Abstract
Chromosomal abnormalities are relevant causes of human infertility, affecting 2 –14 % of infertile males. Patients with seminal anomalies could be affected by improper meiotic recombination and increased sperm chromosome aneuploidy. Since the transmission of a haploid chromosomal asset is fundamental for embryo vitality and development, the study of sperm chromosomes has become fundamental because intracytoplasmic sperm injection allows fertilization in cases of severe male infertility.
In this chapter we summarize the data on the incidence of sperm aneuploidy, detected by fluorescence in situ hybridization (FISH), in infertile men with normal or abnormal karyotype. The possibility of reducing sperm chromosomal imbalance is also reported.
Among control males, the lowest aneuploidy rate was detected (range: 0.09 –0.14 % for autosomes; 0.04 –0.10 % for gonosomes). In infertile patients with normal karyotype, the severity of semen alteration is correlated with the frequency of aneuploidy, particularly for X and Y chromosomes. Among patients with abnormal karyotype, 47,XXY and 47,XYY carriers showed a high variability of sperm aneuploidy both for gonosomes and autosomes. In Robertsonian translocation carriers, the increase in aneuploidy rate was particularly evident for total sex disomy, and resulted mainly from interchromosomal effect (ICE). In reciprocal translocation carriers, a high percentage of unbalanced sperm (approximately 50 %) was detected, perhaps mostly related to ICE.
Sperm chromosomal constitution could be analyzed to obtain more accurate information about the causes of male infertility. It would be worthwhile to evaluate the benefits of a therapy with recombinant Follicle Stimulating Hormone (rFSH) on sperm chromosome segregation in selected infertile males.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Chromosomal abnormalities are a relevant cause of human infertility, affecting 2–14 % of infertile males and have been clearly demonstrated to increase proportionally with the severity of the spermatological phenotype. Both numerical and structural chromosomal aberrations are major contributors to pregnancy loss (Egozcue et al. 2000a), perinatal death, congenital malformations, mental retardation, and behavioral anomalies (Hook 1985; Hecht and Hecht 1987), the latter accounting for the 0.8–1 % of live births (Gardner and Sutherland 2004).
Among the abnormalities, trisomy of chromosomes 13, 18, and 21 and aneuploidies of the sex chromosomes constitute the most important load of congenital abnormalities. In most cases, autosomal trisomies originate in maternal germ cells, whereas sex chromosome aneuploidies are frequently of paternal origin, occurring during spermatogenesis (Sloter et al. 2004).
The term spermatogenesis indicates the processes by which primordial germ cells, namely spermatogonia, become haploid sperm cells. Spermatogonia divide by mitosis, giving rise to primary spermatocytes, which undergo a meiotic process. Meiosis includes two successive cell divisions without DNA replication. During the first and second meiotic divisions, homologous chromosomes separate to form haploid gametes. At the end of spermatogenesis, the haploid spermatid nucleus contains 23 chromosomes with one chromatid. Soon after, they are transformed into spermatozoa by a morphogenetic process, spermiogenesis. Recently, several lines of evidence have linked unexplained male infertility to meiotic defects in pairing, synapsis, and recombination and to an increase in aneuploid sperm (Tempest and Martin 2009).
Errors during mitotic or meiotic divisions may lead to aneuploid gametes, in which autosomes or the sex chromosomes are affected. Aneuploidy, the most frequently detected cytogenetic abnormality, is defined as the condition of having fewer or more than the euploid number of chromosomes. Aneuploidies in male gametes may be caused by two main mechanisms: (1) nondisjunction of chromatid pairs during mitosis or meiosis II or nondisjunction of homologous chromosomes during meiosis I; (2) chromosome lagging near the equator at anaphase followed by chromosome loss (Ford et al. 1988).
The incidence of sperm aneuploidy increases proportionally with the severity of the male-factor sterility, including Y chromosome microdeletions, as confirmed by various studies suggesting that, in selected cases, the paternal contribution to aneuploidy in the developing conceptus could be more relevant than expected from general data on aborted fetuses and live births (Gianaroli et al. 2005; Magli et al. 2009; Mateu et al. 2010; Kahraman et al. 2006; Harton and Helen 2012).
This is particularly true in cases of assisted reproductive techniques, such as intracytoplasmic sperm injection (ICSI), that have improved the chances of achieving pregnancy also for patients with severe seminal anomalies (Van Steirteghem et al. 1993, 1996), despite an increased incidence of embryo aneuploidies (Verpoest and Tournaye 2006; Tesarik and Mendoza 2007). In particular, it appears that men with severe factor infertility treated by ICSI have an increased risk of generating offspring with unbalanced chromosomal constitution (Rimm et al. 2004; Wen et al. 2012).
The clinical use of aneuploidy screening should be recommended in patients affected by Klinefelter syndrome, structural rearrangement of karyotype, severe teratozoospermia, nonobstructive azoospermia, as well as in patients with recurrent pregnancy loss or unexplained repeated in vitro fertilization (IVF) failures.
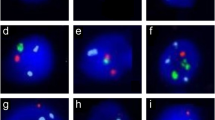
The development of the FISH technique, which uses a chromosome-specific DNA probe detected by fluorescence microscopy, and its application to the study of sperm aneuploidy has made possible the screening of a large number of germ cells in a relatively short time. In addition, the simultaneous use of different probes allows for the evaluation of aneuploidy frequency for different human chromosomes in normal (Fig. 3.1) and pathological conditions (Figs. 3.2 and 3.3) (Egozcue et al. 1997; Downie et al. 1997; Guttenbach et al. 1997a; Rives et al. 1999; Carrel 2008; McLachlan and O’Bryan 2010). Since 1990, this technique has been used to analyze chromosome aneuploidies in sperm, and many papers have been published on sperm aneuploidies even in control individuals (Downie et al. 1997; Guttenbach et al. 1997a; Egozcue et al. 1997; Rives et al. 1999). Nevertheless, FISH has its limits due, for example, to the degree of chromatin condensation since condensation efficiency is directly correlated with fluorescent signal quality (Vidal et al. 1993; Egozcue et al. 1997). Moreover, FISH does not enable one to appreciate the difference between nullisomy (the absence of a chromosome) and the absence of hybridization, despite the fact that if one of the probes gives a correct signal, the absence of a signal from the other probe may be considered evidence of nullisomy (Holmes and Martin 1993; Bischoff et al. 1994). Furthermore, FISH on decondensed sperm head only provides information about anomalies in the chromosome number, while the analysis of chromosome structural rearrangement is still complex due to the use of locus-specific probes not always able to hybridize. In these latter cases, sperm karyotyping, single-sperm polymerase chain reaction, or single-sperm typing should be applied to improve the identification of recombination in specific genome areas (Martin 2008).
Sperm karyotyping through a fusion assay was originally used, with the drawback of the technique being that it is laborious, technically difficult, and only allows for studying sperm that are able to fuse with a hamster oocyte, yielding results on a relatively small number of cells. The advent of FISH revolutionized the study of sperm chromosome constitution, mitigating many of these problems, except that it no longer allowed the whole chromosome complement to be studied in a single cell or structural aberrations to be picked up without the use of specially designed probes.
Nevertheless, not all 24 chromosomes (22 autosomes plus X and Y chromosomes) can be easily assessed in a single cell, and a limited number of fluorocromes are available; therefore 3–5 signals at most are technically feasible.
FISH Analysis in Normozoospermic Males as Reference Value for Aneuploidy Frequency
Sperm chromosomal aneuploidies have been investigated by FISH in normal donors by various authors. Unfortunately, it is not always easy to compare the results of these studies and identify a reference value, mainly because of the differences in the frequency and distribution of aneuploidies. This heterogeneity can be ascribed to differences in application of the technique, such as sperm decondensation, scoring criteria, storage of samples, types of probe, number of sperm scored, or data analysis and reporting and donor selection criteria (e.g., normozoospermic, normozoospermic and fertile, fertile, or healthy men).
To provide some useful information on this topic, we compared data from 19 different studies selected on the basis of donor characteristics and techniques (Gambera et al. 2011). According to donor selection criteria, we distinguished three subgroups: normozoospermic – a total of 55 donors with reported normal semen parameters according to the World Health Organization (WHO) (1999, 2010) or Kruger morphology (1986) criteria but without information about fertility; normozoospermic and fertile – including 57 normozoospermic donors of proven fertility; fertile – including 37 donors with proven fertility but without information about semen parameters. In these studies sperm aneuploidies were evaluated using specific probes for all chromosomes, with the most investigated being 13, 18, 21, X, and Y chromosomes mainly because aneuploidies of these chromosomes do not preclude embryo development and survival. Overall information about sperm chromosome quantitative alteration was given as the total frequency of aneuploidies. The weighted mean of autosomal disomy for each chromosome ranged from a minimum of 0.05 % to a maximum that varied somewhat in the different groups: 0.14 ± 0.06 % in normozoospermic and fertile, 0.23 ± 0.08 % in the normozoospermic group, and 0.09 ± 0.09 % in fertile (Table 3.1).
