Abstract
The effect of forest fragmentation on arboreal species can be measured and quantified at various scales using a variety of technical approaches. Multidisciplinary studies or networks of studies that integrate information across scales and fields of expertise provide the most comprehensive understanding of fragmentation. We illustrate the use of a multifaceted approach to assess the threats, and conservation status, of golden-headed lion tamarins (Leontopithecus chrysomelas, GHLTs), an endangered primate residing in a highly complex landscape of Southern Bahia, Brazil. Most remaining habitat is in the hands of private landowners. In the west, the cattle industry has contributed to the severe fragmentation of forests and led to small and extremely isolated fragments. Local GHLT extinctions are occurring quickly. In the east, declining market prices of cocoa and the rapid spread of a fungal disease have devastated cocoa production, and once rather contiguous expanses of shade-cocoa forests are rapidly being converted to unsuitable habitat. GHLTs have been studied at the population level, with increasingly more information being generated on their behavior, ecology, demographics, habitat, genetics, and health. GHLTs (and their landscapes) have also been studied at broader levels, yielding vital information regarding habitat change and fragmentation trends over time, predictors of the presence and absence, and viability and threat analysis via simulation modeling. Collectively, this information is giving rise to a more integrated sense of the mechanisms by which anthropogenic pressures are affecting GHLTs. Additional factors regarding the rich history of GHLT conservation efforts are discussed in this chapter. In an environment as spatially and temporally dynamic as Southern Bahia, a conservation management approach involving evaluation, adaptation, synthesis, and prioritization is critical towards developing efficient conservation action plans sensitive to the continuously changing socioeconomic context.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Human-induced habitat degradation and fragmentation can influence the behavior, health, and ecology of animals, and lead to changes in the genetic structure, demography, and dynamics of animal populations—often in detrimental ways (Marsh 2003; Clarke and Young 2000). Documenting the changes that occur as a result of habitat degradation and fragmentation is essential for understanding a species’ status and enabling conservation actions to ensure a species’ long-term survival (Chapman and Peres 2001). Collective information on the landscape, habitat, genetics, health, population demographics, ecology, community interactions, behavior, and general biology of animals and their populations serve to better characterize the effects of habitat fragmentation and degradation than any one factor alone. Multidisciplinary studies integrating information across scales and implementing diverse techniques may provide the best understanding of fragmentation (Soulé 1985; Lindenmayer and Peakall 2000). Factors impacting species survival interact across scales and disciplines are cumulative in their influence on population dynamics (Gilpin and Soulé 1986). Studies that are spatially and temporally explicit, comparative in nature, and incorporate species perspective further aide in a holistic approach to understanding the factors affecting a threatened species. In this chapter we illustrate the use of such a comprehensive approach to assessing the conservation status of the golden-headed lion tamarin (GHLT, Leontopithecus chrysomelas), an endangered primate residing in the highly complex landscape of Southern Bahia, Brazil (Fig. 19.1).
GHLTs are small-bodied arboreal primates threatened by extreme habitat fragmentation and loss of the Atlantic Forest in Southern Bahia (Pinto and Rylands 1997; IUCN 2011). They are frugi-faunivores, live in small groups (5–7 individuals on average), and maintain home ranges that can be quite large (20–200 ha) (Rylands 1993; Raboy and Dietz 2004; Kierulff et al. 2002b; Oliveira et al. 2010). Generally one female per group breeds once or twice a year, usually producing twins, and infants are reared cooperatively by the group (French et al. 2002; Tardif et al. 2002; Raboy 2002). Populations range mostly in areas of secondary forest in various stages of degradation and some remaining tracts of mature forests (Pinto and Rylands 1997; Zeigler et al. 2010). In addition to mature and secondary forest, they use shade-cocoa plantations (Oliveira 2010). Pasture is generally unusable to GHLTs and anything more than a small field likely serves as a barrier for movement. The GHLTs’ preference for epiphytic bromeliads for prey foraging and tree holes for sleeping likely limit their use of types of degraded habitats that lack such resources (Rylands 1996).
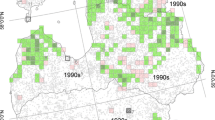
The geographic range of GHLTs is characterized by two distinct vegetation types: coastal humid forest in the east and semideciduous and mesophytic forest in the west (Pinto and Rylands 1997). These two distinct vegetation types coincide with two different predominant economic activities, cocoa cultivation in the east and cattle ranching in the west, resulting in different levels of forest fragmentation and disturbance (Figs. 19.2 and 19.3). The cattle industry has contributed to severe fragmentation of the western portion of the species’ range (Pinto and Rylands 1997). Only small and extremely isolated fragments remain, yet they have considerable edge effect, and local GHLT extinctions are occurring quickly (Zeigler et al. 2010; Raboy et al. 2010; Fig. 19.2). Shade-cocoa plantations predominate in the east where some relatively large forest fragments still exist (Fig. 19.2). However, the decline of cocoa prices and the rapid spread of a fungal disease (witches’ broom) devastated cocoa production, and many landowners are steadily converting their shade-cocoa plantations into cattle ranches or other crops, increasing the level of forest degradation and fragmentation and decreasing the amount of suitable interconnecting matrix habitat (Schroth and Harvey 2007; Cassano et al. 2009). Currently, over 90 % of the species’ range is in the hands of private landowners. Shade-cocoa is the predominant usable habitat remaining for the GHLTs (Raboy et al. 2004; Oliveira 2010).
Map of the GHLT distribution indicating main vegetation types. Bahia state is indicated in gray on the inset. Vegetation types were based on a reclassification of land cover at 30 m resolution published in Landau et al. (2003) from 1996 to 1997 Landsat data
Techniques from diverse disciplines such as ecology (population, community, landscape), animal behavior, botany, veterinary science, and geography have all been used to study GHLTs. The more techniques and disciplines implemented, the more profound our understanding of the species has become.
Scale
Choice of scale can be critical for accurately documenting the effects of habitat fragmentation at relevant levels and reveal previously unknown aspects of a species’ biology. Primates may be studied at all scales including individuals, groups, populations, or metapopulations—or at levels within an individual such as cells and DNA. Choice of scale is often inextricably linked to limitations of time, funding, techniques being implemented, and project feasibility, although ultimately it should be determined by the research questions. Table 19.1 lists past and current research projects focused on in situ GHLT biology, the scale at which they were (or are) being implemented and the techniques used.
