Abstract
Influenza infection represents a major socioeconomic burden worldwide. Skin represents a new target that has gained much attention in recent years for delivery of influenza vaccine as an alternative to the conventional intramuscular route of immunization. In this review we describe different microneedle vaccination approaches used in vivo, including metal and dissolving microneedle patches that have demonstrated promising results. Additionally we analyze the immunological basis for microneedle skin immunization and targeting of the skin’s dense population of antigen presenting cells, their role, characterization, and function. Additionally we analyze the importance of inflammatory signaling in the skin after microneedle delivery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Influenza Virus and Influenza Vaccination
13.1.1 Influenza Virus and Disease
Influenza virus represents one of the most common respiratory viral pathogens and is a major cause of morbidity and mortality worldwide [1, 2]. The virus is responsible for annual epidemics of influenza with seasonal outbreaks in the USA from October through April. The CDC estimates that more than 200,000 hospitalizations in the USA are attributed to influenza infection, annually [3, 4]. In several cases, the magnitude of lung inflammation and respiratory distress can lead to serious complications and even death. It is estimated that more than 40,000 deaths in the USA alone are related to influenza infection or complications following the infection [5–7], while the number of deaths associated with influenza infection account up to 1.5 million worldwide [8–10]. The World Health Organization (WHO) estimates that each year 10–20% of the world’s population is being infected by influenza virus [11]. Seasonal influenza infection can affect all age groups and genders [12]. The severity of influenza infection or complications associated with it are greater in certain high risk groups [13–15]. According to the CDC these groups include children younger than 5 years of age and particularly affected the ones younger than 2 years old [16–18], elderly individuals 65 years old and above [19–23], pregnant women [24, 25], and people with certain underlying medical conditions such as asthma [26, 27], chronic lung disease [28, 29], heart disease, diabetes [22, 30–32], immunocompromised individuals [33–35], and some others [36]. Additionally, people who live in nursing homes and long-term care facilities [30, 37, 38] as well as health care workers [39–42] are at high risk from influenza infection.
Influenza virus is a single-stranded negative sense RNA virus. There are three different serotypes of influenza viruses that can cause disease in humans, A, B, and C, distinguished by their antigenic differences in their nucleocapsid (NP) and matrix (M) protein. Influenza types A and B have eight separate segments encoding at least ten different proteins, they can spread easily among human population and are responsible for seasonal epidemics every year [43–58]. Influenza type C is very rare, it has seven separate different segments encoding nine proteins, and although it may cause mild respiratory disease it is not responsible for epidemics [59]. Influenza type A viruses have common internal antigens but can be divided into several different subtypes based on the antigenic properties of the two major proteins in their surface, the hemagglutinin (H) and neuraminidase (N) proteins. These two proteins also represent the two major surface antigens of influenza viruses. So far 17 different hemagglutinin and 10 neuraminidase proteins have been identified circulating in nature [60–63]. The two influenza A subtypes that cause seasonal influenza infections in humans are the H1N1 and H3N2 influenza viruses [63, 64]. Influenza B viruses have a limited host range (humans and seals) and are not divided into subtypes like influenza A subtypes but are classified based on their strain differences [65].
Influenza viruses exhibit a great ability to introduce minor or major changes in their two major surface proteins, the hemagglutinin and neuraminidase. Minor changes in the influenza virus genome are more common and are induced by the constant selective pressure caused by the host immune responses. These minor changes (antigenic drift) are characterized by point mutations in the HA and NA genes. Due to these changes, the host’s preexisting immunity may only partially recognize the HA and NA proteins of a new strain resulting in decreased protection and subsequently higher infection rate [66–68]. Influenza A viruses circulate among humans as well as different animals, including ducks, chickens, pigs, horses, etc. This constant circulation of influenza viruses among different species results occasionally in genome recombination inside a reservoir host between different strains and in the appearance of an antigenically new influenza virus (antigenic shift) [69–72] that the human immune system has never encountered before and hence has no or little protection against it. Due to lack of preexisting immunity, the new virus spreads quickly causing pandemics and affecting millions worldwide. The five major pandemics of the twentieth century and the first pandemic of the twenty-first century, the swine origin A/California/07/09 strain, resulted from such antigenic shifts. Influenza represents a significant socioeconomic burden, leading to increased health care cost, high levels of work absenteeism, disruption in work, and productivity loss [73].
In the USA, annual influenza epidemics result in an average of 3.1 million hospitalization days and 31.4 million outpatient visits, while the total direct and indirect economic burden of annual influenza epidemics amounts to 87.1 billion dollars [74]. The WHO places the number of infected individuals at high risk from influenza infection as more than 1.2 billion worldwide including 385 million elderly, 140 million infants, 700 million adults and children with underlying health conditions including pregnant women, and approximately 24 million health care workers [11].
