may vary considerably according to all the aforementioned stimulation parameters. Stimulation doses may also vary due to individual skull and brain anatomy [15, 23]. Confusion has persisted concerning the degree to which various devices stimulate brain structures by electrical fields directly reaching brain tissue, or through stimulation of afferent fibers of cranial nerves. Recent research, from modeling [15, 23, 30, 31] and human [24, 25, 32] data indicates that CES devices can modulate cortical and subcortical functions. However, the modeling studies do not account for afferent neural input, and human studies cannot establish whether that modulation is the result of direct cortical stimulation or cranial nerve afferent stimulation.
Despite the long history of CES use in Europe and the USA, there have not been large, well-controlled clinical trials to establish efficacy for neuropsychiatric and other indications. Clinical studies to date have been primarily open trials or randomized trials limited by low subject number, poor subject characterization, inclusion of subjects with mixed diagnoses or subclinical levels of symptoms, inactive sham controls, inadequate blinding, and lack of systematic collection of side effects and adverse events [11, 38]. As devices, stimulation parameters and outcome measures also vary, this makes comparisons and meta-analyses difficult. Even the few well-controlled human studies that do exist often have small numbers of subjects and thus provide constrained evidence of effectiveness for some indications (insomnia, depression, withdrawal from drug addiction) and safety overall.
Earlier in the twentieth century, CES was attempted as a treatment for a variety of of psychosomatic and “psychophysiological” disorders, including encephalitis, preeclampsia, enuresis, acid-peptic disease, essential hypertension, neurodermatitis [33]; and wound healing [9]. In recent decades, the research and clinical focus has been on withdrawal from addictive substances, anxiety, depression, headache, pain, and sleep disorders. More recent studies finally are bringing mainstream clinical trial methodologies to the study of CES, and new indications are under study. However, scientifically unsupported claims continue to be promoted.
There is an extensive literature of animal data using transcranial, auricular and implanted electrodes, supporting efficacy of CES for treatment of pain, drug dependency, and for anesthesia. This literature is not summarized here, except to note that as of 2005, Gilula and Kirsch [34] reported 29 CES animal studies in the literature. Typical examples are the study of Dougherty et al. [35], who found that auricular transcranial electrical stimulation attenuated the severity of naloxone-precipitated morphine withdrawal in rats; and the study of Mantz et al. [36] who found that TCES significantly reduced halothane (a general anesthetic) requirements in a rat model.
Contemporary Devices and Clinical Applications
The previous most active period of interest in research on CES was in the 1960s and 1970s, evidenced by the International Symposia for Electrotherapeutic Sleep and Electroanesthesia, held in Graz, Austria, in 1966 and 1969 [11, 37]. In 1975, there were at least seven American-made commercially available CES devices. In 1995, eight commercial CES devices were on the market [11]. The two most widely used CES devices today in the USA are the Alpha-Stim® devices (Electromedical Products Int. Inc.), which deliver stimulation up to 0.6 mA through earclip electrodes with pulsed frequencies which can be set at 0.5, 1.5, or 100 Hz; and the Fisher-Wallace Cranial Stimulator (Fisher-Wallace Laboratories), which delivers pulsed higher frequency stimulation through sponge electrodes placed at both temples. Current range for the Fisher Wallace device is 1.0–4 mA and frequencies are 15, 500, and 15,000 Hz. The Fisher-Wallace device uses the same patented frequencies as the former Liss Cranial Stimulator. CES-Ultra (Neuro-Fitness LLC), also marketed in the USA, delivers an adjustable current amplitude from 0 to 1.5 mA, a current intensity up to 1 A, as a 100 Hz square wave, with 2 ms pulse duration. CES Ultra gives the option to use ear clip electrodes or gel electrodes placed on the mastoid processes. These three devices have 510 K approval status from the FDA to be marketed for treatment of anxiety, depression, and insomnia. They are available in the USA only with a prescription from a licensed health care practitioner (Fig. 11.1).
The “Limoge’s current” was reportedly satisfactorily used for several years mostly in France and Russia, to produce anesthesia (electroanesthesia) and pain control [18]. Lefaucheur [17] has described the highly specific stimulation parameters of Limoge’s current, primarily used for electroanesthesia. Trains of stimuli are applied at 77–100 Hz, each train composed of positive sharp pulses, delivered at 125–167 kHz and separated by large negative pulses of smaller intensity but with the same area as the positive pulses. This yields a non-polarized stimulus train of 3–4 ms in duration and 30–35 V (200–350 mA) in peak-to-peak amplitude. A specifically engineered device delivers the Limoge’s current into the brain, using a cathode placed between the eyebrows and two anodes on each posterior mastoid region. In the years prior to 1990, high frequency (166 kHz) intermittent Limoge-current transcutaneous cranial electrical stimulation (TCES) was used in cardiac, thoracic, abdominal, urological, and micro-surgery, based on observed benefits of reduced requirement for analgesic drugs, particularly opiates, and long-lasting postoperative analgesia [39, 40].
The CES devices commercially available in the USA and CES devices used in Europe and the USSR are intended to deliver non-targeted stimulation to the head and brain. Some more recent CES-like devices, currently only available abroad, are proposed to modulate the brain indirectly via stimulation of cranial nerve afferent fibers. Specific cranial nerves are chosen to treat specific disorders, for example, supraorbital stimulation of the trigeminal nerve for migraine relief, as in the Cefaly® device [41], and to mitigate epileptic seizures, and possibly treat other conditions, as in the Monarch™ external Trigeminal Nerve Stimulation (eTNS™) system [42]. However, the stimulation parameters are very similar to those of older CES devices, raising the question to what degree these devices and CES devices also act at least in part through direct stimulation of brain tissue vs. stimulation of afferent cranial nerves.
The Cefaly® device (STX-Med, Liège, Belgium) uses a single frontal self-adhesive electrode contained within a rigid headband that is placed horizontally over the forehead and over the ears. Cefaly’s model indicates the device works by stimulating the bifurcation of the trigeminal nerve centrally just above the orbits (the supratrochlear and supraorbital nerves); this cranial nerve transmits sensation from the face and scalp to the brainstem. The device is thought to stimulate endorphin release, and stimulation of sensory afferents is thought to block headache or migraine pain pathways into the central nervous system. Cefaly® asserts that its technology is very safe. The Cefaly® device generates biphasic rectangular impulses with 250 μs pulse width, 60 Hz frequency, and 16 mA current intensity. Stimulation sessions are recommended to last 20 min, once/day. Case reports and research papers are available on the Cefaly® site, which states that more than 5,000 treatment sessions occurred in the cited 25 laboratory, case, pilot, and blinded studies of Cefaly’s clinical effectiveness and safety (http://www.cefaly.ca/site/studies). The Cefaly® device has been submitted for FDA approval in the USA and currently is available in Canada and Europe without a prescription.
The NeuroSigma Monarch™ external Trigeminal Nerve Stimulation (eTNS™) system, also designed to stimulate the trigeminal nerve at both the infraorbital and supraorbital branches. Based on prior research [42–44], FDA has just permitted initiation of a Phase III clinical trial of the Monarch™ system for epilepsy. The manufacturer points out that trigeminal nerve afferents project indirectly to multiple brain areas playing key roles in seizure inhibition and initiation, but also implicated in depression, anxiety, and pain circuits: the nucleus solitarius, locus coeruleus, anterior cingulate, and cerebral cortex. Based on mood improvement in patients treated for epilepsy, this device now also is being investigated for treatment of depression [45]; a Phase II clinical trial is underway in the USA. In addition, a Phase I clinical trial has just been begun of the Monarch ™ device for Post-Traumatic Stress Disorder (PTSD), and for Attention Deficit Hyperactivity Disorder (ADHD) in children. An implantable form of the same technology also is being developed, sTNS™, using subcutaneous electrodes and an implantable pulse generator. The Monarch™ eTNS™ System, not yet available in the USA, is available in Canada and the European Union but only with a physician’s prescription (www.neurosigma.com; http://www.monarch-etns.com).
Finally, the Transair device (abbreviated from (TRANscranial electrotherapy Stimulator for Analgesia, Immunity and Reparation), created at the Pavlov Institute of Physiological Sciences of the Russian Academy of Sciences, Center TES (http://neurotes.com) and marketed in Russia and Eastern Europe (see e.g., Onkocet), is reportedly widely used in clinics in those regions. The Transair devices stimulate via four electrodes, two on the mastoids and two on the forehead. Five devices are mentioned on the site. They have multiple-programmed settings to treat a very wide range of illnesses and conditions. Four devices are for clinic use, including one device specialized for audiological use, and one is for home use. Two Transair devices are described with some detail. The types of electric current used are: TRANSAIR-05: pulsed monopolar current and pulsed bipolar current with frequency modulation control, direct current in combination with pulsed monopolar current, and direct current, with intensity up to 5 mA, at a frequency of 50 Hz; TRANSAIR-04: pulsed bipolar current, pulsed monopolar current and combination monopolar and direct currents in 1:1 ratio, with intensity up to 5 mA, at a frequency of 50 Hz (Table 11.1).
Additional novel electrode sites may be used in future forms of cranial and transcranial stimulators. Drawing on earlier research [46, 47], Kraus and colleagues [48] investigated BOLD fMRI effects in response to transcutaneous electrical stimulation of two different zones in the left outer auditory canal. This area is rich in vagal afferents. Stimulation parameters were pulse width 20 ms, frequency 8 Hz, individually titrated to be well tolerated; and mean stimulation intensity was 32.6 V (min 14 V, max 57 V). They found robust BOLD signal decreases in limbic structures and the brain stem during electrical stimulation of the left anterior auditory canal, including BOLD signal decreases in the area of the nuclei of the vagus nerve, which may indicate an effective stimulation of vagal afferents. Stimulation at the posterior wall of the auditory canal resulted in changes of the BOLD signal within the nucleus solitarius, a key relay station of vagal neurotransmission. Kraus and colleagues concluded that there is promise in this specific novel method of cranial nerve X or vagal stimulation, and that it could be beneficial for treatment of psychiatric conditions. A similar in-ear electrode location was demonstrated by Datta et al. [23] in a modeling study to produce higher induced electrical field magnitudes in the midbrain, pons, hypothalamus, and insula than some conventional CES stimulation sites.
Evidence of CES Efficacy from Open and Randomized Clinical Trials
Clinical conditions for which there is preliminary evidence of CES benefit from human data include, e.g., anxiety [49–51], review; [11], meta-analysis for anxiety indications [52], anxiety in addicts [53] and dental patients [54]; bipolar II disorder [55]; depression [51, 56–58]; hypertension [59, 60]; fibromyalgia [61]; insomnia [62, 63]; migraine headache [41] and tension headache [64]; nightmares, aggression/irritability [62, 65]; pain [66–68]; surgical and post-surgical analgesia [69–71] and anesthesia [18]; Parkinson’s disease [25] and pain in PD [72]; substance abuse withdrawal and relapse prevention [21, 53, 73–78, Smith, 1982]; and visual field deficits after optic nerve injury [79, 80].
Some focused [50, 38, 81–83] and fairly comprehensive reviews of CES [11, 13, 18] also have appeared in recent years. According to Klawansky[11], as of 1995, evidence for efficacy, as measured by effect size based on the 14 included pooled studies, was strongest for anxiety disorders (CES > sham, p < 0.05).
The more robust clinical trials among the above include studies of CES for fibromyalgia [61, Taylor, 2011]; migraine headache [41]; addictions (e.g., [53, 74]); dental procedure anxiety [54]; surgical analgesia [70, 71]; pain [66] and visual field deficits after optic nerve injury [79, 80].