Disomies for chromosomes 13, 18, and 21 have been extensively studied and provide a statistically significant background for the interpretation of results. The disomy rate for each chromosome varied only slightly in the three groups and between groups. However, chromosome 21 disomy had a significantly higher incidence in the normozoospermic group, suggesting greater susceptibility to nondisjunction for this chromosome.
Sex chromosome disomies were also investigated, and XY disomy frequency increased more than twice in comparison with XX and YY disomies. Therefore, it seems that errors in meiosis I, giving rise to sperm with XY chromosomes, should be more frequent than errors in meiosis II, which generate X or Y disomic sperm (Fig. 3.2). On the other hand, it must be considered that errors in meiosis I give rise to two disomic and two nullisomic sperm, while errors in meiosis II may produce a disomic sperm and a nullisomic one. The diploidy rate, evaluated by three fluorescent probes, one for autosomes and two for sex chromosomes, was not statistically different among the three groups.
Few extensive studies have reported high aneuploidy frequencies for chromosomes 14, 21, 22, X, and Y (Shi and Martin 2000a; Templado et al. 2005). A review of the available literature revealed only the disomy frequency of 21, X, and Y in reference subjects increased. This finding could be explained by the hypothesis that chromosomes 21, X, and Y may be prone to recombination reduction or failure during meiosis (Shen et al. 1998) because they have a single chiasmata (Sun et al. 2004), which increases the probability of incorrect segregation during meiosis I (Koehler et al. 1996; Templado et al. 2005).
A lower incidence of the mean of total aneuploidies in male normozoospermic and fertile subjects (0.50 ± 0.25 %) is not unexpected since normozoospermia alone does not necessarily indicate fully fertile status. Moreover, it is well known that donor age and lifestyle, including aspects such as smoking, alcohol consumption, and exposure to toxic substances, can affect semen quality. Therefore, the sperm chromosomal aneuploidy rate probably varies significantly in time; in addition, the fertile status cannot be considered constant throughout a person’s life, according to several published studies on the effects of age and environmental factors on sperm aneuploidy rate (Bosch et al. 2001, 2003; Naccarati et al. 2003; Templado et al. 2011b).
FISH Analysis in Men with Alterations of Sperm Parameters
Nearly all studies investigating sperm aneuploidy in infertile men have demonstrated a significant increase in aneuploidy levels compared to their fertile counterparts (Templado et al. 2005; Sarrate et al. 2005; Miharu 2005). The majority of studies report around a threefold increase in the sperm aneuploidy rate in infertile men.
Increases in sperm aneuploidy are strongly correlated with an increasing severity of infertility: the highest level of aneuploidy was reported in men with severe oligoasthenoteratozoospermia and in cases of nonobstructive azoospermia where sperm are retrieved from testicular tissue (Vidal et al. 2001; Egozcue et al. 2003; Miharu 2005).
Furthermore, FISH data have been reported for all seminal phenotypes, including oligozoospermia (low concentration), asthenozoospermia (poor motility), and teratozoospermia (poor morphology) in infertile males with normal karyotype (Bernardini et al. 1997, 1998; Lahdetie et al. 1997; McInnes et al. 1998; Storeng et al. 1998; Pang et al. 1999; Ushijima et al. 2000; Vegetti et al. 2000; Calogero et al. 2001a; Shi and Martin 2001; Vidal et al. 2001; Egozcue et al. 2003; Rives et al. 2004; Miharu 2005; Sarrate et al. 2005; Templado et al. 2005).
In particular, a negative correlation has been reported between sperm aneuploidy rate and progressive motility (Ushijima et al. 2000; Vegetti et al. 2000; Celik-Ozenki et al. 2004; Collodel et al. 2007), normal morphology (Bernardini et al. 1998; Calogero et al. 2001a; Ryu et al. 2001; Lewis-Jones et al. 2003; Carrell et al. 2004), and nuclear maturity (Kovanci et al. 2001).
Nevertheless, seminal alterations such as oligozoospermia, asthenozoospermia, and teratozoospermia are often detected simultaneously in the same ejaculate because a damaged seminiferous epithelium produces fewer sperm, generally with abnormal morphology and, therefore, with decreased motility, as in oligoasthenoteratozoospermic (OAT) patients. A high percentage of aneuploid sperm could be produced as a result of the negative influence of any testicular pathology on spermatogenetic processes (Martin et al. 1993; Bischoff et al. 1994; Spriggs et al. 1995; Calogero et al. 2001a).
Among sperm alterations, reduced sperm concentration is reported to be the most strongly associated with chromosomal aneuploidies (Ohashi et al. 2001; Martin et al. 2003a; Nagvenkar et al. 2005) because severe quantitative impairment of spermatogenesis has been related to qualitative alterations of chromosome recombination and segregation during spermatogenesis (Egozcue et al. 2005; Miharu 2005; Sarrate et al. 2005).
Among chromosomes, gonosomes are the most susceptible to nondisjunction because X and Y are generally involved in only one crossover in the pseudoautosomal region, and thus if this process remains incomplete, normal disjunction does not occur.
Among autosomes, disomies of chromosomes 13, 18, and 21 were found to increase in OAT patients with a frequency of more than 3 times higher in comparison with normozoospermic and fertile controls (Table 3.1). Sex chromosomes were particularly affected by OAT condition: the mean disomy rates resulted in significantly increases in all gonosomes and mainly for XY disomy, which was approximately eight times higher than in normozoospermic and fertile controls (Table 3.1).
Taken together, all these data suggest that men with impaired spermatogenesis should have reduced genome-wide recombination leading to chromosome-specific sperm defects.
Sperm morphology is considered one of the main criteria for sperm selection before an assisted reproductive procedure. Lee et al. (1996) analyzed the chromosomal constitution of human sperm injected into mouse oocytes. Sperm with abnormal head morphology showed a frequency of structural chromosomal aberrations approximately four times higher than those with normal morphology. The statement that teratozoospermic patients have an aneuploidy rate significantly higher than controls has been confirmed by several authors using multicolor FISH analysis: the frequency of chromosome 18 disomy was approximately eight times greater than in normozoospermic and fertile controls (Table 3.1). Teratozoospermic samples also showed a significant increase in the frequency of disomy for sex chromosomes: some morphological abnormalities may be more closely associated with chromosome imbalance, particularly those involving the sperm head (Sun et al. 2006).
When a high percentage of macrocephalic, multinucleate, and multiflagellate sperm are detected, autosome and gonosome frequencies show an approximately tenfold increase, as reported by various authors (Table 3.1). Globozoospermia, a peculiar sperm head alteration of genetic origin, seems to be strictly associated with a higher incidence of sperm aneuploidies (Carrell et al. 1999, 2001; Moretti et al. 2005).
While a consensus exists on the role played by severe oligozoospermia and teratozoospermia on sperm aneuploidy and diploidy, whether isolated asthenozoospermia affects sperm aneuploidy is less clear. It is often difficult to group data from asthenozoospermic samples into separate categories due to the concomitant alteration of other sperm parameters, such as concentration and morphology.
Isolated asthenozoospermia has been reported by few authors (Aran et al. 1999; Bernardini et al. 2005; Collodel et al. 2007) analyzing a range of autosomes (1, 4, 8, 12, 13, 18, and 21) and sex chromosomes: on the whole, increased sperm disomy and diploidy rates were detected with respect to the controls.
In other cases of absolute asthenozoospermia characterized by systematic sperm anomalies of the flagella, such as stump tail syndrome and Kartagener syndrome, abnormal aneuploidy and diploidy rates were confirmed by different authors (Rives et al. 2005). In the case of fibrous sheath dysplasia, some studies reported that the mean frequency of diploidy (0.43 ± 0.23 %) and sex chromosome aneuploidies increased sharply in comparison to controls group (Baccetti et al. 2005; Moretti and Collodel 2006; Piomboni et al. 2007).
Sperm Aneuploidy in Infertile Male ICSI Candidates to ICSI
The advent of ICSI (Palermo et al. 1992) revolutionized the treatment of male infertility by allowing patients with severely compromised semen parameters to achieve fatherhood. Although sperm with the “best” morphological features are selected for injection into the oocyte, this is not an absolute indicator of a normal genetic constitution (Ryu et al. 2001; Burrello et al. 2004; Celik-Ozenci et al. 2004), and the transmission of chromosomal abnormalities to offspring is possible.
Various researches have shown that prenatal karyotypes of embryos obtained by ICSI have higher sex chromosome aneuploidy rates (0.6 % versus 0.2 %) and higher autosomal structural alterations (0.4 % versus 0.07 %) than the general neonatal population (Veld et al. 1997; Bonduelle et al. 1998, 2002; Van Steirteghem et al. 2002). Several clinical studies suggested a strong correlation between the aneuploidy rate of male gametes and ICSI outcome: implantation failure, decreased pregnancy, and increased miscarriage rates after ICSI have been reported in OAT male when FISH analysis demonstrated abnormal aneuploidy and diploidy frequencies.
