Abstract
Gangliosides are important cell surface molecules in tumors of the nervous system. Initially thought of particularly as diagnostic disease markers, the biological properties and in vivo roles of tumor gangliosides have been recognized more recently. The latter affect the formation and progression of tumors of many types, including neural tumors. This chapter focuses on a prominent and almost universal dynamic property of tumor cells, the rapid synthesis and shedding of gangliosides, and consequences on the host response to tumors. In contemporary terms, these effects are understood to be functional properties of tumor cells affecting normal cells in the tumor microenvironment. Some of the most comprehensive studies have been possible in the human neuroectodermal tumors, neuroblastomas, including first observations of the shedding and activities of human tumor gangliosides. Citing other selected examples from tumors of different types, the impact and mechanisms of action of tumor gangliosides in facilitating tumor formation and progression will be delineated, leading to consideration of clinical relevance and therapeutic implications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Gangliosides
- Shedding
- Neuroectodermal tumors
- Angiogenesis
- Immunosuppression
- Tumor microenvironment
- Tumor progression GD2
- Cerebrospinal fluid gangliosides
- Growth factor signaling
23.1 Introduction
Cellular gangliosides are a component of the cell plasma membrane, present and concentrated on the extracellular surface. A glycosphingolipid, the ganglioside molecule consists of ceramide linked to a sialic acid-containing oligosaccharide. To place these amphiphatic molecules into the context of cancer, it is important to recognize that malignant transformation is a multistep process consisting of both genetic and epigenetic factors that collectively lead to increasing autonomy of malignant tumor cells (Foulds 1965). The tumor cell develops increasing independence from the multiple host control mechanisms for regulating cell growth (e.g., loss of normal cell cycle checkpoint controls, resistance to apoptosis, and increased expression of autocrine growth factors or receptors) (Hanahan and Weinberg 2000). Of particular importance, molecular interactions with the host (Hendrix et al. 2003), initiated by the tumor cell and causing alterations in the tumor microenvironment (TME), are also necessary to promote tumor progression. It is in this context that tumor gangliosides, which are among tumor-derived factors that may favor tumor cell survival, are increasingly being shown to be integral to the process of tumor formation and progression.
As cell surface molecules, initially tumor gangliosides were studied for their role in enabling tumor recognition and diagnosis, mainly by antibody or lectin detection. Their roles in cell-cell interactions by direct contact were also elucidated. These concepts and findings have been substantially developed and addressed elsewhere, e.g., by Hakomori (Hakomori 1985, 1996), and are outside the scope of this chapter. As cell surface molecules, acting as targets for antibody-mediated immunotherapy is also a characteristic of gangliosides on the tumor cell surface. This has been successfully applied in human neuroblastoma, in which therapy with a chimeric anti-GD2 ganglioside antibody in combination with GM-CSF and interleukin-2 caused significantly improved outcome as compared with standard therapy in patients with high-risk neuroblastoma (Yu et al. 2010).
This chapter addresses the observations that gangliosides are actively shed from the cell surface membranes of many tumor cells (Ladisch et al. 1983), but essentially not from normal nonproliferating cells. This dynamic process embodies these molecules with the ability to influence the functions of other cells in the tumor microenvironment and thereby to strongly influence tumor formation and progression. It should be noted that many of the studies defining the roles of gangliosides have been performed in systems other than tumors of the nervous system. However, since the multiple functional characteristics and activities of gangliosides are not dependent on tumor type (but rather on ganglioside structure, as will be discussed further), these diverse examples are included in this chapter with the intent of providing a global overview of dynamic properties of tumor gangliosides.
23.2 Tumor Cell Ganglioside Metabolism
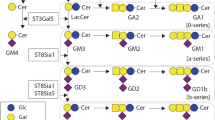
The change from normal tissue to malignancy involves changes in ganglioside composition. These changes both mark the transition from normal to tumor tissue and may affect the outcome, i.e., tumor progression. Tumors may have highly variable composition of individual ganglioside species, evident both between tumor types and among individual tumors. Considering first the question of marking the transition, several examples, although not providing an explanation of cause, underline the likely significance of the details of altered characteristics of ganglioside metabolism resulting in qualitative changes in cell gangliosides. First, the ganglioside patterns of a series of gliomas demonstrated a greater proportion of structurally simpler gangliosides than those of the normal brain (in which GM1, GD1a, GD1b, and GT1b are the major gangliosides). The proportion of simpler gangliosides GM3 and GD3 was higher in tumors of higher grade (grade 3–4) than in those of lower grade (grade 1–2) (Nakamura et al. 1987). Analogous observations have been made in human neuroblastoma tumors. Specifically, higher proportions of complex gangliosides of the “b” pathway (GD1b, GT1b, and GQ1b) are associated with a less aggressive phenotype and a better clinical outcome (progression-free survival). In contrast, the simple “b” pathway gangliosides (GD3 and GD2) have been associated with tumors of poorer prognosis, including the aggressive forms of neuroblastoma (Hettmer et al. 2003; Kaucic et al. 2001; Schengrund et al. 1985).