The Cefaly device reduced migraine frequency during daily treatment for 2 months [41]. Compared to stimulation with a 30 μs pulse width, 1 Hz frequency and 1 mA current intensity. The Cefaly device also was found to promote a sedative effect [84].
The NeuroSigma Monarch™ external Trigeminal Nerve Stimulation (eTNS™) currently is marketed for treatment of epilepsy and depression in Canada and the European Union, but published data supporting efficacy is weak. DeGiorgio [42, 43, 85] conducted a double-blind randomized active-control trial in drug-resistant epilepsy to test the suitability of the NeuroSigma type of CES treatment, and to try to establish control parameters in preparation for a phase III multicenter clinical trial. Fifty subjects with long-term epilepsy (mean age approx. 22 years) and two or more partial onset seizures per month first had a 6-week baseline period, and then were evaluated at 6, 12, and 18 weeks during the acute treatment period. Participants were randomized to treatment (eTNS™ 120 Hz) or control (eTNS™ 2 Hz) parameters, and were matched on key variables; they were highly drug-resistant, having failed on average more than three antiepileptic drugs prior to enrollment. eTNS™ (NeuroSigma) was well-tolerated; side effects included anxiety (4 %), headache (4 %), and skin irritation (14 %). The responder rate (50 % reduction in seizure frequency) was 30 % for the treatment group vs. 21 % for the active control group for the 18-week treatment period (n.s., p = 0.31). However, the treatment group experienced a significant within-group improvement in responder rate over the 18-week treatment period (from 18 % at 6 weeks to 41 % at 18 weeks, p < 0.01). eTNS™ also was associated with improvements in mood on the Beck Depression Inventory (p < 0.02). The authors concluded that this Class II evidence suggests that eTNS™ is safe and may be effective in subjects with drug resistant epilepsy. A larger multicenter phase III clinical trial is being planned.
Using methods and stimulation parameters similar to those in the NeuroSigma epilepsy studies [43, 85]. Schrader et al. [45], examined the effects of the Neuro Sigma device as an adjunct to pharmacotherapy for major depression. Five adults (mean age 47 years), all with persistent depressive symptoms despite adequate pharmacotherapy, participated in an 8-week open-label outpatient trial. Nightly stimulation for a minimum of 8 h over the V1 branch of the trigeminal nerve was well tolerated, although some participants developed skin irritation under the device contact site. The clinician-rated Hamilton Depression Rating Scale (p = 0.006) and self-rated Beck Depression Inventory (p = 0.0004) detected significant symptomatic improvement over baseline. The authors concluded that eTNS™ may be a useful adjunct to pharmacotherapy in major depressive disorder, and call for larger trials. It should be noted that nightly stimulation for 8–12 h is much more extensive than stimulation periods in most CES studies for mood-related problems, of typically 20–30 min/day.
A number of important treatment parameters remain to be investigated for all CES devices. Some studies suggest that response to CES stimulation can be rapid, occurring after 2–10 sessions [8], but it is not clear how long benefits persist after cessation of treatment. Feighner et al. [33] examined the duration of clinical benefit after terminating use of CES for indications such as depression and anxiety. Their double blind, randomized controlled study tested the efficacy of electrosleep on patients with chronic (>2 years) psychiatric illness refractory to treatment, with symptoms of anxiety, insomnia and depression not caused by medical illness. In a crossover design, patients were randomly assigned to either Group I, ten active electrosleep treatments followed by ten sham treatments over a 4-week period, or Group II, ten sham electrosleep treatments, followed by ten active electrosleep treatments over a 4-week period. Repeated, blinded objective and subjective ratings were acquired to assess clinical improvement, and follow-up ratings were done on a monthly basis for 6 months. Results indicated that active electrosleep treatments significantly improved sleep, anxiety, depression, and psychosocial adjustment. However, only one patient had sustained remission; all other patients who initially responded relapsed during the first month following treatment cessation, and of these, only two responded to a further intensive course of electrosleep therapy, and did well with maintenance treatments.
Further research is needed to identify optimal schedules and duration of treatment—daily use for a specific duration in months, or brief bursts of CES application for a few days, then cessation of use for a specific period of time, then repeated, or simply used ad libitum as desired. The effects of tapering of CES treatment either at initiation or termination on efficacy, adverse events, or relapse have not been studied, and should be. A rat model study [27] investigated the effect of varying transcranial AC stimulus frequency, pulse width, charge balance and polarity, electrode placement, and time of day of stimulation on tail flick response to heat. A biphasic, charge balanced waveform with a first phase duration of 2 ms, current 10 mu Amp and repetition rate 10 Hz was found to induce maximum tail flick latency changes from baseline.
There has been almost no systematic examination of whether and how severity of depression or anxiety affects response to CES. In the study of Feighner et al. [33], patients diagnosed as having primary depression (major depressive disorder) did worse with active electrosleep treatment. They concluded that in patients with primary depression, electrosleep therapy should be used with caution, and may be contraindicated. Whether there are sustained or only short term benefits of CES requires much more extensive scientific study.
Lebedev et al. [8] reported that fatigue, stress, and related psycho-physiological disturbances were significantly improved or abolished after 2–5 transcranial electrical stimulation (TES) sessions (TRANSAIR device), in mixed groups of stressed workers, military members, patients with PTSD and other conditions, and others (total N = 808), and according to Lebedev et al., more noticeably in cases of more serious disturbance. Better response in patients with more severe illness seems to contradict the report of Feighner et al. of worse response in more severe depression, but not enough detail is provided in either study to compare severity of illness.
Many patients increasingly seek less invasive and less expensive forms of treatment.
Because CES has not been adequately tested in individuals with major depression or specific anxiety disorders [38], there is appropriate concern that more severely ill individuals may avoid proven interventions in favor of CES self-treatment. Schrader et al. [45] are investigating the NeuroSigma eTNS™ trigeminal nerve stimulating device for major depression, but as an adjunct to pharmacotherapy. A more appropriate role for CES might be to help maintain remission after a course of a proven treatment, for example for depression, but little data is available to address the question of efficacy for more severe symptoms.
A new approach which could be particularly productive for clinical use of CES is to target pain, depression, insomnia and fatigue as a group of symptoms, which commonly co-occur in inflammatory disorders, other medical illnesses, and in situations of chronic stress [57, 86]. Anecdotally, CES users often have reported feelings of increased energy, mild euphoria, and a lack of concern about minor problems [87, 88]. Anecdotal documentation of this response to CES is widespread in many studies over the decades of its use, and also can be found on commercial device Web sites that post consumer endorsements and informal tabulations of benefits and side effects. There also is some evidence that a single session of CES can attenuate acute stress responses [8], reduce physiological and psychological arousal in healthy subjects, and reduce vigilance and increase drowsiness in healthy volunteers [84]. Concerning other applications of CES for stress reduction, some human resources professionals have suggested that CES might be used in nonclinical populations to help alleviate workplace stress [89].
Interestingly, relaxation benefits of CES also are reported in animals. It was Pavlov’s early observation of the soporific effects on dogs in his experiments that stimulated early Russian interest in electrosleep [3]. Fisher-Wallace Laboratories also offers an equine version of a CES device called the Happy Halter, which is marketed to veterinarians and trainers of high performance horses. It reputedly is useful in calming nervous horses and in pain reduction [90]. The Alpha-Stim device reportedly is also successfully being used to calm horses [91].
Taylor and Lee [92], in a double-blind protocol, administered to ninety healthy volunteers 30 min of constant current sine-wave cranial transcutaneous electrical nerve stimulation (TENS) of 5, 100, or 2,000 Hz frequency (current maintained below 0.5 mA for safety), placebo TENS, or no treatment. The five groups were compared on pretreatment to posttreatment changes in blood pressure, heart rate, peripheral temperature, and anxiety. Analysis showed significant reductions in systolic and diastolic blood pressure and heart rate after 100 Hz cranial TENS as compared to the other groups. No other differences achieved significance.
The military has shown interest in CES, in particular for treatment of PTSD [93–95]. Both the Alpha-Stim and Fisher-Wallace company Web sites indicate military use of the devices and Armed Forces funding of clinical trials.
Clinicaltrials.gov posts the following trials of CES as of July 2013: Cranial Stimulation for Chemotherapy Symptoms in Breast Cancer (Virginia Commonwealth University, National Cancer Institute); Efficacy and Safety of Cranial Electrical Stimulation (CES) for Major Depressive Disorder (MDD) (Massachusetts General Hospital, Fisher Wallace Labs, LLC); Cranial Electrical Stimulation Effects on Symptoms in Persons With Fibromyalgia (University of Virginia); Use of Alpha-Stim Cranial-Electrotherapy Stimulation (CES) in the Treatment of Anxiety (Wyndhurst Counseling Center, Liberty University); A Pilot Study of Cranial Electrotherapy Stimulation [CES] for Generalized Anxiety Disorder (University of California, Los Angeles); Cranial Electrotherapy Stimulation (CES) to treat PTSD (CES-fMRI-PTSD) (McLean Hospital, Mending Minds Foundation).
Contraindications for Use and Safety of CES
There are few contraindications for use of CES on the device manufacturers’ Web sites. Interestingly, the Russian company Transair is the only one that lists extensive contraindications (see Table 11.2 below). This makes sense in that Transair seeks to treat much more varied conditions. Transair TES therapy is contraindicated in: seizures, epilepsy; acute brain injuries and tumors, central nervous system infections; stage III hypertension, hypertensive emergency; hydrocephalus; acute psychiatric disorders; thyrotoxicosis; atrial fibrillation; broken or damaged skin on forehead, area of electrode application; implanted electrostimulators; in children under 5 years of age.
No serious adverse events have been reported in the past 50 years of CES use in clinical and research settings. However, few trials to date have systematically and prospectively recorded side effects. In 1974, a review of the research on safety of cranial electrotherapy stimulation (CES) was commissioned by FDA and conducted by the National Research Council, Washington, DC. The NRC reviewers concluded that “significant adverse events or complications attributable” to the application of electric current of approximately 1 mA or less for “therapeutic effect to the head” (cranial electrotherapy stimulation) were “virtually nonexistent” [96].
Electronic Products International (EPI), the manufacturer of the Alpha-Stim device, indicates that consumer reports to EPI in 2007–2011 concerning adverse events were associated with <1 % of a reported 58,030 Alpha-Stim units sold in that same period. Also drawing from 14 published studies using the Alpha-Stim device and involving a total of 2,389 subjects who had active treatment, they further reported that adverse events occurred in less than <1 % of all study treatments. Side effects included pain or itching at the earlobes, vertigo, drowsiness, nausea, headache, tinnitus and others. However, for many of these studies current was set at 0.1 mA, for 60 min, to reduce the chance that subjects could discriminate active treatment from sham. Recently, studies have more systematically collected data on side effects, detecting higher rates of side effects. Even at the low 0.1 mA intensity of stimulation, a recent controlled study with the Alpha-Stim device found that 30 % of subjects reported ear pain or itching at the electrode sites [97]. Of note, at that low stimulation intensity, recent well-controlled trials found no reduction in target symptoms of neuropathic pain [97], insomnia or depression [98].
The NeuroSigma trigeminal nerve stimulation device, intended to be used for 24 h continuously, was associated with mild to moderate skin irritation under the electrodes in eight of 13 subjects [99]. Irritation was relieved by hydrocortisone cream, reduction of length of exposure to stimulation from 24 to 12 h, and alternation of the location from supraorbital to infraorbital.
