Abstract
How is sex determined? In the animal kingdom, there are diverse sets of mechanisms for determining organismal sex, with the predominant ones being chromosomally based, either a dominant-acting sex chromosome or the ratio of the number of X chromosome to autosomes, which lead to oocyte-producing females and sperm-producing males. The resulting germline sexual phenotype is often the logical consequence of somatic sex determination. In this respect however, the Caenorhabditis elegans hermaphrodite is different from mammals and Drosophila. In fact in the C. elegans hermaphrodite germline, male gametes are transiently produced in a female body during larval development. To override chromosomal signals, sex determination of germ cells strongly depends on post-transcriptional regulation. A pivotal role for male gamete production (spermatogenesis) is played by the fem-3 mRNA, which is controlled through FBF and other RNA-binding proteins or splicing factors. Thanks to its powerful genetics, transparent body, small size, and the ability to make sperm and oocytes within one individual, C. elegans represents an excellent system to investigate cellular differentiation and post-transcriptional control.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Reproduction is one of the life’s essential features. While vegetative growth or fission generates clonal progeny, sexual reproduction enhances genetic variation and therefore offers an additional possibility for adaptation. Sexual reproduction uses gametes that are generally of two types: sperm and oocytes. These specialized cells are produced through meiosis, allowing two different haploid genomes to combine at fertilization. This chapter describes the processes of sex determination in the germline.
3.1.1 Sex Determination Among Species
Although sex determination leads to a clear-cut decision, male or female, the mechanisms that are behind this process vary widely. These are directed through chromosomal signals as well as environmental cues such as temperature, hormones, and nutrition. In this chapter we focus on the mechanisms in Caenorhabditis elegans. Of course, much is also known in other model organisms, such as the fruit fly Drosophila melanogaster and vertebrates. These will be shortly introduced to point at possible links with C. elegans. In this chapter we have not included Danio rerio (zebrafish), a well-studied vertebrate model organism. In fact, in contrast to other fish species with male (XY) or female (ZW) heterogamety, zebrafish has no sex chromosomes. Instead, its sex determination is influenced by environmental cues and the presence of the gonad, which is crucial for female sex (Slanchev et al. 2005).
3.1.1.1 Mammals
As in many cases, important findings have come from investigation of disorders in humans. The role of the Y chromosome in mammalian sex determination is a classical example. More than 50 years ago, Ford and colleagues identified an abnormal karyotype in patients with Turner’s syndrome (Ford et al. 1959). Individuals affected by this syndrome lack parts of, or the entire Y heterosome. Moreover, all patients are phenotypically female, indicating that the Y chromosome is not required for female development. Nevertheless, such genotypic X0 females are sterile due to gonadal dysfunction (Ford et al. 1959). Additional evidence for Y chromosome function came from patients with Klinefelter syndrome (XXY karyotype; Jacobs and Strong 1959). In spite of an additional X chromosome, Klinefelter individuals develop as males. Both disorders led to the identification of the Y chromosome as a major determinant for male sex determination in mammals.
Thirty years later, the discovery of the Sry (Sex determining region on Y) gene further illuminated the dominantly acting function of the Y chromosome in sex determination (Sinclair et al. 1990; Gubbay et al. 1990). The role of the Sry gene as a major effector for sex determination was confirmed with transgenic XX mice which developed into phenotypic males when bearing a copy of this gene (Koopman et al. 1991). SRY is a transcription factor of the SOX family (SRY-related HMG box). The HMG box is crucial for its action because mutations in this domain lead to XY females (reviewed in Harley and Goodfellow 1994).
The general process of sex determination in mammals features two main events. Primary sex determination is strictly chromosomal and establishes the sexual identity of the gonads. At a later step, the sexual phenotype is shaped through hormones secreted by the sexually determined gonad. This second event is referred to as secondary sex determination. Mammalian gonads first arise as bipotential organs which later develop into either ovaries or testes. In response to Sry, the bipotential gonad is switched to the male fate. The female fate instead does not require Sry and is therefore considered as a default state (Ford et al. 1959; Sinclair et al. 1990; Jacobs and Strong 1959). In males, Sry activation results in upregulation of Sox9 (SRY box containing gene 9) and repression of Wnt4 which would otherwise activate the female sex-determining pathway in the genital ridge (Kent et al. 1996; Tamashiro et al. 2008; Vaiman and Pailhoux 2000). Therefore, different genes are expressed thus leading to development of testis instead of ovary (for a review see Kashimada and Koopman 2010). In mammals, secondary sex determination is triggered by sex-specific hormones secreted by the testis or the ovary. It leads to somatic male or female phenotypes (reviewed in Ostrer 2000).
In mammals, germ cells are indistinguishable prior to colonization of the genital ridges (McLaren 1984; Donovan et al. 1986). Germ cell sex is determined through the sexual phenotype of the somatic environment, rather than their own heterochromosomal content (for review, see Ewen and Koopman 2010).