23.2.1 Ganglioside Metabolism Is Upregulated in Tumor Cells During Tumor Progression
What could be the cause for overexpression of tumor gangliosides during tumor progression? Considering those genes that are responsible for ganglioside synthesis, genomic studies have revealed that certain genes are upregulated in tumors. This is particularly exemplified by recent findings regarding GM3 synthase (GM3S). Gene expression analysis of human melanoma showed GM3 synthase to be highly overexpressed in comparison to its expression in benign nevi or normal skin (Talantov et al. 2005). Since GM3 synthase itself is the key entry enzyme to the pathway for most human ganglioside synthesis, this may be the critical element in ganglioside expression in tumors. Conversely, other evidence comes from correlations between metastatic potential and the expression of sialidase, which results in the conversion of a ganglioside into a neutral glycosphingolipid; levels of sialidase are inversely correlated with the metastatic potential of mouse colon adenocarcinoma (Sawada et al. 2002). This kind of indirect correlative evidence from gene expression studies strongly suggests that the resulting ganglioside levels are associated with tumor growth and tumor progression. Together with direct ganglioside analyses, the findings point to control of ganglioside synthesis as important in tumor progression, making ganglioside synthesis also a potential target for therapeutic intervention in cancer.
23.2.2 Tumor Gangliosides Are Actively Shed into the Tumor Microenvironment
Over the years both by in vitro studies and in vivo, it has become clear that active synthesis and release of high levels of gangliosides into the tumor microenvironment are characteristic of many tumor cells. Our initial studies led us to propose the hypothesis (Ladisch et al. 1983), subsequently widely confirmed, that many tumors shed tumor gangliosides and that these molecules can then bind to other cells in this microenvironment (Fig. 23.1). There, they can affect the function of otherwise normal cells. This shedding and binding to normal host cells in the tumor microenvironment (TME) enhances tumor progression by modulating the function of these normal host cells critical in facilitating tumor progression enhances tumor progression. Characteristics of most types of tumors, i.e., not specifically and not only of neural tumors, since originally reported in 1983, ganglioside shedding by tumor cells has been widely documented (e.g., leukemias, lymphomas, neuroectodermal tumors such as neuroblastoma and melanoma, brain tumors, ovarian carcinoma, and renal cell carcinoma) (Biswas et al. 2006; Ladisch et al. 1983, 1997; Ladisch and Wu 1985; Merritt et al. 1994; Portoukalian et al. 1993; Ravindranath et al. 2007). And the rapid rate of ganglioside metabolism is associated with rapid shedding of these molecules, with rates of up to 0.5–1.0 %/24 h having been seen (Ladisch et al. 1983). This is of importance because of the multiple potential functional roles of gangliosides acting upon normal cells, predominantly shown by in vitro studies in which normal cells found in the TME are enriched with exogenously added purified gangliosides.
23.2.3 Clinical Evidence for Shedding by Human Neural Tumors
Two specific examples of ganglioside shedding by neural tumors are highlighted here. The first is the human neuroectodermal tumor, neuroblastoma, which is characterized by poor prognosis and extensive ganglioside shedding. In fact, this tumor offers the most comprehensive example of the potential importance of tumor ganglioside in tumor progression and its treatment. This was the first human tumor in which the shedding of gangliosides into the peripheral circulation was clearly documented. This detection was possible because the characteristic ganglioside of neuroblastoma, GD2, is not detectable in normal human plasma, and yet ubiquitous in neuroblastoma tumors and shed by neuroblastoma cells (Fig. 23.2, top panels), making the ganglioside an excellent tumor marker (Ladisch and Wu 1985). Detection of circulating GD2 was further facilitated by the application of the new method of ganglioside isolation which overcame the problem of separating and purifying the gangliosides found in the presence of high concentrations of protein, such as in plasma (Ladisch and Gillard 1985). This approach complements antibody detection of tumor gangliosides, which is very useful in tumors but not so much in plasma.
