Abstract
Understanding the ways in which primates have adapted to tolerate harsh environments, such as temperate forests found at high altitudes with extreme variations in temperature and food supply, may help us to understand the persistence of particular primate species when many others are in decline. In this chapter, I review the main results from the study of the ecology and behavior of an endangered monkey (Rhinopithecus bieti) in a harsh environment at Xiaochangdu, Tibet. The monkeys varied their diet throughout the year to cope with strongly seasonal food availability. They prefer to feed on leaves or fruit whenever they can be obtained, suggesting that lichens serve as a staple or a fallback food, not the resource of choice for this population. They had longer daily travel length (DTL) in summer and spring than in winter. The behavioral responses of the group were site-specific to the temperate zone and generally in accordance with current theories of primate ecology that both travel requirements to find fruit and the time and energy available for traveling increase as the proportion of fruit in the diet increases. The monkeys ranged at elevations between 3,550 and 4,300 m; however, they did not generally prefer any particular altitudinal zone. This vertical ranging behavior may be a site-specific phenomenon resulting from peculiarities of the monkeys’ habitat such as human disturbance patterns. Mating events occurred between July and October; births occurred between February and mid-March. Variation in food availability, temperature, and photoperiod may be ecological influences on the timing of reproductive events.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
An organism’s physical environment is one of the strongest determinants of the selective forces to which it will be subject. This includes both abiotic factors, such as temperature, rainfall, and length of day, and biotic interactions, such as availability of food throughout the year, predation risk, and infection by diseases or parasites. Species frequently adapt to survive and reproduce within a particular habitat or ‘niche’. Animals which are able to exploit marginal environments may enjoy a particular advantage from doing so if few potential competitors can successfully colonize the area. Therefore, understanding the ways in which primates have adapted to tolerate harsh environments such as high altitude temperate forests, which show extreme seasonal variation in temperature and food availability, may help us to understand the persistence of particular primate species when many others are in decline. Comparative studies which utilize data on the adaptive responses of primates living at different altitudes may provide insight into behavioral flexibility with regard to feeding ecology and social organization, since both are theorized to be influenced by environmental gradations.
There are 23 primate species which occur in China, of which five (Macaca thibetana, Macaca cyclopis, Rhinopithecus roxellana, Rhinopithecus brelichi, and Rhinopithecus bieti) are endemic (Smith and Xie 2009). Within the Chinese members of genus Rhinopithecus, the black-and-white snub-nosed monkey (R. bieti) displays the most extreme adaptations to high altitude living. It occupies a limited geographic range within the provinces of Yunnan and Tibet and occurs at altitudes of 3,000–4,300 m (Long et al. 1994). At present, only 15 groups totaling approximately 2,500 individuals remain in the wild (Long YC, pers. Comm.); the monkeys’ habitat is threatened by selective logging for timber to build houses as well as the collection of firewood (Xiang et al. 2007a; Xiao et al. 2003; Zhao 1996).
The Xiaochangdu group of R. bieti occupies the northernmost tip of the species’ distribution and endures many meteorological extremes in rainfall patterns and temperature (Xiang et al. 2007b). At Xiaochangdu, the total annual precipitation is 740 mm, but monthly averages show an uneven distribution of rainfall through the year. About 60 % of yearly precipitation occurs from June to August, often accompanied by dense fog. Snow accumulation begins in late November and may persist in the forest until mid-May. Regarding monthly mean temperature, the highest occurs in August (12.5 °C), and the lowest in January (−3.6 °C). The highest (26.9 °C) and lowest (−15.4 °C) single recorded temperatures also occurred in August and January, respectively. The mean temperature remained negative for 4 months out of the year. The resident monkey group is able to make use of only four habitat types (Xiang et al. 2011): (1) Primary conifer forest, composed primarily of Picea likiangensis and Abies squamata trees interspersed with bushes less than 2 m in height; (2) Secondary conifer forest, a habitat type artificially created by selective logging on the part of local people, where Picea likiangensis and Abies squamata trees along with dense bushes dominate the vegetation; (3) Larch forest, chiefly comprised of Larix griffithiana and Rhododendron spp. trees with a thick undergrowth of typically Rhododendron spp. bushes taller than 2 m; and (4) Evergreen broadleaf forest/montane scerophyllous oak forest, in which Quercus aquifolioides trees dominated the flora, interspersed with bushes which rarely grew over 2 m and remained at low density.