3.1.1.2 Drosophila melanogaster
Almost 100 years ago, Calvin Bridges observed that genotypic XXY flies developed as females and X0 flies developed as somatic sterile males (Bridges 1916). He concluded that the Y chromosome was required for male fertility in Drosophila, but that it was not necessary for sex determination. Thus, contrary to mammals, the Drosophila Y chromosome does not function in sex determination. Rather, the ratio between the number of X chromosomes and sets of autosomes (X:A) proved to be the main determinant for Drosophila sex (Bridges 1916). It took 60 years to identify the sex-lethal gene as the “reader” of the X:A ratio (Cline 1978). The expression of Sxl depends on at least four X-linked genes (scute, sisA, runt, and unpaired) which encode transcription factors that activate the early promoter of Sxl (SxlPe). The current working model postulates that activation of SxlPe is initiated when a threshold concentration of X-linked signal elements (XSE) is reached (reviewed in Salz and Erickson 2010). Upon activation of SxlPe, the late promoter of Sxl (SxlPm) is activated in all somatic cells of both sexes (Gonzalez et al. 2008). The activation of this second promoter ensures the maintenance of Sxl action in females (Gonzalez et al. 2008). Furthermore, action of the Sxl protein is restricted to females through alternative splicing of its pre-mRNA: an autoregulatory feedback mechanism leads to skipping of the third exon, which contains a stop codon (Cline 1984; Bell et al. 1991).
Sxl protein binds near the 3′ splice site and modulates splicing efficiency of specific introns in other pre-mRNAs that code for downstream effectors. For example, Sxl causes exon skipping in the female transformer (tra) mRNA, which results in female-specific splicing of doublesex (Hoshijima et al. 1991). The tra genes regulate the alternative splicing of the switch gene doublesex (dsx), which produces either the male or female isoform of the Doublesex protein (Baker and Wolfner 1988). dsx is responsible for somatic sexual development in most cells, including the somatic gonad (Coschigano and Wensink 1993; Ryner et al. 1996).
In Drosophila how somatic tissues influence the sexual fate of germ cells is not well understood. The Jak/Stat pathway appears to be involved, perhaps being largely responsible for establishing the male fate in male gonads. Signals emanating from the female soma remain to be identified (Wawersik et al. 2005). While the somatic gonad participates in determining germ cell sexual fate, germ cells also determine their fate autonomously. In this respect, Sxl and other genes such as Ovo and Otu (ovarian tumor) contribute directly to female identity in XX germ cells (reviewed in Casper and Van Doren 2009).
3.1.1.3 C. elegans
The primary signals for chromosomal sex determination are similar in C. elegans and in Drosophila. The balance between the number of X chromosomes and the number of sets of autosomes also determines the sexual phenotype in worms (Nigon 1952). Albeit essential for male fertility, the Y chromosome in Drosophila does not control sex determination (Bridges 1916). In C. elegans there is no Y chromosome. The “heterogametic” sex is therefore denoted as X0, as opposed to the XX “homogametic” sex. In C. elegans, low X dosage (X0) leads to activation of the male-specific switch gene xol-1 (X0 lethal) (Miller et al. 1988). xol-1 is on top of the sex determination pathway and promotes male development. In contrast, C. elegans XX embryos undergo female development as a result of xol-1 inactivation through the X-linked determinants sex-1 and fox-1 (Carmi et al. 1998; Hodgkin et al. 1994) and possibly others. It should be noted at this point that C. elegans females are truly protandric hermaphrodites because they transiently produce sperm (Miller et al. 1988).
The absence of xol-1 in females allows sdc-2 expression (sex and dosage compensation) (Nusbaum and Meyer 1989). sdc-2 functions together with sdc-1 and sdc-3 in hermaphrodites (DeLong et al. 1993; Villeneuve and Meyer 1987; Trent et al. 1991). In addition to their role in sex determination, sdc genes also function in dosage compensation (Lieb et al. 2000). The remaining part of the sex determination pathway consists of a cascade of negative regulatory interactions that alternately activate or repress male- or female-specific genes. More precisely, the SDC proteins repress her-1 to prevent male development (Trent et al. 1991). In the absence of HER-1, TRA-2 blocks the action of the FEM proteins and, as a result, the active TRA-1 transcription factor determines the female fate. In contrast, X0 individuals have active HER-1, which represses TRA-2. Further downstream, the FEM proteins inactivate TRA-1 and therefore control male development by activating fog-1 and fog-3 (reviewed in Zarkower 2006). Activation of fog-1 and fog-3 takes place either indirectly, through tra-1, or directly through the fem genes. In fact fem-3;tra-1 double mutants produce oocytes and not sperm (Hodgkin 1986).
In C. elegans, the ratio of sex chromosomes to autosomes influences sex determination of the germ cells, as X0 animals exclusively make sperm and adult XX individuals continuously produce oocytes. Interestingly, the developmental balance is transiently shifted to the opposite sex during the “female” development, so that XX animals become self-fertile hermaphrodites. The determination of germ cell sex in the C. elegans hermaphrodite thus represents an extremely interesting system for the study of cell fate specification (Fig. 3.1).