When results from lower scales are combined, they can provide detailed and multilayered insight into the large-scale impact of habitat fragmentation, particularly when multidisciplinary studies are involved. For example, a feeding ecology study can be relevant in a specific habitat and population, providing information on the type of food resources (and limitations therein) for that specific region (Rylands 1982; Raboy et al. 2004; Guidorizzi 2008; Catenacci 2008). But studying or comparison of data on the feeding ecology in different habitats and populations provides insight into the range of resources used by the various populations that compose the metapopulation, improves our understanding of how habitat fragmentation alters this aspect of the species’ biology, and provides information to develop conservation actions both for specific populations and the metapopulation in general. Comparative work on ecology and demographics is in progress for five field locations where GHLTs have been studied (Table 19.2). Likewise, assessing a population’s health status through biological sampling can lead to specific recommendations for that population (Monteiro et al. 2007), while comparing samples across populations can provide additional understanding on the impact of larger scale landscape factors such as proximity of human populations across the metapopulation (e.g., health assessment study by Catenacci et al.; Table 19.1).
Effective programs promoting primate conservation generally operate on larger spatial and temporal scales than those typically addressed by a single scientist, e.g., embracing a species’ entire geographic region, or including a number of generations or scales sufficient to monitor ecosystem change (Chapman and Peres 2001). Setting up collaborative projects to analyze data or samples collected by studies at a smaller scale can prove useful to clarify issues at a larger scale and meet the demand for information at the scale needed for conservation purposes, provided comparability is maintained across studies. Examples of such collaborative projects for GHLTs that combine samples collected through studies conducted at the population level to investigate metapopulation questions include Grativol and Magro’s genetic study and Catenacci et al.’s health assessment (Table 19.1) and spatiotemporal metapopulation modeling recently published (Zeigler et al. 2013) and in progress by authors drawing on multiple population studies.
Fragmentation: Past, Present, and Future
Southern Bahia has had a complex series of socioeconomic stressors that created its current fragmented habitat (Câmara 2003; Young 2003). Historically, the landscape of Southern Bahia was influenced by timber harvesting, agriculture, livestock, and an increasing human population (Galindo-Leal and Câmara 2003). Cocoa cultivation in the traditional cabruca system has on one hand slowed down deforestation through preserving part of the native shade tree cover, but at the same time also caused conversion of large tracts of mature forest into secondary forest or agroforestry systems (Leão 2010). Since 1965, Brazil’s forest legislation prohibiting logging of Atlantic forest and enforcing the establishment of legal reserves has allowed for a partial recovery of the Atlantic forest, though mostly in the form of secondary forest, while the area of mature forest continues to contract (Gonzalez and Marques 2008).
Estimates for the remaining amount of Atlantic forest cover vary greatly depending on the source, from 7–8 to 27 % (SOS Mata Atlântica/INPE 1993; 2008; Galindo-Leal and Câmara 2003; IESB et al. 2007). Ribeiro et al. (2009) analyzed fragmentation in the Atlantic forest in eight geographical subregions including a “Bahia region” (delineated as eastern Bahia and almost all of Espirito Santo and Sergipe) based on centers of endemism published in Silva and Casteleti (2003). In the Bahia region 16.7 % of original forest remained, and the largest fragment was 29,000 ha. Also within this region, 40–50 % of the forest was within 100 m of an edge (Ribeiro et al. 2009). SOS Mata Atlantica did similar analyses for the entire state of Bahia. They estimated 1.6 million ha of the remaining forest representing 8.38 % of the original Atlantic forest biome (Mata Atlântica and de Pesquisas Espaciais 2008). Raboy et al. (2010) characterized the forest located within the range of GHLTs. They found that 94 % of the patches (N = 784) were <1,000 ha, and 52 % were <100 ha in size. Twelve patches reached sizes >10,000 ha. Comparisons of current and past forest cover convey a picture of recent pressures on the landscape. Zeigler et al. (2010) showed that forest cover loss in the GHLT distribution area between 1987 and 2007 was 145,796 ha (13 %). Zeigler et al. (2010) also noted 1,419 less fragments over the 20 years, and that mean patch size decreased by 10 ha.
In terms of how forest loss has affected population trends, survey work in combination with landscape analysis (Raboy et al. 2010) documented a range reduction for the GHLTs, in particular, from the west where fragments were found to be smaller and more isolated from one another. Predictive work demonstrated that Core Area Index (the proportion of core area to total patch size) and area of fragments were variables that could potentially explain GHLT’s presence or absence. Both forest cover across the landscape and the GHLTs ranging on it have showed significant decline.
Predicting future trends is a vital tool for conservation practitioners. Changes in landscape structure as well as a species’ response to such changes can be modeled to predict outcomes under various scenarios of future change. In the case of GHLTs, the likelihood of continued habitat loss is high. Recent changes to the Brazilian forest code reduce the level of protection for hilltop forests (the majority of the forest left in the western portion of the GHLT distribution area), and the margin of forest necessary to maintain along riverbanks. These changes will have a profound negative effect on the amount of remaining forest in the western of the GHLT range, and forest integrity and connectivity in the east, threatening region-wide conservation (Law No 12.727, of Oct. 17 2012).
Zeigler (2010) looked at predicted future vulnerability of forest loss throughout the GHLT landscape using the most significant predictors of past landscape change (distance from previously cleared areas, elevation, and human population density). Results indicated that most remaining habitat is highly vulnerable to future loss. Additional studies by the same team looked at what might happen to GHLTs in the future by conducting population viability analysis of the species throughout their entire distribution given no future landscape change (Zeigler et al. 2010) and of selected metapopulations in the western half of the species range given likely trends in future deforestation or reforestation (Zeigler et al. 2013). At current or increased rates of deforestation, most metapopulations suffer from increased extinction risk and decreased abundance and genetic diversity, indicating that major efforts to protect populations and tracts of habitat of sufficient size throughout the species’ distribution will be important to protect the species from continuing decline and extinction (Zeigler et al. 2013; Raboy 2008).
Perspective
Better assessment and integration of species perspective into conservation biology and landscape studies may reveal novel ways to interpret fragmentation, its associated stressors, and potential solutions. For example, our impression of good habitat for GHLTs was initially biased by our human-centered view that secondary forest and human-altered habitats were inferior to mature forest for GHLTs. Studies showed though that GHLTs had affinities to degraded habitats for certain resources (Raboy et al. 2004; Catenacci 2008), and that shade-cocoa plantations were suitable habitats (Oliveira 2010). Connectivity is another point where perspective matters. Based on the concept of functional connectivity, traversable habitat (albeit lower quality according to some set specification) actually connects seemingly isolated forest patches into larger mixed-habitat patches. GHLT researchers have in the past used various definitions to classify fragments as isolated, ranging from 30 m to 1 km of isolation (Raboy et al. 2010; Zeigler et al. 2010). Zeigler et al. (2011) varied the resistance levels of the matrix habitat and identified the impact on functionally connected complexes in the GHLT range discovering the landscape becomes somewhat less fragmented the further GHLTs are capable of dispersing in matrix habitat. The maximal dispersal distance is unfortunately currently unknown.