Ganglioside shedding and clinical implications in human neuroblastoma. (a) Neuroblastoma tumor cell (LAN-1) shedding in vitro; (b) detection of GD2 in patient and normal plasma; (c) sequential analysis of circulating GD2 in a neuroblastoma patient, before and after surgical treatment (see text) (Adapted from Ladisch et al. 1987; Li and Ladisch 1991; Valentino et al. 1990)
By tracking circulating GD2 ganglioside levels, it was possible to follow (and even predict) the clinical course of a patient with neuroblastoma. In the specific case shown in Fig. 23.2, bottom panel, circulating GD2 was detectable in the patient at the time of diagnosis of neuroblastoma. Following surgical removal of the tumor, GD2 ganglioside disappeared from the circulation. Sequential studies were negative for GD2, until week 21, when traces of the gangliosides were again evident. This preceded a clinically detected relapse of neuroblastoma, evident finally also as a much higher circulating GD2 level in week 25 (Ladisch et al. 1987). Thus, circulating GD2 was a marker of tumor progression (prior to clinical detection), demonstrating the value of this marker and the significance of the shedding process.
23.2.4 Impact on Outcome
In fact, shed GD2 levels have correlated highly with patient clinical outcome. In an expanded study of a large group of patients, 74 patients with advanced stage (III and IV) neuroblastoma were found to have circulating tumor-derived GD2 ganglioside levels at the time of diagnosis that were inversely related to progression-free survival (PFS) (p = 0.018) (Valentino et al. 1990). By Kaplan-Meier analysis (Fig. 23.3), the quartile of patients having the highest circulating GD2 levels (≥568 pmol/mL) had a strikingly worse outcome than the quartile of patients with the lowest GD2 levels (≤103 pmol/mL). Their median PFS was shorter (9 vs. 28 months), and the long-term survival rate lower (2-year PFS of 24 % vs. 70 %) (p = 0.013). In conclusion, more rapid disease progression and lower survival rate are associated with high circulating GD2 levels at diagnosis. This leads to the hypothesis that shed neuroblastoma gangliosides were facilitating this process in the TME.
Kaplan-Meier analysis of progression-free survival (PFS) of neuroblastoma patients. PFS of quartiles of high-risk neuroblastoma patients having the lowest (dashed line) and highest (dotted line) circulating GD2 concentrations at diagnosis (Adapted from Valentino et al. 1990)
Another example addresses ganglioside shedding by a series of CNS tumors. In this case, detection of shed gangliosides is accomplished by examination of the cerebrospinal fluid draining the CNS tumors. CSF of patients with two types of pediatric brain tumors, medulloblastoma and astrocytoma, was evaluated. Using the same approach as for the plasma studies in neuroblastoma, we detected elevated levels of chemically quantified ganglioside GD3 ganglioside in the CSF of both the patients with medulloblastoma and those with astrocytoma (Fig. 23.4), reflecting shedding of the ganglioside by the tumors, themselves shown to be rich in this ganglioside (Ladisch et al. 1997).
Concentration of GD3 in cerebrospinal fluid (CSF) of controls (C-CSF), medulloblastoma patients (M-CSF), or astrocytoma patients (A-CSF) (Adapted from Ladisch et al. 1997)
The findings of these correlations and associations, as well as those of animal studies, clearly show the potential value of gangliosides as cell surface markers of neural tumors, with implications (a) for diagnosis and (b) as targets for therapeutic interventions (mainly antibody-mediated attacks on the tumor cell). The other important characteristic embodied in this shedding process is the dynamic property of these molecules, affecting normal cells in what might be considered a “paracrine” manner. The question is, what cellular functions do they affect?
23.3 Tumor Cell Ganglioside Function
Two important and now well-accepted biological properties of tumor cell gangliosides, potent immunosuppressive activity and proangiogenic activity, will be considered here. Both depend on shedding of tumor gangliosides, the uptake/binding of these gangliosides by other, normal, cells in the tumor microenvironment, and the modulation of the function of these “normal” cells. These cells include immunocytes (inhibition of antitumor immune responses) and vascular endothelial cells (enhancement of signaling and proliferation, leading to angiogenesis).
Historically, immunoinhibitory properties were the first functional characteristics of gangliosides that were associated with tumor cells. Parenthetically, these observations were being made at a time when the normal role of gangliosides in the nervous system was still a puzzle. Since that time, clear demonstrations of linkage of specific gangliosides to brain development have been made (see Chap. 9), and elucidation of distinct functions of endogenous cellular gangliosides continue. The focus here is on the consequences of exogenous ganglioside enrichment of a cell, by the pathophysiological process of tumor cell ganglioside shedding.
