Abstract
In this chapter, we reconsider existing theoretical models of Gene–Environment (GE) interplay and view them through the lens of a lifespan perspective, focusing on the shifting nature of the environments that impact cognitive function throughout adulthood. Existing evidence for GE interplay in cognitive aging is evaluated from this vantage point, including investigations that tap recent advances in genotyping and gene expression. The extent to which genetic factors are actually correlated with environments that provide more or less support for cognitive skills is unknown. However, educational and occupational attainment as well as leisure activities and exercise may reflect GE correlational processes that deserve further examination from a life course perspective. Emerging evidence is perhaps a bit more encouraging with respect to G × E processes: e.g., higher education and participation in leisure and physical activities may lower the risk of cognitive decline in those who already carry the APOE e4 risk allele. Familiality of methylation levels and telomere lengths, suggests that genetically driven differential sensitivities to environments (e.g., stress) may be important to individual differences in cognitive aging, but detailed investigations of specific environmental factors is minimal as yet. Large-scale efforts to study G × E influences on aging outcomes are underway, and predicted to contribute in important ways to the emerging literature on GE interplay using behavioral genetics and molecular methods.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
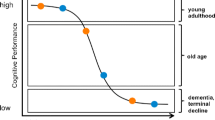
Successful aging is defined, in part, by maintenance of cognitive functioning (Rowe and Kahn 1997) and researchers and entrepreneurs alike are eager to uncover the secrets to slowing or delaying cognitive aging (Buetnner 2008; Fozard et al. 2000). Changes in the relative contribution of environmental factors to cognitive functioning over the course of adulthood suggest that revisiting the concept of Gene–Environment (GE) interplay in middle and late adulthood may increase our understanding of the processes of cognitive aging and provide fertile ground for the development of intervention strategies. Shared environmental influences have a significant impact on individual differences in intelligence in childhood; however, the proportion of variance explained by shared environment drops to negligible levels as early as young adulthood (Plomin et al. 2008). Phenotypic and biometrical studies of cognitive aging provided some early hints that GE interplay may be important to normative cognitive aging. First, variance in cognitive performance tends to increase over the life course for memory , speed of processing, and other fluid abilities but less so for crystallized abilities (Christensen 2001; Morse 1993). Second, twin and adoption studies have both indicated that although heritability increases from childhood into adulthood, increasing from approximately 40 % to a peak of 80 % for general cognitive ability in late adulthood, this increase is followed by a downturn in old-old age to 40–60 % heritability (Finkel and Reynolds 2009; Reynolds 2008a; Reynolds 2008b). These patterns initially result from increasing genetic variance, until about age 65, but are subsequently explained by increasing nonshared environmental variance (Johansson et al. 2004; McGue and Christensen 2002; Reynolds et al. 2005). Some exceptions to this pattern exist: working and episodic memory traits display increases in both genetic and environmental variance (Reynolds 2008a; Reynolds et al. 2005).
Increasing nonshared environmental variance has important implications for investigations of gene by environment interplay. Indeed, if interactions between genes and nonshared environment (denoted G × E) exist but are not formally accounted for in analyses, as is typically the case, G × E effects become part of the nonshared environmental variance estimates (Falconer 1989). Patterns of increasing nonshared environmental variance suggest, therefore, emergent GE interactions. In addition to G × E interactions , correlative associations may arise among genes and environments (GE correlations), which if not accounted for become part of the genetic variance term in biometrical models (Falconer 1989).
In this chapter, we reinterpret existing theoretical models of GE interplay using a lifespan perspective, focusing on the changing nature of the environments that impact cognitive function throughout adulthood. We then evaluate the models in relationship to existing evidence for GE interplay in cognitive aging, including investigations that tap recent advances in genotyping and gene expression that allow researchers to examine GE interplay at molecular levels.
1 Models of Gene–Environment Interplay in Cognitive Aging
Both behavioral genetic and lifespan perspectives provide theoretical models of the interplay of genes and environments applicable to cognitive function in adulthood, both in terms of GE correlation and G × E interaction.
1.1 Gene–Environment Correlation
Scarr and McCartney (1983) placed three forms of GE correlation into an early-life developmental context (infancy through adolescence): passive, evocative, and active. First, passive GE correlation occurs because in nuclear families children receive both their genes and their rearing environment from the same source: their parents. Because of the limited impact of rearing environment on measures of cognitive function in adulthood (Pedersen et al. 1992), it is logical to conclude that passive GE correlation will play a minimal role in adult development and aging (see Fig. 6.1). One possible exception is education, which can play a large role in cognitive functioning in late life, particularly with regard to cognitive reserve hypotheses for dementia (Glymour et al. 2012; Scarmeas and Stern 2003; see Chap. 7). Factors promoting educational achievement are complex but are likely to involve parental education as both an environmental and genetic source of variance. Second, environments and other people react to our (genetically influenced) traits and behaviors, creating evocative or reactive GE correlations. This process can only continue and perhaps even intensify as we move through adulthood experiencing changing societal expectations for cognitive function. Whereas society may expect, and thus promote, high levels of cognitive function in midlife , powerful stereotypes about cognitive decline in late adulthood may produce “social facilitation of the nonuse of competence” (Bieman-Copland et al. 1998). Because of a physical appearance of aging or frailty, older adults may evoke assumptions by others around them of cognitive frailty that inhibit attempts to maintain cognitive function. The competence–environmental press model emphasizes that functioning is maximized when the demands of the environment are sufficiently tailored to the individual’s ability to promote stimulation and maintenance of competence, and even growth (Lawton and Nahemow 1973).
Third, evidence for increasing genetic variance in late adulthood and the acceleration of nonshared environmental variance suggests that the most powerful form of GE interplay in cognitive aging are likely to be active GE correlations . Our choices shape our environment and that environment in turn shapes us; moreover, our choices are—at least to some extent—influenced by our genetic make-up. The environments we choose for ourselves are, by definition, unique to each of us and thus act as sources of nonshared environmental influence on cognitive function. For example, we choose our occupations and our working environments in turn impact our cognitive functioning (Andel et al. 2005; Finkel et al. 2009; Schooler and Caplan 2008). In the old-old age period, however, we predict that evocative GE correlational effects surpass active GE correlation . Increasing frailty and reductions in function are necessarily associated with reduced control over one’s environment (Rodin 1986, 1989) resulting in decreasing opportunities for active GE correlation. Similarly, as frailty and visible signs of aging increase, the response evoked from the environment will intensify, resulting in not only decreased functional independence but also decreased expectations of functioning and narrowing of social contexts.
1.2 G × E Interaction
G × E interaction processes are another set of factors that may bolster cognitive maintenance or precipitate declines in later adulthood. As described earlier, increasing nonshared environmental variance, observed uniformly for cognitive performance across domains (Reynolds 2008a; Reynolds et al. 2005), is a potential indicator of G × E interactions. Given the extant literature three decades ago, Scarr and McCartney (Scarr and McCartney 1983) proposed a relatively limited role of G × E interaction in development in early life (as opposed to GE correlation ), arguing that environmental “treatments” (e.g., adoption) that affect mean level (IQ) performance affect most individuals in like direction rather than altering individuals’ rank ordering. However, we argue for an updated view of the saliency of G × E interaction based on: (1) the qualitatively different impacts of stress during particular developmental periods (Lupien et al. 2009), in particular the “brain maintenance” phase in late adulthood; (2) familiality of epigenetic alterations to gene expression (thus impacting individual differences and potentially rank-order; Boks et al. 2009; Coolen et al. 2011); (3) discordance of monozygotic (MZ) twins in cognitive decline and dementia (e.g., MZ differences in memory trajectories associated with differences in depressive symptoms and moderated by APOE genotype; Reynolds et al. 2007); and (4) growing epidemiological literatures on the APOE gene and measured risk factors (Gatz 2007; Reynolds 2008a, b; see Fig. 6.2).
1.3 Epigenetic Landscape
Lifespan models of GE interplay provide additional means for conceptualizing the environments that impact cognitive aging. For example, we can apply Waddington’s epigenetic landscape (Waddington 1942) to cognitive functioning throughout the lifespan. Waddington emphasized that developmental pathways are shaped by evolution and thus are fairly robust to minor variations in environmental conditions. In contrast, Gottlieb (1991) stressed the influence of environmental variations on the genetic programming. Combining aspects of both genetic canalization (Waddington 1942) and experiential canalization (Gottlieb 1991), development occurs as genetically influenced pathways are impacted by environmental forces, giving rise to individual phenotypes. Although most research on cognition focuses on canalization processes that occur during childhood (e.g., see Chap. 2), there is no reason to assume that the process does not continue throughout the lifespan. Environmental forces (evoked, self-selected, or random) continue to impact cognitive functioning, pushing the individual into particular modes of functioning. Active and even reactive GE correlations can be seen in the context of the epigenetic landscape as continued canalization of cognitive function.
Genetic canalization may imply inflexibility and unmodifiability: once you are headed down a certain canal or path, there is no turning back. Genetic forces have impacted your phenotype and your behavioral options are thus limited. In contrast, with experiential canalization Gottlieb (1991) promoted the concepts of malleability and flexibility. Clearly, these ideas are appealing to researchers looking for interventions to slow or delay cognitive aging. Although Salthouse (2006) has questioned whether there is sufficient support for the disuse theory of cognitive aging, evidence for increases in environmental variance with aging provides hope that experiences may moderate genetic predispositions for cognitive functioning. The SOC model may provide an individual-centered adaptive framework for selecting appropriate goals for cognitive functioning, optimizing the allocation of internal and external resources, and compensating with additional (environmental) resources to counteract loss and decline (Baltes et al. 2006).
1.4 Characterizing the Environment
Clearly, a vital step in the application of any of these models is to determine the aspects of the environment that may have the most impact on the genetically influenced cognitive decline described in Chap. 5.
1.4.1 Compensating and Enhancing Environments
Balte’s SOC model implies an individual-centric view of active compensation for aging-related change and declines. On the whole, whether by individual agency or otherwise, enriched environments may compensate for genetic vulnerabilities, such as the “social context as compensation” model (Shanahan and Hofer 2005). Empirical evidence of environmental compensation has been supported by mouse studies in which enriched environments have mitigated cognitive deficits (Markham and Greenough 2004) such as those due to gene knockouts (NMDAR1; Rampon et al. 2000) or to dietary deficiencies (Lee et al. 2012), perhaps by altering gene expression related to metabolic processes, e.g., GLUT1 expression in cortex and CA1 region of the hippocampus (Harbeby et al. 2012). In humans, evidence for compensation effects can be observed in research on educational attainment and complex social environments on cognitive reserve. For example, social engagement in late adulthood may help to support cognitive functioning in late life despite the increasing presence of age-associated neural pathologies (Bennett et al. 2006), and higher educational attainment is associated with better cognitive performance and to a lesser extent smaller rates of decline (Glymour et al. 2012) and a lower risk of dementia (Ferrari et al. 2012). Moreover, it has been recently observed that high education and participation in leisure activities may lower the risk of dementia otherwise associated with carrying the APOE e4 allele (Ferrari et al. 2012), by serving to delay the onset of symptoms.
Enhancing environments refer to social contexts that interact with genes to promote higher levels of functioning (Shanahan and Hofer 2005). Evidence in support of this concept includes larger heritability estimates for educational attainment across recent generations (perhaps due to more open school access) and higher heritability of cognitive abilities with higher levels of socioeconomic status (SES) (cf., Shanahan and Hofer 2005; Chap. 2 in this volume). As we have noted earlier, heritability for general cognitive abilities increases with age to late adulthood, followed by significant downturns. Whether this pattern is a function of enhancing environments, i.e., reflecting G × Social Contexts (Shanahan and Hofer 2005), or active GE correlational processes (i.e., niche picking) remains to be established. We note, however, that with respect to lifespan development and aging, it is important to go beyond proportions of variance such as that conveyed by heritability statistics and consider “raw” genetic and environmental variances for a more accurate picture of changing genetic and environmental variance (Reynolds 2008a; Reynolds et al. 2005).