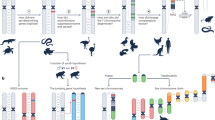
Principal genes controlling sex determination in the C. elegans XX hermaphrodite germline. In response to a double dose of X signal elements, the xol-1 gene is inactivated, thus allowing sdc to repress her-1 and to promote dosage compensation. Post-transcriptional regulation for transient spermatogenesis and the sperm-oocyte switch characterize the tra-2 and fem-3 mRNAs. tra-2 is repressed by GLD-1 and FOG-2, which are required for spermatogenesis. fem-3 is repressed by the FBF and MOG proteins to allow the switch to oogenesis. While TRA-2 activates TRA-1, the FEM proteins repress its action. TRA-1 drives oogenesis and inhibits spermatogenesis by repressing fog-1 and fog-3. Only the main interactions are shown
3.1.2 Sexual Dimorphism in C. elegans
Sexual dimorphism in C. elegans, as in many other animals, is generated through the action of genes acting in the sex determination pathway. Germ cells in C. elegans X0 males are not responsible for their own sexual fate but depend on HER-1, which is mainly produced in the intestine and acts as a secreted ligand to shift the balance towards the male fate (Kuwabara 1996; Perry et al. 1993). HER-1 is only produced in X0 males. In the germline, HER-1 is responsible for the indirect activation of the fem genes, fog-1, and fog-3 which are needed for spermatogenesis (Hodgkin 1986; Ellis and Kimble 1995; Doniach and Hodgkin 1984; Barton and Kimble 1990). tra-1 is the major effector of somatic sex determination in the hermaphrodite. It also promotes sexual dimorphism of the male germline. In fact, loss of tra-1 results in transient spermatogenesis and continuous oogenesis in adult X0 individuals (Schedl et al. 1989; Hodgkin 1987). This indicates that tra-1 is required for maintenance of spermatogenesis in males. Furthermore, partial absence of tra-1 activity in males results in expression of dmd-3 and mab-3 which contribute to the morphogenesis of the male copulatory organ (Mason et al. 2008). Recent studies have shown that in adults, male and hermaphrodite germlines not only differ in their content of mature gametes, but also in terms of the maintenance of germline stem cells (Morgan et al. 2010). Actually, the mitotic region in males is longer and thinner than in hermaphrodites. Moreover, the extent of the cell-cycle length appears to be shorter in males than in hermaphrodites (Morgan et al. 2010). This aspect of sexual dimorphism is caused by the influence of the somatic gonad on the distal germline and suggests that the mitotic region might play a role in early events of germline sex determination and commitment for meiosis (Morgan et al. 2010).
3.1.3 Cell Fate Decisions in the C. elegans Germline
Albeit it is interesting to study how the male fate is maintained throughout the development of X0 individuals, it is much more intriguing to understand how spermatogenesis is achieved in a somatic female environment of a hermaphrodite. Germ cells in C. elegans hermaphrodites develop either into male or female gametes, depending on the worm’s developmental stage. The first three larval stages of C. elegans males and hermaphrodites are characterized by mitotic proliferation. During the L3 stage, germ cells that are located at the proximal end of the gonad begin to differentiate and thus enter the meiotic cell cycle. These cells continue differentiating during the fourth larval stage and are destined to undergo spermatogenesis in both males and hermaphrodites. In contrast, germ cells that enter meiosis in the L4 stage and beyond are destined to female development and differentiate as oocytes in hermaphrodites (reviewed in Schedl 1997 and Kimble and Crittenden 2007). Intriguingly, spermatozoans and oocytes are completely different in size and function, but develop from a common pool of undifferentiated cells.
In summary, the development of gametes in C. elegans hermaphrodites is the result of three fundamental decisions. First, during embryogenesis, cells are specified as germ cells rather than somatic blastomeres. Second, cells proliferate, not only during larval stages L2 and L3, but also throughout later developmental stages, when they keep dividing only in the distal portion of the germline. Third, when germ cells differentiate by entering the meiotic cell cycle, they can develop either as spermatids or as oocytes (Fig. 3.2).
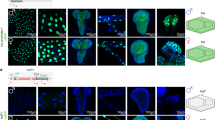
Morphology and protein expression throughout hermaphrodite germline development. (a, b) germline arm of an L4 larva. Chromosomal staining with DAPI shows primary (1°) and secondary (2°) spermatocytes. No spermatids are visible at this step. Immunolocalization of sperm-specific polypeptides in the cytoplasm using anti-SP56 antibodies (Ward et al. 1986). (c) Staining of a dissected gonad at the transition from L4 to adult. Spermatids and spermatozoans are shown (Sp). Fig. 3.2 (continued) The first oocytes are visible (white arrows). The distal end of the germline is indicated by an asterisk. (d) DIC image of an adult germline with the localization of markers for mitosis (GLP-1), meiosis (GLD-1), spermatogenesis (SP56), and oogenesis (RME-2). The inset (bottom, right) with spermatocytes and spermatids comes from a younger worm in which oocytes have not yet pushed all male germ cells into the spermatheca. The dotted line in the inset with GLD-1 expression indicates the transition zone between mitosis (right) and meiosis (left). (e) Drawing of an adult hermaphrodite. The germline has the same color code as in panel (d): yellow = mitosis; green = different stages of the meiotic sequence; red = mature and maturing oocytes; blue = sperm. The somatic gonad is made up of the distal tip cell (DTC), the sheath cells, spermatheca and uterus. Nuclear morphology is shown by DAPI staining. Bar: 10 μm
3.2 The Players in C. elegans Sex Determination