In light of the unusual characteristics of climate and habitat experienced by R. bieti at Xiaochangdu, Tibet, it is valuable to gain an understanding of their behavioral flexibility and adaptive responses. Historically, long-term observational study of R. bieti in its natural habitat has been limited by difficult field conditions, such as the unforgiving topography and strong seasonality of its habitat. This is particularly true for the Tibetan populations. In this chapter, I will first introduce the study site, subjects, and methods of a comprehensive research project at Xiaochangdu, Tibet which has lasted since 2003. I will review results pertaining to diet and range use, then turn to issues of reproduction. Finally, I consider problems pertaining to the conservation of R. bieti.
Study Area and Subjects
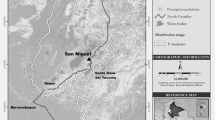
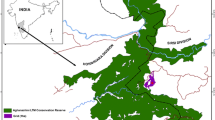
The study area, Xiaochangdu, is located at 29°15′N, 98°37′E within the Honglaxueshan National Nature Reserve, Mangkang County of Southeast Tibet (Fig. 1). The Reserve was founded in 1993 chiefly to protect R. bieti. It is located within the Hengduan Mountains, bounded by the Mekong River to the west and the Heiqu (Gatuo) River to the east. The majority of the mountains in this area rise above 3,500 m (Xiang et al. 2007a).
Seasonality at Xiaochangdu is pronounced. Due to the high altitude and the influence of monsoon winds, it may not be appropriate from a biological standpoint to define the seasons in the manner typical for the rest of the temperate northern hemisphere. I have suggested that winter be defined as those months in which the mean temperature remains below 0 °C, and summer be defined as those months in which the mean temperature is above 10 °C. According to this framework, spring at Xiaochangdu would occur from April to May, summer from June to August, autumn from September to October, and winter from November to the subsequent March.
The study group I observed contained 207 individuals and is comprised of 32 adult males, 71 adult females, 72 juveniles, and 32 infants. Accordingly, the adult sex ratio is 1:2.2. The ratio of adults to immatures (juveniles and infants included) is 1:1, and the ratio of infants to adult females is 1:2.3. These parameters indicate that the Xiaochangdu group is a stable population. This is the only population of R. bieti which has not declined in number since the last survey in 1988 (Xiang et al. 2007a). The basic social group of R. bieti is the multi-female, one-male unit (OMU) (Kirkpatrick et al. 1998). I have also observed a few multi-female, multi-male units, and at least one-male-unit (Xiang 2005). Infants were identified by behavioral characteristics and physical traits. Newborns have white coats except for isolated black hairs on the top of the head, back, and tail tip. They must be constantly carried before the age of 10 days; agile movement begins around day 25 (Zou et al. 1999). The small villages bordering the monkeys’ range, totaling about 60 families, all occur below 3,800 m.
The study group’s contact with humans remains limited. Monkeys have never been seen to take food from farmers or researchers, either directly or indirectly. Hunting is limited due to a religious taboo observed by the Tibetan Buddhist villagers (Xiang et al. 2007a). However, livestock range through the monkeys’ habitat, and local inhabitants collect forest resources during the summer.
Methods
I conducted multiple continuous follows of the study group at Xiaochangdu between June 2003 and March 2005. I employed instantaneous scan sampling (Altmann 1974) at 15 min intervals to record data on diet composition and feeding behavior. Once I spotted an individual processing a food item, I tried to identify both the species and the category of the food. Monthly proportions were then averaged over a year to obtain an overall estimate of the relative contributions of different items to the diet of R. bieti. Food availability was estimated using the crown density method (Marsh 1981; see also Xiang et al. 2007b).
I obtained information on ranging behavior by following the monkeys either for their entire period of diurnal activity or from the time I first established visual contact or auditory contact (vocalization and sound of breaking branches) with the group, lasting until the monkeys ranged out of sight. I used a map of 1:100,000 scale, divided into a grid of 500 m × 500 m2, to record the area used by groups and the position of the center of the group. A “used” grid cell was one in which greater than 50 % of the area was used by the group; habitat type for each cell was determined by whichever occupied the greatest area in the grid. Seasonal home range estimates are derived from plots of new grid cells entered over time, using the area at which the curve reached an asymptote during a certain study period (Kirkpatrick et al. 1998). The tree in which the greatest number of individuals found together was defined as the center of the group. I recorded the location of the group’s centroid every 2 hours using a GPS receiver, unless I was prevented from doing so by dense vegetation or a deep valley that limited signal reception. I marked the group’s location on the map and used these records to estimate the group’s daily travel length (DTL), defined as the distance between the sleeping sites of two consecutive days along the path demarcated by group centroids. On days in which the monkeys were not followed from the time they departed their sleeping site that morning, I estimated DTL from the foraging trails, following signs such as broken branches or freshly fallen feces, so long as the monkeys were tracked to their next sleeping site without losing contact.