1.4.2 Benign Versus Adverse Environments
Both genetic factors and environmental influences may impact susceptibility to cognitive decline with aging. Investigations of GE interplay must be sensitive to the possibility of both benign and adverse effects (e.g., Boardman et al. 2012). For example, a genetic risk factor for cognitive decline (APOE e4) may interact with an environmental factor to exacerbate (head injury) or delay (education) changes in functioning (McArdle and Prescott 2010; Dardiotis et al. 2012). The “social trigger” model proposes that an environmental risk factor (lower education) may be required to elicit the impact of a genetic risk factor like APOE e4 (Reiss and Leve 2007; Shanahan and Hofer 2005). Alternatively, the “social push” model argues that disadvantageous environments may crowd out genetic effects on traits without necessarily playing a causal, or triggering, role (Raine 2002). Thus, according to the social push model, the impact of genetic influences on cognitive decline will only become apparent when disadvantageous social conditions (e.g., low education) are minimized. In contrast, the “social trigger” model posits that genetic influences on cognitive decline will be greater when unfavorable social conditions are maximized (Reiss and Leve 2007; Shanahan and Hofer 2005). It is important to note that the social push model was originally developed for antisocial behavior and related traits and the impact on discussions of cognitive aging has only begun to be explored (Boardman et al. 2012).
1.4.3 Perceived Versus Objective
Is perception reality? It is likely that individual perception moderates the impact of environmental factors. Psychologists attempt to collect objective measurements of environmental factors like SES and availability of social contact, but often the measures used are obtained via self-report; for example, recent evidence suggests that self-reported social participation may drive subsequent changes in perceptual speed (Lovden 2005). However, it is possible that individual perceptions of SES and satisfaction with social engagement color self-reports of ostensibly objective measures, but are equally important in their own rights. Indeed, perceived or subjective environments have been notably referred to as “effective” environments (Rutter 2012). Studies comparing the predictive value of subjective and objective SES for various health outcomes in older adults routinely report that subjective SES is the better predictor (e.g., Singh-Manoux et al. 2005); the same may be true for cognitive decline.
In particular, SES lends itself to the social comparisons inherent in subjective perceptions. As a social species, people are sensitive to the social interactions and disparities that occur within their various social settings. The work place is one example of a social setting in which individuals are organized in a ranked system from dominant to subordinate individuals. Yet, social interactions that highlight resource differences between individuals are prevalent not only in the workplace. At the societal level, social stratification of resources has been associated with a gradient in level of health, with average individual health improving at each level on the social ladder (Adler et al. 1994). This social stratification is not unique to our own species. Primate models suggest well-being may be impacted via hierarchical social systems within primate social groups even when environmental resources are held equal (Sapolsky 2005), an effect that is likely mirrored in our own species (Boyce 2007; Sapolsky 2004). Understanding how these social interactions may impact the development of subjective or “effective” environments should contribute to our understanding of the SES impact on cognitive aging. Social comparisons add a subjective facet to SES, contributing to an individual’s perceptions of their own economic situation. These perceptions, although largely influenced by familial and nonfamilial environmental factors, likely reflect the impact of genetic factors as well (Lichtenstein et al. 1992). Individuals with fewer resources but equally impoverished peers may feel more financial satisfaction than individuals with relatively more resources but surrounded by wealthier peers (Liang and Fairchild 1979). Clearly, the distinction between objective and subjective measures can extend to many of the environmental factors commonly believed to impact cognitive decline: life events, health events, etc.
1.4.4 Proximal Versus Distal
A primary issue in uncovering GE interplay, whether environments are enhancing, compensating, adverse, perceived or otherwise, is one of timing: do proximal or distal environmental factors have a larger impact on cognitive aging? A strict application of Waddington’s epigenetic model , for example, would hypothesize that environmental selection was more salient during early development than during aging; thus the theory would likely nominate distal (early) environmental factors for dominant roles in GE interplay in cognitive aging. Recent longitudinal analyses support this hypothesis, reporting that IQ at age 11 was the strongest predictor of IQ at age 79, over and above concurrent SES and education (Gow et al. 2011). Other research, however, indicates that late-life functioning has many unique aspects and may not relate to variables that predicted functioning even in midlife (e.g., Vaillant 2002). Thus, proximal (or concurrent) environmental factors may play large roles in cognitive aging and may underlie the reported increases in nonshared environmental variance. In mouse models, lifelong enriched environments benefited learning and memory processes across development, whereas enriched exposures appeared to improve performance when introduced in young and middle age, but not when introduced only in late life (Harati et al. 2012), suggesting the saliency of early life as well as later developmental periods.
2 Evidence for Gene–Environment Interplay on Cognitive Aging
Many methods exist for investigating these potential forms of GE interplay in cognitive aging, including analysis of variance components, experimental and epidemiological approaches, and methods that focus on SES and other social and potentially stressful environments. Our review of the literature indicates that some methods remain relatively untapped; thus there is significant potential for future developments.
2.1 Biometrical Approaches to G × E
Variance components approaches consider measured environmental factors as they moderate genetic variance for cognitive aging phenotypes. Typically, such models have been applied to twin data, evaluating information from both MZ and dizygotic (DZ) pairs in an extension of classic biometrical models (Purcell 2002). As described in Chap. 2, higher heritability estimates for childhood and adolescent cognitive ability have been observed as social status and prosperity levels increased suggesting the presence of G × E interaction (Harden et al. 2007). However, these findings have not been fully replicated, and the heterogeneity in findings may be due in part to a lack of consideration of the overlapping variance among SES and cognitive traits (cf., Johnson et al. 2009). Indeed, there is some suggestion that social status may moderate environmental not genetic variance (Hanscombe et al. 2012). Moreover, there have been inconsistent findings in adult twin samples, for example, as to whether parental education modifies heritability of cognitive performance and during what developmental period (i.e., early vs. middle adulthood Grant et al. 2010; Kremen et al. 2005). Applications of the variance components approach to cognitive aging suggest that education moderates both genetic and shared environmental influences in late adulthood (Johnson, et al. 2009). To date, no studies have applied this approach to the longitudinal case. Power of the G × E variance components approach has been evaluated with suggestions that minimally 5,000 twin pairs are required to detect moderation of genetic variance (Hanscombe et al. 2012), which may be a factor in the inconsistent findings. Recent methodological work suggests that false positives are a potential problem with typical G × E models and suggests expansions of the approach to include full appreciation of the genetic and environmental covariance structures of both the putative environmental moderator and phenotype of interest (van der Sluis et al. 2012). The G × E variance components model has been extended to consider gene candidates in an association context (van der Sluis et al. 2008) as well as for the case of ordinal and binary traits (Medland et al. 2009).
A second method for evaluating G × E interaction focuses on MZ twin similarities vs. differences (Fisher 1925; Jinks and Fulker 1970; Martin et al. 1983; van der Sluis, Dolan et al. 2006). Because MZ pairs share identical genotypes, any within-pair differences are attributed to environmental factors unique to the individual pair members, i.e., the nonshared environment . If associations exist between the shared genetic factors and the environmentally driven differences, then it is taken as support for G × E. In terms of cognitive aging, one may use such methods to compare differences in trajectories between MZ pairs and relate these differences to measured gene candidates and environmental exposures. The MZ-only approach inspired by Fisher (1925) considers first whether heterogeneity exists (Fisher 1925; Martin et al. 1983), i.e., whether there are mixtures of within-pair or intrapair difference distributions. If so, presence of gene candidates may then be measured in combination with environmental factors to consider whether they could contribute to the heterogeneity in MZ pair differences (Martin et al. 1983; Reynolds et al. 2007). To avoid false-positive tests of G × E, it is critical that the outcome traits are normally distributed (Jinks and Fulker 1970). A G × E analysis of MZ twins from the Swedish Adoption Twin Study of Aging (SATSA) suggested the presence of GE interactions for cognitive tasks that are particularly dependent upon semantic or episodic memory. Specifically, variations in genes regulating aspects of serotonin (5HTT, HTR2A), estrogen (ESR1a), and cholesterol (APOE e4) interacted with the exposure by those individuals to unique environmental factors that predicted differential semantic or episodic memory change (Reynolds et al. 2007). First, we evaluated and observed significant heterogeneity in within-pair differences, i.e., intrapair variability in memory trajectory features, including performance level at age 65, linear change at age 65, and nonlinear change across age. Moreover, the intrapair variability in memory trajectories differed by genotype whereby those MZ pairs who did not carry the risk alleles showed greater differences in semantic and episodic memory change than those who did carry risk alleles (e.g., APOE e4). Last, the intrapair variability of depression was shown to be associated with the intrapair variability of longitudinal memory change, however, only for noncarriers of either the APOE e4 allele or ESR1a rare allele (rs1801132). This result indicates that noncarriers of these risk alleles for dementia may have greater sensitivity to environmental sequelae that result from depressive symptoms and thereby show differential memory trajectories; however, carriers of risk alleles, who otherwise have an elevated risk of decline (especially vis-à-vis APOE e4), may be less impacted by environmental challenges posed by depression. The findings for APOE are consistent with work on cognitive health and dementia that suggest that non-e4 carriers may be relatively more sensitive to a variety of environmental factors than APOE e4 carriers, while APOE e4 carriers may be more sensitive to vascular risk factors (see Gatz 2007). Indeed, related cognitive aging findings also support this conclusion, as female APOE e4 homozygotes who were more aerobically fit showed better cognitive performance (Etnier et al. 2007). Subsequent studies of physical activity (see later), suggest a range of findings of enhanced effects, compensation effects or no appreciable moderation of physical activity and APOE e4 status on cognitive performance and brain phenotypes (Erickson et al. 2012) indicating further work is necessary to elucidate when and what type of GE interplay is at work.
2.2 Experimental and Epidemiological Approaches
Genetic–epidemiological approaches examining candidate gene variants and environmental exposures in unrelated individuals have also been used to identify G × E associations. For example, nondemented APOE e4 carrying adults ages 16 and 65 years who had sustained head injuries performed worse on verbal memory and attention and perceptual speed tasks 6 months postinjury (Ariza et al. 2006). Untreated hypertension in the presence of positive APOE e4 status was associated with poorer cognitive performance in nondemented women from the Nurses’ Health Study (Kang et al. 2005).
Individuals in deprived neighborhoods are often exposed to more toxins, lower quality housing, and violence; these conditions subject individuals to a higher allostatic load (Evans 2004). Indeed, a study of neighborhood effects and APOE e4 status (Lee et al. 2011) indicated that living in a neighborhood rife with “psychosocial hazards” (e.g., higher crime rates, economic deprivation, familial disruptions, lower educational attainment, poorer infrastructure upkeep, etc.) coupled with carrying the APOE e4 allele predicted worse processing speed and executive performance in adults aged 50–70 years old enrolled in the Baltimore Memory Study. Memory abilities did not show a clear neighborhood environmental effect, although patterns of performance were suggestive of the expected APOE e4 effect. However, recent longitudinal evidence from the Chicago Health and Aging Project supports an interaction, suggesting that APOE e4 coupled with lower ‘neighborhood social disorder’ predicts change in general cognitive functioning (Boardman et al, 2012). Hence, more work is needed.