Few studies published so far have found an effect of sperm aneuploidies on the outcome of ICSI (Colombero et al. 1999; Calogero et al. 2001b) and reported comparable fertilization rates, clinical pregnancy rates, pregnancy losses, and occurrence of neonatal malformations in males with both normal and abnormal semen parameters. However, these authors concluded that, although the overall ICSI outcome was not significantly correlated with sperm aneuploidy, a tendency to a lower aneuploidy rate was underlined in the male partner of pregnant women.
The evaluation of the influence of chromosome abnormalities in men with altered semen parameters undergoing an Assisted Reproductive Technologies (ART) procedure could be biased by semen selection methods. Many reports consistently found an increase in aneuploidy rates in subfertile men, underlining that conventional sperm separation techniques are not able to exclude aneuploid gametes from fertilizing pools (Samura et al. 1997; Pfeffer et al. 1999; Van Dyk et al. 2000). More recently, a selection technique based on hyaluronic acid (HA)–sperm binding was demonstrated as being able to recover a high percentage of euploid sperm: the advantages of HA-mediated sperm selection in relation to ICSI outcome improvement could be due to the decreasing frequency of chromosomal disomy and diploidy, which results in a four- to sixfold reduction in comparison with whole semen samples (Jakab et al. 2005; Huszar et al. 2007).
With regard to clinical practice, sperm aneuploidy screening may be recommended especially in those countries, such as Italy, where preimplantation diagnosis can be performed only in selected cases and therefore FISH on male gametes seems to be the only possible technique for determining the risk of generating unbalanced embryos.
ICSI with Testicular or Epididymal Sperm
Sperm extracted from the epididymis (MESA) or testicular tissue (TESA/TESE) have a substantially increased risk of chromosomal abnormalities, and therefore FISH investigation appears even more suitable. A high aneuploidy rate in testicular sperm recovered from nonobstructive azoospermic (NOA) patients has been widely reported (Bernardini et al. 2000; Levron et al. 2001; Burrello et al. 2002; Mateizel et al. 2002; Palermo et al. 2002; Rodrigo et al. 2004; Gianaroli et al. 2005; Vozdova et al 2012). These data have not been confirmed by Martin et al. (2000), who analyzed aneuploidy frequencies for chromosomes 13, 21, X, and Y in sperm from three men with nonobstructive azoospermia. The authors concluded that NOA patients may not have a substantially increased risk of chromosomally abnormal sperm, in comparison to healthy men. Nevertheless, in these cases it could be take into consideration that only a small number of testicular sperm are available for FISH analysis, and this could affect the accuracy of the estimated aneuploidy rate.
A higher incidence of chromosomal anomalies in epididymal than in ejaculated sperm has also been reported (Bernardini et al. 2000; Burrello et al. 2002; Palermo et al. 2002; Levron et al. 2001; Rodrigo et al. 2004).
We therefore can conclude that chromosomal abnormalities affect the ICSI outcome when sperm are obtained by MESA and TESE, decreasing the fertilization and pregnancy rates and increasing the miscarriage rate.
On the whole, embryos originated by azoospermic patients have an increased rate of chromosomal abnormalities, and therefore appropriate genetic counseling should be offered before ICSI.
Sperm Aneuploidy in Infertile Male Carriers of Chromosomal Alterations
The incidence of constitutional chromosomal abnormalities is approximately 2 % in males with combined indications of infertility (Meschede et al. 1997), 5 % in oligozoospermic, and 14 % in azoospermic men (Johnson 1998). The most common karyotype abnormalities in infertile men include numerical sex chromosome alterations and Robertsonian translocations (Shi and Martin 2001).
Numerical Sex Chromosome Abnormalities
47,XYY
The extra Y chromosome in 47,XYY males may arise by at least two mechanisms: paternal nondisjunction at meiosis II after normal chiasmata in meiosis I (84 %) or postzygotic mitotic error (16 %) (Robinson and Jacobs 1999; Rives et al. 2003a). Males with an extra Y chromosome are generally fertile, and meiotic studies carried out in these patients indicated that the extra Y chromosome is frequently lost during the premeiotic stages (Thompson et al. 1967; Chandley et al. 1976). Nevertheless, in some cases one X and two Y chromosomes have been detected during prophase I as an X univalent plus a YY bivalent (Hulten and Pearson 1971; Speed et al. 1991; Blanco et al. 1997). No increase in the frequency of any category of sex chromosomal aneuploidy was found in 47,XYY patients by Han et al. (1994). In contrast, several authors (Martini et al. 1996; Mercier et al. 1996; Morel et al. 1999; Lim et al. 1999a; Martin et al. 1999; Giltay et al. 2000; Wang et al. 2000; Moretti et al. 2007) reported a moderate increase in sex chromosome disomies.
Globally, the frequencies of sperm with an abnormal number of sex chromosomes range from 0.04% to 19 % depending on the study: the mean XY disomy rate increased sharply (4.43 ± 6.03 %) as shown by an evaluation of comparable data from different studies (Table 3.2). The general finding is that the persistence of an extra chromosome in germ cells of 47,XYY males can impair spermatogenesis, determining a low sperm count. Since most children of 47,XYY fathers have a normal karyotype, the extra Y chromosome may presumably be lost during meiosis (Shi and Martin 2000b, 2001). Nevertheless some XYY germ cells can complete meiosis and produce mature aneuploid sperm.
Recent review studies (Sarrate et al. 2005; Rodrigo et al. 2010) indicated that 3.7 % of the spermatozoa analyzed by FISH carry an extra sex chromosome and that diploid sperm ranges from 0 to 3.35 %.
On the other hand, males with a mosaic 47,XYY/46,XY showed a lower cumulative rate of sex chromosome aneuploidy in sperm than XYY patients. The mean gonosome disomy resulting from comparable data reported in the literature ranged from 0.17 ± 0.16 % for XX to 0.48 ± 0.31 % for XY (Table 3.2). As regards the risk assessment of the transmission of chromosomal aberration to embryos, Gonzalez-Merino et al. (2007) analyzed 47 preimplantation embryos and reported a total aneuploidy rate of 32 %.
47,XXY, Klinefelter Syndrome
Infertile males affected by Klinefelter syndrome (KS) are approximately 3 %, increasing up to 11 % among azoospermic men (Foresta et al. 1999). These subjects are rarely naturally fertile, although assisted reproductive procedures such as ICSI offer them a chance at fatherhood. The sperm phenotype among KS males is widely heterogeneous, ranging from azoospermia to normozoospermia.
The extra X chromosome in males with KS may arise by a paternal nondisjunction at meiosis I in approximately 50 % of cases (Hall et al. 2006). During spermatogenesis, the extra sex chromosome appears to be eliminated (Shi and Martin 2001). On the other end, many studies carried out in 47,XXY males detected marked increases in sex chromosome disomies and diploid sperm (Guttenbach et al. 1997b; Estop et al. 1998; Foresta et al. 1998, 1999; Okada et al. 1999; Rives et al. 2000; Morel et al. 2003; Ferlin et al. 2005; Sarrate et al. 2005; Templado et al. 2011a). The mean disomy rate increased sharply for XX (4.64 ± 2.56 %) and XY (11.1 ± 6.89 %), with an average incidence of 6.3 % (Table 3.2). Diploid sperm in these patients also increased, ranging from 0.03% to 4.2 %, as did autosomal aneuploidies, reaching 6.2 % for chromosome 21 (Templado et al 2011a).
In patients with mosaic KS, the frequency of sperm aneuploidy varied according to the percentage of 47,XXY cells. Various FISH studies (Chevret et al. 1996; Martini et al. 1996; Lim et al. 1999b; Okada et al. 1999; Rives et al. 2000; Ferlin et al. 2005) have demonstrated an increased frequency of sex chromosome disomy, ranging from 0.40% to 1.22 % for XY (Table 3.2).
Chromosomal Translocations
Balanced chromosomal translocations are characterized by breakpoints in two chromosomes and repair of the chromosomal fragments with transpositions of genetic material between them, without loss of genetic material.
Male carriers of these structural alterations generally have a normal phenotype while showing a reduced fertility and an increase in spontaneous miscarriage and birth defects.