13.1.2 Influenza Vaccination
Vaccination represents the best method of prevention and protection from influenza infection and its related complications, improving herd immunity, and reducing morbidity and mortality rates worldwide [2, 75–79]. Currently there are two different types of commercially available influenza vaccines on the market: (a) the trivalent inactivated influenza vaccine (TIV) administered intramuscularly with syringes, approved for use in infants older than 6 months and (b) the live attenuated influenza vaccine (LIV) given as nasal spray, approved for use only in healthy individuals from 2 to 49 years of age who are not pregnant [80]. The trivalent inactivated vaccine is the most widely utilized worldwide. There are three different types of inactivated influenza vaccine: whole virus vaccine, virus vaccine split after detergent treatment, and subunit vaccine consisting of purified HA and NA proteins. In the USA the current influenza vaccines are the split and subunit ones; both contain 15 μg of H1 and H3 hemagglutinins of the circulating seasonal influenza A subtypes and of type B influenza virus. These vaccine formulations were studied in the 1970s and proven to be safe, with reduced reactogenicity when compared to the whole inactivated influenza vaccine used until then [81–86]. Despite the excellent safety profile provided by the split and subunit influenza vaccines, the immune response following vaccination has been proven to be short-lived and not fully protective, especially in high risk groups such as the elderly, children, and immunocompromised individuals [14, 33, 87–90]. Thus studies have shown that the antibody titers to influenza wane within 7–8 months post-vaccination and that children unprimed to influenza require two vaccine doses to elicit protective immune responses. According to FDA guidelines an influenza vaccine is considered protective when the vaccinee develops anti-influenza hemagglutination inhibition titers above 40 [91]. In addition, the efficacy of these vaccines depends on how well matched the influenza strains in the vaccine are with the ones in circulation. According to the CDC, in randomized controlled trials conducted among healthy adults less than 65 years of age, the efficacy of inactivated influenza vaccines has been estimated to be between 50 and 70% during seasons in which the vaccine components were well matched to the circulating influenza viruses. Under conditions of suboptimal match, the efficacy of the inactivated vaccines fluctuates between 48% among high risk groups and 60% among healthy adults. In cases where the influenza vaccine and the circulating influenza viruses are poorly matched, the effectiveness of these vaccines is further reduced [92, 93]. All these facts strongly suggest the need for better vaccines or vaccine delivery approaches to improve protection, duration and breadth of immunity, as well as vaccine acceptance for worldwide coverage.
13.2 Skin as an Immunological Organ
13.2.1 Skin Structure, Functions, and Resident Cell Populations
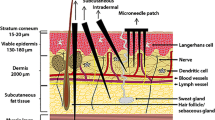
A new vaccine delivery target that has gained more attention in recent years is the skin [94]. The skin is one of the most complex structures and the largest immunological organ of the human body [95]. Its main function is protective, serving as a physical barrier from numerous pathogens but also from injuries and UV radiation. It is also part of the body’s homeostatic mechanism and an important sensory organ. It is composed of two primary layers, the epidermis and dermis [96]. The epidermis represents the most outer layer of the skin. It is 50–100 μm thick and it is divided into several sublayers; (1) stratum corneum which is the outer layer of epidermis, (2) stratum germinativum, (3) stratum lucidum that appears in certain parts of the body, (4) stratum granulosum which contains squamous cells and filaggrin and prevents loss of nutrients, (5) stratum spinosum that further enhances structural support and prevents skin abrasion, and (6) stratum basale that contains epithelial cells which undergo rapid mitosis to replenish dead cells from upper layers. These layers are mostly consisting of keratinocytes, melanocytes, and Langerhans cells (LCs) [96]. Langerhans cells are present in all layers of the epidermis and are in close proximity to the stratum corneum [ 97]. These are immature APCs produced from bone marrow precursors that reach and populate the skin through the peripheral circulation [98]. The dermis lies beneath the epidermis and contains hair follicles, sweat and endocrine glands, lymphatic vessels, blood vessels, and several nerve endings. It is largely populated by dermal dendritic cells (DDCs) that are distinct from the epidermal Langerhans cells populations based on their surface markers. LCs express differential levels of CD11b, CD205int/high, and more specifically CD207 (Langerin) while DCs express CD11bhigh, and CD205low/int and CD207 negative [99, 100]. Additionally, these two populations are characterized by differences in chemokine receptor expression especially during the maturation and migration of LCs from tissues to draining lymph nodes [101–104]. The presence of two types of antigen presenting cells, LCs and DDCs, classify the skin as an immunological organ [105]. Additionally, the expression of Toll-like receptors [106, 107] (TLRs) on LCs, DDCs, and keratinocytes make it an ideal target for vaccine delivery [105]. These two types of APCs, in combination with other immunologically active cells residing in the skin including LC-like DCs, monocytes, and macrophages [108], recognize and take up the antigen upon delivery in the skin, and migrate while undergoing maturation to the proximal lymph nodes where they prime naïve T and B cells thus initiating and shaping the adaptive immune responses [97]. Both LCs and DDCs are involved in the process of T cell activation [97]. Studies have demonstrated that in the absence of a stimulus, epidermal LCs and dermal DCs express low levels of major histocompatibility molecules MHC class I and II and co-stimulatory or adhesion molecules [109]. For LCs it is possible that passive transfer and diffusion is involved in the process of antigen uptake or a more active mechanism has been proposed where LCs reach out and extend their arms in order to capture the antigen [110, 111]. Dermal DCs have also been shown to be actively involved in the antigen presenting process as well and to be immunologically highly active [105, 112]. Two subpopulations have been identified: dermal langerin+ dendritic cells and dermal langerinneg dendritic cells [113]. Dermal DCs occur in higher numbers than epidermal LCs, they express high amounts of MHC class II molecules on their surface, and they are as potent in antigen presentation in naive T cells playing an important role in the regulation of skin immune response [105, 111, 113].