Physical activity may also interact with APOE e4 allele status to predict cognitive functioning (Erickson et al. 2012). As introduced earlier, a study of female APOE e4 homozygotes who performed better on an in-person aerobic fitness test demonstrated higher cognitive performance on a variety of cognitive measures including learning and attention tasks (Etnier et al. 2007). In a recent population-based study of 1,799 participants aged 60 years or older in the NHANES III study with available APOE genotyping (Obisesan et al. 2012), increased self-reported physical activity predicted better cognitive status performance in non-e4 carrying individuals but not e4 carrying individuals between the ages of 60 and 69, with adjustments for illnesses burden and mobility restrictions. However, in those older than 70 years, physical activity benefitted all individuals, including those who carried an e4 allele although the effect dropped when accounting for mobility restrictions (Obisesan et al. 2012). The physical exercise by APOE e4 genotype interaction was supported by recent brain imaging work suggesting that self-reported physical exercise engagement was associated with amyloid plaque deposition in a sample of adults between 45 and 88 years (Head et al. 2012). Sedentariness was most detrimental in terms of increased amyloid plaque deposition in those who were APOE e4 carriers (Head et al. 2012). Although the NHANES III study is cross-sectional , the findings of differential impact of physical activity on cognitive functioning by age suggest an age dependency of GE interplay effects with respect to APOE, perhaps due in part to selectivity resulting from morbidity or mortality. Whether physical activity is particularly beneficial to or merely mitigates risk for APOE e4 carriers is not yet clear (Erickson et al. 2012), but it is likely that a developmental framework taking into account age dependencies is important.
2.3 Socioeconomic Status and Cognitive Aging
In cognitive testing and measures of IQ, researchers have observed differences in cognitive abilities across levels of SES. Often, privileged individuals perform better on cognitive tasks compared to individuals of low SES. Initial explanations of these observed differences attempted to disentangle the impacts of genetic and environmental influences (to some controversy). One might be tempted to conclude that the apparent relationships between perceived SES and health and cognitive aging are generally due to environmental pathways. The etiological factors underlying cognitive performance, educational attainment, and cognitive dysfunction are indicative of the expected complexity. Although environmental pathways may largely influence the association between education and dementia risk (Gatz et al. 2007), one study indicated that the genetic factors that do underlie education and mental status performance among typically aging adults overlap completely with the genetic factors attributed to general cognitive ability (Pedersen et al. 1996). Moreover, Schooler and colleagues propose a person–environment pathway whereby individual difference traits (e.g., self-directedness) and occupational features contribute to later intellectual functioning (Schooler and Caplan 2008). Specifically, higher SES coupled with cognitive ability leads to more demanding and self-directing occupational contexts (Schooler and Caplan 2008), an example of active GE correlation . Such contexts thereby boost cognitive functioning, amplify early-life SES effects and mutually benefit self-directedness and intellectual flexibility. Based on a series of analyses of two-wave data collected 20 years apart, those with occupations that were high on substantive complexity, more self-directed, and less routine predicted positive reciprocal relationships with intellectual flexibility, i.e., mutually increased flexibility and self-directedness, accounting for baseline levels, respectively. Similarly, the complexity of household work may similarly impact intellectual flexibility (Caplan and Schooler 2006; Schooler and Caplan 2008). While these results are in no way definitive of GE processes, the findings provide candidate life course pathways to evaluate from a genetically informative perspective.
Studies of aging in rodent models underscore the positive effect of environmental complexity on dendritic growth, and these benefits seem to obtain throughout the lifespan of the aging rat (Greenough et al. 1986; Markham and Greenough 2004; Mohammed et al. 1993). Animal research continues to show evidence that the surrounding environment can alter the expression of genes in neurons (Harbeby et al. 2012; Mohammed, et al. 1993; Pinaud et al. 2002). With respect to cognitive phenotypes, lifelong enriched environments support maintenance of learning and memory processes, and introducing enriched exposures in young and middle age appear to be restorative though perhaps not in late life (Harati et al. 2012), suggesting that environmental interventions may have a more limited impact as plasticity wanes. While SES as a developmental context is much more complex than the experimental environments of lab animals, the overall implications of the epigenetic forces at play in cognitive development evident from this body of work should not be overlooked.
SES has become an important contextual marker in measuring environmental experiences as a proxy for exposure to toxins, nutrition, education, and leisure activities (Evans 2004). Much of the research in brain functioning and late-life cognition has focused on incidence of dementia (discussed in Chap. 6). Individuals from low SES are at higher risk for developing dementia. One of the theories posited for the relationship between SES, cognitive functioning, and dementia is the hypothesis of cognitive reserve (Staff et al. 2004), with evidence from Swedish studies supporting increased reserve largely via education and occupational complexity (Andel et al. 2005; Andel et al. 2007; Andel et al. 2006). Controlling for education, the complexity of one’s occupation prior to retirement, particularly with respect to working with people (e.g., mentoring roles vs. subordinate roles), supported the relative maintenance of cognitive performance for verbal and spatial skills whereas in postretirement, spatial performance dropped (Finkel et al. 2009). In Sweden, age 65 is a mandatory retirement age and it is uncommon to work formally past this point, which constrains the extent to which GE correlational processes might otherwise play out with continued working. Taken together, empirical findings support the work context as a measureable environmental influence on cognitive aging. As a second example, we note findings that individuals with higher levels of educational attainment may still perform at preclinical levels on the Mini Mental Status Exam even when comparable amounts of brain atrophy are otherwise indicative of Alzheimer’s disease in lower SES individuals (Fotenos et al. 2008). Most interesting are those individuals who remain undiagnosed as demented at time of death. The question stands: What aspects of education and higher SES have afforded these individuals protective cognitive resources that allowed them to function with otherwise biologically compromised brain structures? Additionally, what aspects of genetic endowment have contributed to healthy cognitive aging? To begin answering these questions, we look to current research in brain imaging for a preliminary conjecture until researchers further address these questions in older populations.
SES as a contextual marker of differing environmental conditions underscores the sensitivity of human cognitive aging to variations in environmental conditions. Moreover, perception of SES can augment the impact of objective SES, per se. For example, in a sample drawn from three longitudinal studies of aging in the Swedish Twin Registry, an individual’s perceived SES was predicted of cognitive performance at age 75 for perceptual speed , spatial performance, verbal memory, and episodic memory (Zavala et al. 2013, in preparation). This was particularly true for the oldest cohort, perhaps suggesting the impact of early environments on perceptions of later environments. Overall, individual differences in cognitive performance and decline within and across SES environments highlight the fundamental biological nature of this sensitivity evident in individual brain structure and function. To gain a greater understanding of the mechanisms involved in cognitive aging across the SES spectrum, research in epigenetics may provide clues to possible GE interplay occurring within the human brain, especially with concern to individual differences in plasticity and susceptibility to environmental influences in neuronal gene expression as described later.
2.4 Social/Stressful Environments
We have given primacy to SES and related indices as observable, albeit global, markers of environmental contexts or exposures that may interact with genotype to lead to poorer or better cognitive aging. Physiological and psychological stress may be greater in lower SES contexts (Matthews and Gallo 2011). Recent work on gene expression and childhood SES (Miller et al. 2009) suggest that being raised in disadventageous childhood SES contexts may lead to differential gene programming that potentiates aging-associated dysfunction and disease. Specifically, findings suggested that adversity predicts elevated gene expression in the proinflammatory-immune pathways (CREB/ATF) and reduced expression of glucocorticoid receptor response elements (NF-κ), leading to greater production of stress markers such as cortisol and interleukin 6 (IL-6), a cytokine that is elevated at sites within the body given the presence of acute or chronic inflammation. There was also elevated expression of genes coding for inflammatory mediators (other cytokines or enzymes) such as IL1A, CCL2, CXCL2, CCL20, as well as such as OLR1 and GPR132, which initiate inflammation processes such as macrophage scavenging of oxidized low-density lipoproteins that may lead to accumulations of atherosclerotic plaques and risk of myocardial infarction. The altered gene expression patterns due to exposure to early adversity are presumed to be initiated before adulthood, as controlling for current SES, lifestyle habits, and perceived stress did not alter the described findings (Miller et al. 2009). Inflammatory biomarkers , such as IL-6, as well as CRP, TNF1A, and ICAM-1, have been linked to cardiovascular disease risk and dementia (Dziedzic 2006), as well as normative cognitive aging performance and decline (Gimeno et al. 2008; Jordanova et al. 2007; Krabbe et al. 2009; Mooijaart et al. 2011; Rafnsson et al. 2007; Schram et al. 2007; van den Kommer et al. 2010). Some studies suggest that the presence of cardiovascular disease may be a moderator of the association between inflammatory biomarkers and cognitive decline (Hoth et al. 2008). Last, mouse models directly support the connection between early social adversity and age-associated impairments in spatial memory, which were associated with alterations in hippocampal BDNF expression and synaptophysin immunoreactivity, suggesting both structural and plasticity-related sequlae of the exposure to chronic social stress (Sterlemann et al. 2010).
A growing body of research in both human and animal literatures supports altered brain structures (particularly the hippocampus ) and altered cognitive performance as a consequence of early adversity writ large, not only in terms of socioeconomic adversities but also in other forms of early adversity including childhood maltreatement, combat exposure, and other stress exposures (for review, see Pechtel and Pizzagalli 2011). Last, evidence suggests that perceived (but not objective) social isolation increases gene expression of an array of genes involved in inflammatory processes (Cole 2009). Thus, the perceptions of environmental adversity may be just as salient in some cases, or even more so, than objective adversities (which may indeed become “effective” environments; cf., Rutter 2012).
In sum, the emerging evidence on early adversity would suggest that adverse life experience, objective or perceived, leads to differential gene expression and downstream effects on brain structure and plasticity that may eventually show notable impacts on cognitive performance across the life course and differential impacts on cognitive decline in late life. However, the current findings on early adversity, differential gene expression, and adult outcomes are relatively slim as yet, let alone the findings for cognitive outcomes. Much work is needed from a prospective life course perspective to fully evaluate the direction of effects and extent of impact on adult cognitive performance and aging before strong conclusions can be reached.
3 Biomarkers of Gene–Environment Interplay
3.1 Brain Morphology
As described in Sect. 6.1.3, Gottlieb’s theory of experiential canalization highlights the interaction of biological systems with the surrounding environment (Gottlieb 1991). Variation in brain structure and function among identical twins has been found, suggesting that structure and function are at least partly experience-dependent, and possibly reflective of GE interaction (Thompson et al. 2001). In an adult twin study, average age 48.2 (SD = 3.4 years), 10 pairs of MZ twins (both male and female pairs) had higher similarities in quantity of frontal gray matter than the 10 pairs of DZ twins. Included among the regions examined were cortical language regions, i.e., Broca’s and Wernick’s area (Thompson et al. 2001). Predictably, individual differences in gray matter were related to individual differences in IQ. A subsequent study of MZ and DZ twins from the VETSA study, average age 55.8 (SD = 2.6 years), that was 10-fold larger in sample size, suggested that heritability estimates varied within the frontal cortex, and findings were consistent with respect to a high heritability in Wernicke’s area but not Brocas’s area (Rimol et al. 2010). Changes in GE interplay influencing the frontal cortex and language areas of the brain across the lifespan would be consistent with heritability changes in cognition. The frontal cortex, in particular, may be subject to changes in heritability across the lifespan due to the protracted developmental timeframe (see Giedd et al. 2010). The extended developmental period typical of the frontal cortex may allow for individuals to influence their own development through active GE correlational processes as individuals seek out environments and experiences most consistent with their general cognitive abilities (such as noncompulsory higher education). The role of the environment as an enhancer of potential has been raised in interpreting findings of GE interaction on child and adolescence achievement and IQ as noted earlier (cf., Shanahan and Hofer 2005; Chap. 2 in this volume).
Evidence for continued plasticity within adult brain structure and function suggests an inherent framework by which dynamic genetic and environmental processes may play out to shape cognitive aging trajectories throughout the adult lifespan. In the brains of adult twins (42 years of age and older), magnetic resonance imaging reveals that heritability is not uniform across nor within brain structures (Chen et al. 2012; Giedd et al. 2010; Pfefferbaum et al. 2004; Sullivan et al. 2001; Thompson et al. 2001). In a twin sample of World War II veterans, brain mapping revealed a heritability of about 15–26 %, while other brain structures exhibited evidence of greater genetic influences, including the bilateral temporal horn (38–47 %) and the corpus callosum (46–48 %; Sullivan et al. 2001). For MZ and DZ twin pairs, though genetic influences remained stable across 4 years follow-up, evidence suggested environmental influences on the lateral ventricles increased with age (Pfefferbaum et al. 2004). In particular, the plasticity of the hippocampus is one of the most well-documented phenomena in the study of brain morphology (for a review, see Neves et al. 2008). For example, changes in individual behaviors, such as exercise, can lead to changes in hippocampal brain volume in aging adults (an effect associated with the BDNF gene), leading to increased performance on memory tasks (Erickson et al. 2012). Furthermore, the role that the hippocampus plays in episodic memory (Chadwick et al. 2010) may help to partly explain changes in genetic and environmental variance in memory ability in late life (e.g., Reynolds et al. 2005). For an extended discussion of brain morphology and cognition, see Chap. 8.