Robertsonian Translocations
Robertsonian translocations are the most common chromosomal anomaly among infertile men, characterized by the centric fusion of two acrocentric chromosomes (13, 14, 15, 21, 22) and resulting in a 45 chromosome karyotype. The most frequent reorganization are t(13q;14q) and t(14q;21q), with an estimated frequency of 0.97 and 0.20 %, respectively (Frydman et al. 2001). Before the report of Plymate et al. (1976), testicular function defects were only associated with sex chromosome abnormalities (Paulsen et al. 1968). Since 1976, many studies have shown that carriers of chromosome anomalies, especially translocations, have an altered spermatogenesis characterized by severe oligozoospermia (Chandley et al. 1976; Veld et al. 1997; Ogawa et al. 2000). In addition, unusual ultrastructural sperm anomalies related to immaturity were observed in carriers of Robertsonian translocation (Baccetti et al. 2002). Spermatogenetic alterations could be a consequence of a chromosomal anomaly: the pairing of the reorganized chromosomes during meiotic prophase I gives rise to a trivalent configuration that is prone to segregate in an alternate way, producing normal or balanced sperm (Sybenga 1975; Vidal et al. 1982; Luciani et al. 1984). Unbalanced sperm are generated by an adjacent segregation pattern, and they could be responsible for miscarriages or aneuploid offspring (Egozcue et al. 2000b).
In Robertsonian translocation carriers, FISH analysis demonstrated a percentage of normal or balanced sperm ranging from 73.6 up to 91 % (Escudero et al. 2000; Morel et al. 2001; Anton et al. 2004; Roux et al. 2005; Nishikawa et al. 2008). Contrasting results showing a high percentage of unbalanced sperm derived from adjacent segregation, ranging from 3 % to 36 % (reviewed by Harton and Tempest 2012).
Reciprocal Translocations
Exchanges of genetic material between two or more chromosomes characterize the reciprocal translocation. A wide range of different situations is included in this structural chromosomal anomaly, each of them unique in individual families, depending on the chromosome involved, the size of the translocated regions, and the probability of recombination within these regions (Harton and Tempest 2012). Reciprocal translocations are the most frequent (1/600) structural chromosomal anomalies in humans (Estop et al. 1997). Among infertile males these chromosomal reorganizations are approximately ten times more frequent than in the general population (Van Assche et al. 1996), and a high level of unbalanced gametes are reported in various studies ranging from 29 % up to 81 % (Harton and Tempest 2012), with an average of 50 % (Shi and Martin 2001).
The meiotic behavior of reciprocal translocations depends on the chromosomes involved in the rearrangement, the position of the breakpoints, the presence of crossovers in the translocated chromosomes, and the morphological characteristics of the rearranged chromosomes. During meiosis I, segregation of the quadrivalent formed among the translocated chromosomes and their normal homologs produces a variety of balanced and unbalanced gametes. In the alternate segregation pattern, where homologous centromeres move to opposite poles, chromosomally balanced or normal gametes are produced. Unbalanced gametes are produced by the other segregation patterns, specifically adjacent I, adjacent II, and 3:1 segregation.
Alternate segregation is the most common meiotic behavior, occurring with a frequency of 44–51 %; adjacent I segregants have a frequency of 16–40 %, while adjacent II segregants have a lower mean frequency of approximately 9 % (Shi and Martin 2001), which varied inversely with the length of the shorter centric segment (Faraut et al. 2000). Finally, 3:1 segregants occur with a mean frequency of 11 % (Shi and Martin 2001) even if, in some cases, 3:1 segregation is the preferential pattern (Jalbert et al. 1980; Estop et al. 1999; Van Assche et al. 1999) with an unusually high rate up to 23.5 % as reported in four different reciprocal translocation carriers (Nishikawa et al 2008).
An analysis of familial cases confirmed that segregation patterns were specific for a given translocation, as demonstrated by detection of the same profile of meiotic segregation mode in each family (Rousseaux et al. 1995; Cora et al. 2002; Anton et al. 2004; Morel et al. 2004; Wiland et al. 2007).
Interchromosomal Effect
The possibility that chromosome rearrangements could interfere with the meiotic behavior of chromosomes not involved in translocation led to the concept of interchromosomal effect (ICE), postulated for the first time in humans by Lejeune (1965).
Meiotic segregation of sex chromosomes and autosomes was investigated directly on sperm nuclei by FISH by various authors (Rousseaux et al. 1995; Blanco et al. 2000; Vegetti et al. 2000; Morel et al. 2001; Anton et al. 2004), and the results suggested that ICE was generally restricted to translocation carriers with abnormal semen parameters.
In six carriers of Robertsonian translocations t(13;21) and t(14;22), the interactions between chromosome rearrangements and ICE were studied by evaluating aneuploidy and diploidy frequencies of chromosomes 18, X, and Y: the mean percentage of sex chromosome disomy as well as the frequency of diploid sperm were significantly higher than in controls (Baccetti et al. 2005)
Therefore, the increase in sperm aneuploidies among Robertsonian translocation carriers could be related to ICE, as suggested by many studies (Blanco et al. 2000; Vegetti et al. 2000; Morel et al. 2001; Baccetti et al. 2002, 2005; Anton et al. 2004; Ogur et al. 2006; Chen et al. 2007). However¸ a negative effect of an altered testicular environment on the meiotic process cannot be excluded in any of these studies because none of the enrolled subjects with translocations was classified as normozoospermic.
The question of ICE in reciprocal translocation carriers is still controversial. Some authors did not report any evidence of ICE in several reciprocal translocation carriers (Van Hummelen et al. 1997; Honda et al. 1999; Estop et al. 2000; Rives et al. 2003b; Oliver-Bonet et al. 2004). Some of the analyzed patients had normal semen parameters, and therefore the authors suggested that ICE in translocation carriers could be restricted to patients with abnormal semen parameters (Vegetti et al. 2000; Pellestor et al. 2001).
On the other hand, many reports detected an ICE in different reciprocal translocation carriers (Blanco et al. 2000; Oliver-Bonet et al. 2001, 2002; Baccetti et al. 2003; Douet-Guilbert et al. 2005; Wiland et al. 2007; Vozdova et al. 2008).
All reports support the occurrence of ICE in particular cases of structural chromosome reorganization, depending on the type of reorganization and on the chromosome or chromosomal region involved. However, the increase in aneuploidy and diploidy rates in infertile translocation carriers could be feasibly due to altered semen quality, as previously reported for infertile males of normal karyotype with oligoasthenoteratozoospermia.
Sperm Aneuploidy and Hormone Treatment
As highlighted so far, errors in sperm chromosome segregation are often observed in infertile males, and this is especially negative for candidates for assisted fertilization, increasing the failure rate and risk of generating offspring with chromosome imbalance. Therefore, it would be useful to develop methods for reducing the rate of aneuploidy in sperm.
Follicle stimulating hormone (FSH) is known for its role in the initial development of Sertoli cells and their stimulation to control spermatogenesis. FSH can therefore be used to improve spermatogenesis and fertilizing competence of oligozoospermic males, increasing both spermatogonial population and sperm production (Acosta et al. 1991, 1992; Foresta et al. 1998, 2002, 2005; Baccetti et al. 1997, 2004; Ben-Rafael et al. 2000). The administration of FSH can be useful in hypogonadotropic hypogonadism and when sperm alterations associated with normal gonadotropin levels suggest functional gonadotropin deficit.
In selected male patients with serum FSH less than 8 mIU/ mL and a frequency of sperm aneuploidies greater than 0.6 % according to FISH analysis, 3 months of recombinant FSH therapy improved sperm quality and significantly decreased the frequency of sperm chromosomal alterations. The average percentage of total aneuploidies dropped by 31.8 %. The general improvement in sperm chromosome segregation was predominantly due to the decrease in diploidies and sex chromosome disomies (Piomboni et al. 2009).
The effect of FSH therapy on spermatogenesis may be explained by findings indicating that gonadotropins act as survival factor for spermatogonia and spermatocytes regulating the intrinsic and extrinsic apoptotic pathways, by which germ cells die in normal adult seminiferous epithelium (Ruwanpura et al. 2008).
Conclusions
Multicolor FISH in decondensed sperm nuclei using probes for sex chromosomes and autosomes, particularly chromosomes 1, 13, 18, and 21, allows an accurate evaluation of the incidence of sperm aneuploidy and is an appropriate way to analyze several thousand cells as well as a few cells in the case of severe oligozoospermic or azoospermic patients undergoing testicular biopsies. This technique, developed in the 1990s, may be applied for clinical or research aims. By pooling all published data from FISH analysis on sperm nuclei, it has become a useful tool in reproductive counseling for infertile couples (Gambera et al. 2011).
Using multicolor FISH, errors in chromosomal segregation have been found in sperm from normozoospermic or fertile men with a mean incidence ranging from 0.6 % to 1.45 %. Moreover, the percentage of numerical chromosomal aberrations increases in relation to sperm phenotype as in OAT men, suggesting that the risk of chromosome malsegregation events increases depending on the severity of testicular failure. This is also true for infertile males with abnormal karyotypes, which can produce a high percentage of gametes with unbalanced chromosomes. Sperm carrying chromosome abnormalities generally have a reduced fertilization potential; however, the development of assisted reproductive techniques such as ICSI revolutionized the treatment of male infertility, enabling these patients to procreate but increasing the risk of generating embryos with chromosomal unbalances.
Therefore, on these bases, information about meiosis and the incidence of eventual meiotic abnormalities should be useful in couples undergoing assisted reproduction for male infertility factor.