Studies using higher stimulation frequencies and intensity (4–16 mA) have found that all subjects reported intense paresthesias [41] or flickering lights [24, 100, 101].
Decades ago, electrodes sometimes were applied to the eyes, to bypass skull impedance. but this was associated with blurred vision which persisted for some minutes after treatment.
Other rare, possibly related safety concerns were noted in prior studies. A study of rural law enforcement personnel using CES for depression reported one participant developed increased levels of agitation, and was removed from the study [102]. One participant in a study of CES for chronic mental illness reported an increase in auditory hallucinations but was able to finish the study [103].
Although CES has been suggested as a safer alternative to antidepressant and antianxiety medication during pregnancy, there has been one report of a frequency-dependent reduction in fetal weight and increased fetal death in rats, as a consequence of 1 h of daily CES treatment at 0.125 mA and 0.22 ms pulse width during pregnancy [104].
In 1975, Jordan and Morris investigated safety of a combined AC and DC stimulation paradigm in young male beagle dogs, using an electrosleep (ES) machine manufactured by Hoffman-LaRoche Corporation. This paradigm was based on a human protocol that called for one eye and one occipital electrode at a strength of 1 mA of AC current and 0.33 mA of DC current. The canine protocol involved comparable stimulation sites, with three dogs assigned to each of the three experimental conditions: 1 mA of AC current and 0.33 mA of DC current; 5 mA AC and 1.33 mA DC; and Sham. Frequency was 100 Hz with a pulse width of 5 ms. While the dogs were anesthetized, 13 daily treatments of 1 h duration were applied over a 3-week period, at fixed AM and PM times, with extensive physiologic sampling on days 1, 7, and 13. At the end of the protocol the dogs were sacrificed and both eyes and the brain were examined grossly and microscopically. No clinically significant neurologic signs were observed. Pathological data revealed some suspicious findings (oligodendroglia, areas of calcification) most often in the striate cortex, caudate nucleus and septum, but these were deemed small and of questionable significance, except for one instance. A dose–response relationship was observed, with the high dose condition producing the majority of all lesions (approximately 14/dog), compared to low dose and sham (between approximately 7 and 9/dog), again the majority deemed not likely significant. Other major findings included EEG slowing, depression of B-wave amplitude, and a chronic increase in pulse rate. The authors cited the small number of animals as a reason to replicate the study on a large scale, for valid statistical analysis [105]. Unfortunately this study could not determine whether the AC or DC stimulation was more likely to cause the lesions and other changes observed.
The peak electric field magnitudes generated during CES (<1 V/m) are approximately 100–1,000-fold lower than electric fields induced by transcranial magnetic stimulation (TMS) or electroconvulsive therapy (ECT), which also is AC stimulation [15]. The lack of evidence of brain injury associated with electroconvulsive therapy provides support for the likely safety of CES. ECT uses currents in the range of 2–4 A applied for approximately 30 s per session, designed to induce a seizure. CES uses a 1,000-fold smaller current (0.1–16 mA) for a longer duration (typically, 20–60 min daily) and a greater number of therapeutic sessions (30–60), compared to ECT (6–20). Because CES stimulation is too low intensity to produce seizures, it also does not produce the memory impairment often associated with ECT. There has been no evidence of structural brain injury associated with the far more powerful ECT, as measured by CT or MRI scans [106, 107]. Dwork et al. [108] presented preliminary findings, in what was then the first well-controlled nonhuman primate neuropathological study of ECT to use perfusion fixation, and the first to compare ECT with magnetic seizure therapy (MST); neither modality produced histological lesions in the brain.
There is a literature on transcranial electrical stimulation (TES) used for intraoperative motor evoked potential (MEP) monitoring (although the term TES also has been used by Lebedev for more conventional applications of CES, [7–10]). Journee [22] pointed out that the TES used in intraoperative monitoring differs in several respects from conventional cranial electrical stimulation, for example, it administers brief pulses of several hundreds of volts and currents may exceed 1 A, whereas conventional CES stimulators are limited to <20 mA. Due to the strong scalp pain generated, clinical use of high-intensity TES has been restricted to monitoring of motor pathways under general anesthesia. Transcranial magnetic stimulation, which also causes brief scalp pain in conscious subjects, stimulates a relatively small part of the brain. TES may elicit action potentials in many neural structures in a large volume of the brain, in complex intraoperative stimulation paradigms with increasing numbers of pulses. Therefore, Journee believes that concern about the risk of adverse or irreversible functional changes in the brain is appropriate. High intensity TES would seem to lie on a safety continuum between CES and ECT. MacDonald [109] reviewed the safety of high intensity TES, in comparison with other clinical and experimental brain stimulation methods and in light of clinical experience, in more than 15,000 cases. According to MacDonald, remarkably few adverse events were reported. Journee [22] pointed out that adverse events may have been underreported, but also concluded that with appropriate oversight and stimulation parameters, TES for intraoperative monitoring can be safe and beneficial. The minimal adverse events associated with the more powerful TES device offers some comparative support for the likely safety of the much weaker current of conventional cranial stimulators, although TES is not used chronically.
Research experience with tDCS also provides support for likely safety of CES. The alternating current delivered by CES is of similar amplitude (0.1–16 mA) to the direct current of tDCS. Since the development of tDCS in the 1960s, many hundreds of subjects have participated in studies. tDCS has been very well tolerated, with no significant adverse effects reported after a comprehensive review [110], other than scalp burns. In a more recent review and meta-analysis of studies reporting tDCS-caused adverse events, itching, tingling, burning sensation, headache, and discomfort were reported, more often in older and less healthy subjects and those who got higher current intensities [111]. Of note, scalp burns have never been associated with CES stimulation. Use of alternating current and usually no skin abrasion at the stimulation site are characteristic of CES administration, which may explain why skin burns do not occur with CES, although occasional mild skin reddening does. Additional tDCS safety findings include no elevation of neuron-specific enolase, a sensitive marker of neuronal damage [112]. Bikson et al. [113] discuss animal model data showing brain lesions from use of tDCS at high intensities (higher than would be used therapeutically in humans). The lesions are hypothesized to result from heating of tissue. Further discussion of safety issues for CES and tDCS can be found for example in Bikson et al. [113] and Lefaucheur [16, 17].
Further work will be needed to determine whether there are interactions between CES and neuropsychiatric medications, that could impact efficacy or tolerability of either CES or the concurrent medications. This has not been studied in CES, but has been somewhat examined in tDCS and TMS. Lefaucheur [16] points out that medication is likely to be a major source of changes in cortical function and patients with neuropsychiatric disorders are rarely free of drugs affecting brain excitability. For example, a recent study found that tDCS results improve with concurrent antidepressant administration [114]. The authors’ conclusions were that in major depressive disorder, the combination of tDCS and sertraline increases the efficacy of each treatment; and the efficacy and safety of tDCS and sertraline did not differ. Lefaucheur [16] discusses several kinds of interactions of neurotransmitter agonists and antagonists with tDCS stimulation, and additionally mentions that duration of drug administration and drug plasma levels also influence modulatory effects of cortical stimulation on the excitability of a target area [16]. As mentioned above, Schrader et al. [45] are investigating the NeuroSigma trigeminal nerve stimulating device to treat major depression as an adjunct to pharmacotherapy.
Future work also is needed to determine the risk to patients with bipolar disorder of becoming manic. There is one published report of mania being induced in a bipolar II patient being treated with tDCS [115]. Research on CES for bipolar II is in its infancy [55].
CES Regulatory Status (FDA)
Over the past 35 years, several CES devices were granted 510 K clearance in the USA to be marketed for the treatment of depression, anxiety, and insomnia, because the designs are equivalent to devices which were approved prior to 1976, when FDA began to require evidence of efficacy. In 1989, FDA amended its device regulations to require all devices that had not already done so to go through a formal premarket approval process, including submission of evidence of efficacy, and if requested, safety as well. As of 1993, FDA formally requested that CES device manufacturers comply with this requirement; they did not do so at the time [11]. Despite having received 510 K status in 1991 (e.g., the Fisher-Wallace device) to be marketed for the treatment of depression, anxiety, insomnia, and also chronic pain, due to the revised FDA approval process, CES devices remained in the Class III category. They remain in Class III after FDA hearings in February 2012 (Table 11.3).
Given the rise of interest in all forms of electrical and magnetic stimulation, more and better data for CES should gradually become available. However, because existing devices are close to the end of their patents, there is little incentive for conducting high quality, large-scale clinical trials. Device reclassification for CES types of technologies likely will be revisited in the coming years, particularly given an emerging generations of new low intensity, high frequency, alternating current devices, such as the Cefaly® and Monarch™ eTNS™ devices, and others currently in the experimental stage [15].
Proposed Mechanisms of Action of CES
Though the mechanisms by which CES may have impact on the brain and periphery still are minimally characterized, several have been proposed to date. Below we consider factors that influence the nature of the stimulation, that shape its proposed impact on the brain, and therefore the potential mechanisms of action of CES. We summarize evidence for several biological pathways that may be the source of proposed clinically relevant effects, in the hope as well of identifying clearer targets for scientific study. Proposed mechanisms of action include stimulation of cortical and subcortical regions; effects on endogenous brain oscillations and cortical excitability; impact on neurotransmitters, hormones and endorphins; and impact on autonomic nervous system.
A key question regarding mechanism of action is whether CES can penetrate through the skull (high impedance) and cerebrospinal fluid (CSF; low impedance) to stimulate brain tissue directly, whether CES stimulates peripheral nerves that transmit afferent signals to the central nervous system, or whether CES can stimulate via both pathways. For back pain, stimulation with higher current of 60–100 mA at 50–200 Hz (i.e., a TENS device), at the skin surface is thought to relieve pain by stimulation of afferent sensory nerves. Implanted electrodes for stimulation of peripheral nerves in the spine and forehead have been used for relief of visceral pain [116] and headache [117]. Although device makers claim that cranial nerve stimulation sites are chosen for relief of specific symptoms based on anatomical neural relays, there have been no comparative studies with CES demonstrating differential efficacy based on electrode placement. For example, frontal electrodes have been reported to relieve migraine symptoms [41] and to have sedative effects [84]; but bi-temporal electrodes also have relieved migraine pain [118]. If varied electrode placements have similar clinical effects, this would argue for a more diffuse effect of CES on brain tissue, possibly by modulation of endogenous oscillatory rhythms.
Figure 11.2 illustrates the range of stimulation patterns among published studies using different devices that may differentially shape CES effects [15, 23, 26].
The range of stimulation patterns among published studies using different devices that may differentially shape CES effects (Adapted from [15, 23, 26]). Adapted from: Datta A, Dmochowski JP, Guleyupoglu B, Bikson M, Fregni F (2013a). Cranial electrotherapy stimulation and transcranial pulsed current stimulation: a computer based high-resolution modeling study. Neuroimage. Jan 15;65:280–7
In order to study the biophysical and clinical significance of varying stimulation parameters on the brain, until now, analytical/spherical-based modeling approaches (see below), animal models, resected skulls, and synthetic phantoms all have been used [23]. However, as Datta and colleagues point out, these are of limited value given the need to study the effects of differing patterns of electrical stimulation in vivo on the human anatomy and its material properties. They cite the 1975 research of Dymond et al. [119] as still the only study that employed direct measurement in humans; the impact of DC electrosleep stimulation-induced intra-cortical current flow was studied in patients undergoing presurgical evaluation for epilepsy [23].