3.2 Epigenetic Processes
An individual’s genotype may provide a guide for the development of biological systems, but recent research supports the concept of probabilistic epigenesis (Gottlieb 2007) : a cascade of feedback between genes and the environment that may result in changes in gene expression and cell senescence within the living organism that are not a result of DNA sequence variation or somatic mutations. Advancements particularly in mouse models, but including work on human cognitive disorders, have provided evidence that epigenetic modifications are important to cognition broadly, including learning and memory , and implicated in cognitive disorders such as dementia. Moreover, emerging evidence suggests that DNA biomarkers such as telomere length are associated with cognitive performance and risk of dementia in aging adults.
Epigenetic processes reflect ubiquitous forms of G × E interplay that occur at an environmental-by-molecular level. Epigenetic modifications include acetylation, phosphorylation, and methylation of histone proteins , components of the chromatin, as well as direct methylation of DNA (Day and Sweatt 2010, 2011, 2012). Particular combinations of histone modifications result either in activation or suppression of gene transcription (Day and Sweatt 2011, 2012). Moreover, the persistence of various histone modifications may be of brief duration, while methylation may have a relatively longer time-course (Day and Sweatt 2011). Of particular interest is 5-methylcytosine, i.e., methylation of cytosine-5, occurring at CpG (i.e., CG sequence) rich genomic regions denoted “islands” that occur in or near gene promoter regions. Such methylation has been demonstrated in a variety of human tissues, including brain, muscle, and leukocytes (Fernandez et al. 2012) and it is associated with aging and neurological disease (Boks et al. 2009; Christensen et al. 2009; Fernandez et al. 2012). Moreover, specific methylation patterns may be associated with some forms of neurological disease (e.g., dementia with Lewy bodies ), although particular patterns for Alzheimer disease are thus far elusive in one of the largest studies of methylation across tissue types and across 1505 CpG sites (Fernandez et al. 2012).
Recent evidence suggests that histone modifications may be relevant to learning and memory processes as well to disease risk spanning “susceptibility to stress,” depression , addictions, and cognitive disorders including Alzheimer’s disease and Huntington’s disease (Day and Sweatt 2012; Graff and Mansuy 2009), suggesting that such modifications may be important to cognitive aging. Epigenetic dysregulation of the amyloid precursor protein may explain beta-amyloid production or deposition (Maekawa and Watanabe 2007; Scarpa et al. 2006; Wang et al. 2008; Wu et al. 2008), processes implicated in Alzheimer’s disease neuropathology. Moreover, epigenetic modifications including methylation have been linked to the formation of memories vis-à-vis alterations of hippocampal gene expression in mouse models (Day and Sweatt 2010), such as BDNF (Day and Sweatt 2010; Lubin et al. 2008). While methylation processes in the hippocampus appear to be relatively dynamic, relatively lasting methylation processes underlying remote memory storage in cortical regions have been implicated in the anterior cingulate cortex (Day and Sweatt 2012; Miller et al. 2010).
G × E interplay may be seen in the environmental factors that impact the extent of global DNA methylation . Again, while empirical evidence for cognitive aging outcomes is not yet available, the available findings suggest that such mechanisms could play a role. For example, Fraga et al. (2005) highlighted the increasing differences in DNA methylation profiles for identical twins in a cross-sectional study . The oldest twins with the most divergent self-reported health histories had more divergent acetylation of histones as well as indices of global methylation (Fraga et al. 2005). Moreover, the chromosomal locations of divergent methylation patterns in normal metaphase chromosomes included telomeric regions among twins who differed in global methylation (Fraga et al. 2005). Methylation of 88 gene loci assayed from saliva samples has been shown to be linearly related to chronological age and touted as a biomarker of biological age (Bocklandt et al. 2011). The primary analysis was conducted on 34 male twin pairs and replicated in unrelated male and female individuals. Results highlighted methylation in the promoter regions of the EDARADD and TOM1L1 genes were strongly associated with age in both males and females. This emergent work suggests that environmental factors associated with loci-specific methylation may be important to consider for cognitive aging. Indeed, calorie restriction has been shown to relate to epigenetic processes in the hippocampus in mouse models (Chouliaras et al. 2010b). Additionally, physical exercise is proposed as a promising environmental factor given the numerous studies linking exercise and cognitive performance in older adults and mouse models showing altered expression of genes involved in learning and memory, including BDNF (Kaliman et al. 2011).
The extent to which epigenetic processes, particularly in basic learning and memory processes, are indicative of GE interplay for cognitive aging writ large remains to be addressed. While epigenetic processes are separate from DNA sequence variation by definition, genetic influences on methylation are evident from examinations of significant twin concordance for methylation (Coolen et al. 2011); indeed, heritability of DNA methylation patterns may be gene-specific (Boks et al. 2009). Hence, a full understanding of epigenetic mechanisms is not yet within grasp (Feil and Fraga 2012), including the extent to which epigenetic alterations promote or are a consequence of cognitive aging or dementing processes (Chouliaras et al. 2010a); this necessitates longitudinal measurement of DNA methylation or other epigenetic markers as well as cognitive performance.
3.3 Telomere Shortening
Telomeres are segments of DNA bases that cap the ends of chromosomes. Telomeres become shorter and shorter over the course of thousands of cell divisions and are associated with cellular senescence (Shawi and Autexier 2008). With the loss of telomere length, risk of somatic mutations and damage during cell division may increase (Aubert and Lansdorp 2008). With respect to cognitive aging, a study of female twins who averaged 50.6 years in age (range 19–78 years) from the Twins UK sample suggests that longer telomere lengths are associated with better working and episodic memory performance (Valdes et al. 2008). Moreover, in pairs discordant for telomere length, a shorter telomere length was associated with worse performance relative to the cotwin with longer telomere length (Valdes et al. 2008). However, not all studies find associations with telomere length and dementia risk or related neuropathologies (Lof-Ohlin et al. 2008; Lukens et al. 2009; Martin-Ruiz et al. 2006; Zekry et al. 2010; Zekry et al. 2008). In fact, a recent review of telomere lengths as a biomarker of aging suggests additional work is necessary, particularly from a longitudinal perspective, to ascertain its potential importance (Mather et al. 2010). Differences in findings may be attributable to what tissues are sampled, with a recent study suggesting that shorter telomere lengths measured from buccal or white cells were significantly associated with a Alzheimer’s disease diagnosis, but longer telomere lengths among those with Alzheimer’s disease were observed from hippocampal brain tissue samples (Thomas et al. 2008). Moreover, longer telomeres , as measured from leukocytes tissue, have been observed among nondemented adults (age range 41–81 years) who carried the APOE e4 allele than among noncarriers (Wikgren et al. 2010); this result was noted particularly among younger adults in the study. Last, longer telomere lengths among APOE e4 carriers predicted worse episodic memory performance (Wikgren et al. 2010). The study authors suggested that altered cell maintenance processes may be features of APOE e4 carriers. Taken together, tissue type and genotype may underlie the complexity of telomere length findings on cognitive aging traits. Moreover, variation in methods to assess telomere lengths may be a critical consideration as well (Vera and Blasco 2012).
Environmental factors that predict telomere shortening include SES, stress, and inflammation, all factors that are associated with more rapid cognitive aging (see Chap. 5). First, differences in twins’ telomere lengths can be seen as evidence that phenotypic differences in biomarkers cannot be solely attributed to differences in genetic factors. Second, telomere shortening may occur due to exposures to both psychosocial and physical stressors. For example, shorter telomere lengths are associated with greater perceived stress (Epel 2009; Epel et al. 2004), mood disorder (Epel 2009; Epel et al. 2004), and low SES (e.g., Cherkas et al. 2006). Telomere length is related to physical stressors as well, such as cancer, CVD (Gilley et al. 2008), inflammation (Carrero et al. 2008), and oxidative stress (Houben et al. 2008). Of particular interest, Cherkas et al. (2006) illustrated that female identical twins divergent on SES had significantly different telomere lengths after controlling for BMI, physical activity , and smoking profiles. One implication of such research is that low SES may be a salient risk factor for biological aging as well as cognitive aging. Preliminary longitudinal evidence appears to bear out patterns from cross-sectional findings (Biegler et al. 2012), but it is clear that more work remains to be done, particularly with cognitive aging outcomes.
4 Conclusions and Future Directions
In the course of reviewing a diverse set of literatures on GE interplay on cognitive aging, it is apparent that while many threads suggest the potential importance of GE correlation or G × E interaction on cognitive aging there remains a dearth of studies dedicated to addressing these processes directly, particularly from an informative behavioral genetic perspective that can evaluate the etiologies of phenotypes and “environments.” Theories of development and aging suggesting the pertinence of GE processes have been in place while the empirical data are more or less wanting, particularly with respect to normative cognitive aging. For example, as described in Sect. 6.1.3, Baltes’ SOC model (see Baltes et al. 2006) can be framed as an individual-specific active GE model whereby individuals adapt and reinvest energies in order to maximize or maintain (cognitive) skills in the face of increasing functional loss with age. It is also the case that as individuals lose function, their own personal agency decreases and evocative environmental GE correlations may become increasingly important (see Fig. 6.1). Nevertheless, the extent to which genetic factors are actually correlated with environments that provide more or less support for cognitive skills is unknown (e.g., social interaction vs. isolation). Moreover, it is behavior that mediates the relationships between genes and environments (Rutter 2012), and thus genetically mediated behaviors that appropriate or evoke particular environmental contexts in late life, conducive or not to cognitive maintenance, are perhaps ripe for deeper examinations of GE correlational processes. Educational and occupational attainment as well as leisure activities and physical exercise may reflect GE correlational processes and indeed explain why heritability of cognitive ability increases up to late life.
The extent to which environmental interventions in late life support or improve cognitive function, particularly for those predisposed to cognitive decline (or dementia) due to risk genotypes, such as APOE e4, is not yet clear. Emerging evidence is perhaps encouraging: higher education and participation in leisure and physical activities may lower the risk of cognitive decline or dementia otherwise posed by carrying the APOE e4 allele (Ferrari et al. 2012). However, when one begins to engage in beneficial pursuits may matter: interventions introduced in young or middle age may be beneficial but late-life interventions may be met with more limited success (Harati et al. 2012). In contrast, we should not discount the fact that some environmental factors seem to be more pertinent in late old age than earlier (e.g., physical activity; Head et al. 2012; Obisesan et al. 2012). Moreover, GE interplay may differ for APOE across the life course and APOE genotype may relate to which environmental factors are salient (Gatz 2007; Reynolds et al. 2007). Last, the unique impacts of particular activities on cognitive aging outcomes remain to be elucidated, using appropriate and rigorous control groups. The social and cognitive features of particular physical activities may be relevant to unpack to determine the underlying bases of the associations with cognitive performance and change (Miller et al. 2012).
This chapter has focused only on selected gene candidates beyond APOE that may interact with environmental factors and impact cognitive or brain aging, e.g., BDNF as well as others in estrogen or serotonergic neurotransmitter pathways. This focus largely reflects the extant literature. Indeed, epigenetic processes —potential biomarkers of GE interplay —are implicated in basic memory formation and maintenance (e.g., BDNF; Day and Sweatt 2012; Graff and Mansuy 2009), and thus may be critical to day-to-day and even moment-to-moment adaptations to environments. Familiality of methylation levels and telomere lengths (Bakaysa et al. 2007; Bischoff et al. 2005, but see Huda et al. 2007; Bocklandt et al. 2011; Boks et al. 2009; Coolen et al. 2011) suggests that genetically driven differential sensitivities to environments (e.g., stress) may be important to individual differences in cognitive aging. However, evidence from MZ differences or discordancy approaches suggests interaction of G with nonshared E may also be salient (Reynolds et al. 2007; Valdes et al. 2008), although much work remains to be done. Indeed, the consideration of biomarkers of GE interplay processes is relatively recent with a lot of suggestive findings, but not yet a lot of data, particularly for cognitive aging outcomes.