13.2.2 The Role of Inflammation During Skin Vaccination
The inflammatory environment and inflammatory response induced upon antigen entry into the skin seems to be very important and play a crucial role in the immune response. Several studies have demonstrated that LCs and DDCs can produce large amounts of IL-12, TNF-α, and type I interferons (IFNs) as well as attract and activate other innate lymphocytes such as NK cells, NKT cells, and γδ T cells that secrete large amounts of IFN-γ. A recent study by Martin et al. [114] demonstrated the importance of local responses induced after skin vaccine delivery. In this study, Martin et al. observed the upregulation of several important chemokines and cytokines after microneedle delivery and particularly interleukin 1β (IL-1β), macrophage inflammatory protein 1 alpha (MIP-1α), macrophage inflammatory protein 2 (MIP-2), tumor necrosis factor alpha (TNF-α), and monocyte chemoattractant protein 1 (MCP-1). These cytokines have been shown to contribute to the regulation and migration of LCs and DDCs in the draining lymph nodes. Furthermore other cytokines important to the proliferation, activation, and recruitment of neutrophils and monocytes such as granulocyte colony-stimulation factor (G-CSF), interferon gamma induced protein 10 (IP-10), and cytokine-induced neutrophil chemoattractant (CXCL-1) were also increased after skin vaccination for influenza. These data demonstrate the numerous complex mechanisms activated upon delivery of the antigen into the skin that may be important for the improved immunological responses of the vaccine recipient. All these immunological advantages and mechanisms seem to favor skin delivery of influenza antigen compared to the conventional intramuscular immunization. Current inactivated influenza vaccines are administered intramuscularly in the deltoid muscle area. Several studies have demonstrated that myocytes contain low numbers of APCs and lack MHC class II expressing cells leading to poor antigen-dependent T cell activation and reduced humoral and cellular immune responses [115, 116]. All these limitations can potentially be overcome by skin immunization because of the many professional APCs populating the epidermis and the dermis, and thus achieving an improved quantitative and qualitative immune response when compared to intramuscular immunization.
13.3 Microneedle Vaccination
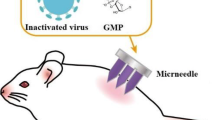
One of the most promising novel vaccine delivery platforms that takes advantage of the skin’s immunological potential is microneedle technology [94, 117–120]. This technology relies on rapid delivery of the antigen into the skin epidermis and/or the dermis layers with high precision, and without causing any discomfort or irritation. The materials of choice used for fabrication are metals or polymers, both FDA approved and already applied in several other medical devices [116, 121–123]. Metal microneedle arrays coated with whole inactivated influenza virus (WIV) or monovalent subunit vaccine and polymer (PVP) microneedles encapsulating WIV have been successfully tested in vivo and have generated promising results for vaccine delivery methods of influenza antigen through the skin [116, 121–123].
13.3.1 Solid Metal Microneedle Arrays
Metal microneedle arrays are fabricated from stainless steel sheets by laser cutting. These are arrays of hundreds of microneedles projecting a few hundred microns from the base of the patch. To deburr and clean the microneedle edges and to make the tips sharp, microneedles are electropolished in an appropriate solution. Each needle is approximately up to 700 μm long. The microneedles are coated using a dip-coating process with different formulated coating solutions that ensure stability of the vaccine. The coating is performed using an appropriate apparatus and monitored by a video camera attached to a microscope. These metal microneedle arrays coated with the antigen, when applied onto the skin, pierce microscopic holes in the skin’s epidermis with a thickness of 10–20 μm for antigen delivery [122, 124–126]. Several studies have demonstrated that by piercing the skin, transdermal permeability increases by as much as four orders of magnitude.
We have previously demonstrated that delivery of whole inactivated influenza vaccine using metal microneedles coated with the antigen can improve the duration of protective immune responses and lead to serological memory [116, 122]. In our latest studies using metal microneedle arrays we demonstrated successful delivery of influenza subunit vaccine in the mouse model in vivo [121], and we observed improved immune responses when compared to the conventional intramuscular administration of the vaccine. Microneedle immunized animals demonstrated enhanced humoral immune responses compared to intramuscularly immunized mice as shown by anti-influenza IgG titers, hemagglutination inhibition titers, and neutralizing antibody titers 9 months after a single dose of vaccine delivery [121] suggesting long-lived immune responses. Their functional antibody titers (HAI and NT) were maintained at levels that are indicative of protection (>40) even at 9 months post-immunization. These findings correlated well with the numbers of bone marrow influenza specific IgG secreting cells which were significantly higher in the microneedle immunized group. Furthermore in the same group the IgG1 and IgG2a isotype profile showed a more balanced response when compared to the isotype profile induced after intramuscular vaccination, which predominantly induced IgG1 responses. The IgG2a isotype profile is indicative of cellular Th1 immune responses. A more balanced IgG1/IgG2a ratio observed after microneedle immunization could indicate the induction of cellular immune responses after vaccination [121]. Overall these data strongly suggest that delivery of subunit influenza vaccine through the skin can lead to improved humoral immune responses.
It is well established that split and subunit influenza vaccines are poor inducers of cellular immune responses [127]. Investigation of IFN-γ cells in the spleen of microneedle immunized animals revealed higher frequency of these cells indicating improved cellular immune responses [121]. Activation of both the humoral and cellular immune system can potentially provide improved protection when compared to the intramuscular route of vaccination. Indeed, studies have demonstrated a much more rapid clearance of the virus from the lungs of mice infected with 10×LD50 of homologous mouse adapted influenza virus after skin vaccine delivery as well as improved longevity of the immune response and improved protection [116, 121].