Datta and colleagues characterized scalp voltages caused by administration of CES, to validate subject-specific finite element method (FEM) models of current flow [23]. Each of the four stimulation electrode configurations tested resulted in a distinct distribution of scalp voltages. The authors suggested that monitoring of scalp voltages may be used to optimize electrode placement and current dose to increase transcranial electrical stimulation safety and reproducibility.
Brain Structures Impacted by CES
Computational modeling has been used to estimate intracranial penetration of electrical stimulation [30]. Two studies used finite element modeling (FEM) to estimate the penetration and focality of alternating current compared to a time invariant direct current stimulation [14, 120]. Using 1 mA and 10 or 100 Hz stimulation, Lopes et al. reported that alternating current stimulation generates cerebral fields that are up to ten times larger and 20 % more focused, in part because alternating current minimizes scalp resistance, with less current shunting between electrodes prior to propagating to deeper layers. Ferdjallah et al. [31] created a four-concentric-spheres simulation of CES with all dimensions and electrical properties of the model adapted from clinical data. Results indicated that, with electrode placement on opposite sides of the head to mimic CES application, the penetrating current density was maximized and a small fraction of the modeled CES reached the thalamus.
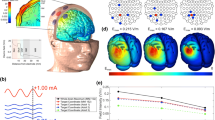
Datta et al. [15], using an updated, more sophisticated form of modeling, have produced new evidence for the proposed cortical and subcortical impacts of CES. They used a high resolution magnetic resonance imaging (MRI)-derived finite element head model including cortical and subcortical structures. Cortical electric field (current density) peak intensities and distributions were analyzed. They evaluated different electrode configurations of CES, or montages both conventional (ear clip) and novel (in-ear, behind ear (ear hook) and over-ear, all similar to headphone devices; see Fig. 11.3 below). All stimulated at 1 mA intensity (distributed across varying numbers of electrodes). Their model confirmed that significant amounts of current pass through the skull to reach cortical and subcortical structures. Depending on the electrode placement, induced currents at subcortical areas—midbrain, pons, thalamus, hypothalamus—can be of similar magnitude to those of cortical areas, and occasionally greater.
Conventional cranial electrotherapy stimulation (CES) ear clip electrode montage and novel transcranial pulsed current stimulation (tPCS) electrode montages. Adapted from: Datta A, Dmochowski JP, Guleyupoglu B, Bikson M, Fregni F (2013a). Cranial electrotherapy stimulation and transcranial pulsed current stimulation: a computer based high-resolution modeling study. Neuroimage. Jan 15;65:280–7
The conventional ear-clip montage resulted in a 0.10 V/m peak induced cortical electric field. Maximal currents were induced in the temporal cortex and in the medulla oblongata, with diffuse activation in the midbrain, pons, thalamus, insula, and hypothalamus. The in-ear placement resulted in a similar spatial profile of induced currents; however the peak induced electric field in the cortex was higher (0.16 V/m) and in the midbrain, pons, hypothalamus, and insula. The behind ear placement led to the highest peak induced cortical electric fields (0.47 V/m) as well as higher electric field in several deeper brain structures, except the medulla oblongata, possibly due to superior current flow through the mid-brain. The over-the-ear montage placement, either two or four contacts, led to similar current activation in sub-cortical and brainstem regions. The models of Datta et al. suggest that even relatively minor changes in CES electrode placement alter peak brain electric field and overall brain current flow patterns.
In another study that modeled multiple tDCS montages across three normal adult participants, Datta et al. [121] also concluded that current flow profile across all subjects and montages was influenced by details in cortical gyri/sulci, suggesting that subject-specific modeling could optimize effects of tDCS. Individual differences in cortical gyri may also influence CES effects.
Although these models predict current flow based on anatomical structures, they do not account for facilitated flow through afferent nerve pathways. For example, the external ear canal is dense with vagal afferents, and a new, less invasive form of vagus nerve stimulation, transcutaneous VNS (tVNS) also is being developed [46–48]. However, it remains to be determined whether these different electrode placements actually yield different clinical effects.
Recent neuroimaging studies in humans support the notion that CES modulates brain activity. Cerebral blood flow (CBF) was measured by xenon-enhanced computed tomography (XeCT™) before and after 2 h of active (n = 17) vs. sham (n = 19) TCES. Globally, CBF was unchanged by TCES; however locally, compared to sham stimulation, TCES caused significant CBF decrease in the brainstem (mesencephalon) and thalamus (diencephalon), structures involved in pain and anxiety.
Two MRI studies have shown CES impact on resting state functional connectivity of the Default Mode Network (DMN), which reflects normal resting state brain activity [24, 29]. In the study by Feusner et al. [29] CES at 0.5- and 100 Hz stimulation was applied to the earlobes at subsensory thresholds during functional magnetic resonance imaging in the resting state. Both 0.5 and 100 Hz stimulation yielded significant deactivation in midline frontal and parietal regions. 100 Hz stimulation was associated with both increases and decreases in connectivity within the default mode network (DMN). In the default mode network, nodes oscillate at the frequency of approximately 0.1 Hz [29]. This suggests that direct current or alternating current of frequency different than the DMN can disrupt DMN oscillations. In another study, both tDCS and CES (5, 500, 15,000 Hz) over the primary motor cortex down-modulated the functional connectivity of the associated resting state motor network in a recent study [24]. In major depression, network abnormalities have been reported for both the resting state default mode network (DMN) and the cognitive control network [122, 123]. Both antidepressants [124] and electroconvulsive therapy [125] have been shown to normalize the DMN in depressed individuals. These reports of CES effects on the default mode network represent significant promise for CES, even in the absence of convincing clinical trials data.
MRI data currently are being acquired in a trial of CES for depression at Massachusetts General Hospital, and a trial of CES for PTSD at McLean Hospital, Belmont, Mass.
Evidence for Effects on Endogenous Brain Oscillations and Cortical Excitability
Recent human laboratory studies have suggested that alternating current electrical stimulation is a useful paradigm to modulate endogenous cortical oscillations in order to study the function of cortical networks. However, underlying mechanisms concerning how periodic, weak global perturbations alter spatiotemporal dynamics of large-scale cortical network dynamics are unclear. Ali and colleagues [126] simulated large-scale networks of spiking neuron models to investigate this question in endogenously rhythmic networks. They also performed multichannel extracellular recordings during alternating current stimulation in anesthetized ferrets, to verify that weak global perturbations can selectively enhance oscillations at the applied stimulation frequency. Their results support future design of alternating current paradigms that dynamically tailor stimulation frequency to the spectral peak of ongoing brain activity.
Marshall et al. [127] studied transcranial application of very low frequency (0.75 Hz) AC during early non-REM sleep (a period of emerging slow wave sleep). This stimulation enhanced the retention of hippocampal-dependent declarative memories acquired prior to sleep onset. The slowly oscillating potential also induced an immediate increase in slow wave sleep, and slow spindle activity in the frontal cortex. Brain stimulation at 5 Hz, a frequency band that normally predominates during REM sleep, reduced slow wave sleep and left declarative memory unchanged.
Using constant low frequency AC stimulation, Kanai et al. [101] demonstrated modulation of phosphene thresholds to single-pulse TMS, in a frequency-dependent manner. Of four frequencies tested (5, 10, 20, and 40 Hz) only stimulation at 20 Hz modulated cortical excitability. However, it is possible that referred CES stimulation of the retina rather than occipital cortex produces phosphenes in this [128] and other studies [129].
Schroeder et al. [130] examined CES-induced EEG changes in 12 healthy right handed males receiving 0.5, 100 Hz, or sham in a randomized, double-blind crossover design, using ear clip electrodes, with CES administered for 20 min., adjusting the current level until the subject could feel sensation at the electrode site. The current was then reduced to a subthreshold level. The current settings for all subjects had a mean of 48 mA and a range of 10–100 mA. Relative to sham, 0.5 and 100 Hz caused the alpha band mean frequency to shift downward, and 100 Hz CES also caused a decrease of the alpha band median frequency and beta band power fraction. Other studies have found changes in resting EEG after a single session [20, 130, 131] and also 2 weeks after completing 14 daily 20 min sessions [132]. Directionality of effects have been conflicting, likely due to wide variation in stimulation parameters among studies.
Zaghi and colleagues [13] conducted an experiment that revealed how important specific stimulation parameters—including electrode size, which influences current density—are to producing neurophysiological effects using CES (here, tACS). They cited a previous study [133] in which tACS was applied for 2 and 5 min with current density of 0.16–0.25 A/m2 (0.4 mA, 10 Hz, 16 cm2 electrodes) that was unable to show robust effects on cortical excitability. Zaghi et al. applied tACS at the significantly higher current density of 0.80 A/m2 (1 mA, 15 Hz, 12.56 cm2 electrodes), for the considerably longer duration of 20 min, and were able to demonstrate measurable changes to cortical excitability. Their results revealed that active 15 Hz tACS of the motor cortex significantly diminished the amplitude of motor evoked potentials and decreased intracortical facilitation (ICF), as compared to baseline and sham stimulation, supporting the notion that AC stimulation with weak currents can induce significant changes in brain excitability. In this study, 15 Hz tACS led to a pattern of inhibition of cortical excitability. They proposed that tACS may have a dampening effect on cortical networks, and perhaps interfere with the temporal and spatial summation of weak subthreshold electric potentials.
In contrast to tDCS which is thought to hyperpolarize or depolarize neurons by electric-field induced changes in the conformation of membrane proteins and thereby change the resting firing rate [134], CES is thought to not hyperpolarize or depolarize neurons, but to modulate endogenous neurophysiologic activity or oscillations [13, 126, 127, 130, 135]. However, recent experimental laboratory studies using targeted electrode placements shown that lower frequency CES can alter visceral and somatosensory perception [136], motor control [137, 138], and memory [127], matched to the synchronized oscillatory activity of cortical areas engaged in specific cognitive and motor processes recorded through EEG [139]. To date, CES laboratory studies of behavioral effects have not examined head-to-head possible differential CES device efficacy based on electrode placement, or any other specific configurations of stimulation parameters. However, in one recent study, at the theta frequency of 6.5 Hz, CES effects were hemisphere-specific for a risk-assessment task [140].
Radman et al. [141] pointed out that it is remarkable that a weak electric field such as that delivered by CES-like devices has the ability to entrain an oscillating brain network.
Abnormalities in oscillatory function have begun to be recognized in depression, schizophrenia, and other neuropsychiatric disorders. CES theoretically has the potential to reactivate hypoactive neuronal circuits or inhibit overactive circuits. In addition, CES may play a therapeutic role by counteracting deleterious, disease-related synchronization between subcortical structures interconnected with the cortex [16].
Evidence for Impact on Neurotransmitters, Hormones, and Endorphins
PET scanning could reveal brain-based changes in particular neurotransmitters’ release and receptor availability as a function of CES stimulation. Although it is frequently suggested that CES raises brain endorphin levels, evidence supporting this assertion still is relatively weak, and primarily based on animal studies.
Two small uncontrolled human studies found increases in cerebrospinal fluid (CSF) beta endorphin and serotonin following CES stimulation [58, 142, 143]. Additional reports of CES-associated changes in urinary or blood plasma level of hormones, neuropeptides, and neurotransmitters, including serotonin, catecholamines, GABA, DHEA, human growth hormone (HGH), cortisol, beta-endorphin, and thyroxine [144–146] are likely to reflect pituitary or peripheral production of these neuromodulators rather than spillover from brain production. However increases in hormones which readily cross the blood brain barrier, such as thyroxine, could impact brain function [144] and peripheral release of neuropeptides could activate the brain through vagal afferents.