Great emphasis has recently been placed on biodiversity conservation in lieu of single species conservation programs. Different species, even sympatric, may be differently affected by continued forest loss and fragmentation. Conservation efforts that are good for one species may not work for others, and such species-specific needs should not be overlooked when developing conservation actions for GHLTs, particularly for actions that affect relatively large regions encompassing diverse network of threatened species. For example, when mounting corridors or restoring degraded areas, factors such as the choice of vertical structure or species of fruit trees planted will have to take into account the needs of many species using the corridor, not just GHLTs. An approach to considering species perspective was taken by Paglia (2003) who considered population viability of three threatened Southern Bahian species including two primates, the GHLT, and the yellow-breasted capuchin (Cebus xanthosternos), as well as a parrot Red-browed Amazon (Amazona rhodocorytha). Forest patches of 2,700–3,600; 5,700–10,000, and 3,300–4,100 ha, respectively, were required to ensure a 95 % chance of surviving for 100 years given several assumptions for each species (Paglia 2003). As such, conserving forest patches, the size necessary for GHLTs, might aide parrots, but would not be sufficient for Cebus. In addition to differing needs in terms of the amount of forest necessary to survive in the future—each species will have varying abilities to traverse matrix habitat, and thus functional connectivity will be different for each.
Comparative Studies
Landscape and survey data show that GHLTs occur in a complex landscape, composed of different vegetation types (evergreen vs. semideciduous), under different forms of economic land use (agriculture vs. cattle ranching) and corresponding degree of fragmentation (Pinto and Rylands 1997). Habitat degradation and varying land use have resulted in a mosaic of various types of unsuitable (pasture, rubber tree plantations, urban and some other agricultural forms of land use) or suitable (swamp, mature, secondary forest in different stages of regeneration) habitat for GHLTs (Pinto and Rylands 1997). This landscape complexity presents a wide variety of threats, each of which impacts the different species in contrasting ways. Obtaining sufficient and comparable data for each of these different landscape and habitat configurations is extremely challenging, given the large spatial scale involved, yet this is imperative for making sound and adequate conservation actions for the entire metapopulation.
Setting up collaborative projects to analyze data or samples collected at the level of populations allows for comparing across populations, and addressing questions at the metapopulation level. Since the 1990s researchers spread across four projects have been studying GHLT behavior and ecology (sometimes simultaneously) in representative habitats through long-term monitoring (Table 19.2). Two projects were set up inside Una Biological Reserve, one in contiguous forest habitat and one in heterogeneous and patchy habitat. Two other projects were established outside of the reserve, one in an isolated semideciduous forest fragment and one in shade-cocoa agroforest, studying groups in shade-cocoa and mosaic forest. The data collection methods from these sites were similar which has thus far offered an insight into the range of food sources and sleeping trees used in each habitat, home ranges, densities, reproductive rates, and demographic parameters among others, across habitats (Table 19.2). Additional collaborative projects have been set up to compare certain aspects across study areas at the metapopulation level (i.e., genetics, vegetation studies and phenology, and health aspects such as infectious diseases and parasite load).
Valuable lessons about the impact of fragmentation on a species can also be learned by evaluating it in context to closely related species that share similar environmental stressors. There are three other species of lion tamarins: black lion tamarins (L. chrysopygus: endangered), golden lion tamarins (L. rosalia: endangered), and black-faced lion tamarins (L. caissara: critically endangered). While each of these species lives in different regions of the Atlantic forest and faces unique threats, basic biology and ecology are comparable (Kleiman and Rylands 2002a). All reside in heavily fragmented habitats (Holst et al. 2006). Of the four species, GHLTs have the largest amount of contiguous forest left (Holst et al. 2006). Looking at the patterns of fragmentation and species response that occurred for other lion tamarin species may help predict the GHLTs’ future fate given further fragmentation. For example, Grativol et al. (2001) demonstrated loss of allelic diversity in and considerable genetic divergence between four recently isolated population of GLTs and Dietz et al. (2000) found that inbreeding increased infant mortality, posing a threat to the viability of all but one of the remaining wild populations of GHLTs. These are possible concerns for GHLTs too (Moraes 2011).
Synthesizing and Evaluating
GHLT researchers are convinced of the need for integrating work across scales and disciplines as evidenced by the wide array of collaborative projects in progress (Table 19.1) or being planned and publications integrating disciplines and scales will be soon available (e.g., Zeigler et al. 2013). Here we present some insights available from looking broadly across available studies.
Data on the species’ feeding ecology and food sources used in different areas and habitats (Raboy and Dietz 2004; Catenacci 2008; Guidorizzi 2008; Oliveira et al. 2010) indicate that GHLTs use a large variety of food sources, which could serve as an advantage in an increasingly unpredictable landscape where plant species composition changes due to habitat modifications. In mature and heterogeneous forest, GHLTs use a large number of plant food species, whereas in semideciduous forest and shade-cocoa agroforest they rely on a smaller number of species as a key feeding resource (i.e., bromeliads in semideciduous forest, Guidorizzi 2008; exotic jackfruit in shade-cocoa agroforest, Oliveira et al. 2011). It’s been suggested that the large plant diversity in the GHLT’s diet reflects a tendency to diversify and use available resources when possible (Catenacci 2008), in which case the floristic composition of areas likely determines the diversity of the GHLT’s diet. This seems true, given that vegetation transect studies have demonstrated a higher species diversity in older growth forest versus regenerating areas (Piedade and Maruim; Pessoa 2008) and the lists of species consumed in those two areas are extensive, but contain relatively few species consumed in both areas (Raboy and Dietz 2004; Catenacci 2008). So, the botanical composition of an area indeed seems to explain variation in the GHLT’s diet composition. But does this mean that diets in semideciduous forest and modified areas, such as shade-cocoa, are nutritionally poorer? GHLTs captured in shade-cocoa weighed more than those in mature forest, suggesting good body condition, and thus adequate diet composition (Oliveira et al. 2011). Do GHLTs in shade-cocoa and semideciduous forest eat a low number of species because they can persist on a less diverse diet in these areas, or because low species richness of the area limits their choice of food plants? Does this then imply that these environments are more challenging for GHLTs, and that GHLTs are potentially more vulnerable to seasonal food shortage and changes in floristic composition in degraded and human-altered habitats? This is hard to answer without data on floristic composition in shade-cocoa and semideciduous forest, the total number of species available in each area, their nutritional value, and existing plant defense mechanisms that may make certain species unsuitable for GHLT consumption.