No technical procedure of sperm selection can guarantee a choice of gamete without chromosomal imbalance; in that case, knowledge of the chromosomal constitution of the male gametes in selected cases might suggest the need for a preimplantation or prenatal genetic diagnosis.
Further information on the relationships between sperm chromosome unbalance and human male infertility could help to promote a correct diagnostic and therapeutic approach to infertile couples, even when the cause of infertility is unknown, as in idiopathic diagnosis.
As regards the progressive improvement of the technique, in the future, the introduction of automated systems for multicolor FISH scoring would save time in the evaluation of results, which actually implies many hours of microscope viewing, which could depend on interoperator variability.
References
Acosta AA, Oehninger S, Ertunc H et al (1991) Possible role of pure human follicle-stimulating hormone in the treatment of severe male-factor infertility by assisted reproduction: preliminary report. Fertil Steril 55:1150–1156
Acosta AA, Khalifa E, Oehninger S (1992) Pure human follicle stimulating hormone has a role in the treatment of severe infertility by assisted reproduction: Norfolk’s total experience. Hum Reprod 7:1067–1072
Anton E, Blanco J, Egozcue J et al (2004) Sperm FISH studies in seven male carriers of Robertsonian translocation t(13;14)(q10;q10). Hum Reprod 19:1345–1351
Aran B, Blanco J, Vidal F et al (1999) Screening for abnormalities of chromosomes X, Y and 18 and for diploidy in spermatozoa from infertile men participating in an in vitro fertilization-intracytoplasmic sperm injection program. Fertil Steril 72:696–701
Baccetti B, Strehler E, Capitani S et al (1997) The effect of follicle stimulating hormone therapy on human sperm structure (Notulae seminologicae 11). Hum Reprod 12:1955–1968
Baccetti B, Capitani S, Collodel G et al (2002) Infertile spermatozoa in a human carrier of Robertsonian translocation 14;22. Fertil Steril 78:1127–1130
Baccetti B, Bruni E, Collodel G et al (2003) 10,15 reciprocal translocation in an infertile man: ultrastructural and fluorescence in-situ hybridization sperm study: case report. Hum Reprod 18:2302–2308
Baccetti B, Piomboni P, Bruni E et al (2004) Effect of follicle-stimulating hormone therapy on sperm quality and pregnancy rate. Asian J Androl 6:133–137
Baccetti B, Collodel G, Marzella R et al (2005) Ultrastructural studies of spermatozoa from infertile males with Robertsonian translocations and 18, X, Y aneuploidies. Hum Reprod 20:2295–2300
Ben-Rafael Z, Farhi J, Feldberg D et al (2000) Follicle stimulating hormone treatment for men with idiopathic oligoteratoasthenozoospermia before in vitro fertilization: the impact on sperm microstructure and fertilization potential. Fertil Steril 73:24–30
Bernardini L, Martini E, Geraedts JP et al (1997) Comparison of gonosomal aneuploidy in spermatozoa of normal fertile men and those with severe male factor detected by in-situ hybridization. Mol Hum Reprod 3:431–438
Bernardini L, Borini A, Preti S et al (1998) Study of aneuploidy in normal and abnormal germ cells from semen of fertile and infertile men. Hum Reprod 13:3406–3413
Bernardini L, Gianaroli L, Fortini D et al (2000) Frequency of hyper, hypohaploidy and diploidy in ejaculate, epididymal and testicular germ cells of infertile patients. Hum Reprod 15:2165–2172
Bernardini LM, Calogero AE, Bottazzi C et al (2005) Low total normal motile count values are associated with increased sperm disomy and diploidy rates in infertile patients. Int J Androl 28:328–336
Bischoff FZ, Nguyen DD, Burt KJ et al (1994) Estimates of aneuploidy using multicolour fluorescence in situ hybridization on human sperm. Cytogenet Cell Genet 66:237–243
Blanco J, Rubio C, Simon C et al (1997) Increased incidence of disomic sperm nuclei in a 47, XYY male assessed by fluorescent in situ hybridization (FISH). Hum Genet 99:413–416
Blanco J, Egozcue J, Vidal F (2000) Interchromosomal effects for chromosome 21 in carriers of structural chromosome reorganizations determined by fluorescence in situ hybridization on sperm nuclei. Hum Genet 106:123–128
Bonduelle M, Aytoz A, Van Assche E et al (1998) Incidence of chromosomal aberrations in children born after assisted reproduction through intracytoplasmic sperm injection. Hum Reprod 13:781–782
Bonduelle M, Ponjaert I, Steirteghem AV et al (2002) Developmental outcome at 2 years of age for children born after ICSI compared with children born after IVF Hum Reprod 18:342–350
Bosch M, Rajmil O, Martínez-Pasarell O et al (2001) Linear increase of diploidy in human sperm with age: a four-colour FISH study. Eur J Hum Genet 9:533–538
Bosch M, Rajmil O, Egozcue J et al (2003) Linear increase of structural and numerical chromosome 9 abnormalities in human sperm regarding age. Eur J Hum Genet 11:754–759
Burrello N, Calogero AE, De Palma A et al (2002) Chromosome analysis of epididymal and testicular spermatozoa in patients with azoospermia. Eur J Hum Genet 10:362–366
Burrello N, Arcidiacono G, Vicari E et al (2004) Morphologically normal spermatozoa of patients with secretory oligo-astheno-teratozoospermia have an increased aneuploidy rate. Hum Reprod 19:2298–2302
Calogero AE, De Palma A, Grazioso C et al (2001a) Aneuploidy rate in spermatozoa of selected men with abnormal semen parameters. Hum Reprod 16:1172–1179
Calogero AE, De Palma A, Grazioso C et al (2001b) High sperm aneuploidy rate in unselected infertile patients and its relationship with intracytoplasmic sperm injection outcome. Hum Reprod 16:1433–1439
Carrel DT (2008) The clinical implementation of sperm chromosome aneuploidy testing: pitfalls and promises. J Androl 29:124–133
Carrell DT, Emery BR, Liu L (1999) Characterization of aneuploidy rates, protamine levels, ultrastructure, and functional ability of round-headed sperm from two siblings and implications for intracytoplasmic sperm injection. Fertil Steril 71:511–516
Carrell DT, Wilcox AL, Udoff LC et al (2001) Chromosome 15 aneuploidy in the sperm and conceptus of a sibling with variable familial expression of round-headed sperm syndrome. Fertil Steril 76:1258–1260
Carrell DT, Emery BR, Wilcox AL et al (2004) Sperm chromosome aneuploidy as related to male factor infertility and some ultrastructure defects. Arch Androl 50:181–185
Celik-Ozenci C, Jakab A, Kovacs T et al (2004) Sperm selection for ICSI: shape properties do not predict the absence or presence of numerical chromosomal aberrations. Hum Reprod 19:2052–2059
Chandley AC, Fletcher J, Robinson JA (1976) Normal meiosis in two 47, XYY men. Hum Genet 33:231–240
Chen Y, Huang J, Liu P et al (2007) Analysis of meiotic segregation patterns and interchromosomal effects in sperm from six males whit Robertsonian translocation. J Assist Reprod Genet 24:406–411
Chevret E, Rousseaux S, Monteil M et al (1996) Increased incidence of hyperhaploid 24, XY spermatozoa detected by three-colour FISH in a 46, XY/47, XXY male. Hum Genet 97:171–175
Collodel G, Capitani S, Baccetti B et al (2007) Sperm aneuploidies and low progressive motility. Hum Reprod 22:1893–1898
Colombero LT, Hariprashad JJ, Tsai MC et al (1999) Incidence of sperm aneuploidy in relation to semen characteristics and assisted reproductive outcome. Fertil Steril 72:90–96
Cora T, Acar H, Kaynak M (2002) Molecular cytogenetic detection of meiotic segregation patterns in sperm nuclei of carriers of 46, XY, t(15;17)(q21; q25). J Androl 23:793–798
Douet-Guilbert N, Bris MJ, Amice V et al (2005) Interchromosomal effect in sperm of males with translocations: report of 6 cases and review of the literature. Int J Androl 28:372–379
Downie SE, Flaherty SP, Matthews CD (1997) Detection of chromosomes and estimation of aneuploidy in humans spermatozoa using fluorescence in situ hybridization. Mol Hum Reprod 3:585–598
Egozcue J, Blanco J, Vidal F (1997) Chromosome studies in human sperm nuclei using fluorescence in-situ hybridization (FISH). Hum Reprod Update 3:441–452
Egozcue S, Blanco J, Vendrell JM et al (2000a) Human male infertility: chromosome anomalies, meiotic disorders, abnormal spermatozoa and recurrent abortion. Hum Reprod Update 6:93–105