3.2.1 xol-1 Is a Master Switch Gene Controlling Sex Determination in C. elegans
As mentioned above, the sex of C. elegans embryos is determined by the ratio between the number of X chromosomes and sets of autosomes (Nigon 1952). Studies by Madl and Herman determined that an X:A ratio of 0.67 leads to males, while a ratio higher than 0.75 results in female development (Madl and Herman 1979). The signals from the X chromosome correspond to at least fox-1 and sex-1 (Carmi et al. 1998; Hodgkin et al. 1994). Other signals originate from the autosomes (the sea genes (Powell et al. 2005; reviewed in Wolff and Zarkower 2008). An X:A ratio equal or below 0.75 results in the activation of xol-1 and therefore leads to the male fate. Conversely an XX genotype leads to inactivation of xol-1 (Miller et al. 1988). Therefore, xol-1 functions as a master switch gene that controls sex determination, analogous to Sxl in Drosophila. In addition to its role in sex determination, xol-1 inhibits dosage compensation in males by repressing the sdc genes (Miller et al. 1988; Rhind et al. 1995; Nusbaum and Meyer 1989). xol-1 is regulated both transcriptionally by the SEA proteins and SEX-1, and post-transcriptionally by the RNA-binding protein FOX-1 (Skipper et al. 1999; Carmi et al. 1998; Powell et al. 2005). Although the exact mechanism of xol-1 regulation is not fully understood, two lines of evidence indicate that xol-1 is the unique target of chromosomal signals. In fact, xol-1 null mutations only affect male development, while hermaphrodites develop normally (Miller et al. 1988). Furthermore, XX animals that ectopically express xol-1 develop as males, indicating that xol-1 acts as an early switch factor for sex determination (Rhind et al. 1995). A recent study proposes that xol-1 is also transcriptionally regulated by the feminizing protein TRA-1, which sits at the end of the sex determination cascade. Therefore, at least in somatic tissues, regulation of xol-1 may include a feedback loop (Hargitai et al. 2009).
3.2.2 Dosage Compensation and Somatic Sex Determination
Although sex determination in C. elegans is initiated by the dose of X chromosomes, normal development requires identical amounts of X-chromosomal gene products in both sexes. Dosage compensation in C. elegans is controlled by three sdc genes that act downstream of xol-1 (Rhind et al. 1995). Among these, sdc-2 plays a predominant role in dosage compensation, as well as promoting hermaphrodite sex determination (Chu et al. 2002; Dawes et al. 1999). SDC-2 belongs to a protein complex containing SDC-1, SDC-3, DPY-21, DPY-26, DPY-27, DPY-28, and DPY-30 (reviewed in Meyer 2005). This dosage compensation complex functions as repressive machinery that reduces by half the transcription on both X chromosomes in hermaphrodites (Meyer and Casson 1986). The mechanism that controls X0 specific her-1 transcription and therefore sex determination requires the same complex, but lacks DPY-21. It also differs in other aspects. First SDC-2 recognizes elements on the X chromosome, while her-1 is located on an autosome and is recognized by SDC-3. Second, in hermaphrodites, the transcription rate of her-1 is reduced by 20-fold and not by 2-fold, as for X-linked genes (Yonker and Meyer 2003). By repressing her-1, sdc genes affect the activity of all genes that are further downstream in the sex determination pathway. They therefore play a dual role in dosage compensation and sex determination.
3.2.3 Germline Sex Determination Genes
HER-1 acts as a secreted ligand for male sex determination by inhibiting the function of the transmembrane receptor TRA-2A (Perry et al. 1993; Hamaoka et al. 2004; Kuwabara 1996). The current model postulates that TRA-2A represses the FEM proteins (FEM-1, -2, -3) in XX animals, thereby leaving transcription factor TRA-1 active. TRA-1 is the terminal activator for female development in somatic tissues (for review, see Zarkower 2006). In X0 individuals, HER-1 binds and represses TRA-2A. As a consequence, the FEM proteins are active and repress TRA-1 to achieve male development (Hodgkin 1986). To do so, the FEM proteins, together with CUL-2, direct proteasome-mediated degradation of TRA-1 (Starostina et al. 2007).
In the germline of hermaphrodites, which produce both spermatids and oocytes, sex determination is more complex because additional factors are required for this process. Nevertheless, C. elegans hermaphrodites offer the possibility to study regulation of spermatogenesis and oogenesis within the same individual. Moreover, both gametes are produced from a single pool of germline stem cells (for a review, see Schedl 1997). This additional regulation requires the control of tra-2 and fem-3 (for a review, see Puoti et al. 2001; Ellis 2010). As a first step, tra-2 is repressed for the onset of spermatogenesis in the L3 larva (Doniach 1986). After a transient period of spermatogenesis, the onset of oogenesis coincides with the repression of spermatogenesis. fem-3 regulation plays a pivotal role in the sperm-oocyte switch (Kimble et al. 1984; Ahringer and Kimble 1991; Barton et al. 1987). While the sex determination pathway culminates with the activation or repression of tra-1 in somatic tissues, fog-1 and fog-3 are also required in the germline for spermatogenesis in both males and hermaphrodites (reviewed in Ellis and Schedl 2007). Moreover, fbf and the mog genes are required for oogenesis by repressing fem-3, but do not influence somatic sex determination (Gallegos et al. 1998; Graham and Kimble 1993; Graham et al. 1993; Zhang et al. 1997). The sexual fate of germ cells in the hermaphrodite therefore depends on a balance between tra-2 and fem-3 activity. In fact tra-2(gf) mutations result in feminized germlines, whereas fem-3(gf) alleles lead to masculinization. Furthermore, gain-of-function mutations in tra-2 and fem-3 suppress each other (Barton et al. 1987).