23.3.1 Immunosuppression
Immunoregulation by gangliosides has been extensively studied and will be briefly summarized here. It should be reiterated that most of these effects are characteristic of tumor cell gangliosides in general, even if not yet demonstrated in neural tumors in vivo. The initial findings of tumor ganglioside-induced immunosuppression were obtained in a murine lymphoma (Ladisch et al. 1983), in which the tumor gangliosides could be isolated, identified, and purified by a then new ganglioside purification method that permitted purification of tumor gangliosides from relatively small amounts of tissue, cells, or plasma (Ladisch and Gillard 1985). In this murine lymphoma model, very low (micromolar) concentrations of purified tumor gangliosides, isolated either from tumor cells in vitro or from the ascitic tumor in vivo, were strikingly inhibitory to lymphocyte proliferative responses to mitogens and antigens, the basis for cell-mediated immunity (Ladisch et al. 1983).
23.3.1.1 Cellular Mechanisms
Following this first demonstration of immunosuppressive activity of tumor gangliosides, subsequent studies identified the antigen-presenting cell as a primary target cell causing ganglioside-induced inhibition of the antigen-induced proliferative response (Ladisch et al. 1984). The affected antigen-presenting cells initially identified were the adherent monocytes (Ladisch et al. 1984) and, more recently, the dendritic cell (DC) (Shen and Ladisch 2002). Inhibition of DC maturation (Shurin et al. 2001) and induction of a state of tolerance of DC to TLR signaling (Shen et al. 2008) have been identified as mechanisms. In addition to these effects on myeloid cells involved in normal lymphoproliferative responses, exogenous gangliosides added in vitro inhibit IL2-dependent cell proliferation (Robb 1986), helper T cell proliferation (Chu and Sharom 1995), and natural killer cell activity (Bergelson et al. 1989; Grayson and Ladisch 1992), promote regulatory T cell development, inhibit T cell cytotoxicity (McKallip et al. 1999), and cause T cell apoptosis (Biswas et al. 2006; Chahlavi et al. 2005; Zhou et al. 1998). In vivo evidence corroborates these effects: in a murine model, tumor gangliosides inhibited immune responses, decreased tumor draining lymph node mass and cell numbers, and suppressed lymphoproliferative responses and the generation of tumor-specific cytotoxic T lymphocytes (Li et al. 1996; McKallip et al. 1999). Thus, a wide range of cellular immune functions are affected by exogenous (shed) gangliosides.
23.3.1.2 Molecular Mechanisms
The molecular mechanisms of ganglioside effects on immune cells, unlike the well-understood mechanisms of effects on fibroblasts or vascular endothelial cells (vide infra), remain to be fully elucidated. What is known is that exogenous tumor cell gangliosides inserted into the target cell (adherent monocyte or dendritic cell) membrane regulate membrane receptor activity and affect Toll-like receptor signaling, subsequently impeding nuclear localization and activation of NF-KB protein (Caldwell et al. 2003). Among mechanisms causing inhibition of T cell effector function (cytotoxicity) most recently, interference with granule exocytosis of CD8 T cells has recently been identified as a cause (Lee et al. 2012). Most likely, new model systems will provide additional insight into immunosuppressive mechanisms, both cellular and molecular, utilized by gangliosides to inhibit the host antitumor immune response.
23.3.1.3 Structure-Activity Relationships
With respect to relationship between immunoinhibitory activity and ganglioside structure, comprehensive studies were undertaken using normal brain gangliosides and human neuroblastoma tumor gangliosides, in the case of which HPLC purification of individual ganglioside ceramide species (Ladisch et al. 1989) allowed structure-activity relationships to be established. Antigen-induced lymphoproliferative responses were the indicator system. These studies revealed that with respect to carbohydrate structure, complexity and increasing number of sialic acid residues were associated with increased activity (Ladisch et al. 1992). With respect to ceramide structure, unsaturation and decreased fatty acyl group chain length of the ceramide portion were similarly associated with increased activity (Ladisch et al. 1994).
23.3.1.4 Brain Tumor Gangliosides
There have been few studies specifically addressing immunomodulation by brain tumor gangliosides. On the one hand, one report suggested that cytotoxic activity of NK cells may be affected by the immunoregulatory disturbances observed in patients with primary tumors in CNS (Peracoli et al. 1999). On the other hand, neurostatins (the O-acetylated forms of gangliosides GD1b and GT1b that are present in normal brain) have also been implicated, but as having an anti-tumorigenic effect (Nieto-Sampedro et al. 2011). Cytostatic for normal astroblasts, they were found to be cytotoxic for rat C6 glioma cells and cells of human astrocytoma grades III and IV, with ID50 values ranging from 200 to 450 nM. In contrast, they did not affect neurons or fibroblasts in concentrations of up to 4 μM or higher. The authors identified at least four different neurostatin-activated, cell-mediated antitumoral processes that they proposed to lead to tumor destruction: (1) inhibition of tumor neovascularization, (2) activation of microglia, (3) activation of natural killer cells, and (4) activation of cytotoxic lymphocytes (Nieto-Sampedro et al. 2011). Thus, there may be a balance between some anti- and pro-tumorigenic effects of specific gangliosides. While this remains to be elucidated, the neurostatin-activated processes clearly do not dominate in vivo in the case of human neural tumors.