13.3.2 Dissolving Microneedle Patches
In contrast to coated metal microneedle arrays where the antigen is being coated on the surface of the needles, the polymer microneedles encapsulate the antigen [123, 128–130]. During delivery into the skin, the whole microneedle array (shaft and tip) dissolves delivering the vaccine cargo into the skin rapidly, eliminating biohazard sharps. This type of needle requires optimal geometry in order to achieve structural rigidity and stability during insertion into the skin [131]. Sullivan et al. designed and fabricated dissolving microneedle patches [123, 131]. The polymers used for microneedle manufacturing were FDA approved and used in several other medical applications. A slurry of vinylpyrollidone was mixed with lyophilized WIV rehydrated to the desired concentration and the mixture was polymerized at room temperature. This process was found to preserve vaccine antigenicity and prolonged shelf life while the microneedles were mechanically strong to ensure skin insertion, rapid dissolution of the needle into the skin, and successful vaccine delivery. Sullivan et al. showed successful dissolution of the microneedles up to 90% within the first 5 min of application into guinea pig skin [123]. This approach has several advantages, delivery of the vaccine to an easily accessible target such as the skin, elimination of biohazard sharps improving public safety and potential for self-administration [128], rendering influenza vaccination more attractive to the population thus ensuring better coverage, stability [124, 132], and rapid distribution of the vaccine. We demonstrated that dissolving microneedle patches induced robust protection in the mouse model after a single immunization with a low antigen dose, at least as good as the one observed after the systemic immunization. Skin delivery of influenza vaccine was followed by higher number of IFN-γ secreting cells in the spleen of microneedle immunized mice when compared to conventional intramuscular vaccine delivery and faster lung virus clearance after infection [123].
Overall, these studies demonstrate that a new platform technology for rapid and easy administration of influenza vaccine through the skin using metal microneedle arrays coated with the antigen or dissolving microneedle patches encapsulating influenza vaccine can be used for successful delivery of the antigen and improved immune responses and protection in vivo [121, 123].
13.3.3 Other Types of Skin Delivery Systems
There are other several types of devices in development from different groups that can be used for delivery of different antigens. One of these designs involves hollow microneedles [120, 131, 133–136]. In this case after delivery and insertion of the microneedles into the target organ the drug/antigen is delivered by a continuous flow into the skin after which the hollow microneedle patch is being removed. Recent studies have demonstrated that a skin penetration depth of 750 mm to 1.5 mm is ideal for intradermal delivery of drugs and antigens including insulin, anthrax vaccine, and even influenza vaccine [120, 121, 131, 134, 137–143]. Another microneedle design that has been successfully tested in vivo is the Nanopatch [144, 145]. This is an array of densely packed projections that are dry-coated with influenza vaccine formulation and applied to the skin for 2 min. In this case delivery of influenza antigen through the skin induced improved immune responses when compared to the conventional intramuscular route of delivery with the additional advantage of a dose sparing effect [145]. Currently there is one FDA approved influenza vaccine in the market for intradermal delivery, Fluzone manufactured by Sanofi Pasteur. Intradermal delivery relies on the same principles of targeting similar populations of antigen presenting cells in the skin that microneedle delivery is based on. Early results from clinical trials demonstrate that intradermal delivery of the Fluzone vaccine through the skin induced similar seroconvertion rates as that induced after intramuscular delivery but with a dose sparing effect; from 15 μg HA per strain (45 μg HA total) used for the conventional intramuscular injection the dose was reduced to 9 μg HA (27 μg HA total) [146]. These results confirm the hypothesis that targeting skin APCs improves immune responses and support the promise of skin vaccination for various drugs and vaccines.
13.4 Conclusions
The complex structure of the skin and the quantity and quality of immunologically active cells that it contains [96, 105] establishes this organ as an ideal target for vaccine delivery. After several years of investigation, significant advances have been made indicating the importance of innate cell populations residing in the skin and the mechanisms behind antigen uptake. Numerous microneedle devices are in development exploiting the unique features of skin. The selection of the best design relies mostly on the type of drug to be delivered and on the type of antigen presenting cells that must be targeted. In the case of influenza vaccination very simple designs have been successfully tested in vivo and show promising results that are in the process to be advanced in clinical trials. Several important advantages make this method ideal for large-scale immunization programs. The simplicity of the method makes it ideal for self-administration. Since the skin is an easily accessed organ, and the method can eliminate biohazardous sharps, it can be completed without the need for highly trained personnel. Additionally preliminary data in humans demonstrate dose sparing further reducing the cost of this vaccination route [146]. Taking under consideration the immunological advantages achieved after microneedle delivery, the data suggest that this method could be an alternative to the conventional intramuscular route of immunization. The logistical advantages such as the ease and the simplicity of administration, the high safety profile, the better acceptance by the public [119, 120, 131, 147], and the immunological advantages [121] make this approach an important future direction in influenza vaccination.
References
Fiore, A.E., et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 57, 1–60 (2008).
Monto, A.S. Seasonal influenza and vaccination coverage. Vaccine 28 Suppl 4, D33–44 (2010).
Osterholm, M.T. Preparing for the next pandemic. N Engl J Med 352, 1839–1842 (2005).
Thompson, W.W., et al. Influenza-associated hospitalizations in the United States. JAMA 292, 1333–1340 (2004).
Beigel, J.H. Influenza. Crit Care Med 36, 2660–2666 (2008).