3.3 Regulation of Germline Sex Determination in C. elegans
3.3.1 Transcriptional Control of her-1
After xol-1 which is controlled by the X:A ratio, her-1 is the next gene in line for male sex determination. Ectopic expression of her-1 in XX animals results in partial masculinization (Trent et al. 1991). How does XOL-1 determine the activity of her-1 in males? While the activation of her-1 in X0 animals remains mysterious, there is evidence that her-1 must be repressed in hermaphrodites. When comparing X0 and XX animals, her-1 mRNA levels are clearly reduced in the latter, indicating that her-1 is transcriptionally regulated (Trent et al. 1991). Furthermore, her-1 gain-of-function alleles correspond to mutations in the her-1 promoter region and lead to increased transcription (Trent et al. 1991; Perry et al. 1994). Finally, elevated levels of her-1 RNA are also observed in sdc-1 or sdc-2 mutants, suggesting that the latter are involved in transcriptional repression of her-1 in hermaphrodites (Trent et al. 1991). Together with SDC-1 and SDC-2, SDC-3 preferentially recognizes the her-1 promoter region (Yonker and Meyer 2003). After recognition by SDC-3, the whole dosage compensation machinery, except DPY-21, is recruited by SDC-2 to the regulatory region of her-1. This results in her-1 silencing (Yonker and Meyer 2003; Chu et al. 2002). In the male germline, her-1 expression is constantly maintained to ensure continuous spermatogenesis (Hodgkin 1980).
3.3.2 Role of the Transcription Factor TRA-1 in the Germline
tra-1 loss-of-function (lf) XX mutant animals are somatically transformed into males (Hodgkin and Brenner 1977). tra-1 is thus required for the female fate. It regulates, among other genes, two homologs of the Drosophila melanogaster doublesex gene: mab-3 and dmd-3 (Raymond et al. 1998; Mason et al. 2008). In the germline the situation is less clear since tra-1 (lf) XX or X0 gonads transiently produce sperm and then switch to oogenesis (Schedl et al. 1989). Therefore, XX tra-1 null mutants have normal germlines making both sperm and oocytes. In contrast, tra-1 X0 mutants produce ectopic oocytes, indicating that tra-1 is required for continuous production of sperm in males (Hodgkin 1987). The tra-1 gene encodes two mRNAs that are translated into two transcription factors containing either two or five zinc-finger motifs (Zarkower and Hodgkin 1992). The larger transcript is present at constant levels throughout development, while the shorter peaks during the second larval stage (Zarkower and Hodgkin 1992). Nevertheless, the levels of both tra-1 mRNAs are similar in males and hermaphrodites, indicating that tra-1 is regulated post-transcriptionally (Zarkower and Hodgkin 1992). All tra-1 activity is attributed to the larger transcript that encodes the TRA-1A protein, while the shorter TRA-1B isoform has no obvious function (Zarkower and Hodgkin 1992). TRA-1A, referred to as TRA-1 in this report, belongs to the GLI family of transcriptional repressors, but it also includes potential activators of transcription (Koebernick and Pieler 2002). TRA-1 is activated by TRA-2 and TRA-3, and repressed through CUL-2 and three FEM proteins, which target TRA-1 for proteasome-mediated degradation (Starostina et al. 2007; Schvarzstein and Spence 2006; Sokol and Kuwabara 2000). TRA-1 activation by TRA-2 is achieved through the intracellular part of TRA-2, which binds to TRA-1 (Lum et al. 2000; Wang and Kimble 2001). In addition, TRA-3 can cleave the intracellular part of TRA-2 to generate an active peptide that binds and represses FEM-3, thus preventing degradation of TRA-1 (Starostina et al. 2007; Sokol and Kuwabara 2000). Nuclear TRA-1 represses the transcription of fog-3, a terminal regulator for spermatogenesis (Chen and Ellis 2000). Additional results show that TRA-1 is also involved in positive regulation of fog-3, possibly explaining the requirement of TRA-1 for continuous spermatogenesis in males (Chen and Ellis 2000). These results place TRA-1 as a regulator for both somatic and germline sex determination in males and hermaphrodites (Chen and Ellis 2000).
TRA-1 is also regulated at the level of its protein, which is cleaved differently in males and in hermaphrodites (Schvarzstein and Spence 2006). First, the TRA-1 levels are higher in hermaphrodites as compared to males. Second, a shorter product, TRA-1100 accumulates in adult hermaphrodites, while in masculinized animals, which produce only sperm, the larger isoform is predominant (Schvarzstein and Spence 2006). Taken together, full-length TRA-1 promotes spermatogenesis in adult males, and the shorter TRA-1100 is required for oogenesis (Schvarzstein and Spence 2006, reviewed in Ellis 2008). Finally, in feminized fem-1 and fem-3 mutants both isoforms of TRA-1 accumulate, indicating that the FEM complex controls TRA-1 degradation (Schvarzstein and Spence 2006).