23.3.2 Angiogenesis
The other functional property of gangliosides and particularly of tumor gangliosides released into the tumor microenvironment that has now been well characterized is the effect on angiogenesis. Here, the shedding of tumor gangliosides into TME may regulate the normal cells (vascular endothelial cells and fibroblasts) that are the structure for new blood vessels. This involves the processes of proliferation, migration, and tube formation in vivo. Initially, this effect on angiogenesis was observed in the laboratories of Gullino (Gullino 1995) and of Seyfried (Manfredi et al. 1999), who found a correlation between complex ganglioside expression and tumor proangiogenic activity, in the latter case in a murine brain tumor model in which a tumor rich in complex gangliosides had enhanced angiogenesis, compared to the parallel tumor containing only GM3 ganglioside.
23.3.2.1 In Vitro Findings
Direct observations of ganglioside promotion of tumor angiogenesis in vitro are using purified ganglioside, GD1a, pointed to a mechanism underlying the effect on responses of normal human umbilical vein endothelial cells (HUVEC) to VEGF (Lang et al. 2001). Preincubation of HUVEC with GD1a enhanced VEGF-induced cell proliferation; 10 μM GD1a caused a twofold increase in DNA synthesis. The migration of HUVEC across a VEGF gradient was also enhanced by 50 %, even with only a brief (1 h) preexposure of the cells to the same concentration of GD1a (Liu et al. 2006). These findings suggested that gangliosides shed by tumor cells can promote tumor angiogenesis by enhancing the VEGF response of endothelial cells in the tumor microenvironment.
23.3.2.2 In Vivo Observations
Further in vivo study has cemented this conclusion. Most recently, the development of a ganglioside knockout model allowed study of tumor formation in the complete absence of ganglioside in the tumor cell itself and without exogenous manipulation of the cells (e.g., pharmacologically). The model was developed by oncogene transformation of murine embryonic fibroblasts in which GM2 synthase and GM3 synthase had been knocked out (Liu et al. 2010). The resulting cells (termed DKO) were consequently constitutively, selectively, specifically, and completely devoid of gangliosides. Injecting these cells in vivo, tumor growth was strikingly affected. The DKO cells formed ganglioside-deficient tumors, much smaller than those formed by the corresponding litter-mate ganglioside-rich wild-type (WT) cells. Importantly, the DKO tumors had a striking paucity of tumor blood vessels (tenfold less than WT tumors), while the level of intrinsic tumor cell VEGF secretion was not reduced (Liu et al. 2013). This study definitively linked ganglioside depletion to impaired tumor angiogenesis and supports the interpretation of an effect of the gangliosides directly on the target (vascular endothelial) cells.
23.3.2.3 Molecular Mechanisms
A molecular mechanism by which gangliosides have this direct effect on angiogenesis has been elucidated. This relates to earlier studies of fibroblast proliferation in which we found that enrichment of the normal fibroblast cell membrane with exogenous gangliosides resulted in enhanced receptor binding affinities for their ligand (EGF and a series of other receptor tyrosine kinases) and enhanced the proliferation of these cells (Li et al. 2000, 2001). In this case of EGF-induced EGFR activation, in normal human dermal fibroblasts, membrane enrichment with GD1a enhanced the EGF-induced EGFR activity by increasing EGFR dimerization and the effective number of high-affinity EGFR, without increasing total receptor protein. Unexpectedly, GD1a enrichment also triggered increased EGFR dimerization in the absence of growth factor. This resulted in enhanced activation of the EGFR signal transduction cascade when EGF was added (Liu et al. 2004). Another recent example of regulating EGF-induced neural cell proliferation and differentiation by gangliosides (in this case, intrinsic cell membrane gangliosides, not shed molecules) is the regulation by ganglioside GD3 of neural stem cell survival. Using a GD3-synthase knockout mouse, the authors showed that GD3 upregulated EGF-EGFR signaling to sustain NSC self-renewal (Wang and Yu 2013).
