Abstract
Antibody response constitutes one of the key immune protection mechanisms. T follicular helper (Tfh) cells represent the major CD4+ T cell subset that provides help to B cells to induce antibody response. How Tfh cells develop and how Tfh cells or their associated subsets regulate antibody response in humans remains largely unknown. In this review, we will summarize the recent discoveries on the biology of Tfh cells, with a particular focus on human Tfh cells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Human
- T follicular helper cells
- CD4+ T cell subsets
- Antibody response
- Tonsils
- Blood
- CXCR5
- Autoimmune diseases
10.1 Introduction
T follicular helper (Tfh) cells are the major CD4+ T cell subset that provides help to B cells to induce antibody response [1, 2]. Tfh cells are essential for the formation of germinal centers (GCs), the site of the selection of high-affinity B cells, and their differentiation into memory B cells or long-lived plasma cells. Similar to other CD4+ T cell subsets such as Th1, Th2, and Th17 cells, the magnitude and the duration of Tfh response need to be controlled by the immune system, because exaggerated Tfh response causes autoimmunity [3]. Recent studies including ours identified similarities and differences in the biology of Tfh cells between humans and mice. Determining the biology and developmental pathways of human Tfh cells is of great significance, as it will provide direct insights regarding the development of novel vaccine design and therapeutic strategies for human autoimmune diseases.
10.2 T Follicular Helper Cells: The CD4+ T Cell Subset Specialized for B Cell Help
In secondary lymphoid organs, CD4+ T cells primed by dendritic cells (DCs) loaded with antigens interact with antigen-primed naïve B cells at extrafollicular sites [4], typically at the border of T cell zone and primary follicles [5]. This interaction initiates the B cell differentiation process towards two different paths: extrafollicular plasma cells and cells forming GCs [6–8]. Extrafollicular plasma cells contribute to the early generation of specific antibodies after antigen exposure [9]. Germinal center B (GC-B) cells subsequently differentiate into either high-affinity long-lived plasma cells or memory B cells after an extensive selection step [10, 11] (Fig. 10.1).
Generation of human Tfh cells. In secondary lymphoid organs, naïve CD4+ T cells interact with DCs loaded with antigens. IL-12 secreted by the DCs induces naïve CD4+ T cells to initiate the program to differentiate into the Tfh lineage. These cells migrate towards B cell follicles, and interact with B cells. T and B cell interaction initiates the B cell differentiation process towards two different paths: extrafollicular plasma cells and cells forming GCs. How Tfh precursors and extrafollicular helper (EF) T cells overlap remains unclear in humans. Inside the GCs, Tfh cells provide help to high-affinity B cells and support their differentiation into long-lived plasma cells or memory B cells
Together with GC-B cells and follicular dendritic cells, Tfh cells constitute essential cell compartments for GC formation. In GCs, Tfh cells play an important role in the selection of high-affinity B cells and in the induction of the differentiation of selected B cells. While no unique markers have been reported, Tfh cells can be identified by the combination of several markers that are directly associated with their functions. The chemokine (C-X-C) receptor 5 (CXCR5) is important for their initial migration into B cell follicles [12–15]. Tfh cells express PD-1, which was shown to play a role in the selection of high-affinity B cells in GCs [16]. Inducible co-stimulator (ICOS) is critical for the development [17, 18] and functions [15, 19, 20] of Tfh cells. CD40 ligand (CD40L) expressed by Tfh cells provides essential signals to B cells through CD40 for their differentiation and class-switching [21]. Tfh cells and their precursors secrete IL-21 [22–24], a γc-family cytokine which potently promotes the growth, differentiation, and class-switching of B cells [25]. IL-21 delivers activation signals to B cells via STAT3 (signal transducer and activator of transcription 3), and accordingly STAT3-deficient patients show severely impaired antibody responses including a decreased generation of memory B cells [26].