After finishing the period of 15 min instantaneous scan sampling to collect diet and foraging records, I chose a clearly observable OMU as a focal group and noted any copulatory events. A copulatory event consists of solicitation, mount, intromission, pelvic thrusts, ejaculation, and dismount. External indications of ejaculation included cessation of thrusting with maximal insertion and a notable tensing of body musculature (Cui and Xiao 2004). As individual identification was generally not possible in primary or secondary conifer forest due to limited visibility, I censused adult females and new infants in the group either by scanning observation with a field scope (Nikon ED II, 25–56X) from 50 to 1,000 m away, or by close observation of the animals at distances of 15–50 m (with or without binoculars) (Xiang and Sayers 2009). Close observation, when possible, provides superior count data to that of long-range scans.
Dietary Adaptations to Extreme Seasonality
R. bieti at Xiaochangdu feeds upon at least 25 different species of trees, shrubs, and ground plants from 19 genera and 13 families (Xiang et al. 2007b). They also consume a minimum of three species of arboreal lichens, two species of grasses, three types of invertebrates retrieved from decayed wood or overturned rocks, and resin from two tree species. Although monkeys preferentially consumed leaves, fruits, and nuts when they were available, lichens were the most dependable and, hence, most frequently consumed resource, especially during periods of scarcity. Feeding records reveal a dietary composition as follows: 82.1 % lichens; 12.1 % buds and leaves; 1.1 % flowers, fruits, and nuts; 0.6 % invertebrates; 4.2 % other foods including resin, bark, and herbs.
The estimate of lichen consumption (82.1 %) from the feeding records of Xiaochangdu monkeys is slightly higher than the figure obtained from Kirkpatrick’s (1996) observations of the Wuyapiya group. Compared to these two populations, the relative amount of lichen (ca. 60 %) consumed by monkeys at Tacheng, located centrally within the species’ range, is low (Ding and Zhao 2004). The estimated percentage of lichens in the diet (ca. 5 %) at Jinsichang, to the south of those sites mentioned above, is lowest of all (Yang and Zhao 2001); however, this value was derived from analysis of fecal contents rather than direct observation. Thus, it may not be commensurate with figures reported from feeding behavior—though Ding and Zhao (2004) observed 60 % of food consumed to be lichen, fecal analysis revealed only 9 % lichen composition. The figure of 5 % lichen content from feces at Jinsichang may suggest a true dietary lichen content closer to 30 %. If this is the case, then the proportion of lichens consumed, from the northernmost group to the southernmost, decreases from 82.2 % (Xiaochangdu) to 75 % (Wuyapiya), 60 % (Tacheng), and lastly 30 % (Jinsichang). These observations suggest that lichens serve as a staple or fallback food rather than a preferred resource of choice for this species (Xiang et al. 2007b), since the proportion of lichen in the diet displays a direct relationship with altitude (and hence an inverse one with habitat richness).
The diet of colobine monkeys responds to environmental factors such as elevation and habitat composition (Bennett and Davies 1994). In general, habitats at lower elevations display more complex floral communities than those found at higher elevations. R. bieti displays considerable variability in habitat diversity and diet composition between groups living at higher or lower latitudes and altitudes. Xiaochangdu offers the poorest food resources of all studied sites in terms of number of different species consumed and plant parts eaten (Table 1). Traveling southward within R. bieti’s range, monkeys tend to feed on more plant species and plant parts and to consume fewer lichens. In addition to being the least botanically diverse locality, Xiaochangdu has the most challenging weather conditions experienced by this species (Table 1).
Ranging Use Pattern and Response to Changing Environment
Home Range, DTL, and Population Density
Through a grid system, we estimated the home range of the Xiaochangdu group to be 16.75 km2 in summer, 10.50 km2 in winter, and 21.25 km2 over a two-year observation period. Ranging data showed clear seasonal trends; during the summer, the monkeys expanded their range and decreased the intensity of use for particular areas (as measured by a lower reuse rate for each grid cell). The mean DTL of the study group was 765 m over the study period. Mean DTL was shortest between December and March, when lichen was the primary food consumed. Maximum DTL approached 1 km in May; this coincided with a period of abundant high-quality food and high human activity (mushroom and Chinese medicine collection) within the forest, making it difficult to distinguish which factor may have been chiefly responsible. Similar to observed ranging behavior, mean DTL also changed with the season. R. bieti had a significantly longer DTL in both summer and spring than in winter (Xiang 2005). Using an estimate for mean adult body mass of 9.1 kg (Kirkpatrick 1996), the population density and biomass of R. bieti at Xiaochangdu were calculated to be 9.7 individuals/km2 and 88.6 kg/km2, respectively.