In the case of angiogenesis, it is another receptor tyrosine kinase, VEGF, which was similarly affected as fibroblast signaling in our previous studies, resulting in enhanced proliferation, migration, and differentiation. In this system, gangliosides act to sensitize vascular endothelial cells to respond to subthreshold levels of VEGF (Liu et al. 2006). Ganglioside enrichment of human umbilical vein vascular endothelial cells (HUVEC) caused very low, normally barely stimulatory, VEGF concentrations to trigger robust VEGF receptor dimerization and autophosphorylation (Fig. 23.5), as well as activation of downstream signaling pathways, and cell proliferation and migration. Overall, by lowering the threshold for growth factor activation of these normal stromal cells, shed tumor gangliosides promote tumor progression, causing normal vascular endothelial cells to become increasingly autonomous from growth factor requirements by a process that we termed tumor-induced progression of the microenvironment (Liu et al. 2006).
Enhancement of VEGFR phosphorylation by membrane enrichment in GD1a ganglioside. Note particularly the amplification of receptor phosphorylation caused at low (<1 ng/ml) VEGF concentrations (Adapted from Liu et al. 2006)
23.4 Conclusion
With the identification of tumor cell gangliosides as potent immunosuppressive and proangiogenic molecules, the clinical relevance of tumor cell gangliosides, both extraneural and neural, is established. Their importance lies in the probability that the many properties elucidated through in vivo/in vitro experimental systems are operative or eventually will be proven to be so in human cancer. To facilitate further discovery, a simple, i.e., practical, sensitive, and specific approach to detecting shed tumor gangliosides in vivo would be valuable. This could provide more insight into prognosis, as the example of neuroblastoma demonstrates. Much remains to be discovered about antitumor immune responses in the central nervous system, and their modulation by tumor gangliosides, but the foundation for these studies has now been laid; it is likely that the mechanisms are analogous to those in other systems. In vivo tumor models in which ganglioside synthesis is constitutively and selective altered will undoubtedly also provide further insights. The murine DKO model may help because it is syngeneic and it allows study of tumor host interactions without exogenous manipulation, comparing ganglioside-poor to ganglioside-rich tumor cells that are genetically identical (except for knockout of the ganglioside synthesis enzymes).
The overall findings that gangliosides facilitate tumor progression suggest that strategies to interrupt tumor cell ganglioside metabolism should be pursued. Targeting synthesis (and elimination of shedding) of tumor gangliosides is likely to be a fruitful approach in neural tumors as in other tumors. This might be accomplished by metabolic or siRNA inhibition, or by otherwise suppressing the activity particularly of GM3 synthase, a key enzyme in human ganglioside synthesis. This approach could be operationally selective for the tumor cells and effective since ganglioside turnover in tumors is much more rapid than in most normal tissues.
References
Bergelson LD, Dyatlovitskaya EV, Klyuchareva TE, Kryukova EV, Lemenovskaya AF, Matveeva VA, et al. The role of glycosphingolipids in natural immunity. Gangliosides modulate the cytotoxicity of natural killer cells. Eur J Immunol. 1989;19(11):1979–83.
Biswas K, Richmond A, Rayman P, Biswas S, Thornton M, Sa G, et al. GM2 expression in renal cell carcinoma: potential role in tumor-induced T-cell dysfunction. Cancer Res. 2006;66(13):6816–25.
Caldwell S, Heitger A, Shen W, Liu Y, Taylor B, Ladisch S. Mechanisms of ganglioside inhibition of APC function. J Immunol. 2003;171(4):1676–83. Epub 2003/08/07.
Chahlavi A, Rayman P, Richmond AL, Biswas K, Zhang R, Vogelbaum M, et al. Glioblastomas induce T-lymphocyte death by two distinct pathways involving gangliosides and CD70. Cancer Res. 2005;65(12):5428–38. Epub 2005/06/17.
Chu JW, Sharom FJ. Gangliosides interact with interleukin-4 and inhibit interleukin-4-stimulated helper T-cell proliferation. Immunology. 1995;84(3):396–403.
Foulds L. Multiple etiologic factors in neoplastic development. Cancer Res. 1965;25(8):1339–47. Epub 1965/09/01.
Grayson G, Ladisch S. Immunosuppression by human gangliosides. II. Carbohydrate structure and inhibition of human NK activity. Cell Immunol. 1992;139(1):18–29. Epub 1992/01/01.
Gullino PM. Prostaglandins and gangliosides of tumor microenvironment: their role in angiogenesis. Acta Oncol. 1995;34(3):439–41. Epub 1995/01/01.
Hakomori S. Aberrant glycosylation in cancer cell membranes as focused on glycolipids: overview and perspectives. Cancer Res. 1985;45(6):2405–14. Epub 1985/06/01.
Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56(23):5309–18. Epub 1996/12/01.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. Epub 2000/01/27.