3.3.3 Post-transcriptional Regulation: 3′UTR-Mediated Control in the Germline
Many germline genes encode RNA-binding proteins, and most of them are required for germline development (reviewed in Lee and Schedl 2006; Nousch and Eckmann 2012, Chap. 8). In addition, the 3′untranslated regions (UTR) are primary regulatory elements for the expression of many germline mRNAs (Merritt et al. 2008). In fact, based on GFP reporter genes flanked by specific 3′UTRs, most germline genes are faithfully expressed, therefore suggesting that post-transcriptional mechanisms play a major role in the germline. This does not, however, apply to genes that are specifically expressed in sperm (Merritt et al. 2008). One of the best characterized translational regulators is the STAR/KH domain protein GLD-1, which has three distinct functions in the germline: initiation of meiosis, progression through the meiotic prophase in oocytes, and spermatogenesis in hermaphrodites (Kadyk and Kimble 1998; Jones et al. 1996; Francis et al. 1995a,b). The GLD-1 protein physically interacts with several mRNAs, including the tra-2 mRNA (Jan et al. 1999; Jungkamp et al. 2011; Lee and Schedl 2001, 2004).
3.3.4 Transitory Spermatogenesis and Translational Control of tra-2 by GLD-1 and FOG-2
tra-2 promotes female fates in both germline and somatic tissues (Hodgkin and Brenner 1977). The discovery of tra-2 gain-of-function (gf) mutant alleles led to the identification of cis-acting negative regulatory elements in its 3′UTR. These elements (TGE, Tra and GLI element; also known as DRE, direct repeat element) correspond to two 28-nucleotide tandem repeats (Goodwin et al. 1993). Dominant mutations in the TGEs transform XX animals, but not X0 males, into females producing only oocytes (Doniach 1986). tra-2(gf) mutations do not affect steady-state levels of tra-2 transcripts suggesting that the TGEs neither control transcription nor stability of their mRNA. Rather, tra-2(gf) mRNAs are preferentially associated with polyribosomes indicating that gain-of-function mutated tra-2 mRNAs are more actively translated than the wild-type transcripts (Goodwin et al. 1993). To allow transient spermatogenesis during the fourth larval stage, the TGEs are bound and repressed by FOG-2 and a dimer of GLD-1 (Jan et al. 1999; Clifford et al. 2000; Ryder et al. 2004). Repression of tra-2 and transient spermatogenesis is abrogated in both tra-2(gf) and fog-2(lf) mutants, indicating that the binding of GLD-1/FOG-2 to the TGEs is abolished (Schedl and Kimble 1988; Jan et al. 1999; Clifford et al. 2000). Since HER-1 is not transcribed in hermaphrodites, tra-2 expression must be post-transcriptionally repressed in hermaphrodites for spermatogenesis. In males, her-1 is active and represses tra-2, allowing continuous spermatogenesis (Kuwabara 1996). Therefore post-transcriptional regulation of tra-2 is critical for transient spermatogenesis in hermaphrodites.
3.3.5 The Sperm-Oocyte Switch and Repression of fem-3 by FBF, MOGs, DAZ-1
The role of fem-3 in the germline of XX animals is particularly intriguing. On the one hand, in the absence of fem-3, XX animals develop as fertile females (Hodgkin 1986). On the other hand, fem-3 gain-of-function alleles lead to masculinized germlines that are genotypically XX (Barton et al. 1987; Hodgkin 1986). Again, fem-3 is regulated post-transcriptionally through its 3′UTR. More specifically, a five-nucleotide element (Point Mutation Element, PME) acts in cis to achieve repression of fem-3 and therefore the switch from spermatogenesis to oogenesis (Ahringer and Kimble 1991). The PME is bound by the conserved RNA-binding proteins FBF-1 and FBF-2 (Zhang et al. 1997). fem-3(gf) mutations abolish FBF-binding and could therefore abrogate repression of fem-3 (Zhang et al. 1997; Zanetti et al. 2012). The FBF proteins belong to the Puf family and include members in all eukaryotes. The most prominent example is Drosophila Pumilio, which prevents the translation of the hunchback mRNA (Murata and Wharton 1995; Wharton et al. 1998). Additional factors that are involved in regulation of fem-3 are the translational activator DAZ-1 and the MOG proteins (Otori et al. 2006; Graham et al. 1993). DAZ-1 (Deleted in Azoospermia) binds and increases FBF translation and is therefore required for oogenesis.
Six mog genes (masculinization of the germline) are also strictly implicated in the post-transcriptional regulation of the fem-3 mRNA via its 3′UTR. mog loss-of-function mutations lead to masculinized germlines, but do not affect the XX soma (Graham and Kimble 1993; Graham et al. 1993). X0 mog mutant males are largely normal in that they produce sperm, but have occasional somatic defects. Two observations suggest that the mog genes are possible regulators of fem-3. fem-3 is epistatic to mog because mog(lf); fem-3(lf) double mutants produce oocytes and fem-3(gf) and mog(lf) have masculinized germlines (Graham and Kimble 1993). Therefore, mog genes are not essential for oogenesis, but rather for the switch from spermatogenesis to oogenesis. Additional evidence comes from somatic expression of a reporter transgene flanked by the fem-3 3′UTR, which is derepressed in the absence of mog (Gallegos et al. 1998).