Importantly, the most recent work in gene-finding efforts using genome-wide association (GWAS) approaches affirms the polygenic nature of general intelligence traits (Davies et al. 2011) and cognitive decline (Davies et al. submitted), with few “hits” beyond APOE and neighboring genes such as TOMM40. That is, genes of very small effect contribute to cognitive abilities, with up to 51 % of the variance in spatial/fluid cross-sectional performance accounted for by thousands of SNPs included in the GWAS (Davies et al. 2011). Moreover, 24 % of genetic influences on general cognitive ability may differ (i.e., new genes) between childhood and late adulthood (Deary et al. 2012), which would be consistent with previous SATSA longitudinal work reporting evidence of increasing genetic variation up to age 65 (Reynolds et al. 2005). That working and episodic memory traits display both increasing genetic and environmental variance (Reynolds et al. 2005; Reynolds 2008a) is consistent with the putative time-dependent impact of APOE on dementia risk as well as the variety of significant APOE × E effects. Moreover, it has been argued that APOE is one example of a “plasticity” gene (Chen et al. 2010; Holtzman and Fagan 1998; Myers and Nemeroff 2012; Nichol et al. 2009; Petit-Turcotte et al. 2005; Teter 2004; Weeber et al. 2002); BDNF (Fritsch et al. 2010; Li Voti et al. 2011) and dopaminergic or serotonergic candidates (cf., Belsky and Beaver 2011; Belsky et al. 2009; Rutter 2012) could be added to the argument. Certainly, pleiotropic effects appear to be evident. Nonetheless, taking a broader approach to consider multiple genetic variants within and across genes will be more informative, compared to single markers, given the current status of genetics research to date (Dick 2011). The paucity of strong genetic signals from candidates, apart from APOE, likely a consequence of SNP-by-SNP evaluations, may also suggest the importance of considering GE interplay, albeit with much caution in an essentially postGWAS era (Dick 2011).
It is also important to consider differential GE interplay for men and women, particularly in the timing of GE associations. All too often sex is treated as a covariate to be controlled rather than considered as a moderator. Recent studies suggest men may be at greater risk than women for mild cognitive impairments (Roberts et al. 2012), while it has been long established that the prevalence of dementia among women is greater (Alzheimer's Association 2012). Twin studies have not found appreciable evidence for differential sex effects on longitudinal trajectories for normative cognitive aging of most traits, with the exception of verbal ability (Finkel et al. 2006). However, risk factors such as serum lipids may differentially predict cognitive decline after age 50 (Reynolds et al. 2010), which may be in part attributable to differential life course trajectories in cholesterol and other lipids and lipoproteins. Thus, distinctive age-related risk profiles may be important to consider in evaluating G × E interplay for cognitive aging for men and women.
Large-scale efforts to study G × E influences on aging outcomes are afoot that will add to emerging literature on GE interplay using behavioral genetics methods. The IGEMS project (Interplay of Genes and Environment across Multiple Studies) is a new collaboration among nine existing longitudinal twin and family studies in Sweden, Denmark, and the United States (Pedersen et al. 2013). The central focus is to harmonize social–environmental data that can be related to physical functioning and health, psychological well-being (emotional stability/depression), and cognitive health outcomes in midlife and old age in order to address GE interplay, both GE correlation and G × E interactions. Through this and similar efforts, the several threads suggestive of GE interplay may become clearer in the near future as research begins to illuminate the dynamic pathways to variation in cognitive maintenance and aging in late life.
References
Adler, N. E., Boyce, T., Chesney, M. A., Cohen, S., Folkman, S., Kahn, R. L., & Syme, S. L. (1994). Socioeconomic status and health: The challenge of the gradient. American Psychologist, 49(1), 15–24. doi:10.1037/0003-066x.49.1.15.
Andel, R., Crowe, M., Pedersen, N. L., Mortimer, J., Crimmins, E., Johansson, B., & Gatz, M. (2005). Complexity of work and risk of Alzheimer’s disease: a population-based study of Swedish twins. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Twin Study). The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 60(5). P251–258.
Andel, R., Vigen, C., Mack, W. J., Clark, L. J., & Gatz, M. (2006). The effect of education and occupational complexity on rate of cognitive decline in Alzheimer’s patients. (Research Support, N.I.H., Extramural). Journal of the International Neuropsychological Society, 12(1), 147–152. doi:10.1017/S1355617706060206.
Andel, R., Kareholt, I., Parker, M. G., Thorslund, M., & Gatz, M. (2007). Complexity of primary lifetime occupation and cognition in advanced old age. (Research Support, Non-U.S. Gov’t). Journal of Aging and Health, 19(3), 397–415. doi:10.1177/0898264307300171.
Ariza, M., Pueyo, R., Matarin Mdel, M., Junque, C., Mataro, M., Clemente, I., et al. (2006). Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. (Research Support, Non-U.S. Gov’t). Journal of Neurology, Neurosurgery, and Psychiatry, 77(10), 1191–1193. doi:10.1136/jnnp.2005.085167.
Alzheimer’s Association (2012). Alzheimer’s disease facts and figures. Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association, 8, 131–168.
Aubert, G., & Lansdorp, P. M. (2008). Telomeres and aging. Physiological Reviews, 88(2), 557–579. doi:10.1152/physrev.00026.2007.
Bakaysa, S. L., Mucci, L. A., Slagboom, P. E., Boomsma, D. I., McClearn, G. E., Johansson, B., & Pedersen, N. L. (2007). Telomere length predicts survival independent of genetic influences. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Twin Study). Aging Cell, 6(6), 769–774. doi:10.1111/j.1474-9726.2007.00340.x.
Baltes, P. B., Lindenberger, U., & Staudinger, U. M. (2006). Life span theory in developmental psychology. In R. M. Lerner & W. Damon (Eds.), Handbook of Child Psychology: Vol. 1, Theoretical Models of Human Development (6th ed, pp. 569–664). Hoboken: Wiley.
Belsky, J., & Beaver, K. M. (2011). Cumulative-genetic plasticity, parenting and adolescent self-regulation. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t). Journal of Child Psychology and Psychiatry, and Allied Disciplines, 52(5), 619–626. doi:10.1111/j.1469-7610.2010.02327.x.
Belsky, J., Jonassaint, C., Pluess, M., Stanton, M., Brummett, B., & Williams, R. (2009). Vulnerability genes or plasticity genes? (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’tReview). Molecular Psychiatry, 14(8), 746–754. doi:10.1038/mp.2009.44.
Bennett, D. A., Schneider, J. A., Tang, Y., Arnold, S. E., & Wilson, R. S. (2006). The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. (Research Support, N.I.H., Extramural). Lancet Neurology, 5(5), 406–412. doi:10.1016/S1474-4422(06)70417–3.
Biegler, K. A., Anderson, A. K., Wenzel, L. B., Osann, K., & Nelson, E. L. (2012). Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: Implications for cancer prevention. (Research Support, N.I.H., Extramural). Cancer Prevention Research, 5(10), 1173–1182. doi:10.1158/1940-6207.CAPR-12-0008.
Bieman-Copland, S., Ryan, E. B., & Cassano, J. (1998). Responding to challenges of late life. In D. M. Stack & D. R. White (Eds.), Improving competence across the lifespan: Building interventions based on theory and research (pp. 141–157). New York: Plenum.
Bischoff, C., Graakjaer, J., Petersen, H. C., Hjelmborg, J. V. B., Vaupel, J. W., Bohr, V., et al. (2005). The heritability of telomere length among the elderly and oldest-old. Twin Research and Human Genetics, 8(5), 433–439. doi:10.1375/183242705774310141.
Boardman, J. D., Barnes, L. L., Wilson, R. S., Evans, D. A., & de Leon, C. F. M. (2012). Social disorder, apoe-e4 genotype, and change in cognitive function among older adults living in Chicago. Social Science & Medicine, 74(10), 1584–1590. doi: http://dx.doi.org/10.1016/j.socscimed.2012.02.012.
Bocklandt, S., Lin, W., Sehl, M. E., Sanchez, F. J., Sinsheimer, J. S., Horvath, S., & Vilain, E. (2011). Epigenetic predictor of age. (Twin Study). PLoS ONE, 6(6), e14821. doi:10.1371/journal.pone.0014821.
Boks, M. P., Derks, E. M., Weisenberger, D. J., Strengman, E., Janson, E., Sommer, I. E., Ophoff, R. A., et al. (2009). The relationship of DNA methylation with age, gender and genotype in twins and healthy controls. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Twin Study). PLoS ONE, 4(8), e6767. doi:10.1371/journal.pone.0006767.
Boyce, W. T. (2007). A biology of misfortune: Stress reactivity, social context, and the ontogeny of psychopathology in early life. In A. Masten (Ed.), Multilevel dynamics in developmental psychopathology: Pathways to the future (34th ed., pp. 45–82). Minneapolis: University of Minnesota.
Buetnner, D. (2008). The Blue Zones: Lessons for living longer from the people who’ve lived the longest. Washington, DC: National Geographic Press.
Caplan, L. J., & Schooler, C. (2006). Household work complexity, intellectual functioning, and self-esteem in men and women. Journal of Marriage and Family, 68, 883–900.
Carrero, J. J., Stenvinkel, P., Fellstrom, B., Qureshi, A. R., Lamb, K., Heimburger, O., et al. (2008).Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. (Research Support, Non-U.S. Gov’t). Journal of Internal Medicine, 263(3), 302–312. doi:10.1111/j.1365-2796.2007.01890.x.
Chadwick, M. J., Hassabis, D., Weiskopf, N., & Maguire, E. A. (2010). Decoding individual episodic memory traces in the human hippocampus. (Research Support, Non-U.S. Gov’t). Current Biology, 20(6), 544–547. doi:10.1016/j.cub.2010.01.053.
Chen, Y., Durakoglugil, M. S., Xian, X., & Herz, J. (2010). ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. (In Vitro Research Support, Non-U.S. Gov’t). Proceedings of the National Academy of Sciences of the United States of America, 107(26), 12011–12016. doi:10.1073/pnas.0914984107.
Chen, C.-H., Gutierrez, E. D., Thompson, W., Panizzon, M. S., Jernigan, T. L., Eyler, L. T., et al. (2012). Hierarchical genetic organization of human cortical surface area. Science, 335(6076), 1634–1636. doi:10.1126/science.1215330.
Cherkas, L. F., Aviv, A., Valdes, A. M., Hunkin, J. L., Gardner, J. P., Surdulescu, G. L., et al. (2006). The effects of social status on biological aging as measured by white-blood-cell telomere length. (Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t). Aging Cell, 5(5), 361–365. doi:10.1111/j.1474-9726.2006.00222.x.
Chouliaras, L., Rutten, B. P., Kenis, G., Peerbooms, O., Visser, P. J., Verhey, F., et al. (2010a). Epigenetic regulation in the pathophysiology of Alzheimer’s disease. Progress in Neurobiology, 90(4), 498–510. doi:S0301-0082(10)00013-4 [pii] 10.1016/j.pneurobio. 2010. 01.002.
Chouliaras, L., van den Hove, D. L., Kenis, G., Dela Cruz, J., Lemmens, M. A., van Os, J., et al. (2010b). Caloric restriction attenuates age-related changes of DNA methyltransferase 3a in mouse hippocampus. Brain, Behavior, and Immunity, 25(4), 616–623. doi:S0889-1591(10)00571-4 [pii] 10.1016/j.bbi.2010.11.016.