A number of animal studies implicate the endogenous opioid system in the analgesic effects of CES [36, 39, 40].
Further animal studies report CES effects on hormones and neurotransmitters [147, 148]. Warner and colleagues [147] found that serotonin (5-HT) was involved in analgesia induced by low current transcranial electrostimulation (TE), 10 mu-Amp, 10 Hz, pulsed current via electrodes in the rat ear, in a tail pressure paradigm. This involves putting progressively increasing pressure on the rat tail 1/4 in. from the tip with a pneumatically driven, right angle wedge. The amount of pressure at which the rat moved its tail was measured both before and after TE, or sham TE, and recorded as the difference in tolerated peak pressure (DTPP). TE produced analgesia as manifested by a 613 % increase in DTPP compared with sham TE treatment values. Among TE-treated rats, pretreatment with pCPA (para-chlorophenylalanine, a synthetic amino acid which is a selective, irreversible inhibitor of tryptophan hydroxylase, a rate-limiting enzyme in biosynthesis of serotonin) decreased DTPP 91.5 % compared with saline control values, indicating 5HT involvement. 5HTP restored TE-induced analgesia in pCPA-treated rats to the level of saline treated control animals, confirming 5HT involvement. Warner et al. also reported [148] on anesthetized rats exposed either to a 10 Hz, 10 muAmp transcranial electrostimulation treatment (TCET) current for 30 min, via electrodes placed in the ears, or to 0 muAmp sham stimulation. Post-sacrifice, brain levels of several neurotransmitters and their metabolites were measured in selected homogenized brain areas by high performance liquid chromatography. Levels of norepinephrine (NE) and dopamine (DA) were significantly higher in the hypothalamic region of stimulated rats compared to control rats; midbrains of TCET rats contained significantly elevated levels of DA, MHPG (3-Methoxy-4-hydroxyphenylglycol, a metabolite of norepinephrine), and 5HT and 5HIAA (5-Hydroxyindoleacetic acid, the primary metabolite of serotonin); in the hindbrain no significant differences were observed. They did not find any change in serum endorphin levels which they suggest indicated that TCET-induced opioid activity may be confined to the central nervous system.
A small randomized trial of low-frequency (0.5 Hz) CES to try to improve the rest/activity pattern of patients with Alzheimer’s disease did not find an effect of CES on salivary cortisol [149].
Evidence for Impact on Autonomic Nervous System
An open trial of CES for hypertensive subjects found an increase in heart rate variability during treatment with CES, suggesting changes in sympathetic and parasympathetic tone [60]. Many studies and consumer anecdotes report relaxation and meditation-like experiences, post-CES, which are in keeping with reduced sympathetic tone and increased parasympathetic tone [20, 88, 145, 146]. An increase in parasympathetic tone, or a decrease in sympathetic tone, could play a role in many of the proposed clinical benefits of CES, including improvements in insomnia, anxiety, and pain.
Whether or not CES methodologies can be developed to target specific brain areas, non-focal modulation of endogenous brain activity also may be an effective approach to depression treatment. ECT is non-focal, and investigators in Denmark have been conducting human clinical studies with a technology called T-PEMF [150, 151], a device using multichannel low voltage transcranial pulsed electromagnetic fields generated by seven coils (R/L anterior temporal, R/L posterior temporal, R/L parietal, and midline occipital) which has shown efficacy in treatment resistant depression [151]. Wires in a housing create a magnetic field orders of magnitude weaker than that generated by TMS; the neural impact of this stimulation is non-focal, similar to CES. Results show a statistically significant benefit for patients with treatment resistant depression treated with T-PEMF plus antidepressant medication [150, 151].
CES and Alternative Medicine
Since the beginnings of the alternative medicine movement in the USA in the 1960s, up to the present, some practices have been mainstreamed, such as acupuncture, meditation, and healthful dietary patterns, while others, including CES, have remained marginalized. The reasons for the failure of CES to enter the mainstream along with acupuncture, yoga, and meditation are not entirely clear but the relatively lower number of individuals, and physicians, aware of and using CES may be a factor. In addition, although we live in an era of ever-proliferating electronic gadgetry, both medical and nonmedical, which now extends to new brain stimulating devices, the decades-old negative reputation of ECT may have biased many against even much more gentle electrical devices to directly stimulate the head and brain. This bias against electrical devices may be receding as new knowledge reaches the alternative medicine community [152] and the wider public.
Of note, sometimes CES is linked to alternative and complementary medicine, and sometimes it is not. The National Center for Complementary and Alternative Medicine (NCCAM) of the National Institutes of Health (NIH) does not list CES as a therapy on its Web site. Perhaps this is because it is an FDA sanctioned device, because it does not fall into existing categories such as interventions derived from World traditional medicine systems, or is not seen as a “natural” treatment. The Wikipedia page for CES (http://en.wikipedia.org/wiki/Cranial_Electrotherapy_Stimulation), a primary source for many people researching the topic, lists it under the heading “Alternative medicine /fringe therapies.”
The association of CES with alternative medicine also may have contributed to the relative lack of academic neuropsychiatric research interest over the past 50 years. CES has had longstanding acceptance within the alternative medicine community due to interest in therapies perceived to be gentler, less invasive, and more likely to support the body’s endogenous systems and properties [153, 154], including Chi (Xi), the body’s endogenous life force as understood in Chinese and other Eastern medical traditions [155].
Another factor contributing to the popularity of CES in the alternative medicine community is that it can be prescribed by non-M.D. practitioners, including nurses, acupuncturists, chiropractors, and psychologists, in contrast to pharmaceuticals and more invasive devices and procedures which require prescription by a physician. For medical professionals, even if CES might be helpful, this understandably raises the concern that non-physician practitioners will use CES for more severely ill individuals who would be better served by pharmaceuticals or more powerful electric or magnetic interventions.
A related issue is that CES, because of its minimal side effects, and availability to nonmedical practitioners, often has been applied to subclinical conditions, which have not been well characterized, and often fall outside diagnostic categories of conventional medicine. While this use is mostly not scientifically documented, patient testimonials and other information on company and alternative medicine Web sites and online communities offer evidence of benefit. In addition, many who are coping with hard-to-diagnose or treat conditions, such as withdrawal from addictive substances and fibromyalgia, have turned to CES and other alternative medicine approaches for symptom relief, for a sense of personal control, and to avoid side effects of mainstream treatments [151].
Rehabilitation medicine has long employed both mainstream and alternative low intensity, high frequency, alternating current devices for peripheral nerve stimulation, typically for pain relief, although their efficacy and optimal stimulation sites and parameters continue to be debated: e.g., transcutaneous electrical nerve stimulation at noncranial sites (TENS); implantation of subcutaneous electrodes or percutaneous electrical nerve stimulation (PENS); and electroacupuncture, a form of acupuncture in which a weak alternating electric current is passed between pairs of acupuncture needles [156]. Both PENS and electroacupuncture have been applied to sites on the head, which could be considered a form of CES stimulation as well.. Several trials of electroacupuncture for conditions such as depression are listed on the Web site Clinicaltrials.gov. Stimulated acupuncture points, via traditional needling and electroacupuncture, have been shown to activate diverse brain regions and physiological pathways detectable by brain imaging [156].
Ultimately, major reasons why CES has failed to garner mainstream interest comparable to that in tDCS, despite a century of evidence of therapeutic potential, are circular: the absence of large scale wellcontrolled clinical trials, systematic safety studies, and standardization of parameters of stimulation [155]; proprietary devices with patented frequencies that are not embraced by scientists; the mostly non-targeted nature of CES treatment and therefore non- focal brain effects, if any; and unclear mechanism(s) of action. In addition, because the existing CES devices are coming to the end of existing patents, there is limited motivation to invest in large-scale randomized clinical trials.
Interest in CES devices now may be increasing, as the effects of differing electrode configurations and stimulation parameters are investigated for their varied cranial nerve and brain stimulating effects, and as a new interest in therapeutic cranial nerve stimulation develops. Renewed interest in CES also is being swept along by greatly increased academic research interest in tDCS, and rTMS, which are subject to the current more stringent FDA efficacy and safety criteria for approval, and restricted to use by physicians.
Barriers and Future Directions
It is remarkable that CES has not gained traction in the world of modern brain stimulation research. This has continued to puzzle many, given considerable evidence that it may be therapeutically useful. Another major reason why CES never gained traction—companies were understandably concerned to market their “special patented” waveform, but this then greatly limits the interest of neural scientists. Commercial CES over many decades has been characterized by changing waveforms, as different devices were engineered and patented, therefore any safety and efficacy data applies only to the characteristics of that device, and to the specific dose used in a given study using a specific CES device [26, 158]. By comparison, tACS, where the waveforms are simple, i.e., constant sinusoidal alternating current and no special patented waveforms, allows for replication of studies using any tACS device or paradigm. Laboratory-designed tPCS stimulation similarly could be controlled and studied just as any other scientifically investigated protocol or device. Put another way—we cannot establish what CES does or does not do, until we can control what CES is.
While regulators (FDA) allow any similar low intensity AC device to call itself CES, to scientists these various devices are not the same and will have different neural and clinical impacts [26]. That said, increasingly there are valid scientific and clinical motivations to systematically study a range of low intensity electrical devices, and vary a range of stimulation parameters, using both constant (i.e., tACS) and pulsed (i.e., tPCS) alternating current stimulation, as well as DC stimulation (tDCS), and low intensity magnetic devices such as T-PEMF (Table 11.4).
It remains to be seen whether CES will attain the degree of scientific and commercial interest which has been focused on tDCS, which is undergoing continued technical development with an aim to target specific brain areas more effectively [134]. The ability to localize tDCS stimulation has made it more attractive to researchers. The degree to which transcranial CES/external and cranial nerve stimulation can have well-documented localized and therapeutically focal effects within the brain remains to be determined, as does the potential usefulness of deliberately using more generalized stimulation for therapeutic ends.
As of October 2013, searching Pubmed.org for “direct and current and stimulation and treatment and human and brain” yields 637 references, one quarter of them published in 2012–2013. Substituting “alternating” for “direct” yields only 40 references, 11 of them published in 2012–2013, although this particular search does not capture all CES papers in part due to the highly variable nomenclature for AC devices. Among the numerous publications for tDCS are several reporting positive results of double-blind randomized sham-controlled trials for depression [159], Parkinson’s disease [160], epilepsy [161], memory function [162], and addiction [163]. Additional open label evidence exists for pain and fibromyalgia [164].
Although bioengineering modeling studies indicate that electrode placement can affect how CES impacts the brain with respect to which cortical and subcortical areas are stimulated, with what intensity and magnitude [15, 23, 165], it remains to be determined whether different electrode placements actually yield different clinical effects. In Fig. 11.3 above, schematic images depict one conventional CES electrode placement and several novel ones. Placement of CES electrodes bilaterally in the ear canal yielded, as one might expect, enhanced transcranial stimulation [15]. Even if CES stimulation has less potential for localization compared to tDCS, synchronization of brain oscillatory currents by CES may have unique therapeutic effects, such as ability to beneficially modulate aberrant CNS activity patterns, and enhanced anti-inflammatory and autonomic action. Much work needs to be done to identify any such generalizing neural and physiological effects of CES.