Both ecological and human-induced differences in resource availability exist among habitats, forcing GHLTs to adapt in different ways. Vegetation studies document lower species richness and more pronounced seasonal changes in fruit availability in the mesophytic forest in the western part of the species range compared to the coastal humid forest of the east (Guidorizzi 2008; Pessoa 2008; Pessoa et al. 2012). The existing ecological differences between east and west might have prompted GHLTs to evolve mechanisms to deal with food shortage. Landscape data have demonstrated more severe human-induced fragmentation of western compared to eastern forests (Raboy et al. 2010), and thus edge effects are expected to be more pronounced, all contributing to the floristic composition of an area and the choice of plants available to GHLTs. In shade-cocoa agroforest, agricultural management practices strongly reduce species diversity among shade trees (Sambuichi and Haridasan 2007; Schroth et al. 2011), affecting GHLT diet choice. In all, dietary flexibility versus specialization and the particular importance of key food species may have different relevance in the western mesophytic forest vs. the eastern humid forest, and in poor or modified habitats where species richness is low, due to ecological factors, human factors, or a combination of both. Integrated analyses of overall floristic composition and data on feeding ecology may answer some of these issues. Such comparisons will be instrumental for understanding whether in semideciduous forests and in degraded and modified habitats, GHLTs rely on key species because they prefer to, or because they have to, and improve our understanding of the species’ vulnerability to alterations in vegetation composition due to ecological and human-induced habitat differences.
Ecological, demographic, and survey studies indicate that GHLTs are able to survive and reproduce in a variety of habitats, including older growth forest, heterogeneous degraded forests, shade-cocoa agroforest, and smaller isolated forests (Raboy et al. 2004; Oliveira 2010). Rapid degradation and increased amount of human-altered habitat may have forced GHLTs into using some of these areas more than they would have if more pristine habitat (the habitat in which they evolved in) still existed. While it is hard to tease apart preference from necessity, researchers have been documenting the extent to which GHLTs can use and thrive in different habitats. Birth rates and individual weights are higher in shade-cocoa (Oliveira et al. 2010). Yet, does this mean the species thrives there? More births seem like a benefit but population growth also requires low mortality and high reproductive success. How do differences in resource availability and predation risk between habitats affect birth and mortality rates? Mature forest may provide safer habitat, with lower predation risk and improved survival, whereas shade-cocoa provides abundant resources but at the cost of increased predation (Oliveira and Dietz 2011). Slower reproductive rates, steadier population growth, and long-term stability may be characteristics of more advanced forest, whereas high reproductive rates but larger population turnover may be more typical of modified habitats, leaving GHLTs (in particular small populations) in modified habitats more vulnerable to random factors affecting population growth. GHLT presence in isolated and degraded forest fragments in the west is probably the clearest case of GHLTs’ use of suboptimal habitat and landscape. Comparative work comparing surveys 15 years apart indicates that local extinctions are common in the west; however, we still see GHLTs in surprisingly small and isolated fragments with extreme edge effect. The patches on which they persist may no longer be able to support healthy reproducing populations and those populations still present have entered a downward spiral to local extinction—a phenomena known as the extinction debt (Tilman et al. 1994).
Studies on seed dispersal and interspecies interactions have improved our understanding of the role of GHLTs in their community. Studies on associations between GHLTs and Wied’s marmosets tested hypotheses on the benefits of interspecies associations in relation to ecological factors such as predation and food resources (Raboy 2002; Raboy and Dietz 2000; Oliveira and Dietz 2011). Interspecific associations with Wied’s marmosets in shade-cocoa may be determined by predation risk, whereas those in more undisturbed habitat may be shaped largely by foraging benefits. Further, GHLTs seem crucial for the dispersal of bromeliad seeds, and thus may well be instrumental for maintaining one of their own principal food and foraging resources (Fontoura et al. 2010). GHLTs also help regenerate degraded forest, by dispersing seeds across vegetation types (Catenacci et al. 2009; Cardoso 2008; Cardoso et al. 2011). More studies on community ecology are particularly important for understanding how changes in behavior and population dynamics of GHLTs as a consequence of habitat modification might affect the ecological community. As we are beginning to understand the complex network of which GHLTs are part, we will gain a better understanding of how conservation actions directed towards one species may affect others, which will help us in developing species conservation plans that are adequate for not only GHLTs but also other Atlantic Forest species.
Integrated landscape and GIS research have helped dispel a false notion that GHLTs are relatively safe from threat. The last population estimates available for GHLTs (6,000–15,000; Pinto and Rylands 1997) presented an optimistic population estimate and implied a relative security in numbers for the species. Moreover, increased visibility of GHLTs and other forest species near farms and towns added to this notion that the species are more abundant than previously thought. However, increased sightings may actually be an effect of habitat modification, with GHLTs increasingly forced towards the limits of urban centers and agricultural zones, where they are more likely to be noticed. Thus, it can be harder to convince scientists, conservationists, and the general public that conservation actions are as pressing as formerly believed. Consequently, mounting conservation programs is deemed less critical and conservation funding is directed elsewhere. Recent multidisciplinary GIS and modeling studies have demonstrated GHLT range reduction, continuing and projected habitat degradation and destruction, decreased functional connectivity of habitat, and larger number of local extinctions for the species. The western populations of GHLTs have already reached the stage where intense management of individuals will likely be needed, but the eastern populations may be at a great disadvantage due to continued economic troubles, causing conversion of shade-cocoa to non-habitat. Eastern populations may still be saved principally through carefully thought-out landscape management, but actions are slow to take place. As Kleiman and Rylands (2002b) indicated, “we cannot, yet again, watch and wait as a threatened species reaches numbers so small that the species’ survival becomes critically endangered.”
The future is likely to provide us with many more insights, and more examples of the value of a comprehensive approach to GHLT conservation. For now, perhaps the most compelling example of how being comprehensive has helped us and can help us in the future is Raboy and Zeigler’s integrated studies (Table 19.1) employing a range of different data, including GIS, demographic, and ecological data from a wide range of areas, and landscape analyses to provide not only an image of GHLT absence and presence in the current landscape, but also a basis for exploring future scenarios of landscape change, and potential conservation actions.