Food availability is considered an important determinant of daily travel distance among primate species (Milton 1980; Champman 1988). When food items are widely dispersed, group-living primates should have to travel farther to satisfy nutritional requirements (Champman and Champman 1990; Oates 1987). Primates may also pursue a high-risk, high-reward strategy when the most preferred foods are abundant, responding to these conditions with increased daily travel as well (Norberg 1977). Lichens are low in protein, reducing their desirability as a resource (Kirkpatrick 1996). Compensatory feeding on fruits, nuts, and leaves may be a strategy to cope with this deficit, in addition to the capacity of colobine monkeys to get limited amounts of protein from intestinal bacteria during the process of proliferation accompanied with fermentation of celluloses (Kay and Davies 1994; Chivers 1994). Monkeys at Xiaochangdu show a strong preference for eating young leaves, flowers, fruits, and nuts (Xiang et al. 2007b). These items are only available in the forest understory from May to September (Xiang et al. 2007b). The results discussed above are consistent with the prediction that a proportional increase in fruits/leaves consumption will also increase both the travel requirement to find such fruits/leaves and the energy available to do so (Strier 2003).
Range size is typically influenced by a species’ body size, average group size, and population density (Clutton-Brock and Harvey 1979; Olupot et al. 1994; Butynski 1990). Primates that occupy the largest home ranges tend to have large bodies, large social groups, or both (Altmann 1974; Chapman et al. 1995; Janson and Goldsmith 1995; Gillespie and Chapman, 2001). Black-and-white snub-nosed monkeys are relatively massive, especially by colobine standards, and often live in social groups exceeding 100 individuals. The home range of the Xiaochangdu group is approximately 21.25 km2, consistent with other studies of R. bieti (Kirkpatrick et al. 1998; Liu et al. 2004; Huo 2005; Grueter et al. 2008; Ren et al. 2009) (Table 2).
Temperate primates are predicted to have larger home ranges than tropical primates due to increased environmental seasonality and the resulting patchiness of productive habitat (Bishop 1979; Takasaki 1981). This pattern has been observed in the odd-nosed colobines, a group which contains R. bieti (Kirkpatrick 1996). However, the species itself does not show any clear indications of latitudinal trends in range size. At Wuyapiya, about 100 km south of Xiaochangdu, the resident monkey group had a home range of 25 km2 (Kirkpatrick et al. 1998). Another group of >410 individuals living to the south had a home range of 25 km2 (Grueter et al. 2008), while a group of approximately 180 individuals occupied 23.3 km2 (Ren et al. 2009).
Food availability and consequent population density for leaf-eating monkeys tend to increase with the species richness of their habitat’s plant community. Within the range of black-and-white snub-nosed monkeys, plant diversity increases with decreasing latitude (Xiang et al. 2007b). Consequently, Xiaochangdu features the lowest botanical species richness of any recorded site, as well as the harshest weather conditions. However, results indicate that monkey groups occupying this site have greater population density and biomass than all southerly populations with the exception of Samage (Table 2). This could be attributed to more severe anthropogenic change in those areas, causing continual stress on monkey populations (Mt Longmashan: Huo 2005; Mt Fuhe: Liu et al. 2004). Xiaochangdu may support the only currently stable subpopulation of R. bieti (Xiang et al. 2007a), having reached local carrying capacity. On the other hand, other groups may experience a recovery over the near term, as conservation policies put into place during the 1990s have allowed for habitat recovery during the 2000s (Liu et al. 2004; Huo 2005).
Altitudinal Range
The monkeys range between elevations of 3,550 and 4,300 m, with a mean of 4,060 ± 105 m. Additionally, 92 % of all location records are distributed in a narrower altitude band, between 3,800 m and 4,200 m. The altitude of the monthly mean daily range did not vary significantly across the seasons (ANOVA, F = 2.22, p > 0.05). The results indicate that the monkeys do not generally prefer any particular altitudinal zone. However, we found that the monkeys can respond to snowstorms by vertical migration. From November 11 to December 8, 2004 the monkeys had remained between 4,100 and 4,300 m for about 2 weeks, with mean altitude 4,207, and then descended to about 3,800 m. The monkeys were observed searching for the fallen seeds of oak trees (Quercus aquifolioides) on the ground and feeding on lichens during this time. However, when a heavy snowstorm took place over 4 days in February 2005, the monkeys descended to 3,500 m, to lower altitude forests near the Lancang River, where they stayed for about 5 weeks.