Despite remaining barriers, the future of minimally invasive, low intensity electric therapeutics looks bright. Bioelectronics is a rapidly developing collaboration among engineers, computer scientists, and biomedical scientists. Proponents envision more rapid development of new and potentially more effective electrical and magnetic therapeutics [86]. Medical treatment of the future increasingly may include what some now are calling electroceuticals, disease-specific low intensity electrical therapeutics designed as an alternative to pharmaceuticals ([86, 166]. The proliferation of neuromodulating devices may increase, not decrease, as some classes of devices become sub-specialized for specific therapeutic tasks and targets. CES devices recently developed (Cefaly®, Neuro Sigma Monarch eTNS™) to stimulate cranial afferents for relief of specific ills (migraine; epilepsy and depression, respectively) are an example. Modulating cranial nerves for narrow therapeutic purposes has been called the “low-hanging fruit” of the next generation of brain stimulation [86]).
Conclusion
In summary, CES variants and other low intensity brain stimulation technologies such as tDCS, tVNS and T-PEMF, may hold significant promise for the treatment of neuropsychiatric disorders. Given the proliferation of these devices, and unclear mechanisms of action, much work lies ahead to establish possible therapeutic rationales and roles for each of them in mainstream psychiatry, neurology, and rehabilitation medicine. Experimental and modeling studies are providing new insights into putative mechanism(s) of action of CES, which should shed light on pathophysiology of target conditions and at the same time help to refine CES device designs and treatment protocols. Rapidly growing interest, industry funding [167] and academic scientific involvement in investigating the so-called electroceuticals [86, 152] should hasten the emergence of better science and new understanding of the multiple ways therapeutic electricity can be used to modulate brain function [15, 23, 26].
If efficacy is established, CES could be an attractive primary or augmentation treatment for psychiatric and neurological conditions, with potentially fewer side effects than medications, and potentially lower cost than medications and more invasive forms of brain stimulation (ECT, TMS, VNS), and psychotherapy. CES devices are inexpensive and can be used in the home, making this treatment approach relatively affordable and convenient. Because of low side effect burden and expense, CES may be very useful when it would be preferable to avoid use of medications, such as in the elderly, in individuals with substance abuse disorders, and for pregnant and nursing women. In addition to a possible role in treating diagnosed neuropsychiatric conditions, CES also may be useful for less disabling degrees of anxiety, depression, and insomnia, to help prevent clinical levels of illness from developing in the first place, and to help maintain remission. These potential therapeutic uses have not yet been studied.
In conclusion, a great deal more research on CES—mechanistic studies, well-powered, rigorously designed clinical trials, and studying updated technologies – is very much needed. All accumulated evidence to date would suggest it is very warranted.
References
Stillings D. A survey of the history of electrical stimulation for pain to 1900. Med Instrum. 1975;9:255–9.
Zaghi S, Acar M, Hultgren B, Boggio PS, Fregni F. Noninvasive brain stimulation with low-intensity electrical currents: putative mechanisms of action for direct current and alternating current stimulation. Neuroscientist. 2009;16(3):285–307.
Pavlov IP. Experimental psychology and other essays. New York: Philosophical Library; 1957.
Sergeev GV. The use of electrosleep in clinical medicine. Proceedings of the 1st International Symposium on Electrosleep and Electroanesthesia. 1967;1:115-120.
Robinovitch LG. Electrical analgesia, sleep, and resuscitation. In: Gwathmey JT, editor. Anesthesia. New York, NY: Appleton; 1914.
Anan’ev MG, Golubeva IV, Gurova EV, Kaschevskaia LA, Levitskaia LA, Khudyi YB. Preliminary data on experimental electronarcosis induced with apparatus of the Scientific Research Institute of Experimental Surgical Apparatus and Instruments. Anesthesiology. 1960;21:215–9.
Lebedev VP. [Transcranial electrostimulation: a new approach (experimental and clinical bases and equipment)]. Med Tekh. 1997;2:7–13. Article in Russian.
Lebedev VP, Malygin AV, Kovalevski AV, Rychkova SV, Sisoev VN, Kropotov SP, Krupitski EM, Gerasimova LI, Glukhov DV, Kozlowski GP. Devices for noninvasive transcranial electrostimulation of the brain endorphinergic system: application for improvement of human psycho-physiological status. Artif Organs. 2002;26(3):248–51.
Lebedev VP, Il’inskiĭ OB, Savchenko AB, Kolosova LI, Kovalevskiĭ AV, Tsirul’nikov EM, Rychkova SV, Malikhova MV, Aleksandrov VA, Gerasimova LI, Pavlov VA. [Noninvasive transcranial electrostimulation of endorphin structures of the brain as a reparation activator: experimental and clinical parallels]. Med Tekh. 2002;6:32–5. Article in Russian.
Lebedev VP, Bilichenko SV, Ordyan NE, Pivina SG, Nechiporenko SP, Puzyrev AA, Mikheeva EA, Kubacheva KK. Transcranial electrostimulation activates reparative regeneration and the insulin-producing function of pancreatic B-cells in alloxan diabetes in rats. Neurosci Behav Physiol. 2007;37(4):341–7.
Klawansky S, Yeung A, Berkey C, Shah N, Phan H, Chalmers TC. Meta-analysis of randomized controlled trials of cranial electrostimulation: efficacy in treating selected psychological and physiological conditions. J Nerv Ment Dis. 1995;183(7):478–84. Comment in, J Nerv Ment Dis. 1997 Dec;185(12):766–7.
Joy ML, Lebedev VP, Gati JS. Imaging of current density and current pathways in rabbit brain during transcranial electrostimulation. IEEE Trans Biomed Eng. 1999;46(9):1139–49.
Zaghi S, de Freitas RL, Machado de Oliveira L, El-Nazera R, Menning S, Tadini A, Fregni B. Inhibition of motor cortex excitability with 15 Hz transcranial alternating current stimulation (tACS). Neurosci Lett. 2010;479:211–4.
Lopes S, Davies N, Toumazou C, Grossman N. Theoretical investigation of transcranial alternating current stimulation using laminar model. Conf Proc IEEE Eng Med Biol Soc. 2012;4152–5.
Datta A, Dmochowski JP, Guleyupoglu B, Bikson M, Fregni F. Cranial electrotherapy stimulation and transcranial pulsed current stimulation: a computer based high-resolution modeling study. Neuroimage. 2013;65:280–7.
Lefaucheur JP. Principles of therapeutic use of transcranial and epidural cortical stimulation. Clin Neurophysiol. 2008;119(10):2179–84.
Lefaucheur JP. Methods of therapeutic cortical stimulation. Neurophysiol Clin. 2009;39(1):1–14.
Limoge A, Robert C, Stanley TH. Transcutaneous cranial electrical stimulation (TCES): a review 1998. Neurosci Biobehav Rev. 1999;23:529–38.
Flemenbaum A. Cerebral electrotherapy (electrosleep): a review. Curr Psychiatr Ther. 1975;15:195–202.
Kennerly R. QEEG analysis of cranial electrotherapy: a pilot study. J Neurother. 2004;112–113.
Krupitsky EM, Burakov AM, Karandashova GF, Katsnelson JS, Lebedev VP, Grinenko AJ, Borodkin JS. The administration of transcranial electric treatment for affective disturbances therapy in alcoholic patients. Drug Alcohol Depend. 1991;27(1):1–6.
Journée HL. Electrical safety in intraoperative monitoring. Proceedings, Intraoperative Neurophysiology 2003, Symposium on Intraoperative Neurophysiology, with the 19th Dr. Janez Faganel Memorial Lecture, Oct 17–18, Ljubljana, Slovenia.
Datta A, Zhou X, Su Y, Parra LC, Bikson M. Validation of finite element model of transcranial electrical stimulation using scalp potentials: implications for clinical dose. J Neural Eng. 2013;10(3):036018.
Alon G, Roys SR, Gullapalli RP, Greenspan JD. Non-invasive electrical stimulation of the brain (ESB) modifies the resting-state network connectivity of the primary motor cortex: a proof of concept fMRI study. Brain Res. 2011;1403:37–44.
Alon G, Yungher DA, Shulman LM, Rogers MW. Safety and immediate effect of noninvasive transcranial pulsed current stimulation on gait and balance in Parkinson disease. Neurorehabil Neural Repair. 2012;26(9):1089–95. Epub 2012 May 10.
Guleyupoglu B, Schestatsky P, Edwards D, Fregni F, Bikson M. Classification of methods in transcranial Electrical Stimulation (tES) and evolving strategy from historical approaches to contemporary innovations. J Neurosci Methods. 2013;219(2):297–311.
Wilson OB, Hamilton RF, Warner RL, Johnston CM, deFriece R, Harter L, Schweitzer C, Talaverra J, Hymel CM, Skolnick MH. The influence of electrical variables on analgesia produced by low current transcranial electrostimulation of rats. Anesth Analg. 1989;68(5):673–81.
Kirsch DL. The science behind cranial electrotherapy stimulation. 2nd ed. Edmonton, AL: Medical Scope; 2002.
Feusner JD, Madsen S, Moody TD, Bohon C, Hembacher E, Bookheimer SY, Bystritsky A. Effects of cranial electrotherapy stimulation on resting state brain activity. Brain Behav 2012;1–10.
Bai S, Loo C, Dokos S. A review of computational models of transcranial electrical stimulation. Crit Rev Biomed Eng. 2013;41:21–35.
Ferdjallah M, Bostick Jr FX, Barr RE. Potential and current density distributions of cranial electrotherapy stimulation (CES) in a four-concentric-spheres model. IEEE Trans Biomed Eng. 1996;43(9):939–43.
Gense de Beaufort D, Sesay M, Stinus L, Thiebaut R, Auriacombe M, Dousset V. Cerebral blood flow modulation by transcutaneous cranial electrical stimulation with Limoge’s current. J Neuroradiol. 2011;39(3):167–75.
Feighner JP, Brown SL, Olivier JE. Electrosleep therapy. A controlled double blind study. J Nerv Ment Dis. 1973;157(2):121–8.
Gilula MF, Daniel L. Kirsch DL. Cranial electrotherapy stimulation review: a safer alternative to psychopharmaceuticals in the treatment of depression. J Neurotherapy. 2005;9(2).
Dougherty PM, Dafny N. Trans-cranial electrical stimulation attenuates the severity of naloxone-precipitated morphine withdrawal in rats. Life Sci. 1989;44(26):2051–6.
Mantz J, Azerad J, Limoge A, Desmonts JM. Transcranial electrical stimulation with Limoge’s currents decreases halothane requirements in rats. Evidence for the involvement of endogenous opioids. Anesthesiology. 1992;76:253–60.
Wageneder FM, Iwanovsky A, Dodge CH. Electrosleep (cerebral electrotherapy) and electroanesthesia: the international effort at evaluation. Foreign Sci Bull. 1969;5:1–104.
Kavirajan HC, Lueck K, Chuang K. Alternating current cranial electrotherapy stimulation (CES) for depression. Cochrane Database Syst Rev. 2014;7, CD010521.
Auriacombe M, Tignol J, Le Moal M, Stinus L. Transcutaneous electrical stimulation with Limoge current potentiates morphine analgesia and attenuates opiate abstinence syndrome. Biol Psychiatry. 1990;28(8):650–6.
Stinus L, Auriacombe M, Tignol J, Limoge A, Le Moal M. Transcranial electrical stimulation with high frequency intermittent current (Limoge’s) potentiates opiate-induced analgesia: blind studies. Pain. 1990;42(3):351–63.
Schoenen J, Vandersmissen B, Jeangette S, Herroelen L, Vandenheede M, Gérard P, Magis D. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80(8):697–704.