Christensen, H. (2001). What cognitive changes can be expected with normal ageing? (Research Support, Non-U.S. Gov’t Review). The Australian and New Zealand Journal of Psychiatry, 35(6), 768–775.
Christensen, B. C., Houseman, E. A., Marsit, C. J., Zheng, S., Wrensch, M. R., Wiemels, J. L., et al. (2009). Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t). PLoS Genetics, 5(8), e1000602. doi:10.1371/journal.pgen.1000602.
Cole, S. W. (2009). Social regulation of human gene expression. Current Directions in Psychological Science, 18(3), 132–137. doi:10.1111/j.1467-8721.2009.01623.x.
Coolen, M. W., Statham, A. L., Qu, W., Campbell, M. J., Henders, A. K., Montgomery, G. W., et al. (2011). Impact of the genome on the epigenome is manifested in DNA methylation patterns of imprinted regions in monozygotic and dizygotic twins. (Research Support, Non-U.S. Gov’t Twin Study). PLoS ONE, 6(10), e25590. doi:10.1371/journal.pone.0025590.
Dardiotis, E., Grigoriadis, S., & Hadjigeorgiou, G. M. (2012). Genetic factors influencing outcome from neurotrauma. (Review). Current Opinion in Psychiatry, 25(3), 231–238. doi:10.1097/YCO.0b013e3283523c0e.
Davies, G., Tenesa, A., Payton, A., Yang, J., Harris, S. E., Liewald, D., et al. (2011). Genome-wide association studies establish that human intelligence is highly heritable and polygenic. (Meta-Analysis Research Support, Non-U.S. Gov’t). Molecular Psychiatry, 16(10), 996–1005. doi:10.1038/mp.2011.85.
Day, J. J., & Sweatt, J. D. (2010). DNA methylation and memory formation. Nature Neuroscience, 13(11), 1319–1323. doi:10.1038/nn.2666.
Day, J. J., & Sweatt, J. D. (2011). Epigenetic mechanisms in cognition. Neuron, 70(5), 813–829. doi:S0896-6273(11)00433-8 [pii] 10.1016/j.neuron.2011.05.019.
Day, J. J., & Sweatt, J. D. (2012). Epigenetic treatments for cognitive impairments. Neuropsychopharmacology, 37(1), 247–260. doi:10.1038/npp.2011.85 npp201185 [pii].
Deary, I. J., Yang, J., Davies, G., Harris, S. E., Tenesa, A., Liewald, D., Visscher, P. M., et al. (2012). Genetic contributions to stability and change in intelligence from childhood to old age. (Research Support, Non-U.S. Gov’t). Nature, 482(7384), 212–215. doi:10.1038/nature10781.
Dick, D. M. (2011). Gene-environment interaction in psychological traits and disorders. (Research Support, N.I.H., Extramural Review). Annual Review of Clinical Psychology, 7, 383–409. doi:10.1146/annurev-clinpsy-032210-104518.
Dziedzic, T. (2006). Systemic inflammatory markers and risk of dementia. American Journal of Alzheimer’s Disease and Other Dementias, 21(4), 258–262.
Epel, E. S. (2009). Psychological and metabolic stress: A recipe for accelerated cellular aging? (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review). Hormones, 8(1), 7–22.
Epel, E. S., Blackburn, E. H., Lin, J., Dhabhar, F. S., Adler, N. E., Morrow, J. D., & Cawthon, R. M. (2004). Accelerated telomere shortening in response to life stress. (Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.). Proceedings of the National Academy of Sciences of the United States of America, 101(49), 17312–17315. doi:10.1073/pnas.0407162101.
Erickson, K. I., Miller, D. L., Weinstein, A. M., Akl, S. L., & Banducci, S. (2012). Physical activity and brain plasticity in late adulthood: A conceptual and comprehensive review (Vol. 3).
Etnier, J. L., Caselli, R. J., Reiman, E. M., Alexander, G. E., Sibley, B. A., Tessier, D., & McLemore, E. C. (2007). Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t). Medicine and Science in Sports and Exercise, 39(1), 199–207. doi:10.1249/01.mss.0000239399.85955.5e.
Evans, G. W. (2004). The environment of childhood poverty. (Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Review). The American Psychologist, 59(2), 77–92. doi:10.1037/0003-066X.59.2.77.
Falconer, D. S. (1989). Introduction to Quantitative Genetics. (3rd ed.). London: Longman, Scientific & Technical.
Feil, R., & Fraga, M. F. (2012). Epigenetics and the environment: Emerging patterns and implications. (10.1038/nrg3142). Nature Reviews Genetics, 13(2), 97–109.
Fernandez, A. F., Assenov, Y., Martin-Subero, J. I., Balint, B., Siebert, R., Taniguchi, H., & Esteller, M. (2012). A DNA methylation fingerprint of 1628 human samples. (Research Support, Non-U.S. Gov’t). Genome Research, 22(2), 407–419. doi:10.1101/gr.119867.110.
Ferrari, C., Xu, W.-L., Wang, H.-X., Winblad, B., Sorbi, S., Qiu, C., & Fratiglioni, L. (2012). How can elderly apolipoprotein E Œµ4 carriers remain free from dementia? Neurobiology of Aging, 34(1), 13–21. doi:10.1016/j.neurobiolaging.2012.03.003.
Finkel, D., & Reynolds, C. A. (2009). Behavioral genetic investigations of cognitive aging. In Y.-K. Kim (Ed.), Handbook of Behavior Genetics (pp. 101–112). New York: Springer.
Finkel, D., Reynolds, C. A., Berg, S., & Pedersen, N. L. (2006). Surprising lack of sex differences in normal cognitive aging in twins. International Journal of Aging and Human Development, 62(4), 335–357.
Finkel, D., Andel, R., Gatz, M., & Pedersen, N. L. (2009). The role of occupational complexity in trajectories of cognitive aging before and after retirement. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Twin Study). Psychology and Aging, 24(3), 563–573. doi:10.1037/a0015511.
Fisher, R. A. (1925). The resemblance between twins, a statistical examination of Lauterbach’s Measurements. Genetics, 10(6), 569–579.
Fotenos, A. F., Mintun, M. A., Snyder, A. Z., Morris, J. C., & Buckner, R. L. (2008). Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t). Archives of Neurology, 65(1), 113–120. doi:10.1001/archneurol.2007.27.
Fozard, J. L., Reitsema, J., Bouma, H., & Graafmans, J. A. M. (2000). Gerontechnology: Creating enabling environments for the challenges and opportunities of aging. Educational Gerontology, 26, 331–344.
Fraga, M. F., Ballestar, E., Paz, M. F., Ropero, S., Setien, F., Ballestar, M. L., et al. (2005). Epigenetic differences arise during the lifetime of monozygotic twins. Proceedings of the National Academy of Sciences, 102(30), 10604–10609.
Fritsch, B., Reis, J., Martinowich, K., Schambra, H. M., Ji, Y., Cohen, L. G., & Lu, B. (2010). Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. (Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t). Neuron, 66(2), 198–204. doi:10.1016/j.neuron.2010.03.035.
Gatz, M. (2007). Genetics, dementia, and the elderly. Current Directions in Psychological Science, 16, 123–127.
Gatz, M., Mortimer, J. A., Fratiglioni, L., Johansson, B., Berg, S., Andel, R., et al. (2007). Accounting for the relationship between low education and dementia: A twin study. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Twin Study). Physiology & Behavior, 92(1–2), 232–237. doi:10.1016/j.physbeh.2007.05.042.
Giedd, J., Stockman, M., Weddle, C., Liverpool, M., Alexander-Bloch, A., Wallace, G., et al. (2010). Anatomic magnetic resonance imaging of the developing child and adolescent brain and effects of genetic variation. Neuropsychology Review, 20(4), 349–361. doi:10.1007/s11065-010-9151-9.
Gilley, D., Herbert, B. S., Huda, N., Tanaka, H., & Reed, T. (2008). Factors impacting human telomere homeostasis and age-related disease. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review). Mechanisms of Ageing and Development, 129(1–2), 27–34. doi:10.1016/j.mad.2007.10.010.
Gimeno, D., Marmot, M. G., & Singh-Manoux, A. (2008). Inflammatory markers and cognitive function in middle-aged adults: The Whitehall II study. Psychoneuroendocrinology, 33(10), 1322–1334.
Glymour, M. M., Tzourio, C., & Dufouil, C. (2012). Is cognitive aging predicted by one’s own or one’s parents’ educational level? Results from the three-city study. American Journal of Epidemiology, 175(8), 750–759. doi:10.1093/aje/kwr509.
Gottlieb, G. (1991). Experiential canalization of behavioral development: Theory. Developmental Psychology, 27, 4–13.
Gottlieb, G. (2007). Probabilistic epigenesis. Developmental Science, 10(1), 1–11. doi: DESC556 [pii] 10.1111/j.1467–7687.2007.00556.x
Gow, A. J., Johnson, W., Pattie, A., Brett, C. E., Roberts, B., Starr, J. M., & Deary, I. J. (2011). Stability and change in intelligence from age 11 to ages 70, 79, and 87: The Lothian Birth Cohorts of 1921 and 1936. (Research Support, Non-U.S. Gov’t). Psychology and Aging, 26(1), 232–240. doi:10.1037/a0021072.
Graff, J., & Mansuy, I. M. (2009). Epigenetic dysregulation in cognitive disorders. European Journal of Neuroscience, 30(1), 1–8. doi:EJN6787 [pii] 10.1111/j.1460-9568.2009.06787.x.
Grant, M. D., Kremen, W. S., Jacobson, K. C., Franz, C., Xian, H., Eisen, S. A., et al. (2010). Does parental education have a moderating effect on the genetic and environmental influences of general cognitive ability in early adulthood? (Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S. Twin Study). Behavior Genetics, 40(4), 438–446. doi:10.1007/s10519-010-9351-3.
Greenough, W. T., McDonald, J. W., Parnisari, R. M., & Camel, J. E. (1986). Environmental conditions modulate degeneration and new dendrite growth in cerebellum of senescent rats. Brain research, 380(1), 136–143.
Hanscombe, K. B., Trzaskowski, M., Haworth, C. M., Davis, O. S., Dale, P. S., & Plomin, R. (2012). Socioeconomic status (SES) and children’s intelligence (IQ): In a UK-representative sample SES moderates the environmental, not genetic, effect on IQ. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Twin Study). PLoS ONE, 7(2), e30320. doi:10.1371/journal.pone.0030320.
Harati, H., Barbelivien, A., Herbeaux, K., Muller, M. A., Engeln, M., Kelche, C., et al. (2012). Lifelong environmental enrichment in rats: impact on emotional behavior, spatial memory vividness, and cholinergic neurons over the lifespan. Age. doi:10.1007/s11357-012-9424-8.
Harbeby, E., Jouin, M., Alessandri, J. M., Lallemand, M. S., Linard, A., Lavialle, M., et al. (2012).n-3 PUFA status affects expression of genes involved in neuroenergetics differently in the fronto-parietal cortex compared to the CA1 area of the hippocampus: Effect of rest and neuronal activation in the rat. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 86(6), 211–220. doi:10.1016/j.plefa.2012.04.008.
Harden, K. P., Turkheimer, E., & Loehlin, J. C. (2007).Genotype by environment interaction in adolescents’ cognitive aptitude. Behavior Genetics, 37(2), 273–283. doi:10.1007/s10519-006-9113-4.
Head, D., Bugg, J. M., Goate, A. M., Fagan, A. M., Mintun, M. A., Benzinger, T., et al. (2012). Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Archives of neurology, 69(5), 636–643. doi:10.1001/archneurol.2011.845.
Holtzman, D. M., & Fagan, A. M. (1998). Potential role of apoE in structural plasticity in the nervous system; implications for disorders of the central nervous system. Trends in cardiovascular medicine, 8(6), 250–255.