In mountainous areas, ecological zones are observed to display altitudinal as well as climatic gradients (Wu 1991). As a result, primates living along these altitudinal gradients have been known to exhibit seasonal vertical migration, following the distribution of food (Bishop 1979; Hu et al. 1980; but see Kirkpatrick and Long 1994). Zhong et al. (2008) found, through analysis of R. bieti fecal samples collected along an elevational transect at Baimaxueshan North, that monkeys consistently roamed in higher altitudes but could travel to lower altitudes during spring to eat young sprouts and leaves and during winter to avoid bad snowstorms. Li et al (2008) observations at Baimaxueshan South are also consistent with this migration pattern. Yang (2003), Liu et al. (2004) found the use of lower elevations as a refuge from severe winter weather as well. However, except for severe snowstorms, we did not observe the same responsiveness to the environment in the monkeys’ vertical ranging behavior that we did in their daily range size and travel distance. It is possible that there is a lower bound to the group’s descent from the mountains because human occupation and disturbance become noticeable below 3,800 m, with 90 % of the home range at this altitude near a village. Measurement difficulty might also play a role—fine-grained distinctions in seasonal migration patterns could not be detected within a fairly narrow range of about 400 m generally pitched between 25 and 35o (Xiang ZF, unpublished data). We would expect the monkeys to range lower in the spring and summer in order to spend more time in the diverse secondary conifer forest habitat they seem to prefer (Xiang et al. 2011). However, human disturbance in the lower altitudes, particularly during good weather when animals roam free and villagers collect resources, may drive the monkeys back toward higher areas where people are less likely to go. Therefore, the monkeys’ altitudinal ranging pattern at Xiaochangdu is a site-specific example of an adaptive response to a particular habitat.
Habitat Preference
Black-and-white snub-nosed monkeys are thought to depend on the accessibility of primary alpine fir forest (Zhao et al. 1988; Li et al. 1981) in the northern part of their distribution. In contrast, Li et al. (2008) found a preference for mixed deciduous broadleaf and conifer forest in the center of the monkeys’ distribution. Further south, Huo (2005) has argued that the monkeys’ preferred forest type is mixed deciduous broadleaf forest. However, the monkeys at Xiaochangdu spent more time in primary and secondary conifer forest than expected, avoiding other habitat types (Xiang et al. 2011). Monkeys appeared to ignore deciduous broadleaf forest and alpine shrubs. In fact, I never observed the monkeys entering shrub forest even in those instances when it was nearby.
Lichen constitutes the bulk of R. bieti’s diet during winter, when other options are scarce (Grueter et al. 2009). However, when young leaves and fruits are available, these foods are obtained preferentially (Xiang et al. 2007b). Primary conifer forest, with its typically higher lichen cover, may provide a comparatively rich resource patch during the winter months. However, secondary conifer forest still offers enough lichens to sustain monkeys through the lean season (Xiang et al. 2011). During other seasons, these lightly disturbed forest areas could actually contain the highest quality food resources, due to greater species richness. From the perspective of survival over the entire year, having a mix of habitat types in their range may provide R. bieti with the best opportunity for survival (Xiang et al. 2011).
Reproduction and Response to Changing Environment
Mating behavior was difficult to observe due to the dense tree and bush cover limiting visibility within the habitat. I was able to record the occurrence of only 10 mounts with intromission and pelvic thrusting between July and October, of which there were 8 ejaculatory mounts. In the seven instances in which the solicitor of copulation could be identified, it was a female in six instances compared to only one instance for a male. One mating event with ejaculation was observed in August at 1,625 h, and another without ejaculation in October at 1,100 h. Births were synchronized across the group, with newborns appearing only during February and March (Fig 2). The birth season of R. bieti at Xiaochangdu fits the pulse model, with a standard deviation (SD) of 6.5 days (Xiang and Sayers 2009). However, the timing of the birth season is earlier than that of two other wild populations, Wuyapiya (Kirkpatrick et al. 1998) and Mt. Longma (Huo 2005). Seasonal birthing may be an adaptation to harsh environmental conditions (day length, ambient temperature, food supply, nutritional state, or a combination thereof). Winter, which generally arrives earlier at higher than lower latitudes, is the leanest season for R. bieti (Xiang et al. 2007b). Low resource availability can affect the survival of infants directly and indirectly, via the nutritional state of the lactating mother. If infants have not reached a given threshold of development before the onset of winter, they may be unlikely to survive on the limited, nutrition-poor foods available during that time. Therefore, birth timing appears to be a strategy to maximize offspring survival, as reported previously for captive golden snub-nosed monkeys (Rhinopithecus roxellana) (Zhang et al. 2000) and Japanese macaques (Macaca fuscata) (Cozzolin et al. 1992).