DeGiorgio CM, Soss J, Cook IA, Markovic D, Gornbein J, Murray D, Oviedo S, Gordon S, Corralle-Leyva G, Kealey CP, Heck CN. Randomized controlled trial of trigeminal nerve stimulation for drug-resistant epilepsy. Neurology. 2013;80(9):786–91.
DeGiorgio CM, Murray D, Markovic D, Whitehurst T. Trigeminal nerve stimulation for epilepsy: long-term feasibility and efficacy. Neurology. 2009;72(10):936–8.
Editors. Medgadget Sep 7, 2012 • 2:38 pm. http://www.medgadget.com/2012/09/neurosigmas-monarch-etns-system-for-epilepsy-depression-cleared-in-europe.html (2012). Accessed 5 Oct 2013.
Schrader LM, Cook IA, Miller PR, Maremont ER, DeGiorgio CM. Trigeminal nerve stimulation in major depressive disorder: first proof of concept in an open pilot trial. Epilepsy Behav. 2011;22(3):475–8.
Fallgatter AJ, Neuhauser B, Herrmann MJ, Ehlis AC, Wagener A, Scheuerpflug P, Reiners K, Riederer P. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J Neural Transm. 2003;110(12):1437–43. Epub 2003.
Kraus T, Hösl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm. 2007;114(11):1485–93.
Kraus T, Kiess O, Hösl K, Terekhin P, Kornhuber J, Forster C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal – a pilot study. Brain Stimul. 2013;6(5):798–804.
Bystritsky A, Kerwin L, Feusner J. A pilot study of cranial electrotherapy stimulation for generalized anxiety disorder. J Clin Psychiatry. 2008;69:412–7.
DeFelice EA. Cranial electrotherapy stimulation (CES) in the treatment of anxiety and other stress-related disorders: a review of controlled clinical trials. Stress Med. 1997;13(1):31–42.
Barclay TH, Barclay RD. A clinical trial of cranial electrotherapy stimulation for anxiety and comorbid depression. J Affect Disord. 2014;164:171–7.
Overcash SJ. Cranial electrotherapy stimulation in patients suffering from acute anxiety disorders. Am J Electromed. 1999;16(1):49–51.
Schmitt R, Capo T, Boyd E. Cranial electrotherapy stimulation as a treatment for anxiety in chemically dependent persons. Alcohol Clin Exp Res. 1986;10(2):158–60.
Winick RL. Cranial electrotherapy stimulation (CES): a safe and effective low cost means of anxiety control in a dental practice. Gen Dent. 1999;47(1):50–5.
Koppolu SS, Kazariants G, Varvara M, McClure D, Yaseen Z, Lee AMR, Galynker I. A single blind, randomized, sham controlled study of Cranial Electrical Stimulation in Bipolar II disorder. Poster presented at the Annual Meeting of the American Psychiatric Association, May 10, 2013.
Hearst ED, Cloninger CR, Crews EL, Cadoret RJ. Electrosleep therapy: a double-blind trial. Arch Gen Psychiatry. 1974;30(4):463–6.
Lyon DE, Schubert C, Taylor AG. Pilot study of cranial stimulation for symptom management in breast cancer. Oncol Nurs Forum. 2010;37(4):476–83.
Shealy CN, Cady RK, Wilkie RG, Cox R, Liss S, Clossen W. Depression: a diagnostic, neurochemical profile and therapy with cranial electrical stimulation (CES). J Neurolo Orthop Med Surg. 1989;10(4):319–21.
Danilova I, Orekhova E.[The use of sinusoidal modulated currents as a method of electrosleep] Russian. Vopr Kurortol Fizioter Lech Fiz Kult 6;1989.
Podzolkov VI, Mel’nikova TS, Suvorova IA, Churganova LI, Starovoĭtova SP. [Cranial electrostimulation–a new nondrug method of treating the initial stage of hypertension]. Ter Arkh. 1992;64(1):24–7. Article in Russian.
Lichtbroun AS, Raicer MC, Smith RB. The treatment of fibromyalgia with cranial electrotherapy stimulation. J Clin Rheumatol. 2001;7(2):72–8.
Koegler RR, Hicks SM, Barger JH. Medical and psychiatric use of electrosleep: transcerebral electrotherapy. Dis Nerv Syst. 1971;32:100–4.
Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8–13.
Mousavi SA, Mirbod SM, Khorvash F. Comparison between efficacy of imipramine and transcutaneous electrical nerve stimulation in the prophylaxis of chronic tension-type headache: a randomized controlled clinical trial. J Res Med Sci. 2011;16:923–7.
Childs A, Price L. Cranial electrotherapy stimulation reduces aggression in violent neuropsychiatric patients. Prim Psychiatr. 2007;14:50–6.
Capel ID, Dorrell HM, Spencer EP, Davis MW. The amelioration of the suffering associated with spinal cord injury with subperception transcranial electrical stimulation. Spinal Cord. 2003;41:109–17.
Gabis L, Shklar B, Baruch YK, Raz R, Gabis E, Geva D. Pain reduction using transcranial electrostimulation: a double-blind “active placebo” controlled trial. J Rehabil Med. 2009;41:256–61.
Holubec JT. Cumulative response from Cranial Electrotherapy Stimulation (CES) for chronic pain. Prac Pain Manag. 2009;9(9):80–3.
Mignon A, Laudenbach V, Guischard F, Limoge A, Desmonts JM, Mantz J. Transcutaneous cranial electrical stimulation (Limoge’s currents) decreases early buprenorphine analgesic requirements after abdominal surgery. Anesth Analg. 1996;83:771–5.
Stanley TH, Cazalaa JA, Atinault A, Coeytaux R, Limoge A, Louville Y. Transcutaneous cranial electrical stimulation decreases narcotic requirements during neurolept anesthesia and operation in man. Anesth Analg. 1982;61(10):863–6.
Stanley TH, Cazalaa JA, Limoge A, Louville Y. Transcutaneous cranial electrical stimulation increases the potency of nitrous oxide in humans. Anesthesiology. 1982;57(4):293–7.
Rintala DH, Tan G, Willson P, Bryant MS, Lai ECH. Feasibility of using Cranial Electrotherapy Stimulation for pain in persons with Parkinson’s Disease. Parkinsons Dis. 2010;2010:569154.
Eidelman WS. Control of cigarette cravings with cranial electrotherapy stimulation. The Townsend Letter for Doctors. 2009;311:81–5.
Gomez E, Mikhail AR. Treatment of methadone withdrawal with cerebral electrotherapy (electrosleep). Br J Psychiatr. 1978;134:111–3.
Overcash SJ, Siebenthall A. The effects of cranial electrotherapy stimulation and multisensory cognitive therapy on the personality and anxiety levels of substance abuse patients. Am J Electromed. 1989;105–111.
Patterson MA, Firth J, Gardiner R. Treatment of drug, alcohol and nicotine addiction by neuroelectric therapy: analysis of results over 7 years. J Bioelectr. 1984;3(1&2):193–221.
Patterson MA, Patterson L, Patterson SI. Electrostimulation: addiction treatment for the coming millennium. J Altern Complement Med. 1996;2(4):485–91.
Schmitt R, Capo T, Frazier H, Boren D. Cranial electrotherapy stimulation treatment of cognitive brain dysfunction in chemical dependence. J Clin Psychiatry. 1984;45:60–063.
Gall C, Sgorzaly S, Schmidt S, Brandt S, Fedorov A, Sabel BA. Noninvasive transorbital alternating current stimulation improves subjective visual functioning and vision-related quality of life in optic neuropathy. Brain Stimul. 2011;4:175–88.
Schmidt S, Mante A, Rönnefarth M, Fleischmann R, Gall C, Brandt SA. Progressive enhancement of alpha activity and visual function in patients with optic neuropathy: a two-week repeated session alternating current stimulation study. Brain Stimul. 2013;6(1):87–93.
Gilula MF. Cranial electrotherapy stimulation and fibromyalgia. Expet Rev Med Dev. 2007;4(4):489–95.
Kirsch DL. Cranial electrotherapy stimulation: a safe and effective treatment for anxiety. Med Scope Mon. 1996;3:1–26.
O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2010;9, CD008208. Review.
Piquet M, Balestra C, Sava SL, Schoenen JE. Supraorbital transcutaneous neurostimulation has sedative effects in healthy subjects. BMC Neurol. 2011;11:135.
DeGiorgio CM, Shewmon DA, Whitehurst T. Trigeminal nerve stimulation for epilepsy. Neurology. 2003;61(3):421–2.
Solon O. Electroceuticals. WIRED Magazine. 28 May http://www.wired.co.uk/news/archive/2013-05/28/electroceuticals (2013). Accessed Jul 22 2013.
Rosenthal SH, Wulfsohn NL. Electrosleep: a preliminary communication. J Nerv Mental Dis. 1970;151(2):146–51.
Schappi J. Aging and Parkinson’s and me. http://parkinsonsand5htp.blogspot.com/2012/06/cranial-electrotherapy-stimulation.html(2012). Accessed Jul 24 2013.
Matteson MT, Ivancevich JM. An exploratory investigation of CES as an employee stress management procedure. J Health Hum Resour Admin. 1986;9(1):93–109.
Fisher-Wallace Labs. http://www.happyhalter.com/uploads/Brochure.pdf (undated).
Clarke N, Mills D, Marchant J. Evaluation of the potential efficacy of the Alpha-Stim SCS in the horse. Caythorpe, UK: De Montfort University Lincoln; 2000.
Taylor DN, Lee CT. Frequency-dependent effects of sine-wave cranial transcutaneous electrical nerve stimulation in human subjects. Acupunct Electrother Res. 1992;17(3):221–7.
Kirsch DL, Price LR, Nichols F, Marksberry JA, Platoni KT. Efficacy of Cranial Electrotherapy Stimulation for anxiety, PTSD, insomnia and depression: US Military service members and veterans self-reports. Poster presented at the Annual Meeting of the Chinese Society of Psychiatry; co-sponsored by the Chinese Medical Association, Psychiatry Branch of the Chinese Medical Association and the Nanjing Brain Hospital. October 18–21, Nanjing, Jiangsu Province, China; 2012.
Kirsch DL, Price LR, Nichols F, Marksberry JA, Platoni KT. Efficacy of cranial electrotherapy stimulation for anxiety, PTSD, insomnia and depression: US M3litary service members and veterans self-reports. The Army Medical Dept Journal. 2014;(In Press).
Xenakis SN. It’s time to move beyond the cliches. October 16 Huffington Post. http://www.huffingtonpost.com/brigadier-general-stephen-n-xenakis-md/veterans-ptsd_b_4099070.html (2013). Accessed Oct 17 2013
National Research Council (December 14, 1974, p. 42). Petitioner presentation to Neurological Devices Panel for reclassification of Alpha-Stim® CES devices from Class III to Class II. In: Alpha-Stim. http://www.alpha-stim.com/wp-content/uploads/EPIs-fda-presentation.pdf(2012). Accessed Aug 1 2013.
Tan G, Rintala D, Jensen MP, Richards JS, Holmes SA, Parachuri R, Lashgari-Saegh S, Price LR. Efficacy of cranial electrotherapy stimulation for neuropathic pain following spinal cord injury: a multi-site randomized controlled trial with a secondary 6-month open-label phase. J Spinal Cord Med. 2011;34(3):285–96.