Hoth, K. F., Haley, A. P., Gunstad, J., Paul, R. H., Poppas, A., Jefferson, A. L., et al. (2008). Elevated C-reactive protein is related to cognitive decline in older adults with cardiovascular disease. Journal of the American Geriatrics Society, 56(10), 1898–1903.
Houben, J. M., Moonen, H. J., van Schooten, F. J., & Hageman, G. J. (2008).Telomere length assessment: Biomarker of chronic oxidative stress? (Review). Free Radical Biology & Medicine, 44(3), 235–246. doi:10.1016/j.freeradbiomed.2007.10.001.
Huda, N., Tanaka, H., Herbert, B. S., Reed, T., & Gilley, D. (2007).Shared environmental factors associated with telomere length maintenance in elderly male twins. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Twin Study). Aging Cell, 6(5), 709–713. doi:10.1111/j.1474-9726.2007.00330.x.
Jinks, J. L., & Fulker, D. W. (1970). Comparison of the biometrical genetical, MAVA, and classical approaches to the analysis of human behavior. Psychological bulletin, 73(5), 311–349.
Johansson, B., Hofer, S. M., Allaire, J. C., Maldonado-Molina, M. M., Piccinin, A. M., Berg, S., et al. (2004).Change in cognitive capabilities in the oldest old: The effects of proximity to death in genetically related individuals over a 6-year period. (Research Support, U.S. Gov’t, P.H.S. Twin Study). Psychology and Aging, 19(1), 145–156. doi:10.1037/0882-7974.19.1.145.
Johnson, W., Deary, I. J., McGue, M., & Christensen, K. (2009). Genetic and environmental transactions linking cognitive ability, physical fitness, and education in late life. Psychology and Aging, 24, 48–62.
Jordanova, V., Stewart, R., Davies, E., Sherwood, R., & Prince, M. (2007). Markers of inflammation and cognitive decline in an African-Caribbean population. International Journal of Geriatric Psychiatry, 22(10), 966–973.
Kaliman, P., Parrizas, M., Lalanza, J. F., Camins, A., Escorihuela, R. M., & Pallas, M. (2011). Neurophysiological and epigenetic effects of physical exercise on the aging process. Ageing Research Reviews, 10(4), 475–486. doi:S1568-1637(11)00046-8 [pii] 10.1016/j.arr.2011.05.002.
Kang, J. H., Logroscino, G., De Vivo, I., Hunter, D., & Grodstein, F. (2005).Apolipoprotein E, cardiovascular disease and cognitive function in aging women. Neurobiology of Aging, 26(4), 475–484. doi:10.1016/j.neurobiolaging.2004.05.003.
Krabbe, K. S., Mortensen, E. L., Avlund, K., Pilegaard, H., Christiansen, L., Pedersen, A. N., et al. (2009). Genetic priming of a proinflammatory profile predicts low IQ in octogenarians. Neurobiology of Aging, 30(5), 769–781.
Kremen, W. S., Jacobson, K. C., Xian, H., Eisen, S. A., Waterman, B., Toomey, R., et al. (2005).Heritability of word recognition in middle-aged men varies as a function of parental education. (Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S. Twin Study). Behavior Genetics, 35(4), 417–433. doi:10.1007/s10519-004-3876-2.
Lawton, M. P., & Nahemow, L. (1973). Ecology of the aging process. In C. Eisdorfer & M. P. Lawton (Eds.), The psychology of adult development and aging (pp. 619–674). Washington, DC: American Psychological Association.
Lee, B. K., Glass, T. A., James, B. D., Bandeen-Roche, K., & Schwartz, B. S. (2011). Neighborhood psychosocial environment, Apolipoprotein E genotype, and cognitive function in older adults. Archives of General Psychiatry, 68(3), 7.
Lee, S., Doulames, V., Donnelly, M., Levasseaur, J., & Shea, T. B. (2012). Environmental enrichment can prevent cognitive decline induced by dietary oxidative challenge. (Research Support, Non-U.S. Gov’t). Journal of Alzheimer’s Disease, 28(3), 497–501. doi:10.3233/JAD-2011-111562.
Li Voti, P., Conte, A., Suppa, A., Iezzi, E., Bologna, M., Aniello, M. S., et al. (2011).Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. (Comparative Study). Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale, 212(1), 91–99. doi:10.1007/s00221-011-2700-5.
Liang, J., & Fairchild, T. J. (1979). Relative deprivation and perception of financial adequacy among the aged. Journal of Gerontology, 34(5), 768–759.
Lichtenstein, P., Harris, J. R., Pedersen, N. L., & McClearn, G. E. (1992). Socioeconomic status and physical health, how are they related? An empirical study based on twins reared apart and twins reared together. Social Science & Medicine, 36(4), 441–450.
Lof-Ohlin, Z. M., Hagnelius, N. O., & Nilsson, T. K. (2008).Relative telomere length in patients with late-onset Alzheimer’s dementia or vascular dementia. Neuroreport, 19(12), 1199–1202. doi:10.1097/WNR.0b013e3283089220 00001756-200808060-00006 [pii].
Lovden, M., Ghisletta, P., & Lindenberger, U. (2005).Social participation attenuates decline in perceptual speed in old and very old age. Psychology and Aging, 20(3), 423–434. doi:10.1037/0882-7974.20.3.423.
Lubin, F. D., Roth, T. L., & Sweatt, J. D. (2008).Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. The Journal of Neuroscience, 28(42), 10576–10586. doi:28/42/10576 [pii] 10.1523/JNEUROSCI.1786-08.2008.
Lukens, J. N., Van Deerlin, V., Clark, C. M., Xie, S. X., & Johnson, F. B. (2009). Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer’s disease. Alzheimers Dement, 5(6), 463–469. doi:S1552-5260(09)02014-7 [pii] 10.1016/j.jalz.2009.05.666.
Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. (2009).Effects of stress throughout the lifespan on the brain, behaviour and cognition. (Review). Nature reviews. Neuroscience, 10(6), 434–445. doi:10.1038/nrn2639.
Maekawa, M., & Watanabe, Y. (2007). Epigenetics: Relations to disease and laboratory findings. Current Medicinal Chemistry, 14(25), 2642–2653.
Markham, J. A., & Greenough, W. T. (2004). Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biology, 1(4), 351–363. doi:10.1017/s1740925×05000219.
Martin, N. G., Rowell, D. M., & Whitfield, J. B. (1983). Do the MN and Jk systems influence environmental variability in serum lipid levels? (Comparative Study). Clinical Genetics, 24(1), 1–14.
Martin-Ruiz, C., Dickinson, H. O., Keys, B., Rowan, E., Kenny, R. A., & Von Zglinicki, T. (2006).Telomere length predicts poststroke mortality, dementia, and cognitive decline. Annals of Neurology, 60(2), 174–180. doi:10.1002/ana.20869.
Mather, K. A., Jorm, A. F., Parslow, R. A., & Christensen, H. (2010).Is telomere length a biomarker of aging? A review. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 66(2), 202–213. doi:glq180 [pii] 10.1093/gerona/glq180.
Matthews, K. A., & Gallo, L. C. (2011). Psychological perspectives on pathways linking socioeconomic status and physical health. (Research Support, N.I.H., Extramural Review). Annual Review of Psychology, 62, 501–530. doi:10.1146/annurev.psych.031809.130711.
McArdle, J. J., & Prescott, C. A. (2010).Contemporary modeling of Gene-by-Environment effects in randomized multivariate longitudinal studies. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 5(5), 606–621. doi:10.1177/1745691610383510.
McGue, M., & Christensen, K. (2002).The heritability of level and rate-of-change in cognitive functioning in Danish twins aged 70 years and older. Experimental Aging Research, 28(4), 435–451. doi:10.1080/03610730290080416.
Medland, S. E., Neale, M. C., Eaves, L. J., & Neale, B. M. (2009).A note on the parameterization of Purcell’s G x E model for ordinal and binary data. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t). Behavior Genetics, 39(2), 220–229. doi:10.1007/s10519-008-9247-7.
Miller, C. A., Gavin, C. F., White, J. A., Parrish, R. R., Honasoge, A., Yancey, C. R., et al. (2010).Cortical DNA methylation maintains remote memory. Nature Neuroscience, 13(6), 664–666. doi:nn.2560 [pii] 10.1038/nn.2560.
Miller, G. E., Chen, E., Fok, A. K., Walker, H., Lim, A., Nicholls, E. F., et al. (2009). Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. PNAS Proceedings of the National Academy of Sciences of the United States of America, 106(34), 14716–14721.
Miller, D. I., Taler, V., Davidson, P. S., & Messier, C. (2012). Measuring the impact of exercise on cognitive aging: Methodological issues. (Research Support, Non-U.S. Gov’t Review). Neurobiology of Aging, 33(3), 622, e629–643. doi:10.1016/j.neurobiolaging.2011.02.020.
Mohammed, A. H., Henriksson, B. G., Söderström, S., Ebendal, T., Olsson, T., & Seckl, J. R. (1993). Environmental influences on the central nervous system and their implications for the aging rat. Behavioural Brain Research, 57(2), 183–191.
Mooijaart, S. P., Sattar, N., Trompet, S., Polisecki, E., de Craen, A. J. M., Schaefer, E. J., et al. (2011). C-reactive protein and genetic variants and cognitive decline in old age: The PROSPER Study. PLoS One, 6(9), e23890.
Morse, C. K. (1993). Does variability increase with age? An archival study of cognitive measures. Psychology and Aging, 8(2), 156–164.
Myers, A. J., & Nemeroff, C. B. (2012).APOE: A risk factor for multiple disorders. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 20(7), 545–548. doi:10.1097/JGP.0b013e318259b9a5.
Neves, G., Cooke, S. F., & Bliss, T. V. P. (2008). Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nature Reviews Neuroscience, 9(1), 65–75.
Nichol, K., Deeny, S. P., Seif, J., Camaclang, K., & Cotman, C. W. (2009). Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. (Research Support, N.I.H., Extramural). Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 5(4), 287–294. doi:10.1016/j.jalz.2009.02.006.
Obisesan, T. O., Umar, N., Paluvoi, N., & Gillum, R. F. (2012). Association of leisure-time physical activity with cognition by apolipoprotein-E genotype in persons aged 60 years and over: The National Health and Nutrition Examination Survey (NHANES-III). (Comparative Study Research Support, N.I.H., Extramural). Clinical Interventions in Aging, 7, 35–43. doi:10.2147/CIA.S26794.
Pechtel, P., & Pizzagalli, D. A. (2011).Effects of early life stress on cognitive and affective function: an integrated review of human literature. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review). Psychopharmacology, 214(1), 55–70. doi:10.1007/s00213-010-2009-2.
Pedersen, N. L., Plomin, R., Nesselroade, J. R., & McClearn, G. E. (1992). A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychological Science, 3(6), 346–353.
Pedersen, N. L., Reynolds, C. A., & Gatz, M. (1996). Sources of covariation among Mini-Mental State Examination scores, education, and cognitive abilities. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 51(2), P55–63.
Pedersen, N. L., Christensen, K., Dahl, A., Finkel, D., Franz, C. E., Gatz, M., Horwitz, B. N., Johansson, B., Johnson, W., Kremen, W. S., Lyons, M. J., Malmberg, B., McGue, M., Neiderhiser, J. M., Petersen, I., & Reynolds, C. A. (2013). IGEMS: The consortium on interplay of genes and environment across multiple studies. Twin Research and Human Genetics, 16, 481–489.
Petit-Turcotte, C., Aumont, N., Beffert, U., Dea, D., Herz, J., & Poirier, J. (2005).The APOE receptor apoER2 is involved in the maintenance of efficient synaptic plasticity. (Comparative Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.). Neurobiology of Aging, 26(2), 195–206. doi:10.1016/j.neurobiolaging.2004.04.007.
Pfefferbaum, A., Sullivan, E. V., & Carmelli, D. (2004). Morphological changes in aging brain structures are differentially affected by time-linked environmental influences despite strong genetic stability. Neurobiology of Aging, 25(2), 175–183.