3.4 fem-3: A Paradigm to Study RNA Regulation
3.4.1 The RNA-Binding Protein FBF
FBF-1 and FBF-2 specifically bind to the wild-type fem-3 3′UTR (Zhang et al. 1997). Both proteins are highly similar and contain a conserved RNA-binding motif composed of eight tandem repeats that are found throughout the Puf family members (Zhang et al. 1997; Zamore et al. 1997). Drosophila Pumilio plays an essential role in establishing an anterior–posterior gradient of hunchback mRNA throughout the syncytium of the embryo (Murata and Wharton 1995). It binds to the Nanos Responsive Element (NRE) in the hunchback 3′UTR and represses its translation via deadenylation in the presence of the Nanos protein, which is restricted to the posterior of the embryo (Wreden et al. 1997; Wharton et al. 1998; Sonoda and Wharton 1999). Another deadenylation-independent mechanism has been proposed but also leads to translational repression of the hunchback mRNA (Chagnovich and Lehmann 2001; Wreden et al. 1997). To date, FBF has been found to be a broad-spectrum gene regulator potentially targeting 7 % of all protein coding genes in C. elegans (Kershner and Kimble 2010). In addition to its role in RNA binding, FBF interacts with poly(A) polymerase GLD-2 and with deadenlyase CCF-1/Pop2p, therefore suggesting two different roles of FBF in mRNA regulation: activation through poly(A) tail extension and repression through deadenylation (Suh et al. 2009). At least in vitro, the action of FBF on the gld-1 RNA is predicted to occur through deadenylation and translational silencing (Suh et al. 2009; see also Nousch and Eckmann 2012, Chap. 8). FBF binds to the fem-3 3′UTR to achieve the switch from spermatogenesis to oogenesis in hermaphrodites (Zhang et al. 1997). This switch requires, in addition, the binding of NOS-3, a worm homolog of Drosophila Nanos (Kraemer et al. 1999). Therefore, parts of the mechanism that governs embryonic polarity in Drosophila and germ cell sex determination in C. elegans have been conserved throughout evolution (Kraemer et al. 1999; Zhang et al. 1997). Even if the mechanism of FBF action is not fully understood, it is hypothesized that FBF reduces the expression of fem-3 via repression of translation, as shown for Pumilio and Nanos on hunchback mRNA (Crittenden et al. 2002; Hansen et al. 2004b; Wharton et al. 1998; Wreden et al. 1997).
To date, two additional mechanisms of fem-3 regulation have been proposed. FEM-1 and FEM-3 might be subjected to degradation by the proteasome when targeted by the F-box protein SEL-10 (Jager et al. 2004). Furthermore, sel-10 functions upstream of fem-3 and loss-of-function mutations for sel-10 cause weak masculinization of hermaphrodites. Therefore SEL-10 might be required for female fates via degradation of FEM-3, at least in somatic tissues (Jager et al. 2004). Another interesting possibility is that fem-3 might be regulated through the stabilization of its mRNA (Zanin et al. 2010; Zanetti et al. 2012). In fact, fem-3 mRNA levels are increased in the absence of larp-1, suggesting that larp-1 controls the switch from spermatogenesis to oogenesis by decreasing fem-3 mRNA levels (Zanin et al. 2010). larp-1 codes for a protein with an ancient La RNA-binding motif, and functions in oogenesis by lowering Ras-MAPK signaling (Nykamp et al. 2008). Masculinization of the germline is more pronounced in larp-1(lf);fem-3(q22) double mutants than in fem-3(q22) animals (Zanin et al. 2010). In addition, in masculinized fem-3(gf)/+ heterozygotes fem-3(gf) mRNA is more abundant than its wild-type counterpart indicating that the former is stabilized (Zanetti et al. 2012).
3.4.2 Do Splicing Factors Regulate fem-3?
As mentioned above, the MOG proteins are implicated in the post-transcriptional regulation of fem-3 (Graham and Kimble 1993; Graham et al. 1993; Gallegos et al. 1998). Many MOG proteins are homologs of well-known splicing factors: MOG-1, -4, and -5 are, for example, the worm homologs of yeast PRP16, PRP2, and PRP22, respectively (Puoti and Kimble 1999; Puoti et al. 2001). This finding suggests that MOG might regulate fem-3 through the processing of fem-3 itself, or via other transcripts, which in turn are required for fem-3 repression (Kasturi et al. 2010; Belfiore et al. 2002; Puoti and Kimble 1999). General splicing, however, and in particular the splicing of fem-3 is normal in most mog null mutants (Kasturi et al. 2010). The first direct evidence for mog action in splicing came with mog-2, which encodes the worm homolog of the well-conserved U2A′ protein (Zanetti et al. 2011). The U2A′ protein is a component of the spliceosomal U2snRNP complex, which includes the protein U2B′′ and the U2snRNA (Mattaj et al. 1986; Scherly et al. 1990; Sillekens et al. 1989). C. elegans mog-2 mutants are not defective in general splicing, but are less efficient in processing cryptic splice sites (Zanetti et al. 2011). Nonetheless, fem-3 mRNA splicing is unaffected in mog-2 animals. It is therefore possible that the Mog phenotype is caused by splicing detects in mRNAs that code for repressors of fem-3. Although the splicing targets of mog remain to be found, it is tempting to compare MOG-2 with Drosophila SNF (Sans-fille), the unique fly homolog of both U1A and U2B′′ (Nagengast and Salz 2001). SNF functions as an accessory factor in somatic and germ cell sex determination (Oliver et al. 1988; Steinmann-Zwicky 1988).