Rose K, Taylor AG, Bourguignon C. Effects of cranial electrical stimulation on sleep disturbances, depressive symptoms, and caregiving appraisal in spousal caregivers of persons with Alzheimer’s disease. Appl Nurs Res. 2009;2:119–25.
Pop J, Murray D, Markovic D, DeGiorgio CM. Acute and long-term safety of external trigeminal nerve stimulation for drug-resistant epilepsy. Epilepsy Behav. 2011;22(3):574–6.
Kanai R, Chaieb L, Antal A, Walsh V, Paulus W. Frequency-dependent electrical stimulation of the visual cortex. Curr Biol. 2008;18:1839–43.
Kanai R, Paulus W, Walsh V. Transcranial alternating current stimulation (tACS) modulates cortical excitability as assessed by TMS-induced phosphene thresholds. Clin Neurophysiol. 2010;121(9):1551–4. Epub 2010 Apr 9.
Mellen RR, Mackey W. Reducing sheriff’s officers’ symptoms of depression using cranial electrotherapy stimulation (CES): a control experimental study. The Correctional Psychologist. 2009;41(1):9–15.
Strentzsch JA. An examination of cranial electrotherapy stimulation (CES) on alpha-amylase levels, cortisol levels and state-trait anxiety scores in the chronically mentally ill. Doctoral dissertation. Saint Mary’s University, San Antonio, Texas; 2008.
Little B, Patterson MA. Embryofetal effects of neuroelectric therapy (NET). Electro Magnetobiol. 1996;15(1):1–8.
Jordan JE, Morris D. Evaluation of the electrosleep machine. Dis Nerv Syst. 1975;36(12):661–5, 14.
Devanand DP, Dwork AJ, Hutchinson ER, Bolwig TG, Sackeim HA. Does ECT alter brain structure? Am J Psychiatry. 1994;151:957–70.
Weiner R, Lisanby SH, Husain MM, Morales OG, Maixner DF, Hall SE, Beeghly J, Greden JF, National Network of Depression Centers. Electroconvulsive therapy device classification: response to FDA advisory panel hearing and recommendations. J Clin Psychiatry. 2013;74:38–42.
Dwork AJ, Arango V, Underwood M, Ilievski B, Rosoklija G, Sackeim HA, Lisanby SH. Absence of histological lesions in primate models of ECT and magnetic seizure therapy. Am J Psychiatry. 2004;161:576–8.
MacDonald DB. Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2002;19(5):416–29.
Iyer MB, Mattu MA, Grafman J, Lomarev M, Sato M, Wasserman E. Safety and cognitive effects of frontal DC brain polarization in healthy individuals. Neurology. 2005;64:872–5.
Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 2011;14(8):1133–45.
Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114(11):2220–2.
Bikson M, Datta A, Elwassif M. Establishing safety limits for transcranial direct current stimulation. Clin Neurophysiol. 2009;120(6):1033–4.
Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, Boggio PS, Lotufo PA, Benseñor IM, Fregni F. The sertraline vs electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatr. 2013;70:383–91.
Galvez V, Alonzo A, Martin D, Mitchell PB, Sachdev P, Loo CK. Hypomania induction in a patient with bipolar II disorder by transcranial direct current stimulation (tDCS). J ECT. 2011;27:256–8.
Kapural L, Narouze SN, Janicki TI, Mekhail N. Spinal cord stimulation is an effective treatment for the chronic intractable visceral pelvic pain. Pain Med. 2006;7(5):440–3.
Bartsch T, Paemeleire K, Goadsby PJ. Neurostimulation approaches to primary headache disorders. Curr Opin Neurol. 2009;22:262–8.
Brotman P. Low-intensity transcranial electrostimulation improves the efficacy of thermal biofeedback and quieting reflex training in the treatment of classical migraine headache. Am J Electromed. 1989;6:120–3.
Dymond AM, Coger RW, Serafetinides EA. Intracerebral current levels in man during electrosleep therapy. Biol Psychiatry. 1975;10:101–4.
Neuling T, Wagner S, Wolters CH, Zaehle T, Herrmann CS. Finite-element model predicts current density distribution for clinical applications of tDCS and tACS. Front Psychiatr. 2012;3:83.
Datta A, Truong D, Minhas P, Parra LC, Bikson M. Inter-individual variation during transcranial Direct current stimulation and normalization of dose using MRI-derived computational models. Front Psychiatry. 2012;3(91).
Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–37.
Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, Yao S. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71:611–7.
Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, McGrath PJ, Stewart JW, Peterson BS. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatr. 2013;70:373–82.
Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, Schwarzbauer C. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci U S A. 2012;3:5464–8.
Ali MM, Sellers KK, Fröhlich F. Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J Neurosci. 2013;33(27):11262–75.
Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3.
Schwiedrzik CM. Retina or visual cortex? The site of phosphene induction by transcranial alternating current stimulation. Front Integr Neurosci. 2009;3:6.
Schutter DJ, Hortensius R. Retinal origin of phosphenes to transcranial alternating current stimulation. Clin Neurophysiol. 2010;121(7):1080–4.
Schroeder MJ, Barr RE. Quantitative analysis of the electroencephalogram during cranial electrotherapy stimulation. Clin Neurophysiol. 2001;112(11):2075–83.
Zaehle T. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS One. 2010;5:e13766.
Yanmin L, Guiqing Z. qEEG study on the treatment of ADHD with CES. Chinese Journal of Clinicians. 2011;5:2462–3. Electronic Edition.
Antal A, Boros K, Poreisz C, Chaieb L, Terney D, Paulus W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008;1(2):97–105.
Kuo H-I, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, Nitsche MA. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimul. 2012;6(4):644–8.
Reato D, Gasca F, Datta A, Bikson M, Marshall L, Parra LC. Transcranial electrical stimulation accelerates human sleep homeostasis. PLoS One. 2013;9:e1002898.
Feurra M, Paulus W, Walsh V, Kanai R. Frequency specific modulation of human somatosensory cortex. Front Psychol. 2011;2:13.
Feurra M, Bianco G, Santarnecchi E, Del Testa M, Rossi A, Rossi S. Frequency-dependent tuning of the human motor system induced by transcranial oscillatory potentials. J Neurosci. 2011;31:12165–70.
Pogosyan A, Gaynor LD, Eusebio A, Brown P. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr Biol. 2009;19:1637–41.
Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–39.
Sela T, Kilim A, Lavidor M. Transcranial alternating current stimulation increases risk-taking behavior in the balloon analog risk task. Front Neurosci. 2012;6:22.
Radman T, Datta A, Peterchev AV. In vitro modulation of endogenous rhythms by AC electric fields: syncing with clinical brain stimulation. J Physiol. 2007;584(Pt 2):369–70.
Kuzin MI, Avrutskiĭ MI, Shliuznikov BM, Lakhter MA, Panchenko LF. [Effect of transcutaneous transcerebral electrostimulation as electroanesthesia on the beta-endorphin content of the cerebrospinal fluid and blood plasma]. Biull Eksp Biol Med. 1984;97:515–6. Russian.
Shealy CN, Cady RK, Culver-Veehoff et al. Cerebrospinal fluid and plasma neurochemicals: response to cranial electrical stimulation. J Neurol Orthop Med Surg. 1988;18:94-97
Rosenthal SH. Alterations in serum thyroxine with cerebral electrotherapy (electrosleep). Arch Gen Psychiatry. 1973;28:28–9.
Liss S, Liss B. Physiological and therapeutic effects of high frequency electrical pulses. Integr Physiol Behav Sci. 1996;31(2):88–95.
Liss S, Liss B. Modulated electric energy stimulators. Report presented at the American Academy of Pain Management Conference, Las Vegas, NV, Sep; 1999.
Warner R, Hudson-Howard L, Johnston C, Skolnick M. Serotonin involvement in analgesia induced by transcranial electrostimulation. Life Sci. 1990;46(16):1131–8.
Warner RL, Johnston C, Hamilton R, Skolnick MH, Wilson OB. Transcranial electrostimulation effects on rat opioid and neurotransmitter levels. Life Sci. 1994;54(7):481–90.
Scherder E, Knol D, van Someren E, Deijen JB, Binnekade R, Tilders F, Sergeant J. Effects of low-frequency cranial electrostimulation on the rest-activity rhythm and salivary cortisol in Alzheimer’s disease. Neurorehabil Neural Repair. 2003;17(2):101–8.
Bech P, Gefke M, Lunde M, Lauritzen L, Martiny K. The pharmacopsychometric triangle to illustrate the effectiveness of T-PEMF concomitant with antidepressants in treatment resistant patients: a double-blind, randomised, sham-controlled trial revisited with focus on the patient-reported outcomes. Depress Res Treat. 2011;806298.
Martiny K, Lunde M, Bech P. Transcranial low voltage pulsed electromagnetic fields in patients with treatment-resistant depression. Biol Psychiatry. 2010;68:163–9.
HMDI Editors. Heart MD Institute 08 April. http://www.heartmdinstitute.com/health-topics/alternative-medicine/99-electroceuticals (2010). Accessed 5 Oct 2013.
Ruggie M. Marginal to mainstream: alternative medicine in America. New York: Cambridge University Press; 2004.
Schneirov M, Geczik J. A diagnosis for our times: alternative health, from lifeworld to politics. New York, NY: State University Press of New York; 2003.
McCusker C. Cranial Electrotherapy Stimulation (CES), Chi, homeostasis, and the bioelectrical system. http://charlesmccusker.com (2005). Accessed Apr 22 2013.
Zhang Z-J, Wang X-M, McAlonan GM. Neural acupuncture unit: a new concept for interpreting effects and mechanisms of acupuncture. Evidence-based complementary and alternative medicine. 2012;2012(429412): 23 pages. Review.
Edelmuth RC, Nitsche MA, Battistella L, Fregni F. Why do some promising brain-stimulation devices fail the next steps of clinical development? Expet Rev Med Dev. 2010;7(1):67–97.
Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, Pascual-Leone A, Bikson M. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul. 2012;5(4):435–53. Review.
Martin DM, Alonzo A, Mitchell PB, Sachdev P, Gálvez V, Loo CK. Fronto-extracephalic transcranial direct current stimulation as a treatment for major depression: an open-label pilot study. J Affect Disord. 2011;134(1–3):459–63.
Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, Silva MT, Barbosa ER, Nitsche MA, Pascual-Leone A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov Disord. 2006;21:1693–702.
Fregni F, Otachi PT, Do Valle A, Boggio PS, Thut G, Rigonatti SP, Pascual-Leone A, Valente KD. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol. 2006;60:447–55.
Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarization induced inhibition of the human motor cortex. Clin Neurophys. 2003;114:600–4.
Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, Basaglia A, Fregni F. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend. 2008;92:55–60.
Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, Bravo R, Rigonatti SP, Freedman SD, Nitsche MA, Pascual-Leone A, Boggio PS. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54:3988–98.
Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng. 2011;8(4):046011.
Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M. Comment: a jump-start for electroceuticals. Nature. 2013;496(7444):159–61.
Staff, medcitynews.com. GSK plans $1 M prize to jump-start “electroceuticals.” Medcitynews, April 10, 2013 1:00 pm. http://medcitynews.com/2013/04/gsk-plans-1m-prize-to-jump-start-electroceuticals/(2013). Accessed Jul 22 2013.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Mindes, J., Dubin, M.J., Altemus, M. (2015). Cranial Electrical Stimulation. In: Knotkova, H., Rasche, D. (eds) Textbook of Neuromodulation. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1408-1_11
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1408-1_11
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1407-4
Online ISBN: 978-1-4939-1408-1
eBook Packages: MedicineMedicine (R0)