Hendrix MJ, Seftor EA, Kirschmann DA, Quaranta V, Seftor RE. Remodeling of the microenvironment by aggressive melanoma tumor cells. Ann N Y Acad Sci. 2003;995:151–61. Epub 2003/06/20.
Hettmer S, Malott C, Woods W, Ladisch S, Kaucic K. Biological stratification of human neuroblastoma by complex “B” pathway ganglioside expression. Cancer Res. 2003;63(21):7270–6. Epub 2003/11/13.
Kaucic K, Etue N, LaFleur B, Woods W, Ladisch S. Neuroblastomas of infancy exhibit a characteristic ganglioside pattern. Cancer. 2001;91(4):785–93. Epub 2001/03/10.
Ladisch S, Gillard B, Wong C, Ulsh L. Shedding and immunoregulatory activity of YAC-1 lymphoma cell gangliosides. Cancer Res. 1983;43(8):3808–13.
Ladisch S, Ulsh L, Gillard B, Wong C. Modulation of the immune response by gangliosides. Inhibition of adherent monocyte accessory function in vitro. J Clin Invest. 1984;74(6):2074–81. Epub 1984/12/01.
Ladisch S, Gillard B. A solvent partition method for microscale ganglioside purification. Anal Biochem. 1985;146(1):220–31. Epub 1985/04/01.
Ladisch S, Wu ZL. Detection of a tumour-associated ganglioside in plasma of patients with neuroblastoma. Lancet. 1985;1(8421):136–8. Epub 1985/01/19.
Ladisch S, Wu ZL, Feig S, Ulsh L, Schwartz E, Floutsis G, et al. Shedding of GD2 ganglioside by human neuroblastoma. Int J Cancer. 1987;39(1):73–6. Epub 1987/01/15.
Ladisch S, Sweeley CC, Becker H, Gage D. Aberrant fatty acyl alpha-hydroxylation in human neuroblastoma tumor gangliosides. J Biol Chem. 1989;264(20):12097–105. Epub 1989/07/15.
Ladisch S, Becker H, Ulsh L. Immunosuppression by human gangliosides: I. Relationship of carbohydrate structure to the inhibition of T cell responses. Biochim Biophys Acta. 1992;1125(2):180–8. Epub 1992/04/23.
Ladisch S, Li R, Olson E. Ceramide structure predicts tumor ganglioside immunosuppressive activity. Proc Natl Acad Sci U S A. 1994;91(5):1974–8. Epub 1994/03/01.
Ladisch S, Chang F, Li R, Cogen P, Johnson D. Detection of medulloblastoma and astrocytoma-associated ganglioside GD3 in cerebrospinal fluid. Cancer Lett. 1997;120(1):71–8.
Lang Z, Guerrera M, Li R, Ladisch S. Ganglioside GD1a enhances VEGF-induced endothelial cell proliferation and migration. Biochem Biophys Res Commun. 2001;282(4):1031–7. Epub 2001/05/16.
Lee HC, Wondimu A, Liu Y, Ma JS, Radoja S, Ladisch S. Ganglioside inhibition of CD8+ T cell cytotoxicity: interference with lytic granule trafficking and exocytosis. J Immunol. 2012;189(7):3521–7. Epub 2012/09/08.
Li R, Gage D, McKallip R, Ladisch S. Structural characterization and in vivo immunosuppressive activity of neuroblastoma GD2. Glycoconj J. 1996;13(3):385–9.
Li R, Manela J, Kong Y, Ladisch S. Cellular gangliosides promote growth factor-induced proliferation of fibroblasts. J Biol Chem. 2000;275(44):34213–23. Epub 2000/06/22.
Li R, Liu Y, Ladisch S. Enhancement of epidermal growth factor signaling and activation of SRC kinase by gangliosides. J Biol Chem. 2001;276(46):42782–92. Epub 2001/09/06.
Li RX, Ladisch S. Shedding of human neuroblastoma gangliosides. Biochim Biophys Acta. 1991;1083(1):57–64. Epub 1991/04/24.
Liu Y, Li R, Ladisch S. Exogenous ganglioside GD1a enhances epidermal growth factor receptor binding and dimerization. J Biol Chem. 2004;279(35):36481–9. Epub 2004/06/25.
Liu Y, McCarthy J, Ladisch S. Membrane ganglioside enrichment lowers the threshold for vascular endothelial cell angiogenic signaling. Cancer Res. 2006;66(21):10408–14. Epub 2006/11/03.
Liu Y, Yan S, Wondimu A, Bob D, Weiss M, Sliwinski K, et al. Ganglioside synthase knockout in oncogene-transformed fibroblasts depletes gangliosides and impairs tumor growth. Oncogene. 2010;29(22):3297–306. Epub 2010/03/23.