10.3 Development of Tfh Cells
Differentiation of naïve CD4+ T cells towards conventional helper T cell (Th) subsets (including Th1, Th2, and Th17 cells) is regulated by the signals that they receive from DCs and from microenvironment [27, 28]. Recent mouse studies indicate that this is also the case for Tfh cell generation. Tfh cells express large amounts of B cell lymphoma 6 (Bcl-6) [23], the transcription repressor that is necessary and sufficient for Tfh cell generation in vivo [29–31]. Initial commitment towards Tfh cells occurs by upregulation of Bcl-6 expression when CD4+ T cells encounter with DCs [32–35]. The subsequent interaction between T and B cells appears to be important for the maintenance of Bcl-6 expression in Tfh cells. However, Bcl-6 does not regulate IL-21 secretion in mouse [30, 31] or human CD4+ T cells [36]. This is in contrast to other transcription factors engaged in the differentiation of other conventional Th subsets, regulating the secretion of cytokines typical of each subset. Thus, Tfh cell generation occurs through a highly orchestrated process, and likely requires other transcription factors in addition to Bcl-6.
Which types of DCs do promote Tfh cell generation? DCs are endowed with enormous functional plasticity, which permits them to induce different immune responses according to the microenvironment. In addition, the DC system is composed of subsets associated with the induction of different types of immunity [37, 38]. Our study on human skin DC subsets demonstrated that CD14+ dermal DCs are one of the most efficient DC subsets at inducing human naïve CD4+ T cells to become Tfh-like cells in vitro [39]. CD4+ T cells primed by CD14+ dermal DCs, but not by epidermal Langerhans cells, strongly induce naïve B cells to become antibody secreting plasma cells producing IgM, as well as to switch isotypes towards IgG and IgA [39]. Furthermore, in vitro-generated DCs sharing properties with CD14+ dermal DCs induce the differentiation of CD40-activated naïve B cells into IgM-producing plasma cells through direct DC and B cell interactions [40]. These observations suggest that CD14+ dermal DCs display unique properties to promote the development of antibody responses in humans. Notably, in mice, activated dermal DCs migrate into the outer paracortex just beneath the B cell follicles, whereas LCs migrate into the T cell rich inner paracortex [41]. This suggests that also in mice, dermal DCs, rather than LCs, are one of the major DC subsets associated with the development of humoral immunity.
Mouse studies showed that STAT3 signaling delivered by IL-6 and IL-21 contributes to Tfh cell development [42–45]. Similarly, STAT3-deficient human subjects (Hyper IgE syndrome) were shown to have altered Tfh response [46], although whether this is T cell intrinsic or secondary to defective B cell response [26] remains unclear. We and others showed that the IL-12-STAT4 pathway is one of the major pathways in humans by which DCs promote the development of IL-21-producing Tfh-like cells [47, 48]. IL-6 and IL-21 are much less potent than IL-12 in vitro at inducing human naïve CD4+ T cells to express Tfh-associated molecules, including IL-21, CXCR5, ICOS, and Bcl-6 [47, 49, 50]. Indeed, dermal CD14+ DCs, but not LCs, express IL-12 upon CD40L stimulation [39], which explains at least in part why dermal CD14+ DCs are efficient at inducing Tfh-like cells. Interestingly, there is evidence that the IL-12-STAT4 pathway contributes to the development of Tfh cells also in mice [51, 52]. Thus both STAT3 and STAT4 are involved in the generation of Tfh cells in mice and humans, while the extent of contribution by each pathway and/or cytokine might be different. It will be important to address whether IL-12 and/or IL-23 indeed contribute to in vivo Tfh and GC response in humans. Also it will be important to determine how the initial lineage commitment towards Th1 and Tfh cells is regulated in humans, because the IL-12/STAT4 axis also potently promotes Th1 cell generation through the upregulation of T-bet [53]. In mice, the effect of IL-12 on CD4+ T cells for the expression of Tfh-associated molecules is short-lived and eventually dominated by T-bet-driven Th1 cell generation [51].