Evaluating, Prioritizing, and Creating an Action Plan
Brazil is one of the world’s richest biodiversity regions and a world leader in biodiversity conservation (Mittermeier et al. 2005). The increasing cadre of conservation professionals has resulted in a national protected area system, the elaboration of threatened species lists, a large number of conservation NGOs, and a conservation system based on sound science involving capacity building of conservation scientists (Mittermeier et al. 2005). These elements have greatly facilitated lion tamarin conservation. As a result of a process of adaptation and reevaluation, over the years, GHLT research has moved from a generalized understanding of the ecology and demographics of GHLTs in the wild to understanding more specifically the influence of habitat on GHLT biology and the role landscape plays in all of this. In particular, conservation efforts for lion tamarins have been closely guided and evaluated through the International Committee for the Conservation and Management of the Lion Tamarins (ICCM; an official advisory organ to the Brazilian Government) and Population and Habitat Viability Assessments (PHVAs; Seal et al. 1990; Ballou et al. 1998; Holst et al. 2006). The PHVA Workshops use detailed data on species biology, genetics, and ecology integrated with estimates of human-based threats, such as current and projected land use patterns and sophisticated computer models to evaluate the risk of wildlife population decline or extinction under alternative future management scenarios. These models serve as tools for scientists and wildlife managers to develop detailed recommendations for conservation action focused on the most urgent problems. The resulting Action Plans (Seal et al. 1990; Ballou et al. 1998; Holst et al. 2006) served as a guideline for implementing both new research projects and conservation plans.
In response to the research needs defined during the 1990 PHVA, the first long-term research project in the relatively pristine eastern part of Una Biological Reserve was initiated, providing information on basic ecological parameters, in addition to detailed behavioral data. Following additional recommendations during the 1997–2006 PHVAs, Project BioBrasil was initiated in degraded and heterogeneous habitat in 2002, a project in semideciduous forest in 2006, and a project in shade-cocoa in 2008. During the 1990 workshop, conservationists were still under the assumption that the number of GHLTs in the wild was quite low. Conservation actions deemed necessary focused on securing and protecting habitat, conducting inventories and protecting wild populations, and establishing scientifically managed self-sustainable captive populations as a future source of animals to restock suitable habitat lacking tamarins. Then, following findings that the species also used shade-cocoa agroforest (Alves 1990), and a detailed species survey conducted in 1993–1994 (Pinto and Rylands 1997) suggesting a higher number of GHLTs remaining in the wild, conservation priorities defined for the GHLT conservation program during the 1997 PHVA changed considerably. Reintroduction no longer became an objective, the captive population was reduced in size, and it was decided that conservation actions should concentrate on forest protection and increasing forest connectivity (Ballou et al. 1998). In case suitable habitat became available, given the complications of reintroducing captive-held animals (Kierulff et al. 2002a; Beck et al. 2002), translocations, rather than the reintroduction of captive animals, would be preferred. In turn, research priorities as defined in 1997 and 2005 became strongly determined by the need to obtain the necessary data to develop adequate guidelines for landscape management, such as the identification of important forest fragments, and the construction of corridors as a means of increasing connectivity, and information on the use of shade-cocoa agroforest (Ballou et al. 1998; Holst et al. 2006). Further, the 2005 PHVA also emphasized the importance of research in other understudied habitats (semideciduous forest), as well as at the level of the landscape, in addition to genetic and health studies. Additional comparative research projects were set up in response to this (Table 19.1).
The work of the ICCM has been interrupted since the reorganization of IBAMA and ICMBio in 2007, prompting the lion tamarin programs to find other means of directing and evaluating its research and conservation actions. For example, in 2011, the GHLT research community organized a Research Symposium at the State University of Santa Cruz (UESC; Ilhéus, Bahia, Brazil) with the aim of sharing recent work and discussing potential future avenues for research. This allowed for the dissemination of information to the global GHLT community, compilation of recent advances in research, and identification of gaps in knowledge of GHLT biology, ecology, and conservation, which resulted in a list of research topics considered high priority for future research (Vleeschouwer et al. 2012).
Currently, the principal conservation planning instrument used by Instituto Chico Mendes, the Brazilian Federal institute for the Conservation of Biodiversity (formerly part of IBAMA), is the National Action Plans (NAPs), comprehensive action plans that address the conservation of Brazil’s endangered species (ICMBio 2012). These NAPs propose and monitor the implementation of a series of actions that allow for a harmonic relationship between regional development and the preservation of regional biodiversity. Some are limited to one taxon only (e.g., Brachyteles; Jeruzalinsky et al. 2011), and others are cross-species action plans encompassing entire regions and their corresponding subset of endangered species, e.g., the NAP for the Conservation of Southeastern Mammals encompassing 27 species, including GHLTs and a series of actions from Southern Bahia, over Minas Gerais, Espirito Santo, Rio de Janeiro, and São Paulo states, up to the North of Paraná (PAN-MAMAC 2012). Obviously, this and other NAPs will have to incorporate species perspective and reconcile the needs of all species as a basis for formulating measures that benefit all species together and their landscape.
Conclusions: Future Directions in GHLT Conservation
Our current knowledge on the conservation status of GHLTs suggests that, due to population numbers in the wild still in the low thousands, conservation strategies to secure the long-term survival of the species are likely to be concerned primarily with the management of the landscape, ensuring safe-guarding of representative habitats and improving forest connectivity, and less with the management of individuals (Holst et al. 2006). This holds at least for the eastern part of the distribution that contains most of the remaining GHLT populations in the wild (Pinto and Rylands 1997). In the west, the intense degree of forest fragmentation means that extinctions are imminent (Zeigler et al. 2010) and conservation strategies aiming at restoring a healthy genetically viable western population of GHLTs will be more intense, almost certainly involving management of individual GHLTs and groups (e.g., translocations), in addition to management of forest and habitat linkage areas. This difference between east and west affects research priorities for each area within the distribution range (Vleeschouwer et al. 2012).
Recently, GHLT landscape and population data have been serving as a test case for the development of new conservation biology modeling paradigms and specific software in creation by the Chicago Zoological Society and the Conservation Breeding Specialist Group of the IUCN (Lacy 2012). A diverse network of collaborators have been working on ways in which to synthesize and computationally share knowledge across disciplines, linked through population viability analysis (PVA). Collaborators are in the process of developing a metamodel interface to run concurrent discipline-specific individually based simulation programs such as animal movement, epidemiology, PVA, and a social or life-history simulator, using shared and modifiable input/output and all connected to a GIS platform with supporting data that are spatially structured. For GHLTs the program is being implemented to model the consequences of developing corridors for forest connectivity in certain areas over others as measured by the long-term chances of population persistence and maintenance of genetic diversity given the species movement rules and demographic factors across a complex landscape. Such modeling paradigms and software will assist many conservation practitioners across the world and may have particular utility in multidisciplinary workshop settings.