Egozcue S, Vendrell JM, Garcia F et al (2000b) Increased incidence of meiotic anomalies in oligoasthenoteratozoospermic males preselected for intracytoplasmic sperm injection. J Assist Reprod Genet 17:307–309
Egozcue J, Blanco J, Anton E, Sarrate Z et al (2003) Genetic analysis of sperm and implications of severe male infertility-a review. Placenta 24:S62–S65
Egozcue J, Sarrate Z, Codina-Pascual M et al (2005) Meiotic abnormalities in infertile males. Cytogenet Genome Res 111:337–342
Escudero T, Lee M, Carrel D et al (2000) Analysis of chromosome abnormalities in sperm and embryos from two 45, XY, t(13;14)(q10;q10) carriers. Prenat Diagn 20:599–602
Estop AM, Cieply KM, Aston CE (1997) The meiotic segregation pattern of a reciprocal translocation t(10;12)(q26.1;p13.3) by fluorescence in situ hybridization sperm analysis. Eur J Hum Genet 5:78–82
Estop AM, Cieply K, Munne S et al (2000) Is there an interchromosomal effect in reciprocal translocation of carriers? Sperm FISH studies Hum Genet 106:517–524
Estop AM, Munne S, Cieply KM et al (1998) Meiotic products of a Klinefelter 47, XXY male as determined by sperm fluorescence in situ hybridization analysis. Hum Reprod 13:124–127
Estop AM, Cieply KM, Munne S et al (1999) Multicolor fluorescence in situ hybridization analysis of the spermatozoa of a male heterozygous for a reciprocal translocation t(11;22)(q23;q11). Hum Genet 104:412–417
Faraut T, Mermet M-A, Demongeot J et al (2000) Cooperation of selection and meiotic mechanisms in the production of imbalances in reciprocal translocations. Cytogenet Cell Genet 88:15–21
Ferlin A, Garolla A, Foresta C (2005) Chromosome abnormalities in sperm of individuals with constitutional sex chromosomal abnormalities. Cytogenet Genome Res 111:310–316
Ford JH, Schultz CJ, Correll AT (1988) Chromosome elimination in micronuclei: a common cause of hypoploidy. Am J Hum Genet 43:733–740
Foresta C, Betella A, Ferlin A et al (1998) Evidence for a stimulatory role of follicle-stimulating hormone on the spermatogonial population in adult males. Fertil Steril 69:1–7
Foresta C, Galeazzi C, Bettella A et al (1999) Analysis of meiosis in intratesticular germ cells from subjects affected by classic Klinefelter’s syndrome. J Clin Endocrinol Metab 84:3807–3810
Foresta C, Betella A, Merico M et al (2002) Use of recombinant human follicle-stimulating hormone in the treatment of male factor infertility. Fertil Steril 77:238–244
Foresta C, Betella A, Garolla A et al (2005) Treatment of male idiopathic infertility with recombinant human follicle-stimulating hormone: a prospective, controlled, randomized clinical study. Fertil Steril 84:654–661
Frydman N, Romana S, Le Lorc’h M et al (2001) Assisting reproduction of infertile men carrying a Robertsonian translocation. Hum Reprod 16:2274–2277
Gambera L, Morgante G, Serafini F et al (2011) Human sperm aneuploidy: FISH analysis in fertile and infertile men. Expert Rev Obstet Gynecol 6:609–627
Gardner RJ, Sutherland GR (2004) Chromosome abnormalities and genetic counselling, 3rd edn. Oxford University Press, New York
Gianaroli L, Magli MC, Cavallini G et al (2005) Frequency of aneuploidy in sperm from patients with extremely severe male factor infertility. Hum Reprod 20:2140–2152
Giltay JC, van Golde RJ, Kastrop PM (2000) Analysis of spermatozoa from seven ICSI males with constitutional sex chromosomal abnormalities by fluorescent in situ hybridization. J Assist Reprod Genet 17:151–155
Gonzalez-Merino E, Hans C, Abramowicz M et al (2007) Aneuploidy study in sperm and preimplantation embryos from nonmosaic 47, XYY men. Fertil Steril 88:600–606
Guttenbach M, Engel W, Schmid M (1997a) Analysis of structural and numerical chromosome abnormalities in sperm of normal men and carriers of constitutional chromosome aberrations. Rev Hum Genet 100:1–21
Guttenbach M, Michelmann HW, Hinney B et al (1997b) Segregation of sex chromosomes into sperm nuclei in a man with 47, XXY Klinefelter’s karyotype: a FISH analysis. Hum Genet 99:474–477
Hall H, Hunt P, Hassold T (2006) Meiosis and sex chromosome aneuploidy: how meiotic errors cause aneuploidy; how aneuploidy causes meiotic errors. Curr Opin Genet Dev 16:323–329
Han TH, Ford JH, Flaherty SP et al (1994) A fluorescent in situ hybridization analysis of the chromosome constitution of ejaculated sperm in a 47, XYY male. Clin Genet 45:67–70
Harton GL, Helen G (2012) Chromosomal disorders and male infertility. Asian J Androl 14:32–39
Harton GL, Tempest HG (2012) Chromosomal disorders and male infertility. Asian J Androl 14(1):32–39
Hecht F, Hecht BK (1987) Aneuploidy in humans: dimensions, demography and dangers of abnormal number of chromosomes. In: Vig BK, Sandberg AA (eds) Aneuploidy. Part A: incidence and etiology. Alan R. Liss, New York, pp 9–49
Holmes JM, Martin RH (1993) Aneuploidy detection in human sperm nuclei using fluorescence in situ hybridization. Hum Genet 91:20–24
Honda H, Miharu N, Ohashi Y et al (1999) Analysis of segregation and aneuploidy in two reciprocal translocation carriers, t(3;9)(q26.2;q32) and t(3;9)(p25;q32), by triple-color fluorescence in situ hybridization. Hum Genet 105:428–436
Hook EB (1985) In: Dellarco VL, Voytek PE, Hollander A (eds) Aneuploidy: etiology and mechanism. Plenum, New York, pp 7–33
Hulten M, Pearson PL (1971) Fluorescent evidence for spermatocytes with two Y chromosomes in an XYY male. Ann Hum Genet 34:273–276
Huszar G, Jakab A, Sakkas D et al (2007) Fertility testing and ICSI sperm selection by hyaluronic acid binding: clinical and genetic aspects. Reprod Biomed Online 14:650–663
Jakab A, Sakkas D, Delpiano E et al (2005) Fertil Steril 84:1665–1673
Jalbert P, Sele B, Jalbert H (1980) Reciprocal translocations: a way to predict the mode of imbalanced segregation by pachytene-diagram drawing. Hum Genet 55:209–222
Johnson MD (1998) Genetic risks of intracytoplasmic sperm injection in the treatment of male infertility: recommendations for genetic counseling and screening. Fertil Steril 70:397–411
Kahraman S, Findikli N, Biricik A et al (2006) Preliminary FISH studies on spermatozoa and embryos in patients with variable degrees of teratozoospermia and a history of poor prognosis. Reprod Biomed Online 12(6):752–761
Koehler KE, Hawley RS, Sherman S et al (1996) Recombination and non disjunction in humans and flies Hum Mol Genet 5:1495–1504
Kovanci E, Kovacs T, Moretti E et al (2001) FISH assessment of aneuploidy frequencies in mature and immature human spermatozoa classified by the absence ore presence of cytoplasmic retention. Hum Reprod 16:1209–1217
Kruger TF, Menkveld R, Stander FS et al (1986) Sperm morphologic features as a prognostic factor in vitro fertilization. Fertil Steril 46:1118–1123
Lahdetie J, Saari N, Ajosenpää-Saari M et al (1997) Incidence of aneuploid spermatozoa among infertile men studied by multicolor fluorescence in situ hybridization. Am J Med Genet 71:115–121
Lee JD, Kamiguchi Y, Yanagimachi R (1996) Analysis of chromosome constitution of human spermatozoa with normal and aberrant head morphologies after injection into mouse oocytes Hum Reprod 11:1942–1946
Lejeune J (1965) The meiotic consequences of chromosome modifications. Ann Genet 8:9–10
Levron J, Aviram-Goldring A, Madgar I et al (2001) Sperm chromosome abnormalities in men with severe male factor infertility who are undergoing in vitro fertilization with intracytoplasmic sperm injection. Fertil Steril 76:479–484
Lewis-Jones I, Aziz N, Seshadri S et al (2003) Sperm chromosomal abnormalities are linked to sperm morphologic deformities. Fertil Steril 79:212–215
Lim AS, Fong Y, Yu SL (1999a) Analysis of the sex chromosome constitution of sperm in men with a 47, XYY mosaic karyotype by fluorescence in situ hybridization. Fertil Steril 72:121–123
Lim AS, Fong Y, Yu SL (1999b) Estimates sperm sex chromosome disomy and diploidy rates in a 47, XXY/46, XY mosaic Klinefelter patient. Hum Genet 104:405–409
Luciani JM, Guichaoua MR, Mattei A et al (1984) Pachytene analysis of a man with a 13q;14q translocation and infertility. Behavior of the trivalent and nonrandom association with the sex vesicle. Cytogenet Cell Genet 38:14–22