Dushoff, J., Plotkin, J.B., Viboud, C., Earn, D.J. & Simonsen, L. Mortality due to influenza in the United States--an annualized regression approach using multiple-cause mortality data. Am J Epidemiol 163, 181–187 (2006).
Heron, M. Deaths: leading causes for 2007. Natl Vital Stat Rep 59, 1–95 (2011).
Simonsen, L. The global impact of influenza on morbidity and mortality. Vaccine 17 Suppl 1, S3–10 (1999).
Palese, P. Influenza: old and new threats. Nat Med 10, S82–87 (2004).
Cox, N.J. & Subbarao, K. Influenza. Lancet 354, 1277–1282 (1999).
WHO. Immunization, Vaccines and Biologicals-Influenza. (2008).
Zhou, H., et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis 54, 1427–1436 (2012).
Longini, I.M., Jr. & Halloran, M.E. Strategy for distribution of influenza vaccine to high-risk groups and children. Am J Epidemiol 161, 303–306 (2005).
Ompad, D.C., Galea, S. & Vlahov, D. Distribution of influenza vaccine to high-risk groups. Epidemiol Rev 28, 54–70 (2006).
Loerbroks, A., Stock, C., Bosch, J.A., Litaker, D.G. & Apfelbacher, C.J. Influenza vaccination coverage among high-risk groups in 11 European countries. Eur J Public Health (2011).
O’Brien, M.A., et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics 113, 585–593 (2004).
Izurieta, H.S., et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 342, 232–239 (2000).
Nolan, T., et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA 303, 37–46 (2010).
Avelino-Silva, V.I., et al. Campaign, counseling and compliance with influenza vaccine among older persons. Clinics (Sao Paulo) 66, 2031–2035 (2011).
Miraglia, J.L., et al. Immunogenicity and reactogenicity of 2009 influenza A (H1N1) inactivated monovalent non-adjuvanted vaccine in elderly and immunocompromised patients. PloS one 6, e27214 (2011).
Mullooly, J.P., et al. Influenza vaccination programs for elderly persons: cost-effectiveness in a health maintenance organization. Ann Intern Med 121, 947–952 (1994).
Nichol, K.L., et al. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med 348, 1322–1332 (2003).
Reber, A.J., et al. Immunosenescence and Challenges of Vaccination against Influenza in the Aging Population. Aging Dis 3, 68–90 (2012).
Myers, E.R., Misurski, D.A. & Swamy, G.K. Influence of timing of seasonal influenza vaccination on effectiveness and cost-effectiveness in pregnancy. Am J Obstet Gynecol 204, S128–140 (2011).
Blanchard-Rohner, G. & Siegrist, C.A. Vaccination during pregnancy to protect infants against influenza: why and why not? Vaccine 29, 7542–7550 (2011).
Ford, E.S., Mannino, D.M. & Williams, S.G. Asthma and influenza vaccination: findings from the 1999–2001 National Health Interview Surveys. Chest 124, 783–789 (2003).
Ford, E.S., Williams, S.G., Mannino, D.M. & Redd, S.C. Influenza vaccination coverage among adults with asthma: findings from the 2000 Behavioral Risk Factor Surveillance System. Am J Med 116, 555–558 (2004).
Plans-Rubio, P. Prevention and control of influenza in persons with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2, 41–53 (2007).
Hsu, A.C., See, H.V. & Wark, P.A. Innate immunity to influenza in chronic airways diseases. Respirology (2012).
Iorio, A.M., et al. Influenza vaccination in patients on long-term anticoagulant therapy. Vaccine 24, 6624–6628 (2006).
Shaw, S.M., Williams, S.G., Yonan, N. & Fildes, J.E. Decreased immune responses to influenza vaccination in patients with heart failure. J Card Fail 15, 549; author reply 549–551 (2009).
Ott, S.R., et al. [The impact of viruses in lower respiratory tract infections of the adult. Part II: acute bronchitis, acute exacerbated COPD, pneumonia, and influenza]. Pneumologie 64, 18–27 (2010).
Hakim, H., et al. Immunogenicity and safety of inactivated monovalent 2009 H1N1 influenza A vaccine in immunocompromised children and young adults. Vaccine 30, 879–885 (2012).
Bickel, M., et al. Low rate of seroconversion after vaccination with a split virion, adjuvanted pandemic H1N1 influenza vaccine in HIV-1-infected patients. AIDS 24, F31–35 (2010).
Cooper, C., et al. Immunogenicity is not improved by increased antigen dose or booster dosing of seasonal influenza vaccine in a randomized trial of HIV infected adults. PloS one 6, e17758 (2011).
Jain, S. & Chaves, S.S. Obesity and influenza. Clin Infect Dis 53, 422–424 (2011).
Camilloni, B., et al. An influenza B outbreak during the 2007/2008 winter among appropriately immunized elderly people living in a nursing home. Vaccine 28, 7536–7541 (2010).
Potter, J.M., O’Donnel, B., Carman, W.F., Roberts, M.A. & Stott, D.J. Serological response to influenza vaccination and nutritional and functional status of patients in geriatric medical long-term care. Age Ageing 28, 141–145 (1999).
Clarke, C.E. & McComas, K. Seeking and processing influenza vaccine information: a study of health care workers at a large urban hospital. Health Commun 27, 244–256 (2012).
Potter, J., et al. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis 175, 1–6 (1997).
Carman, W.F., et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet 355, 93–97 (2000).