Birth distribution of black-and-white snub-nosed monkeys (Rhinopithecus bieti at Xiaochangdu data are calculated from the observations from 25 January to 9 April, 2005). The mean birth date, median birth date, and SD are shown on the bar above the histogram. Each period code corresponds to 7 days after 4 February, 2005
Conservation Implications
The Xiaochangdu population of R. bieti has enjoyed greater protection from anthropogenic disturbance than snub-nosed monkeys at other sites for both cultural and practical reasons. Villagers in the areas surrounding monkey habitat within the reserve practice Tibetan Buddhism. If a Living Buddha (a senior Tibetan monk) declares a mountain to be sacred, the killing of animal life on the mountain becomes taboo. Honglaxueshan’s status as one such sacred mountain contributes to its suitability as a refuge for monkeys and other wildlife living there. Another factor is that qingke, or slash-and-burn agriculture, is largely unproductive at altitudes above 3,800 m. This practice, which has degraded a multitude of primate habitats both within China (Zhao 1996; Xiang et al. 2004) and elsewhere (Vargas et al. 2002), does not occur within this reserve (Xiang et al. 2007a). Lastly, the composition of the floral community at Xiaochangdu appears to have a mediating effect on human disturbance. Despite a reduction in canopy cover by approximately 35 % as a result of logging, forest structure as measured by height and density of trees exceeding ≥33 cm circumference at breast height is relatively unchanged (Xiang et al. 2011). Selective logging by locals who take oak trees (Quercus aquifoliodes) for firewood and fir trees for construction has been going on for at least 50 years (Jiang Q, pers. comm.). At subsistence levels, selective logging may increase species diversity in localized patches by reducing the density of the most competitively dominant species. This may allow other species which the monkeys find desirable as food to grow more readily within the disturbed areas. Xiaochangdu may be a rare case in which certain anthropogenic disturbances actually benefit the monkey group.
References
Altman, J. (1974). Observational study of behavior: Sampling methods. Behaviour, 49, 227–267.
Bennett, L. E., & Davies, A. G. (1994). The ecology of Asian colobines. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour, and evolution (pp. 129–171). Cambridge: Cambridge University Press.
Bishop, N. H. (1979). Himalayan langurs: Temperate colobines. Journal Human Evolution, 8, 251–281.
Butynski, T. M. (1990). Comparative ecology of blue monkeys (Cercopithecus mitis) in high- and low-density subpopulation. Ecology Monography, 60, 1–26.
Chapman, C. A. (1988). Patterns of foraging and range use by three species of neotropical primate. Primates, 29, 177–194.
Chapman, C. A., & Chapman, L. J. (1990). Dietary variability in primate populations. Primates, 31(1), 121–128.
Chapman, C. A., Wrangham, R. W., & Chapman, L. J. (1995). Ecological constraints on group size: An analysis of spider monkey and chimpanzee subgroups. Behavior Ecology and Sociobiology, 36(1), 59–70.
Chivers, D. J. (1994). Functional anatomy of the gastrointestinal tract. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour, and evolution (pp. 205–227). Cambridge: Cambridge University Press.
Clutton-Brock, T. H., & Harvey, P. H. (1979). Home range size, population density and phylogeny in primates. In I. S. Bernstein & E. O. Smith (Eds.), Primate ecology and human origins: ecological influences on social organization (pp. 201–214). New York: Garland STPM Press.
Cozzolino, R., Cordischi, C., Aureli, F., & Scucchi, S. (1992). Environmental temperature and reproductive seasonality in Japanese macaques (Macaca fuscata). Primates, 33, 329–336.
Cui, L. W., & Xiao, W. (2004). Sexual behavior in one-male unit of Rhinopithecus bieti in captivity. Zoo Biology, 19, 11–25.
Ding, W., & Zhao, Q. K. (2004). Rhinopithecus bieti at Tacheng, Yunan: Diet and daytimes activities. Internitional Journal Primatology, 25, 583–598.
Fernadez-Duque, E., Rotundo, M., & Ramirez-Llorens, P. (2002). Enivironmental determinants of birth seasonality in night monkeys of the Argentinean Chaco. International Journal of Primatology, 23, 639–656.
Gillespie, T. R., & Chapman, C. A. (2001). Determinants of group size in the red colobus monkey (Procolobus badius): An evaluation of the generality of the ecological-constraints model. Behavior Ecology and Sociobiology, 50, 329–338.