Magli MC, Gianaroli L, Ferraretti AP et al (2009) Paternal contribution to aneuploidy in preimplantation embryos. Reprod Biomed Online 18:536–542
Martin RH (2008) Cytogenetic determinants of male fertility. Hum Reprod Update 14:379–390
Martin RH, McInnes B, Rademaker AW (1999) Analysis of aneuploidy for chromosomes 13, 21, X and Y by multicolour fluorescence in situ hybridization (FISH) in a 47, XYY male. Zygote 7:131–134
Martin RH, Greene C, Rademaker A et al (2000) Chromosome analysis of spermatozoa extracted from testes of men with non-obstructive azoospermia. Hum Reprod 15:1121–1124
Martin RH, Ko E, Chan K (1993) Detection of aneuploidy in human interphase spermatozoa by fluorescence in situ hybridization (FISH) Cytogenet Cell Genet 64:23–26
Martin RH, Rademaker AW, Greene C et al (2003a) A comparison of the frequency of sperm chromosome abnormalities in men with mild, moderate, and severe oligozoospermia Biol Reprod 69:535–539
Martini E, Geraedts JP, Liebaers I et al (1996) Constitution of semen samples from XYY and XXY males as analyzed by in situ hybridization. Hum Reprod 11:1638–1643
Mateizel I, Verheyen G, Van Assche E et al (2002) FISH analysis of chromosome X, Y and 18 abnormalities in testicular sperm from azoospermic patients. Hum Reprod 17:2249–2257
Mateu E, Rodrigo L, Martínez MC et al (2010) Aneuploidies in embryos and spermatozoa from patients with Y chromosome microdeletions. Fertil Steril 94:2874–2877
McInnes B, Rademaker A, Greene CA et al (1998) Abnormalities for chromosomes 13 and 21 detected in spermatozoa from infertile men. Hum Reprod 13:2787–2790
McLachlan RI, O’Bryan MK (2010) State of the art for genetic testing of infertile men. J Clin Endocrinol Metab 85:1013–1024
Mercier S, Morel F, Roux C et al (1996) Analysis of the sex chromosomal equipment in spermatozoa of a 47, XYY male using two-colour fluorescence in-situ hybridization. Mol Hum Reprod 2:485–488
Meschede D, Louwen F, Eiben B et al (1997) Intracytoplasmic sperm injection pregnancy with fetal trisomy 9p resulting from a balanced paternal translocation Hum Reprod 12:1913–1914
Miharu N (2005) Chromosome abnormalities in sperm from infertile men with normal somatic karyotypes: oligozoospermia. Cytogenet Genom Res 111:347–351
Morel F, Roux C, Bresson JL (1999) Sex chromosome aneuploidies in sperm of 47, XYY men. Arch Androl 43:27–36
Morel F, Roux C, Bresson JL (2001) FISH analysis of the chromosomal status of spermatozoa from three men with 45, XY, der(13;14)(q10;q10) karyotype. Mol Hum Reprod 7:483–488
Morel F, Bernicot I, Herry A, Le Bris MJ, Amice V, De Braekeleer M (2003) An increased incidence of autosomal aneuploidies in spermatozoa from a patient with Klinefelter’s syndrome. Fertil Steril 79:1644–1646
Morel F, Douet-Guilbert N, Roux C et al (2004) Meiotic segregation of a t(7;8)(q11.21;cen) translocation in two carrier brothers. Fertil Steril 81:682–685
Moretti E, Collodel G (2006) Three cases of genetic defects affecting sperm tail: a FISH study. J Submicrosc Cytol Pathol 38:137–141
Moretti E, Collodel G, Scapigliati G et al (2005) “Round head’” sperm defect. Ultrastructural and meiotic segregation study. J Submicrosc Cytol Pathol 37:297–303
Moretti E, Anichini C, Sartini B et al (2007) Sperm ultrastructure and meiotic segregation in an infertile 47, XYY man. Andrologia 39:229–234
Naccarati A, Zanello A, Landi S et al (2003) Sperm-FISH analysis and human monitoring: a study on workers occupationally exposed to styrene. Mutat Res 537:131–140
Nagvenkar P, Zaveri K, Hinduja I (2005) Comparison of the sperm aneuploidy rate in severe oligozoospermic and oligozoospermic men and its relation to intracytoplasmic sperm injection outcome. Fertil Steril 84:925–931
Nishikawa N, Sato T, Suzumori N et al (2008) Meiotic segregation analysis in male translocation carriers by using fluorescent in situ hybridization. Int J Androl 31:60–66
Ogawa S, Araki S, Araki Y et al (2000) Chromosome analysis of human spermatozoa from an oligoasthenozoospermic carrier for a 13;14 Robertsonian translocation by their injection into mouse oocytes. Hum Reprod 15:1136–1139
Ogur G, Van Assche E, Vegetti W et al (2006) Chromosomal segregation in spermatozoa of 14 Robertsonian translocation carriers. Mol Hum Reprod 12:209–215
Ohashi Y, Miharu N, Honda H et al (2001) High frequency of XY disomy in spermatozoa of severe oligozoospermic men. Hum Reprod 4:703–708
Okada H, Fujioka H, Tatsumi N et al (1999) Klinefelter’s syndrome in the male infertility clinic. Hum Reprod 14:946–952
Oliver-Bonet M, Navarro J, Codina-Pascual M et al (2001) Meiotic segregation analysis in a t(4;8) carrier: comparison of FISH methods on sperm chromosome metaphases and interphase sperm nuclei. Eur J Hum Genet 9:395–403
Oliver-Bonet M, Navarro J, Carrera M et al (2002) Aneuploid and unbalanced sperm in two translocation carriers: evaluation of the genetic risk. Mol Hum Reprod 8:958–963
Oliver-Bonet M, Navarro J, Codina-Pascual M et al (2004) From spermatocytes to sperm: meiotic behaviour of human male reciprocal translocations. Hum Reprod 19:2515–2522
Palermo G, Joris H, Devroey P et al (1992) Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 340:17–18
Palermo GD, Colombero LT, Hariprashad JJ et al (2002) Chromosome analysis of epididymal and testicular sperm in azoospermic patients undergoing ICSI. Hum Reprod 17:570–575
Pang MG, Hoegerman SF, Cuticchia AJ et al (1999) Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in situ hybridization from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Hum Reprod 14:1266–1273
Paulsen CA, Gordon DL, Carpenter RW et al (1968) Klinefelter’s syndrome and its variants: a hormonal and chromosomal study. Recent Prog Horm Res 24:321–363
Pellestor F, Imbert I, Andréo B et al (2001) Study of the occurrence of interchromosomal effect in spermatozoa of chromosomal rearrangement carriers by fluorescence in-situ hybridization and primed in-situ labelling techniques. Hum Reprod 16:1155–1164
Pfeffer J, Pang MG, Hoegerman SF et al (1999) Aneuploidy frequencies in semen fractions from ten oligoasthenoteratozoospermic patients donating sperm for intracytoplsamic sperm injection. Fertil Steril 72:472–478
Piomboni P, Gambera L, Serafini F et al (2007) Displasia of the fibrous sheath sperm defect and outcome of intracytoplasmic sperm injection. Androl Update 1: 268–276
Piomboni P, Serafini F, Gambera L et al (2009) Sperm aneuploidies after human recombinant FSH therapy in infertile males. Reprod BioMed Online 18:622–629
Plymate SR, Bremner WJ, Paulsen CA (1976) The association of D-group chromosomal translocations and defective spermatogenesis. Fertil Steril 27:139–144
Rimm AA, Katayama AC, Diaz M et al (2004) A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. J Assist Reprod Genet 21:437–443
Rives N, Saint Clair A, Mazurier S et al (1999) Relationship between clinical phenotype, semen parameters and aneuploidy frequency in sperm nuclei of 50 infertile males. Hum Genet 105:266–272
Rives N, Joly G, Machy A et al (2000) Assessment of sex chromosome aneuploidy in sperm nuclei from 47, XXY and 46, XY/47, XXY males: comparison with fertile and infertile males with normal karyotype. Mol Hum Reprod 6:107–111
Rives N, Siméon N, Milazzo JP et al (2003a) Meiotic segregation of sex chromosomes in mosaic and non-mosaic XYY males: case reports and review of the literature. Int J Androl 26:242–249
Rives N, Jarnot M, Mousset-Siméon N et al (2003b) Fluorescence in situ hybridisation (FISH) analysis of chromosome segregation and interchromosomal effect in spermatozoa of a reciprocal translocation t(9,10)(q11;p11.1) carrier. J Hum Genet 48:535–540
Rives N, Mousset-Siméon N, Sibert L et al (2004) Chromosome abnormalities of spermatozoa. Gynecol Obstet Fertil 32:771–778
Rives N, Mousset-Simeon N, Mazurier S et al (2005) Primary flagellar abnormality is associated with an increased rate of spermatozoa aneuploidy. J Androl 26:61–69
Robinson DO, Jacobs PA (1999) The origin of the extra Y chromosome in males with a 47, XYY karyotype. Hum Mol Genet 8:2205–2209