Music, T. Protecting patients, protecting healthcare workers: a review of the role of influenza vaccination. Int Nurs Rev 59, 161–167 (2012).
Medina, R.A. & Garcia-Sastre, A. Influenza A viruses: new research developments. Nat Rev Microbiol 9, 590–603 (2011).
Guan, R., et al. Structural basis for the sequence-specific recognition of human ISG15 by the NS1 protein of influenza B virus. Proc Natl Acad Sci USA 108, 13468–13473 (2011).
Sridharan, H., Zhao, C. & Krug, R.M. Species specificity of the NS1 protein of influenza B virus: NS1 binds only human and non-human primate ubiquitin-like ISG15 proteins. J Biol Chem 285, 7852–7856 (2010).
Higgins, R.R., et al. Recovery of Influenza B Virus with the H273Y Point Mutation in the neuraminidase Active site from a Human Patient. J Clin Microbiol (2012).
Ak, O., et al. Influenza B-associated encephalopathy in two adults. J Infect Chemother (2012).
Rath, B., et al. Virus Load Kinetics and Resistance Development during Oseltamivir Treatment in Infants and Children Infected with Influenza A (H1N1) 2009 and Influenza B Viruses. Pediatr Infect Dis J (2012).
Steininger, C., et al. Acute encephalopathy associated with influenza A virus infection. Clin Infect Dis 36, 567–574 (2003).
Baccam, P., Beauchemin, C., Macken, C.A., Hayden, F.G. & Perelson, A.S. Kinetics of influenza A virus infection in humans. J Virol 80, 7590–7599 (2006).
Canini, L. & Carrat, F. Population modeling of influenza A/H1N1 virus kinetics and symptom dynamics. J Virol 85, 2764–2770 (2011).
Russell, C.A. The global circulation of seasonal influenza A (H3N2) viruses. Science 320, 340–346 (2008).
Russell, R.J., Stevens, D.J., Haire, L.F., Gamblin, S.J. & Skehel, J.J. Avian and human receptor binding by hemagglutinins of influenza A viruses. Glycoconj. J. 23, 85–92 (2006).
Skountzou, I. Immunity to pre-1950 H1N1 influenza viruses confers cross-protection against the pandemic swine-origin 2009 A (H1N1) influenza virus. J. Immunol. 185, 1642–1649 (2010).
Xu, R. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science 328, 357–360 (2010).
Perez, D.R. Fitness of pandemic H1N1 and seasonal influenza A viruses during co-infection: evidence of competitive advantage of pandemic H1N1 influenza versus seasonal influenza. PLoS Curr. 1, RRN1011 (2009).
Chen, R. & Holmes, E.C. The evolutionary dynamics of human influenza B virus. J Mol Evol 66, 655–663 (2008).
Hampson, A.W. & Mackenzie, J.S. The influenza viruses. Med J Aust 185, S39–43 (2006).
Racaniello, V.R. & Palese, P. Isolation of influenza C virus recombinants. J Virol 32, 1006–1014 (1979).
Fouchier, R.A. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 79, 2814–2822 (2005).
Tong, S., et al. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci USA 109, 4269–4274 (2012).
Krauss, S., Walker, D. & Webster, R.G. Influenza virus isolation. Methods Mol Biol 865, 11–24 (2012).
CDC. Influenza Type A Viruses and Subtypes. (2011).
Khiabanian, H., Farrell, G.M., St George, K. & Rabadan, R. Differences in patient age distribution between influenza A subtypes. PloS one 4, e6832 (2009).
Pica, N., Chou, Y.Y., Bouvier, N.M. & Palese, P. Transmission of influenza B viruses in the guinea pig. J Virol 86, 4279–4287 (2012).
Sandbulte, M.R., et al. Discordant antigenic drift of neuraminidase and hemagglutinin in H1N1 and H3N2 influenza viruses. Proc Natl Acad Sci USA 108, 20748–20753 (2011).
Stray, S.J. & Pittman, L.B. Subtype- and antigenic site-specific differences in biophysical influences on evolution of influenza virus hemagglutinin. Virol J 9, 91 (2012).
Yewdell, J.W. Viva la revolucion: rethinking influenza a virus antigenic drift. Curr Opin Virol 1, 177–183 (2011).
Salazar, M.I., Lopez-Ortega, O., Leon-Avila, G., Ramirez-Gonzalez, J.E. & Castro-Mussot, M.E. [The origin of the genetic variability of influenza viruses]. Gac Med Mex 146, 199–206 (2010).
Epstein, S.L. & Price, G.E. Cross-protective immunity to influenza A viruses. Expert Rev Vaccines 9, 1325–1341 (2010).
Bouvier, N.M. & Palese, P. The biology of influenza viruses. Vaccine 26 Suppl 4, D49–53 (2008).
Grebe, K.M., Yewdell, J.W. & Bennink, J.R. Heterosubtypic immunity to influenza A virus: where do we stand? Microbes Infect 10, 1024–1029 (2008).
Szucs, T. The socio-economic burden of influenza. J Antimicrob Chemother 44 Suppl B, 11–15 (1999).
Molinari, N.A. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25, 5086–5096 (2007).
Monto, A.S. Seasonal influenza vaccinations: specialized products for different target groups. Vaccine 28 Suppl 4, D14–23 (2010).
Hannoun, C., Megas, F. & Piercy, J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 103, 133–138 (2004).
Fiore, A.E., et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 59, 1–62 (2010).