Grueter, C. C., Li, D. Y., van Schaik, C. P., Ren, B. P., Long, Y. C., & Wei, F. W. (2008). Ranging of Rhinopithecus bieti in the Samage Forest, China. I. Characteristics of range use. International Journal of Primatology, 29, 1121–1145.
Grueter, C. C., Li, D. Y., Ren, B. P., Wei, F. W., & van Schaik, C. P. (2009). Dietary profile of Rhinopithecus bieti and its socioecological implications. International Journal of Primatology, 30, 601–624.
Hu, J. C., Deng, Q. X., Yu, Z. W., Zhou, S. D., & Tian, Z. X. (1980). Research on the ecology and biology of the giant panda, golden monkey, and other rare animals. Journal Nanchong Teacher’s College, 2, 1–39.
Huo, S. (2005). Diet and habitat use of Rhinopithecus bieti at Mt Longma, Yunnan, and phylogeny of the family Viverridae in China. PhD Dissertation of Chinese Academy of Sciences, Kunming Institute of Zoology, Kunming.
Janson, C. H., & Goldsmith, M. L. (1995). Predicting group size in primates: Foraging costs and predation risks. Behavior Ecology, 6, 326–336.
Kay, R. N. B., & Davies, A. G. (1994). Digestive physiology. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour, and evolution (pp. 229–250). Cambridge: Cambridge University Press.
Kirkpatrick, R. C. (1996). Ecology and behavior of the Yunnan Snub-nosed langur (Rhinopithecus bieti, Colobinae). PhD dissertation, University of California, Davis.
Kirkpatrick, R. C., & Long, Y. C. (1994). Altitudinal ranging and terrestriality in the Yunnan snub-nosed monkeys (Rhinopithecus bieti). Folia Primatologica, 63, 102–106.
Kirkpatrick, R. C., Long, Y. C., Zhong, T., & Xiao, L. (1998). Social organization and range use in the Yunnan snub-nosed monkey Rhinopithecus bieti. International Journal of Primatology, 19, 13–51.
Li, Z. X., Ma, S. L., Hu, C. H., & Wang, Y. X. (1981). The distribution and habit of Yunnan Snub-nosed monkey. Zoological Reserach, 2, 9–16.
Li, D. Y., Grueter, C. C., Ren, B. P., Long, Y. C., Li, M., Peng, Z. S., et al. (2008). Ranging of Rhinopithecus bieti in the Samage Forest, China. II. Use of land cover types and altitudes. International Journal of Primatology, 29, 1147–1173.
Liu, Z. H., Ding, W., & Grueter, C. C. (2004). Seasonal variation in ranging patterns of Yunnan snub-nosed monkeys Rhinopithecus bieti at Mt. Fuhe, China. Acta Zoologica Sinica, 50, 691–696.
Long, Y. C., Kirkpatrick, R. C., Zhong, T., & Xiao, L. (1994). Report on the distribution, population, and ecology of the Yunnan snub-nosed monkey Rhinopithecus bieti. Primates, 35, 241–250.
Marsh, C. W. (1981). Ranging behaviour and its relation to diet selection in Tana River red colobus (Colobus badius rufomitratus). Journal Zoology (London), 195, 473–492.
Milton, K. (1980). The foraging strategy of howler monkeys: A study in primate economics. New York: Columbia University Press.
Norberg, P. A. (1977). An ecological theory on foraging time and energetics and costs of optimal food searching method. Journal Animal Ecology, 46, 511–529.
Oates, J. F. (1987). Food distribution and foraging behavior. In B. B. Smuts, D. L. Cheney, R. M. Seyfarth, R. W. Wrangham, & T. T. Struhsaker (Eds.), Primate societies (pp. 197–209). Chicago: University of Chicago Press.
Olupot, W., Chapman, C. A., Brown, C. H., & Waser, P. M. (1994). Mangabey (Cercocebus albigena) population density, group size, and ranging: A 20 year comparison. American Journal Primatology, 32, 197–205.
Ren, B. P., Li, M., Long, Y. C., & Wei, F. W. (2009). Influence of day length, ambient temperature, and seasonality on daily travel distance in the Yunnan snub-nosed monkey at Jinsichang, Yunnan, China. American Journal Primatology, 71, 233–241.
Smith, A. T., & Xie, Y. (2009). A guide to the Mammals of China. Hunan, Changsha: Hunan Education Published Housed.
Strier, K. B. (2003). Food, foraging and females. Boston Allyn and Bacon: Primates behavioural ecology.
Takasaki, H. (1981). Troop size, habitat quality, and home range area in Japanese macaques. Behavior Ecology Sociobiology, 9, 277–281.