Addressing future research and conservation priorities for GHLTs will require broad multidisciplinary research projects on a larger scale (across habitats) as well as the development of focused research questions in specific locations. The latter forms the building blocks of well-integrated research, addressing issues such as the impact of particular threats (e.g., predation risk in different habitats). Creating these building blocks is greatly assisted by long-term monitoring of critical ecological parameters in representative habitats, which can serve to identify specific pressures that may become the subject of shorter term or more focused studies (Wintle et al. 2010). Long-term monitoring is also critical towards investigating medium- to long-term effects of natural environmental variation and human-induced environmental modifications, including climate change, on population dynamics and GHLT behavior.
Finally, as we progress in understanding the factors that affect GHLT populations and what is needed to protect them, we should increasingly include additional disciplines, particularly those addressing social, political, and economic issues. An understanding of the economic factors behind particular threats, such as the impact of cocoa prices on the maintenance of shade-cocoa plantations, or political factors promoting economic development versus sustainability is essential for a full understanding of the underlying causes driving habitat modification. It is neither feasible nor desirable to maintain GHLTs in protected areas only. Saving GHLTs will involve the establishment of a network of collaborating private landowners who adopt sustainable forest management and biodiversity-friendly methods of land use, as a means of ensuring the maintenance and connectivity of critical tracts of forest. It is only through the integration of studies on genetics, ecology, resource management, economics, politics, and sociology that we can ever hope to achieve comprehensive effective long-term management of threatened species (Clarke 2000).
References
Alves MC (1990) The role of cacao plantations in the conservation of the Atlantic forest of Southern Bahia, Brazil. Master thesis, University of Florida
Ballou JD, Kleiman DG, Rylands A, Ellis S (eds) (1998) Leontopithecus II. The second population and habitat viability assessment for lion tamarins (Leontopithecus): final report. Conservation Breeding Specialist Group (SSC/IUCN), Apple Valley, MN
Beck BB, Castro MI, Stoinski TS, Ballou JD (2002) The effects of prerelease environments and postrelease management on survivorship in reintroduced golden lion tamarins. In: Kleiman DG, Rylands AB (eds) Lion tamarins – biology and conservation. Smithsonian Institution Press, Washington, pp 283–300
Câmara IG (2003) Brief history of conservation in the Atlantic Forest. In: Galindo-Leal C, Câmara IG (eds) The Atlantic Forest of South America: biodiversity status, threats and outlook. CABS and Island, Washington
Cardoso N (2008) Frugivoria e dispersão de sementes por mico-leão-da-cara dourada (Leontopithecus chrysomelas) na Reserva Biológica de Una – Bahia. Master thesis, Universidade Estadual de Santa Cruz – UESC, Ilhéus
Cardoso NA, Le Pendu Y, Lapenta MJ, Raboy BE (2011) Frugivory patterns and seed dispersal by golden-headed lion tamarins (Leontopithecus chrysomelas) in Una Biological Reserve, Bahia, Brazil. Mammalia 75:327–337
Cassano CR, Schroth G, Faria D, Delabie JHC, Bede L (2009) Landscape and farm scale management to enhance biodiversity conservation in the cocoa producing region of southern Bahia, Brazil. Biodivers Conserv 18:577–603
Catenacci LS (2008) Ecologia alimentar do mico-leão-da-cara-dourada (primates: Callitrichidae) em áreas degradadas da Mata Atlântica do sul da Bahia. Master thesis, Universidade Estadual de Santa Cruz, Ilhéus, Bahia
Catenacci LS, De Vleeschouwer KM, Nogueiro-Filho SLG (2009) Seed dispersal by Leontopithecus chrysomelas (primates, Callitrichidae) in southern Bahian Atlantic Forest, Brazil. Biotropica 41:744–750
Chapman CA, Peres CA (2001) Primate conservation in the new millennium: the role of scientists. Evol Anthropol 10:16–33
Clarke GM (2000) Inferring demography from genetics: a case study of the endangered golden sun moth, Synemon plana. In: Young AG, Clarke GM (eds) Genetics, demography and viability of fragmented populations. Cambridge University Press, Cambridge
Clarke GM, Young AG (2000) Introduction: genetics, demography and the conservation of fragmented populations. In: Young AG, Clarke GM (eds) Genetics, demography and viability of fragmented populations. Cambridge University Press, Cambridge
Coimbra-Filho AF (1973) Distribution and ecology of the genus Leontopithecus lesson, 1840 in Brazil. Primates 14:47–66
De Vleeschouwer K, Oliveira L, Raboy B, Raghunathan N, Zeigler S (2012) Golden-headed lion tamarin research in the 21st century: recent advances and potential areas of future research. Neotrop Prim 18(2):72–76
Dietz JM, De Sousa SN, Billerbeck R (1996) Population dynamics of golden-headed lion tamarins Leontopithecus chrysomelas in Una Reserve, Brazil. Dodo, J Wildl Preserv Trusts 32:115–122
Dietz JD, Baker AJ, Ballou JD (2000) Demographic evidence of inbreeding depression in wild golden lion tamarins. In: Young AG, Clarke GM (eds) Genetics, demography and viability of fragmented populations. Cambridge University Press, Cambridge
Fontoura T, Cazetta E, Nascimento W, Catenacci L, De Vleeschouwer K, Raboy BE (2010) Frugivory on the bromeliad species Aechmea depressa L.B. Smith (Bromeliaceae) from northeastern Brazil: the prominent role taken by a small forest primate? Biota Neotrop 10:351–354
French JA, De Vleeschouwer K, Bales K, Heistermann M (2002) Reproductive function in female lion tamarins (Leontopithecus). In: Kleiman DG, Rylands AB (eds) Lion tamarins: biology and conservation. Smithsonian Institution Press, Washington