Hanon, E. Vaccination strategies against influenza. Bull Mem Acad R Med Belg 164, 283–287 (2009).
Nichol, K.L. & Treanor, J.J. Vaccines for seasonal and pandemic influenza. J Infect Dis 194 Suppl 2, S111–118 (2006).
CDC. Seasonal Influenza (Flu). (2011).
Beyer, W.E., Nauta, J.J., Palache, A.M., Giezeman, K.M. & Osterhaus, A.D. Immunogenicity and safety of inactivated influenza vaccines in primed populations: a systematic literature review and meta-analysis. Vaccine 29, 5785–5792 (2011).
Gross, P.A. & Ennis, F.A. Influenza vaccine: split-product versus whole-virus types--How do they differ. N Engl J Med 296, 567–568 (1977).
Gross, P.A., et al. A controlled double-blind comparison of reactogenicity, immunogenicity, and protective efficacy of whole-virus and split-product influenza vaccines in children. J Infect Dis 136, 623–632 (1977).
Johansson, B.E. & Brett, I.C. Changing perspective on immunization against influenza. Vaccine 25, 3062–3065 (2007).
Cook, I.F., Barr, I., Hartel, G., Pond, D. & Hampson, A.W. Reactogenicity and immunogenicity of an inactivated influenza vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine 24, 2395–2402 (2006).
Schwartz, B. & Wortley, P. Mass vaccination for annual and pandemic influenza. Current topics in microbiology and immunology 304, 131–152 (2006).
Fredrickson, K., et al. Influenza vaccination coverage among children aged 6-23 months - six immunization information system sentinel sites, United States, 2005-06 influenza season. MMWR Morb Mortal Wkly Rep 55, 1329–1330 (2006).
McElhaney, J.E. Influenza vaccine responses in older adults. Ageing Res Rev 10, 379–388 (2011).
Ellebedy, A.H. & Webby, R.J. Influenza vaccines. Vaccine 27 Suppl 4, D65–68 (2009).
Marcelin, G., et al. Inactivated seasonal influenza vaccines increase serum antibodies to the neuraminidase of pandemic influenza A(H1N1) 2009 virus in an age-dependent manner. J Infect Dis 202, 1634–1638 (2010).
Coudeville, L., et al. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol 10, 18 (2010).
CDC. Vaccine Effectiveness - How Well Does the Flu Vaccine Work? (2011).
CDC. Flu Vaccine Effectiveness: Questions and Answers for Health Professionals. (2011).
Kim, Y.C. & Prausnitz, M.R. Enabling skin vaccination using new delivery technologies. Drug Deliv Transl Res 1, 7–12 (2011).
Jepps, O.G., Dancik, Y., Anissimov, Y.G. & Roberts, M.S. Modeling the human skin barrier - Towards a better understanding of dermal absorption. Adv Drug Deliv Rev (2012).
Skountzou, I. & Kang, S.M. Transcutaneous immunization with influenza vaccines. Current topics in microbiology and immunology 333, 347–368 (2009).
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Romani, N., et al. Morphological and phenotypical characterization of bone marrow-derived dendritic Thy-1-positive epidermal cells of the mouse. J Invest Dermatol 85, 91 s-95 s (1985).
Itano, A.A., et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003).
Valladeau, J., et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity 12, 71–81 (2000).
Allavena, P., et al. The chemokine receptor switch paradigm and dendritic cell migration: its significance in tumor tissues. Immunol Rev 177, 141–149 (2000).
Sozzani, S., Allavena, P., Vecchi, A. & Mantovani, A. Chemokines and dendritic cell traffic. J Clin Immunol 20, 151–160 (2000).
Locati, M., Allavena, P., Sozzani, S. & Mantovanii, A. Shaping and tuning of the chemokine system by regulation of receptor expression and signaling: dendritic cells as a paradigm. J Neuroimmunol 107, 174–177 (2000).
Sozzani, S., et al. In vitro and in vivo regulation of chemokine receptors. Eur Cytokine Netw 11, 502–503 (2000).
Romani, N., et al. Targeting skin dendritic cells to improve intradermal vaccination. Current topics in microbiology and immunology 351, 113–138 (2012).
Miller, L.S. Toll-like receptors in skin. Adv Dermatol 24, 71–87 (2008).
Abdelsadik, A. & Trad, A. Toll-like receptors on the fork roads between innate and adaptive immunity. Hum Immunol 72, 1188–1193 (2011).
Dupasquier, M., Stoitzner, P., van Oudenaren, A., Romani, N. & Leenen, P.J. Macrophages and dendritic cells constitute a major subpopulation of cells in the mouse dermis. J Invest Dermatol 123, 876–879 (2004).
Debenedictis, C., Joubeh, S., Zhang, G., Barria, M. & Ghohestani, R.F. Immune functions of the skin. Clin Dermatol 19, 573–585 (2001).
Kubo, A., Nagao, K., Yokouchi, M., Sasaki, H. & Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med 206, 2937–2946 (2009).
Flacher, V., et al. Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis. J Invest Dermatol 130, 755–762 (2010).
Romani, N., Brunner, P.M. & Stingl, G. Changing views of the role of Langerhans cells. J Invest Dermatol 132, 872–881 (2012).
Flacher, V., et al. Skin Langerin(+) dendritic cells transport intradermally injected anti-DEC-205 antibodies but are not essential for subsequent cytotoxic CD8(+) T cell responses. J Immunol 188, 2146–2155 (2012).
del Pilar Martin, M., et al. Local response to microneedle-based influenza immunization in the skin. MBio 3, e00012–00012 (2012).