Vargas, A., Jiméneza, J., Palomates, F., & Palacios, M. J. (2002). Distribution, status and conservation needs of the golden-crowned sifaka (Propithecus tattaersalli). Biological Conservation, 108, 325–334.
Wu, Z. Y. (1991). The areal-types of Chinese genera of seed plants. Acta Botanica Yunnanica, (4), 1–139.
Xiang, Z. F. (2005). The ecology and behavior of black-and-white snub-nosed monkeys (Rhinopithecus bieti, Colobinae) at Xiaochangdu in Honglaxueshan National Nature Reserve, Tibet, China. PhD Dissertation of Chinese Academy of Sciences, Kunming Institute of Zoology, Kunming.
Xiang, Z. F., & Sayers, K. (2009). Reports on seasonality of mating and birth events in wild black-and-white snub-nosed monkeys (Rhinopithecus bieti) at Xiaochangdu, Tibet. Primates, 50, 50–55.
Xiang, Z. F., Huo, S., Ma, X. F., & Ma, S. L. (2004). Status and threat factors of non-human primates at Mt Huanglian area, Yunnan, China. Chinese Journal of Ecology, 23, 168–171. (in Chinese with an English abstract).
Xiang, Z. F., Huo, S., Xiao, W., Quan, R. C., & Grueter, C. C. (2007a). Diet and feeding behavior of Rhinopithecus bieti at Xiaochangdu, Tibet: Adaptations to a marginal environment. American Journal of Primatology, 69, 1141–1158.
Xiang, Z. F., Huo, S., Wang, L., Cui, L. W., Xiao, W., & Zhong, T. (2007b). Distribution, status and conservation strategies of the black-and-white snub-nosed monkey Rhinopithecus bieti in Tibet. Oryx, 41, 525–531.
Xiang, Z. F., Huo, S., & Xiao, W. (2011). Habitat selection of black-and-white snub-nosed monkeys (Rhinopithecus bieti) in Tibet: Implications for species conservation. American Journal of Primatology, 73, 347–355.
Xiao, W., Ding, W., Cui, L. W., Zhou, R. L., & Zhao, Q. K. (2003). Habitat degradation of Rhinopithecus bieti in Yunnan, China. International Journal Primatology, 24, 389–398.
Yang, S. J. (2003). Altitudinal ranging of Rhinopithecus bieti at Jinsichang, Lijiang, China. Folia Primatology, 74, 88–91.
Yang, S. J., & Zhao, Q. K. (2001). Bamboo leaf-based diet of Rhinopithecus bieti at Lijiang, China. Folia Primatology, 72, 92–95.
Zhang, S. Y., Liang, B., & Wang, L. X. (2000). Seasonality of matings and births in captive Sichuan Golden Monkeys (Rhinopithecus roxellana). American Journal of Primatology, 51, 265–269.
Zhao, Q. K. (1996). Ecological information on statistics of human population and agriculture in Hengduan Mountains from Yunnan. Chinese Biodiversity, 4, 217–221. (in Chinese with an English abstract).
Zhao, Q. K., He, S. J., Wu, B. Q., & Nash, L. T. (1988). Excrement distribution and habitat use in Rhinopithecus bieti in winter. American Journal of Primatology, 16, 275–284.
Zhong, T., Xiao, L., Huo, S., Xiang, Z. F., Xiao, W., & Cui, L. W. (2008). Altitudinal range of black-and-white Snub-nosed Monkeys (Rhinopithecus bieti) at Baima Snow Mountain, China. Zoological Research, 29, 181–188.
Zou, R. J., Yang, S. C., & Ji, W. Z. (1999). Infant development and growth of the Yunnan snub-nosed monkey (Rhinopithecus bieti). Sichuan Journal Zoology, 18, 12–14.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31071937), the Project of Public Benefit for Forestry (201104073), and the State Forestry Administration of China. We thank Ms. Alicia Krzton for useful suggestions and editing of the English manuscript, and the staff at the administrative bureau of Honglaxueshan National Nature Reserve of Mangkang County in Tibet for their support. Without the help of our field assistants, Mr. Ding Z, Ci R, Zhu J, Wang Z, A’Nan, and Deng P, it would have been impossible to complete the fieldwork.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Xiang, Z. (2014). Rhinopithecus bieti at Xiaochangdu, Tibet: Adaptations to a Marginal Environment. In: Grow, N., Gursky-Doyen, S., Krzton, A. (eds) High Altitude Primates. Developments in Primatology: Progress and Prospects. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8175-1_10
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8175-1_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8174-4
Online ISBN: 978-1-4614-8175-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)