Pinaud, R., Tremere, L. A., Penner, M. R., Hess, F. F., Robertson, H. A., & Currie, R. W. (2002). Complexity of sensory environment drives the expression of candidate-plasticity gene, nerve growth factor induced-A. Neuroscience, 112(3), 573–582.
Plomin, R., DeFries, J. C., McClearn, G. E., & McGuffin, P. (2008). Behavioral Genetics (5th ed.). New York: Worth.
Purcell, S. (2002).Variance components models for gene-environment interaction in twin analysis. (Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Twin Study). Twin research: The Official Journal of the International Society for Twin Studies, 5(6), 554–571. doi:10.1375/136905202762342026.
Rafnsson, S. B., Deary, I. J., Smith, F. B., Whiteman, M. C., Rumley, A., Lowe, G. D. O., & Fowkes, F. G. R. (2007). Cognitive decline and markers of inflammation and hemostasis: The Edinburgh Artery Study. Journal of the American Geriatrics Society, 55(5), 700–707.
Raine, A. (2002). Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology, 30, 311–326.
Rampon, C., Tang, Y. P., Goodhouse, J., Shimizu, E., Kyin, M., & Tsien, J. Z. (2000).Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. (Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.). Nature Neuroscience, 3(3), 238–244. doi:10.1038/72945.
Reiss, D., & Leve, L. D. (2007).Genetic expression outside the skin: Clues to mechanisms of Genotype x Environment interaction. (Research Support, N.I.H., extramural review). Development and Psychopathology, 19(4), 1005–1027. doi:10.1017/S0954579407000508.
Reynolds, C. A. (2008a). Genetic and environmental influences on cognitive change. In S. M. Hofer & D. F. Alwin (Eds.), The Handbook on Cognitive Aging: Interdisciplinary Perspectives. Thousand Oaks: Sage.
Reynolds, C. A. (2008b). Genetics of brain aging—twin aging. In L. Squire (Ed.), Encyclopedia of Neuroscience (Network Version ed., pp. 10500): Elsevier.
Reynolds, C. A., Finkel, D., McArdle, J. J., Gatz, M., Berg, S., & Pedersen, N. L. (2005). Quantitative genetic analysis of latent growth curve models of cognitive abilities in adulthood. Developmental Psychology, 41(1), 3–16.
Reynolds, C. A., Gatz, M., Berg, S., & Pedersen, N. L. (2007). Genotype-environment interactions: Cognitive aging and social factors. Twin Research and Human Genetics, 10(2), 241–254. doi:10.1375/twin.10.2.241.
Reynolds, C. A., Gatz, M., Prince, J. A., Berg, S., & Pedersen, N. L. (2010). Serum lipid levels and cognitive change in late life. Journal of the American Geriatrics Society, 58(3), 501–509. doi:JGS2739 [pii] 10.1111/j.1532-5415.2010.02739.x.
Rimol, L. M., Panizzon, M. S., Fennema-Notestine, C., Eyler, L. T., Fischl, B., Franz, C. E., et al. (2010).Cortical thickness is influenced by regionally specific genetic factors. (Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S. Twin Study). Biological Psychiatry, 67(5), 493–499. doi:10.1016/j.biopsych.2009.09.032.
Roberts, R. O., Geda, Y. E., Knopman, D. S., Cha, R. H., Pankratz, V. S., Boeve, B. F., Petersen, R. C., et al. (2012).The incidence of MCI differs by subtype and is higher in men: The Mayo Clinic Study of Aging. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t). Neurology, 78(5), 342–351. doi:10.1212/WNL.0b013e3182452862.
Rodin, J. (1986). Aging and health: Effects of sense of control. Science, 233, 1271–1276.
Rodin, J. (1989). Sense of control: Potentials for intervention. Annals of the American Academy of Political and Social Science, 503, 29–42.
Rowe, J. W., & Kahn, R. L. (1997). Successful aging. (Research Support, Non-U.S. Gov’t Review). The Gerontologist, 37(4), 433–440.
Rutter, M. (2012).Gene–environment interdependence. European Journal of Developmental Psychology, 9(4), 391–412. doi:10.1080/17405629.2012.661174.
Salthouse, T. A. (2006). Mental exercise and mental aging: Evaluating the validity of the “use it or lose it” hypothesis. Perspectives on Psychological Science, 1, 68–87.
Sapolsky, R. M. (2004). Social status and health in humans and other animals. Annual Review of Anthropology, 33, 393–418.
Sapolsky, R. M. (2005). The influence of social hierarchy on primate health. Science, 308, 648–652.
Scarmeas, N., & Stern, Y. (2003). Cognitive reserve and lifestyle. Journal of Clinical and Experimental Neuropsychology, 25, 625–633.
Scarpa, S., Cavallaro, R. A., D’Anselmi, F., & Fuso, A. (2006). Gene silencing through methylation: An epigenetic intervention on Alzheimer disease. Journal of Alzheimer’s Disease, 9, 407–414.
Scarr, S., & McCartney, K. (1983). How people make their own environments: A theory of genotype greater than environment effects. (Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.). Child Development, 54(2), 424–435.
Schooler, C., & Caplan, L. J. (2008). Those who have, get: Social structure, environmental complexity, intellectual functioning, and self-directed orientations in the elderly. In K. W. Schaie & R. P. Abeles (Eds.), Social Structures and Aging Individuals: Continuing Challenges (Vol. 20, pp. 131-153).New York: Springer.
Schram, M. T., Euser, S. M., de Craen, A. J., Witteman, J. C., Frölich, M., Hofman, A., Westendorp, R. G. J. (2007). Systemic markers of inflammation and cognitive decline in old age. Journal of the American Geriatrics Society, 55(5), 708–716.
Shanahan, M. J., & Hofer, S. M. (2005). Social context in gene-environment interactions: Retrospect and prospect. (Review). The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 60(Spec No 1), 65–76.
Shawi, M., & Autexier, C. (2008). Telomerase, senescence and ageing. (Research Support, Non-U.S. Gov’t Review). Mechanisms of Ageing and Development, 129(1–2), 3–10. doi:10.1016/j.mad.2007.11.007.
Singh-Manoux, A., Marmot, M. G., & Adler, N. E. (2005).Does subjective social status predict health and change in health status better than objective status? Psychosomatic Medicine, 67(6), 855–861. doi:10.1097/01.psy.0000188434.52941.a0.
Staff, R. T., Murray, A. D., Deary, I. J., & Whalley, L. J. (2004).What provides cerebral reserve? Brain, 127(5), 1191–1199. doi:10.1093/brain/awh144.
Sterlemann, V., Rammes, G., Wolf, M., Liebl, C., Ganea, K., Muller, M. B., & Schmidt, M. V. (2010).Chronic social stress during adolescence induces cognitive impairment in aged mice. Hippocampus, 20(4), 540–549. doi:10.1002/hipo.20655.
Sullivan, E. V., Pfefferbaum, A., Swan, G. E., & Carmelli, D. (2001). Heritability of hippocampal size in elderly twin men: Equivalent influence from genes and environment. Hippocampus, 11(6), 754–762.
Teter, B. (2004). ApoE-dependent plasticity in Alzheimer’s disease. (Review). Journal of Molecular Neuroscience, 23(3), 167–179. doi:10.1385/JMN:23:3:167.
Thomas, P., O’Callaghan, N. J., & Fenech, M. (2008). Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mechanisms Ageing and Development, 129(4), 183–190. doi:S0047-6374(07)00194-7 [pii] 10.1016/j.mad.2007.12.004.
Thompson, P. M., Cannon, T. D., Narr, K. L., van Erp, T., Poutanen, V. P., Huttunen, M., Toga, A. W. (2001).Genetic influences on brain structure. (Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.). Nature Neuroscience, 4(12), 1253–1258. doi:10.1038/nn758.
Vaillant, G. E. (2002). Aging well: Surprising guideposts to a happier life. Boston: Little, Brown.
Valdes, A. M., Deary, I. J., Gardner, J., Kimura, M., Lu, X., Spector, T. D., Cherkas, L. F. (2008). Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiology of Aging, 31(6), 986–992. doi:S0197-4580(08)00256-X [pii] 10.1016/j.neurobiolaging.2008.07.012
van den Kommer, T. N., Dik, M. G., Comijs, H. C., Jonker, C., & Deeg, D. J. H. (2010). Homocysteine and inflammation: Predictors of cognitive decline in older persons? Neurobiology of Aging, 31(10), 1700–1709.
van der Sluis, S., Dolan, C. V., Neale, M. C., Boomsma, D. I., & Posthuma, D. (2006).Detecting genotype-environment Interaction in monozygotic twin data: Comparing the Jinks and Fulker test and a new test based on Marginal Maximum Likelihood estimation. Twin Research and Human Genetics, 9(3), 377–392. doi:10.1375/twin.9.3.377.
van der Sluis, S., Dolan, C. V., Neale, M. C., & Posthuma, D. (2008). A general test for gene-environment interaction in sib pair-based association analysis of quantitative traits. Behavior Genetics, 38(4), 372–389.
van der Sluis, S., Posthuma, D., & Dolan, C. (2012).A note on false positives and power in G × E modelling of twin data. Behavior Genetics, 42(1), 170–186. doi:10.1007/s10519-011-9480-3.
Vera, E., & Blasco, M. A. (2012). Beyond average: Potential for measurement of short telomeres. Aging, 4(6), 379–392.
Waddington, C. H. (1942). Canalization of development and the inheritance of acquired characters. Nature, 150, 563–565.
Wang, S. C., Oelze, B., & Schumacher, A. (2008). Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS ONE, 3(7), e2698. doi:10.1371/journal.pone.0002698.
Weeber, E. J., Beffert, U., Jones, C., Christian, J. M., Forster, E., Sweatt, J. D., & Herz, J. (2002).Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. (Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.). The Journal of Biological Chemistry, 277(42), 39944–39952. doi:10.1074/jbc.M205147200.
Wikgren, M., Karlsson, T., Nilbrink, T., Nordfjall, K., Hultdin, J., Sleegers, K., et al. (2010). APOE epsilon4 is associated with longer telomeres, and longer telomeres among epsilon4 carriers predicts worse episodic memory. Neurobiology of Aging, 33(2), 335–344. doi:S0197-4580(10)00115-6 [pii] 10.1016/j.neurobiolaging.2010.03.004.
Wu, J., Basha, M., & Zawia, N. (2008). The environment, epigenetics and amyloidogenesis. Journal of Molecular Neuroscience, 34(1), 1–7.
Zavala, C., Gatz, M., Johansson, B., Malmberg, B., Pedersen, N. L., & Reynolds, C. A. (2013, in preparation). Perceived socioeconomic status predicts cognitive trajectories: A cohort comparison.
Zekry, D., Herrmann, F. R., Irminger-Finger, I., Ortolan, L., Genet, C., Vitale, A. M., et al. (2008). Telomere length is not predictive of dementia or MCI conversion in the oldest old. Neurobiology of Aging, 31(4), 719–720. doi:S0197-4580(08)00169-3 [pii] 10.1016/j.neurobiolaging.2008.05.016.
Zekry, D., Herrmann, F. R., Irminger-Finger, I., Graf, C., Genet, C., Vitale, A. M., et al. (2010). Telomere length and ApoE polymorphism in mild cognitive impairment, degenerative and vascular dementia. Journal of the Neurological Sciences, 299(1–2), 108–111. doi:S0022-510X(10)00342-4 [pii] 10.1016/j.jns.2010.07.019.
Acknowledgments
The authors gratefully acknowledge that a portion of this work was supported by the National Institutes of Aging (R01 AG037985).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Reynolds, C., Finkel, D., Zavala, C. (2014). Gene by Environment Interplay in Cognitive Aging. In: Finkel, D., Reynolds, C. (eds) Behavior Genetics of Cognition Across the Lifespan. Advances in Behavior Genetics, vol 1. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7447-0_6
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7447-0_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7446-3
Online ISBN: 978-1-4614-7447-0
eBook Packages: Behavioral ScienceBehavioral Science and Psychology (R0)