10.4 Human Tfh Subsets
10.4.1 Tonsillar Tfh Subsets
Whereas Tfh cells are considered to help the selection and differentiation of B cells in GCs, the identity of CD4+ T cells interacting with B cells outside GCs was unknown in humans. We recently identified a Tfh-committed subset that is exclusively localized outside GCs in human tonsils [24]. This subset can be identified by the expression of IL-7 receptor, and low levels of CXCR5 and ICOS (CXCR5loICOSlo). The expression of BCL6 and PRDM1 transcripts are comparable between CXCR5loICOSlo CD4+ T cells and CXCR5hiICOShi GC-Tfh cells. Interestingly, these two Tfh-lineage subsets differentially help B cells. CXCR5hiICOShi GC-Tfh cells are efficient at helping GC-B cells. Reciprocally, GC-B cells are able to maintain the survival of CXCR5hiICOShi GC-Tfh cells. CXCR5loICOSlo CD4+ T cells are far more efficient than GC-Tfh cells at inducing naïve B cells to proliferate and differentiate into Ig-producing cells. Notably, CXCR5loICOSlo CD4+ T cells lack the capacity to help GC-B cells and induce the apoptosis of GC-B cells through the FAS/FAS-ligand (FAS-L) interaction. Thus, CXCR5loICOSlo CD4+ tonsillar CD4+ T cells likely represent extrafollicular helper cells engaged in inducing the differentiation of B cells into extrafollicular plasma cells and/or represent precursors of GC-Tfh cells (Pre-Tfh cells).
10.4.2 Blood Circulating CXCR5+ CD4+ T Cell Subsets
Human tonsillar Tfh cells display distinct phenotype and gene profiles from other conventional Th subsets [15, 23, 54]. Discovery of Bcl-6 as a “master regulator” of Tfh cell generation further supports the concept that Tfh cells represent an independent CD4+ T cell subset. However, mouse Tfh cells are indeed heterogeneous, and encompass distinct subsets secreting cytokines characteristic of Th1, Th2, and Th17 cells [55–59]. Furthermore, mouse Th2 [57] and T regs [60] were shown to be convertible into Tfh cells in vivo. Thus, the type of Tfh precursors also remains elusive and the relationship between Tfh cells and other Th subsets still remains unclear.
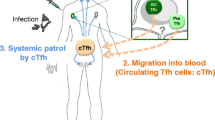
A fraction of human blood memory CD4+ T cells expresses CXCR5 [61]. Several observations suggest the relationship between CXCR5+ CD4+ T cells and Tfh cells. For example, humans who show severely impaired GC formation through deficiency of CD40-ligand or ICOS display significantly less circulating CXCR5+ CD4+ T cells [18]. On the contrary, CXCR5+ CD4+ T cells expressing ICOS are present at a higher frequency in blood of lupus patients [62]. Our studies on human blood CXCR5+ CD4+ T cells conclude that they share functional properties with Tfh cells from secondary lymphoid organs, and likely represent their circulating memory compartment [63]. In concordance with Tfh cells, blood CXCR5+ CD4+ T cells induce naïve and memory B cells to become Ig-producing cells via IL-21, IL-10, ICOS, and secrete CXCL13. At variance with Tfh cells, blood CXCR5+ CD4+ T cells barely express CD69 and ICOS, and express PD-1 only at low levels [14, 48, 62], suggesting that they are in a resting state. Consistently, blood CXCR5+ CD4+ T cells required cell activation to provide help to B cells through cognate interaction. In contrast to GC-Tfh cells, blood CXCR5+ CD4+ T cells express CCR7 and CD62L, suggesting their capacity to migrate into secondary lymphoid organs.
Importantly, blood CXCR5+ CD4+ T cells comprise three subsets; Th1, Th2, and Th17 cells [63] (Fig. 10.2). These subsets can be defined according to the expression of chemokine receptors, expression of transcription factors, and the type of cytokine secretion patterns. Th2 and Th17 cells within CXCR5+ compartment efficiently induce naïve B cells to produce immunoglobulins and to switch isotypes through IL-21 secretion. While CXCR5+ Th2 cells promote IgG and IgE secretion, CXCR5+ Th17 cells are efficient at promoting IgG and, in particular, IgA secretion. In contrast, CXCR5+ Th1 cells lack the capacity to help naïve B cells. These findings suggest that Tfh cells associated with conventional Th subsets differentially shape the quality of human humoral immunity, with CXCR5+ Th2 cells favoring IgE responses and CXCR5+ Th17 cells favoring protective mucosal antibody responses. Whether such Th1, Th2, and Th17-commited Tfh subsets are present in human secondary lymphoid organs is under investigation.