Galindo-Leal C, Câmara IG (2003) Atlantic Forest hotspot status: an overview. In: Galindo-Leal C, Câmara IG (eds) The Atlantic Forest of South America: biodiversity status, threats and outlook. CABS and Island, Washington
Gilpin ME, Soulé ME (1986) Minimum viable populations: the processes of species extinction. In: Soulé ME (ed) Conservation biology: the science of scarcity and diversity. Sinauer Associates, Sunderland, MA
Gonzalez P, Marques A (2008). Forest carbon sequestration from avoided deforestation and reforestation in Mata Atlantica (Atlantic Forest), Sul da Bahia, Brazil. Topical report to the US Department of Energy
Grativol AD, Ballou D, Fleischer RC (2001) Microsatellite variation within and among recently fragmented populations of the golden lion tamarin (Leontopithecus rosalia). Conserv Genet 2:1–9
Guidorizzi CE (2008). Ecologia e comportamento do mico-leão-da-cara dourada, Leontopithecus chrysomelas (Kuhl, 1820) (primates, callitrichidae), em um fragmento de floresta semidecidual em Itororó, Bahia, Brasil. Master thesis, Universidade Estadual de Santa Cruz—UESC, Ilhéus
Holst B, Medici EP, Marino-Filho OJ, Kleiman D, Leus K, Pissinatti A, Vivekananda G, Ballou JD, Traylor-Holzer K, Raboy B, Passos F, Vleeschouwer K, Montenegro MM (eds) (2006) Lion tamarin population and habitat viability assessment workshop 2005, final report. IUCN/SSC Conservation Breeding Specialist Group, Apple Valley, MN
ICMBio (2012). Planos de ação nacionais. Website ICMBio http://www.icmbio.gov.br/portal/biodiversidade/fauna-brasileira/planos-de-acao-nacionais.html. Accessed 20 Nov 2012
Instituto de Estudos Sócioambientais do Sul da Bahia (IESB), Instituto de Geociências da Universidade Federal do Rio de Janeiro (IGEO/UFRJ), Departamento de Geografia da Universidade Federal Fluminence (UFF) (2007). Levantamento da cobertura vegetal nativa do bioma Mata Atlântica. Final report. PROBIO 03/2004, Brasília, p 84
IUCN (2011) 2011 IUCN Red List of Threatened Species. www.iucnredlist.org
Jeruzalinsky L, Talebi M, Melo FR (eds) (2011) Plano de ação nacional para a conservação dos muriquis. Instituto Chico Mendes de Conservação da Biodiversidade, Brasília
Kierulff MCM, Oliveira PP, Beck BB, Martins A (2002a) Reintroduction and translocation as conservation tools for golden lion tamarins. In: Kleiman DG, Rylands AB (eds) Lion tamarins: biology and conservation. Smithsonian Institution Press, Washington
Kierulff MCM, Raboy B, Oliveira PP, Miller K, Passos FC, Prado F (2002b) Behavioral ecology of Leontopithecus. In: Kleiman DG, Rylands AB (eds) Lion tamarins: biology and conservation. Smithsonian Institution Press, Washington
Kleiman DG, Rylands AB (eds) (2002a) Lion tamarins: biology and conservation. Smithsonian Institution Press, Washington
Kleiman DG, Rylands AB (2002b) Lion tamarin biology and conservation: a synthesis and challenges for the future. In: Kleiman DG, Rylands AB (eds) Lion tamarins: biology and conservation. Smithsonian Institution Press, Washington
Lacy RC (2012) Tools for species conservation in a changing world. http://www.vortex10.org/index.html
Landau EC, Hirsch A, Musinsky J (2003) Cobertura vegetal e Uso do Solo do Sul da Bahia-Brasil. In: Prado PI, Landau EC, Moura RT, Pinto LPS, Fonseca GAB, Alger K (eds) Corredor de biodiversidade da Mata Atlântica do Sul da Bahia. IESB/DI/CABS/UFMF/UNICAMP (CD-ROM), Ilheus, Brazil
Leão AC (2010) O cultivo do cacao (Theobroma cacao L.) no Brasil. CEPLAC/CEPEC, Itabuna, BA
Lindenmayer DB, Peakall R (2000) The Tumut experiment—integrating demographic and genetic studies to unravel fragmentation effects. In: Young AG, Clarke GM (eds) Genetics, demography and viability of fragmented populations. Cambridge University Press, Cambridge
Marsh LK (ed) (2003) Primates in fragments. Ecology in conservation. Kluwer Academic/Plenum, New York, NY
Mittermeier RA, Fonseca GAB, Rylands AB, Brandon K (2005) A brief history of biodiversity conservation in Brazil. Conserv Biol 19:601–607
Monteiro RV, Dietz JM, Raboy B, Beck B, Vleeschouwer KD, Baker A, Martins A, Jansen AM (2007) Parasite community interactions: Trypanosoma cruzi and intestinal helminths infecting wild golden lion tamarins Leontopithecus rosalia and golden-headed lion tamarins L. chrysomelas (Callitrichidae, L., 1766). Parasitol Res 101:1689–1698
Moraes AM (2011) Dados genéticos para a avaliação do status de conservação do mico-leão-da-cara-dourada, Leontopithecus chrysomelas (Kuhl, 1820), no sul da Bahia, Brasil. Master thesis, Universidade Estadual do Norte Fluminense, Campos dos Goytacazes, RJ
Oliveira LC (2010) Ecology and demography of golden-headed lion tamarins (Leontopithecus chrysomelas) in Cabruca Agroforest, Bahia State, Brazil. PhD Dissertation, University of Maryland
Oliveira LC, Dietz J (2011) Predation risk and the interspecific association of two Brazilian Atlantic forest primates in Cabruca agroforest. Am J Primatol 73:852–860
Oliveira LC, Hankerson SJ, Dietz J, Raboy B (2010) Key tree species for the golden-headed lion tamarin and implications for shade-cocoa management in southern Bahia, Brazil. Anim Conserv 13:60–70
Oliveira LC, Neves LG, Raboy BE, Dietz JM (2011) Abundance of jackfruit (Artocarpus heterophyllus) affects group characteristics and use of space by golden-headed lion tamarins (Leontopithecus chrysomelas) in cabruca agroforest. Environ Manage 48:248–262
PAN-MAMAC (2012). Sumário executivo do plano de ação nacional para a conservação dos mamíferos da Mata Atlântica Central. http://www.icmbio.gov.br/portal/biodiversidade/fauna-brasileira/plano-de-acao/372-pan-mamiferos-da-mata-atlantica.html. Accessed 20 Nov 2012
Paglia AP (2003) Análise de viabilidade populacional: quantos indivíduos? Serão eles suficientes? Estudo de caso para espécies ameaçadas da Mata Atlântica do sul da Bahia. Corredor de biodiversidade da Mata Atlântica do sul da Bahia. Belo Horizonte, Conserv Int (CD-rom)
Pessoa MS (2008) Comparação da comunidade arbórea e fenologia reprodutiva de duas fisionomias em floresta Atlântica no sul da Bahia. MSc dissertation, Universidade Estadual de Santa Cruz, Ilhéus, Bahia