Raz, E., et al. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA 91, 9519–9523 (1994).
Koutsonanos, D.G., et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. J Infect Dis 204, 582–591 (2011).
Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev 56, 581–587 (2004).
Prausnitz, M.R., Mitragotri, S. & Langer, R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov 3, 115–124 (2004).
Kim, Y.C., Park, J.H. & Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev (2012).
Kim, Y.C., Jarrahian, C., Zehrung, D., Mitragotri, S. & Prausnitz, M.R. Delivery systems for intradermal vaccination. Current topics in microbiology and immunology 351, 77–112 (2012).
Koutsonanos, D.G., et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep 2, 357 (2012).
Koutsonanos, D.G., et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PloS one 4, e4773 (2009).
Sullivan, S.P., et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med 16, 915v920 (2010).
Kim, Y.C., Quan, F.S., Compans, R.W., Kang, S.M. & Prausnitz, M.R. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. Journal of controlled release : official journal of the Controlled Release Society 142, 187v195 (2010).
Gill, H.S. & Prausnitz, M.R. Coating formulations for microneedles. Pharm Res 24, 1369–1380 (2007).
Gill, H.S. & Prausnitz, M.R. Coated microneedles for transdermal delivery. Journal of controlled release : official journal of the Controlled Release Society 117, 227–237 (2007).
Koyama, S., et al. Plasmacytoid dendritic cells delineate immunogenicity of influenza vaccine subtypes. Sci Transl Med 2, 25ra24 (2010).
Chu, L.Y., Choi, S.O. & Prausnitz, M.R. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: Bubble and pedestal microneedle designs. J Pharm Sci 99, 4228–4238 (2010).
Lee, J.W., Park, J.H. & Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 29, 2113–2124 (2008).
Chu, L.Y. & Prausnitz, M.R. Separable arrowhead microneedles. Journal of controlled release : official journal of the Controlled Release Society 149, 242–249 (2011).
Prausnitz, M.R., Mikszta, J.A., Cormier, M. & Andrianov, A.K. Microneedle-based vaccines. Current topics in microbiology and immunology 333, 369–393 (2009).
Choi, H.J., et al. Stability of influenza vaccine coated onto microneedles. Biomaterials 33, 3756–3769 (2012).
Patel, S.R., Lin, A.S., Edelhauser, H.F. & Prausnitz, M.R. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res 28, 166–176 (2011).
Gupta, J., Denson, D.D., Felner, E.I. & Prausnitz, M.R. Rapid local anesthesia in humans using minimally invasive microneedles. Clin J Pain 28, 129–135 (2012).
Wang, P.M., Cornwell, M., Hill, J. & Prausnitz, M.R. Precise microinjection into skin using hollow microneedles. J Invest Dermatol 126, 1080–1087 (2006).
Gupta, J., Felner, E.I. & Prausnitz, M.R. Minimally invasive insulin delivery in subjects with type 1 diabetes using hollow microneedles. Diabetes Technol Ther 11, 329–337 (2009).
Banks, S.L., et al. Transdermal delivery of naltrexol and skin permeability lifetime after microneedle treatment in hairless guinea pigs. J Pharm Sci 99, 3072–3080 (2010).
Gupta, J., Felner, E.I. & Prausnitz, M.R. Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol Ther 13, 451–456 (2011).
Lee, J.W., Choi, S.O., Felner, E.I. & Prausnitz, M.R. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small 7, 531–539 (2011).
Andrews, S., Lee, J.W., Choi, S.O. & Prausnitz, M.R. Transdermal insulin delivery using microdermabrasion. Pharm Res 28, 2110–2118 (2011).
Tas, C., et al. Delivery of salmon calcitonin using a microneedle patch. Int J Pharm 423, 257–263 (2012).
Song, J.M., et al. DNA Vaccination in the Skin Using Microneedles Improves Protection Against Influenza. Mol Ther (2012).
Kim, Y.C., et al. Increased immunogenicity of avian influenza DNA vaccine delivered to the skin using a microneedle patch. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V (2012).
Prow, T.W., et al. Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small 6, 1776–1784 (2010).
Fernando, G.J., et al. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. Journal of controlled release : official journal of the Controlled Release Society 159, 215–221 (2012).
FDA. Influenza Virus Vaccine, Trivalent, Types A and B. (2012).
Norman, J.J. & Prausnitz, M.R. Improving patient acceptance of insulin therapy by improving needle design. J Diabetes Sci Technol 6, 336–338 (2012).
Acknowledgements
We thank for the collaboration Dr. Mark R. Prausnitz, Dr. Vladimyr G. Zarnitsyn, Dr. Harvinder Singh Gill and Dr. Sean P. Sullivan, School of Chemical and Biomolecular Engineering, Georgia Institute of Technology, Atlanta, GA.
Research was supported by 1U01 AI074579-01/NIH and 1U01 EB012495/NIBIB grants.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Koutsonanos, D.G., Compans, R.W., Skountzou, I. (2013). Targeting the Skin for Microneedle Delivery of Influenza Vaccine. In: Katsikis, P., Schoenberger, S., Pulendran, B. (eds) Crossroads Between Innate and Adaptive Immunity IV. Advances in Experimental Medicine and Biology, vol 785. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6217-0_13
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6217-0_13
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6216-3
Online ISBN: 978-1-4614-6217-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)