Rodrigo L, Rubio C, Mateu E et al (2004) Analysis of chromosomal abnormalities in testicular and epididymal spermatozoa from azoospermic ICSI patients by fluorescence in-situ hybridization. Hum Reprod 19:118–123
Rodrigo L, Peinado V, Mateu E et al (2010) Impact of different patterns of sperm chromosomal abnormalities on the chromosomal constitution of preimplantation embryos. Fertil Steril 94:1380–1386
Rousseaux S, Chevret E, Monteil M et al (1995) Meiotic segregation in males heterozygote for reciprocal translocations: analysis of sperm nuclei by two and three colour fluorescence in situ hybridization. Cytogenet Cell Genet 71:240–246
Roux C, Tripogney C, Morel F et al (2005) Segregation of chromosomes in sperm of Robertsonian translocation carriers. Cytogenet Genome Res 111:291–296
Ruwanpura SM, McLachlan RI, Matthiesson KL et al (2008) Gonadotrophins regulate germ cell survival, not proliferation, in normal adult men. Hum Reprod 23:403–411
Ryu HM, Lin WW, Lamb DJ et al (2001) Increased chromosome X, Y and 18 nondisjunction in sperm from infertile patients that were identified as normal by strict morphology: implication for intracytoplasmic sperm injection. Fertil Steril 76:879–883
Samura O, Miharu N, He H et al (1997) Assessment of sex chromosome ratio and aneuploidy rate in motile sperm selected by three different methods. Hum Reprod 12:2437–2442
Sarrate Z, Blanco J, Anton E et al (2005) FISH studies of chromosome abnotmalities in germ cells and its relevance in reproductive counselling. Asian J Androl 7:227–236
Shen JJ, Sherman SL, Hassold TJ (1998) Centromeric genotyping and direct analysis of nondisjunction in humans: Down syndrome. Chromosoma 107:166–172
Shi Q, Martin RH (2000a) Aneuploidy in human sperm: a review of the frequency and distribution of aneuploidy, effects of donor age and lifestyle factors. Cytogenet Cell Genet 90:219–226
Shi Q, Martin RH (2000b) Multicolor fluorescence in situ hybridization analysis of meiotic chromosome segregation in a 47, XYY male and a review of the literature. Am J Med Genet 93:40–46
Shi Q, Martin R (2001) Aneuploidy in human spermatozoa: FISH analysis in men with constitutional chromosomal abnormalities, and in infertile men. Reproduction 121:655–666
Sloter E, Nath J, Eskenazi B et al (2004) Effects of male age on the frequencies of germinal and heritable chromosomal abnormalities in humans and rodents. Fertil Steril 81:925–943
Speed RM, Faed MJ, Batstone PJ et al (1991) Persistence of two Y chromosomes through meiotic prophase and metaphase I in an XYY man. Hum Genet 87:416–420
Spriggs EL, Rademarker AW, Martin RH (1995) Aneuploidy in human sperm: results of two- and three- color fluorescence in situ hybridization using centromeric probes for chromosomes 1, 12, 15, 18, X, and Y. Cytogenet Cell Genet 71:47–53
Storeng RT, Plachot M, Theophile D et al (1998) Incidence of sex chromosome abnormalities in spermatozoa from patients entering an IVF or ICSI protocol. Acta Obstet Gynecol Scand 77:191–197
Sun F, Oliver-Bonet M, Liehr T et al (2004) Human male recombination maps for individual chromosomes. Am J Hum Genet 74:521–531
Sun F, Ko E, Martin RH (2006) Is there a relationship between sperm chromosome abnormalities and sperm morphology? Reprod Biol Endocrinol 4:1–5
Sybenga J (1975) Chromosome structural variants. In: Sybenga J (ed) General cytogenetics. North-Holland, Amsterdam, pp 165–212
Tempest HG, Martin RH (2009) Cytogenetic risks in chromosomally normal infertile men. Curr Opin Obstet Gynecol 21(3):223–227
Templado C, Bosch M, Benet J (2005) Frequencies and distribution of chromosome abnormalities in human spermatozoa. Cytogenet Genome Res 111:199–205
Templado C, Donate A, Giraldo J et al (2011a) Advanced age increases chromosome structural abnormalities in human spermatozoa. J Hum Genet 19:145–151
Templado C, Vidal F, Estop A (2011b) Aneuploidy in human spermatozoa. Cytogenet Genome Res 133:91–99
Tesarik J, Mendoza C (2007) Treatment of severe male infertility by micromanipulation-assisted fertilization: an update. Front Biosci 12:105–114
Thompson H, Melenyk J, Hecht F (1967) Reproduction and meiosis in XYY men. Lancet ii:831
Ushijima C, Kumasako Y, Kihaile PE et al (2000) Analysis of chromosomal abnormalities in human spermatozoa using multi-colour fluorescence in situ hybridization. Hum Reprod 15:1107–1111
Van Assche E, Bonduelle M, Tournaye H et al (1996) Cytogenetics of infertile men. Hum Reprod 11:1–24
Van Assche E, Staessen C, Vegetti W et al (1999) Preimplantation genetic diagnosis and sperm analysis by fluorescence in-situ hybridization for the most common reciprocal translocation t(11;22). Mol Hum Reprod 5:682–690
Van Dyk Q, Lanzendorf S, Kolm P et al (2000) Incidence of aneuploid spermatozoa from subfertile men: selected with motility versus hemizona-bound. Hum Reprod 15:1529–1536
Van Hummelen P, Manchester D, Lowe X et al (1997) Meiotic segregation, recombination, and gamete aneuploidy assessed in a t(1;10)(p22.1;q22.3) reciprocal translocation carrier by three- and four-probe multicolor FISH in sperm. Am J Hum Genet 61:651–659
Van Steirteghem AC, Liu J, Joris H et al (1993) Higher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of second series of 300 consecutive treatment cycles. Hum Reprod 8:1055–1060
Van Steirteghem A, Bonduelle M, Devroey P et al (2002) Follow-up of children born after ICSI Hum Reprod Update 8:111–116
Van Steirteghem A, Nagy P, Joris H et al (1996) The development of intracytoplasmic sperm injection. Hum Reprod 11:59–72
Vegetti W, Van Assche E, Frias A et al (2000) Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in situ hybridization in infertile men. Hum Reprod 15:351–365
Veld PA, Weber RF, Los FJ et al (1997) Two cases of Robertsonian translocations in oligozoospermic males and their consequences for pregnancies induced by intracytoplasmic sperm injection. Hum Reprod 12:1642–1644
Verpoest W, Tournaye H (2006) ICSI: hype or hazard? Hum Fertil 9:81–92
Vidal F, Templado C, Navarro J et al (1982) Meiotic and synaptonemal complex studies in a 14/21 translocation carrier. Int J Androl 5:21–26
Vidal F, Moragas M, Català V et al (1993) Sephadex filtration and human serum albumin gradients do not select spermatozoa by sex chromosome: a fluorescent in-situ hybridization study. Hum Reprod 8:1740–1743
Vidal F, Blanco J, Egoczue J (2001) Chromosomal abnormalities in sperm. Mol Cell Endocrinol 183:S51–S54
Vozdova M, Oracova E, Horinova V et al (2008) Sperm fluorescence in situ hybridization study of meiotic segregation and an interchromosomal effect in carriers of t(11;18). Hum Reprod 23:581–588
Vozdova M, Heracek J, Sobotka V et al (2012) Testicular sperm aneuploidy in non-obstructive azoospermic patients. Hum Reprod 27:2233–2239
Wang JY, Samura O, Zhen DK et al (2000) Fluorescence in situ hybridization analysis of chromosomal constitution in spermatozoa from a mosaic 47, XYY/46, XY male. Mol Hum Reprod 6:665–668
Wen J, Jiang J, Ding C et al (2012) Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis. Fertil Steril 97:1331–1337
Wiland E, Midro AT, Panasiuk B et al (2007) The analysis of meiotic segregation patterns and aneuploidy in the spermatozoa of father and son with translocation t(4;5)(p15.1;p12) and the prediction of the individual probability rate for unbalanced progeny at birth. J Androl 28:262–272
World Health Organization (1999) WHO laboratory manual for the examination of human semen and semen-cervical mucus interactions, 4th edn. Cambridge University Press, Cambridge
World Health Organization (2010) WHO laboratory manual for the examination and processing of human semen, 5th edn. Cambridge University Press, Cambridge
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Piomboni, P., Stendardi, A., Gambera, L. (2014). Chromosomal Aberrations and Aneuploidies of Spermatozoa. In: Baldi, E., Muratori, M. (eds) Genetic Damage in Human Spermatozoa. Advances in Experimental Medicine and Biology, vol 791. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7783-9_3
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7783-9_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7782-2
Online ISBN: 978-1-4614-7783-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)