Hypothetical model in the development of distinct Tfh subsets in humans. We surmise that Tfh-committed cells sharing properties with conventional Th1, Th2, and Th17 cells develop at the Pre-Tfh stage. During the maturation process towards GC-Tfh cells, transcription factor changes including Bcl-6 upregulation suppress the expression and/or function of transcription factors associated with conventional Th subsets. Once Tfh cells (or pre-Tfh cells) differentiate into memory cells, they decrease the expression of Bcl-6 and start to reveal the property of conventional Th subsets while maintaining the identity of the Tfh lineage
Importantly, alteration in the balance of blood CXCR5+ CD4+ T cell subsets, which likely reflects the type of Tfh cells in secondary lymphoid organs, was found to be associated with autoimmunity. Patients with juvenile dermatomyositis, a systemic autoimmune disease, display a profound skewing of blood CXCR5+ CD4+ T cell subsets towards Th2 and Th17 [63]. Significantly, the skewing of subsets correlates with disease activity and frequency of blood plasmablasts. Furthermore, a study on Sjogren’s syndrome patient blood samples shows that CXCR5+ Th17 cells are dominant in this disease, and the increase of these cells correlate with clinical characteristics including autoantibody titers and disease activity [64]. Thus, these studies provide a strong rationale that analysis on blood CXCR5+ CD4+ T cell subsets might provide diagnostic and/or prognostic biomarkers in human autoimmune diseases.
Whether blood CXCR5+ CD4+ T cells originate from cells that migrated out of GCs, Tfh precursors, or Tfh-committed extrafollicular helper cells [9, 65] is an important question, but will be challenging to address in humans.
10.5 Perspectives
Tfh cells in human secondary lymphoid organs and in blood are composed of functionally different subsets. Establishing the mechanisms whereby the DC system induces Tfh cells with different functions will facilitate the design of novel vaccines. In particular, establishing how DC system generates Tfh subsets associated with the induction of mucosal homing plasma cells will provide a significant insight in the development of novel mucosal vaccines.
References
Crotty S. Follicular helper CD4 T cells (TFH). Annual review of immunology. 2011;29:621–63.
King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annual review of immunology. 2008;26:741–66.
Weinstein JS, Hernandez SG, Craft J. T cells that promote B-Cell maturation in systemic autoimmunity. Immunological reviews. 2012;247(1):160–71.
Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281(5373):96–9.
Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS biology. 2005;3(6):e150.
MacLennan IC, Gray D. Antigen-driven selection of virgin and memory B cells. Immunological reviews. 1986;91:61–85.
McHeyzer-Williams MG, McLean MJ, Lalor PA, Nossal GJ. Antigen-driven B cell differentiation in vivo. The Journal of experimental medicine. 1993;178(1):295–307.
Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. The Journal of experimental medicine. 1991;173(5):1165–75.
MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, et al. Extrafollicular antibody responses. Immunological reviews. 2003;194:8–18.
Tarlinton DM. Evolution in miniature: selection, survival and distribution of antigen reactive cells in the germinal centre. Immunology and cell biology. 2008;86(2):133–8.
MacLennan IC. Germinal centers. Annual review of immunology. 1994;12:117–39.
Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. The Journal of experimental medicine. 2000;192(11):1545–52.
Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. The Journal of experimental medicine. 2000;192(11):1553–62.
Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. The Journal of experimental medicine. 2001;193(12):1373–81.
Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. European journal of immunology. 2006;36(7):1892–903.
Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nature immunology. 2010;11(6):535–42.
Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175(4):2340–8.
Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, et al. ICOS Deficiency Is Associated with a Severe Reduction of CXCR5+CD4 Germinal Center Th Cells. J Immunol. 2006;177(7):4927–32.
Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397(6716):263–6.
Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29(1):127–37.
Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, et al. The CD40 antigen and its ligand. Annual review of immunology. 1994;12:881–922.
Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179(12):8180–90.
Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173(1):68–78.
Bentebibel S-E, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proceedings of the National Academy of Sciences. 2011;10.1073/pnas.1100898108.
Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annual review of immunology. 2008;26:57–79.
Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. The Journal of experimental medicine. 2010;207(1):155–71, S1–5.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52.
Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–26.
Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl–6 directs T follicular helper cell lineage commitment. Immunity. 2009;31(3):457–68.
Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–5.
Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–10.
Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34(6):961–72.
Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity. 2011;34(6):932–46.
Baumjohann D, Okada T, Ansel KM. Cutting Edge: Distinct Waves of BCL6 Expression during T Follicular Helper Cell Development. J Immunol. 2011.
Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34(6):947–60.
Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, et al. Bcl6 and Maf Cooperate To Instruct Human Follicular Helper CD4 T Cell Differentiation. J Immunol. 2012.
Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, et al. Dendritic cell subsets in health and disease. Immunological reviews. 2007;219:118–42.
Ueno H, Schmitt N, Klechevsky E, Pedroza-Gonzalez A, Matsui T, Zurawski G, et al. Harnessing human dendritic cell subsets for medicine. Immunological reviews. 2010;234(1):199–212.
Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29(3):497–510.
Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90(4):1458–70.
Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22(5):643–54.
Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29(1):138–49.
Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6(3):e17739.
Lu Kristina T, Kanno Y, Cannons Jennifer L, Handon R, Bible P, Elkahloun Abdel G, et al. Functional and Epigenetic Studies Reveal Multistep Differentiation and Plasticity of In Vitro-Generated and In Vivo-Derived Follicular T Helper Cells. Immunity. 2011;35(4):622–32.
Eddahri F, Denanglaire S, Bureau F, Spolski R, Leonard WJ, Leo O, et al. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood. 2009;113(11):2426–33.
Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119(17):3997–4008.
Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31(1):158–69.
Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, et al. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunology and cell biology. 2009.
Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, et al. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunology and cell biology. 2009;87(8):590–600.
Lund R, Ahlfors H, Kainonen E, Lahesmaa AM, Dixon C, Lahesmaa R. Identification of genes involved in the initiation of human Th1 or Th2 cell commitment. European journal of immunology. 2005;35(11):3307–19.
Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, et al. Early Th1 Cell Differentiation Is Marked by a Tfh Cell-like Transition. Immunity. 2011;35(6):919–31.
Wei L, Vahedi G, Sun HW, Watford WT, Takatori H, Ramos HL, et al. Discrete Roles of STAT4 and STAT6 Transcription Factors in Tuning Epigenetic Modifications and Transcription during T Helper Cell Differentiation. Immunity. 2010;32(6):840–51.
Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–69.
Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104(7):1952–60.
Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature immunology. 2009;10(4):385–93.
King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. The Journal of experimental medicine. 2009;206(5):1001–7.
Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. The Journal of experimental medicine. 2009;206(5):991–9.
Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature immunology. 2009;10(2):167–75.
Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30(3):324–35.
Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323(5920):1488–92.
Forster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein--coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84(3):830–40.
Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis and rheumatism. 2010;62(1):234–44.
Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–21.
Li X-y, Wu Z-b, Ding J, Zheng Z-h, Li X-y, Chen L-n, et al. Role of the frequency of blood CD4+ CXCR5+ CCR6+ T cells in autoimmunity in patients with Sjögren’s syndrome. Biochemical and Biophysical Research Communications. 2012.(http://dx.doi.org/10.1016/j.bbrc.2012.04.133).
Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, et al. In Vivo Regulation of Bcl6 and T Follicular Helper Cell Development. J Immunol. 2010;doi:10.4049/jimmunol.0904023.
Acknowledgments
We thank former and current members of the Institute for their contributions to our progresses. These studies have been supported by the NIH (P01 CA084514, U19 AI057234, U19 AI082715, and U19 AI089987), the Baylor Health Care System; the Baylor Health Care System Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Schmitt, N., Ueno, H. (2013). Human T Follicular Helper Cells: Development and Subsets. In: Katsikis, P., Schoenberger, S., Pulendran, B. (eds) Crossroads Between Innate and Adaptive Immunity IV. Advances in Experimental Medicine and Biology, vol 785. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6217-0_10
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6217-0_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6216-3
Online ISBN: 978-1-4614-6217-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)