Liu Y, Wondimu A, Yan S, Bobb D, Ladisch S. Tumor gangliosides accelerate murine tumor angiogenesis. Angiogenesis 2013. Epub 2013/10/30.
Manfredi MG, Lim S, Claffey KP, Seyfried TN. Gangliosides influence angiogenesis in an experimental mouse brain tumor. Cancer Res. 1999;59(20):5392–7. Epub 1999/10/28.
McKallip R, Li R, Ladisch S. Tumor gangliosides inhibit the tumor-specific immune response. J Immunol. 1999;163(7):3718–26. Epub 1999/09/22.
Merritt WD, Der-Minassian V, Reaman GH. Increased GD3 ganglioside in plasma of children with T-cell acute lymphoblastic leukemia. Leukemia. 1994;8(5):816–22.
Nakamura O, Ishihara E, Iwamori M, Nagai T, Matsutani M, Nomura K, et al. Lipid composition of human malignant brain tumors. No To shinkei. 1987;39(3):221–6. Epub 1987/03/01.
Nieto-Sampedro M, Valle-Argos B, Gomez-Nicola D, Fernandez-Mayoralas A, Nieto-Diaz M. Inhibitors of glioma growth that reveal the tumour to the immune system. Clin Med Insights Oncol. 2011;5:265–314. Epub 2011/11/16.
Peracoli MT, Montelli TC, Soares AM, Parise-Fortes MR, Alquati SA, Ueda A, et al. Immunological alterations in patients with primary tumors in central nervous system. Arq Neuropsiquiatr. 1999;57(3A):539–46. Epub 2000/02/10.
Portoukalian J, David MJ, Gain P, Richard M. Shedding of GD2 ganglioside in patients with retinoblastoma. Int J Cancer. 1993;53(6):948–51.
Ravindranath MH, Muthugounder S, Presser N, Selvan SR, Santin AD, Bellone S, et al. Immunogenic gangliosides in human ovarian carcinoma. Biochem Biophys Res Commun. 2007;353(2):251–8.
Robb RJ. The suppressive effect of gangliosides upon IL 2-dependent proliferation as a function of inhibition of IL 2-receptor association. J Immunol. 1986;136(3):971–6.
Sawada M, Moriya S, Saito S, Shineha R, Satomi S, Yamori T, et al. Reduced sialidase expression in highly metastatic variants of mouse colon adenocarcinoma 26 and retardation of their metastatic ability by sialidase overexpression. Int J Cancer. 2002;97(2):180–5. Epub 2002/01/05.
Schengrund CL, Repman MA, Shochat SJ. Ganglioside composition of human neuroblastomas. Correlation with prognosis. A Pediatric Oncology Group Study. Cancer. 1985;56(11):2640–6. Epub 1985/12/01.
Shen W, Ladisch S. Ganglioside GD1a impedes lipopolysaccharide-induced maturation of human dendritic cells. Cell Immunol. 2002;220(2):125–33. Epub 2003/03/27.
Shen W, Stone K, Jales A, Leitenberg D, Ladisch S. Inhibition of TLR activation and up-regulation of IL-1R-associated kinase-M expression by exogenous gangliosides. J Immunol. 2008;180(7):4425–32. Epub 2008/03/21.
Shurin GV, Shurin MR, Bykovskaia S, Shogan J, Lotze MT, Barksdale Jr EM. Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001;61(1):363–9. Epub 2001/02/24.
Talantov D, Mazumder A, Yu JX, Briggs T, Jiang Y, Backus J, et al. Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin Cancer Res. 2005;11(20):7234–42. Epub 2005/10/26.
Valentino L, Moss T, Olson E, Wang HJ, Elashoff R, Ladisch S. Shed tumor gangliosides and progression of human neuroblastoma. Blood. 1990;75(7):1564–7. Epub 1990/04/01.
Wang J, Yu RK. Interaction of ganglioside GD3 with an EGF receptor sustains the self-renewal ability of mouse neural stem cells in vitro. Proc Natl Acad Sci U S A. 2013;110(47):19137–42. Epub 2013/11/08.
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–34. Epub 2010/10/01.
Zhou J, Shao H, Cox NR, Baker HJ, Ewald SJ. Gangliosides enhance apoptosis of thymocytes. Cell Immunol. 1998;183(2):90–8.
Conflicts of Interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Ladisch, S., Liu, Y. (2014). Dynamic Aspects of Neural Tumor Gangliosides. In: Yu, R., Schengrund, CL. (eds) Glycobiology of the Nervous System. Advances in Neurobiology, vol 9. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1154-7_23
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1154-7_23
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1153-0
Online ISBN: 978-1-4939-1154-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)