Pessoa MS, De Vleeschouwer KM, Talora DC, Rocha L, Amorim AM (2012) Reproductive phenology of Miconia mirabilis (Melastomataceae) within three distinct physiognomies of Atlantic Forest, Bahia, Brazil. Biota Neotrop 12:49–56
Pinto LPS, Rylands AB (1997) Geographic distribution of the golden-headed lion tamarin, Leontopithecus chrysomelas: implications for its management and conservation. Folia Primatol 68:167–180
Raboy BE (2002) The ecology and behavior of wild golden-headed lion tamarins (Leontopithecus chrysomelas). PhD dissertation, University of Maryland, College Park, MD
Raboy BE (2008) The golden-headed lion tamarin connection. CEPF final project completion report. The Critical Ecosystem Partnership Fund, Washington DC. http://www.cepf.net/Documents/Final_Smithsonian_LionTamarin.pdf
Raboy BE, Dietz JM (2000) Patterns of interspecific associations between wild golden-headed lion tamarins and sympatric Wied's marmosets in southern Bahia, Brazil. Am J Primatol 51:83–84
Raboy BE, Dietz JM (2004) Diet, foraging, and use of space in wild golden-headed lion tamarins. Am J Primatol 63:1–15
Raboy BE, Christman MC, Dietz JM (2004) The use of degraded and shade cocoa forests by endangered golden-headed lion tamarins Leontopithecus chrysomelas. Oryx 38:75–83
Raboy BE, Neves LN, Zeigler S, Saraiva NA, Cardoso N, Santos GR, Ballou JD, Leimgruber P (2010) Strength of habitat and landscape metrics in predicting golden-headed lion tamarin presence or absence in forest patches in Southern Bahia, Brazil. Biotropica 42:388–397
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153
Rylands AB (1982) The ecology and behavior of three species of marmosets and tamarins (Callitrichidae, primates) in Brazil. PhD dissertation, University of Cambridge, Cambridge
Rylands AB (1993) The ecology of the lions tamarins, Leontopithecus: some intrageneric differences and comparisions with other callitrichids. In: Rylands AB (ed) Marmosets and tamarins: systematics, behaviour and ecology. Oxford University Press, Oxford
Rylands AB (1996) Habitat and the evolution of social and reproductive behavior in the Callitrichidae. Am J Primatol 38:5–18
Sambuichi RHR, Haridasan M (2007) Recovery of species richness and conservation of native Atlantic forest trees in the cacao plantations of southern Bahia in Brazil. Biodivers Conserv 16:3681–3701
Schroth G, Harvey CA (2007) Biodiversity conservation in cocoa production landscapes: an overview. Biodivers Conserv 16:2237–2244
Schroth G, Faria D, Araujo M, Bede L, Van Bael SA, Cassano CR, Oliveira LC, Delabie JHC (2011) Conservation in tropical landscape mosaics: the case of the cacao landscape of southern Bahia, Brazil. Biodivers Conserv 20:1635–1654
Seal US, Ballou JD, Padua CV (1990) Leontopithecus population viability analysis. Workshop report. Captive Breeding Specialist Group (IUCN/SSC/CBSG)/Species Survival Commission/IUCN
SOS Mata Atlântica, Instituto Nacional de Pesquisas Espaciais (1993) Atlas da evolução dos remanescentes florestais da Mata Atlântica e ecossistemas associados no período de 1985–1990. São Paulo
SOS Mata Atlântica, Instituto Nacional de Pesquisas Espaciais (2008) Atlas dos remanescentes florestais da Mata Atlântica, período de 2000 a 2005. http://www.sosmatatlantica.org.br
da Silva JMC, Casteleti CHM (2003) Status of the biodiversity of the Atlantic Forest of Brazil. In: Galindo-Leal C, Câmara IG (eds) The Atlantic Forest of South America: biodiversity status, threats and outlook. CABS and Island, Washington
Soulé ME (1985) What is conservation biology? Bioscience 11:727–734
Tardif SD, Santos CV, Baker AJ, Van Elsacker L, Feistner ATC, Kleiman DG, Ruiz-Miranda CR, Moura AC, Passos F, Price EC, Rappaport L, De Vleeschouwer K (2002) Infant care and development in lion tamarins. In: Kleiman DG, Rylands AB (eds) Lion tamarins: biology and conservation. Smithsonian Institution Press, Washington
Tilman D, May RM, Lehman CL, Nowak MA (1994) Habitat destruction and the extinction debt. Nature 371:65–66
Vleeschouwer K, Oliveira L, Raboy B, Raghunathan N & Zeigler S (2012) Golden-headed lion tamarin research in the 21st century: recent advances and potential areas of future research. Neotropical Primates 18(2):72–76
Wintle BA, Runge MC, Bekessy SA (2010) Allocating monitoring effort in the face of unknown unknowns. Ecol Lett 13:1325–1337
Young CEF (2003) Socioeconomic causes of deforestation in the Atlantic Forest of Brazil. In: Galindo-Leal C, Câmara IG (eds) The Atlantic Forest of South America: biodiversity status, threats and outlook. CABS and Island, Washington
Zeigler SL (2010) Forest loss and fragmentation in Southern Bahia, Brazil: implications for the extinction risk of golden-headed lion tamarins (Leontopithecus chrysomelas). PhD dissertation, University of Maryland, College Park, MD
Zeigler S, Fagan WF, De Fries R, Raboy BE (2010) Identifying important forest patches for the long-term persistence of the endangered golden-headed lion tamarin (Leontopithecus chrysomelas). Trop Conserv Sci 3:63–77
Zeigler SL, Neel MC, Oliveira LC, Raboy BE, Fagan WF (2011) Conspecific and heterospecific attraction in assessments of functional connectivity. Biodivers Conserv 20:2779–2796
Zeigler SL, De Vleeschouwer KM, Raboy BE (2013) Assessing extinction risk in small metapopulations of golden-headed lion tamarins (Leontopithecus chrysomelas) in Bahia, Brazil. Biotropica. DOI: 10.1111/btp.12037
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
De Vleeschouwer, K.M., Raboy, B.E. (2013). Multilevel and Transdisciplinary Approaches to Understanding Endangered Primates in Complex Landscapes: Golden-Headed Lion Tamarins in Southern Bahia, Brazil. In: Marsh, L., Chapman, C. (eds) Primates in Fragments. Developments in Primatology: Progress and Prospects. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8839-2_19
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8839-2_19
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8838-5
Online ISBN: 978-1-4614-8839-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)