Finally, an RNAi-based survey identified homologs of splicing factors that control germline proliferation and sex determination in C. elegans (Kerins et al. 2010). For example, teg-4, ddx-23 and prp-17 mutants are defective in germline proliferation and sex determination, indicating that reduced splicing efficiency may affect germ cell development, with few consequences on somatic development (Kerins et al. 2010; Zanetti et al. 2011; Konishi et al. 2008; Mantina et al. 2009).
3.5 Making Appropriate Amounts of Sperm: Fine-Tuned Control of the Sperm-Oocyte Switch
In order to produce the appropriate number of sperm per gonadal arm, fem-3 has to be exclusively active at a given time, and in the correct set of cells. GLD-3 is another RNA-binding protein regulator of spermatogenesis (Zhang et al. 1997; Eckmann et al. 2002). gld-3 encodes a homolog of Bicaudal-C family of RNA-Binding proteins with two KH domains (Eckmann et al. 2002). A closer look at the phenotype of gld-3 mutants indicates that GLD-3 is required for continuous spermatogenesis and repression of oogenesis. In hermaphrodites the number of sperms produced is strongly reduced (Eckmann et al. 2002). The possibility of GLD-3 regulating fem-3 in this process has been explored. GLD-3 binds to FBF and functions upstream of FBF (Eckmann et al. 2002). Importantly, this interaction antagonizes the binding of FBF to the fem-3 mRNA. Therefore, it has been proposed that a competition between GLD-3 and FBF promotes spermatogenesis by release of the fem-3 mRNA from FBF (Eckmann et al. 2002). However, additional studies have shown that GLD-3 does not only bind to FBF-1 and FBF-2. In fact GLD-3 has also been found to associate with GLD-2 to form an active cytoplasmic poly(A) polymerase (Wang et al. 2002). This finding leads to an alternative hypothesis for GLD-3: it could antagonize the repressing activity of FBF by promoting polyadenylation of target mRNAs, perhaps of fem-3, and thus favor spermatogenesis (Suh et al. 2009; Wang et al. 2002).
In addition to binding to GLD-3, GLD-2 associates with RNP-8, another RNA-binding protein, and forms a different cytoplasmic poly(A) polymerase that functions in oogenesis (Kim et al. 2009). rnp-8 mutants produce more sperm than normal because the switch from spermatogenesis to oogenesis is delayed (Kim et al. 2009). Depending on its partner, GLD-2 either promotes spermatogenesis with GLD-3 or favors oogenesis when bound to RNP-8 (Kim et al. 2009; Suh et al. 2009). Moreover, GLD-2 also functions in sex determination by binding to either FBF-1 or FBF-2 (Suh et al. 2009). In fact, gld-2; fbf-1 mutants are masculinized, while gld-2; fbf-2 animals are feminized in their germlines (Kim et al. 2009). Putative target mRNAs of the GLD-2/RNP-8 poly(A) polymerase were obtained by co-immunoprecipitation (Kim et al. 2010). These targets include many maternal mRNAs that function in oogenesis of which the most prominent are tra-2, gld-1, gld-3, gld-2, and rnp-8. Remarkably, masculinizing mRNAs such as fem-3, gld-3 and fog-1 were also identified as possible GLD-2/RNP-8 targets, suggesting that polyadenylation is not the only driving force for transient spermatogenesis in hermaphrodites (Kim et al. 2010).
In addition to RNP-8 and GLD-3, which compete for GLD-2 binding (Kim et al. 2009), GLS-1 (germline sterile) antagonizes FBF for GLD-3 binding (Rybarska et al. 2009). At least in vitro, the novel protein GLS-1 recruits GLD-3 from the FBF/GLD-3 duplex (Rybarska et al. 2009). Moreover, gls-1 functions in oogenesis and is epistatic to gld-3 and other genes that are required for spermatogenesis (Rybarska et al. 2009). Taken together, GLS-1 is on the top of a pathway that regulates GLD-3, which in turn counters FBF-directed repression of fem-3 mRNA for the control of appropriate amounts of sperm in hermaphrodites (Rybarska et al. 2009).
3.5.1 FOG-1 and FOG-3 Are Terminal Regulators for Spermatogenesis
fog-1 function is restricted to the germline as it causes transformation of all germ cells into oocytes in both XX and X0 animals, without affecting the somatic phenotype (Barton and Kimble 1990). Additional phenotypic characterization revealed a dose-dependent function of fog-1 and genetic interaction with fbf (Thompson et al. 2005; Barton and Kimble 1990; Ellis and Kimble 1995). In fact, fog-1 is epistatic to fbf and the FBF proteins repress fog-1 mRNA by binding to regulatory elements in its 3′UTR to promote oogenesis (Thompson et al. 2005). Therefore, fog-1 is a target of FBF. High doses of FOG-1 promote spermatogenesis, whereas low FOG-1 levels are required for germ cell proliferation (Thompson et al. 2005). In fact fog-1;fbf-1fbf-2 triple mutants are feminized but only make very few oocyte-like cells. fbf-1;fbf-2 double mutants are masculinized and also largely underproliferative (Crittenden et al. 2002). However, with one dose of fog-1, fog-1/+;fbf-1 fbf-2 germlines are masculinized but show much more proliferation than fbf-1 fbf-2 or fog-1; fbf-1 fbf-2 germlines (Thompson et al. 2005). FOG-1 is a member of the CPEB family of RNA-binding proteins and might therefore control translation by regulating the poly(A) tails of target mRNAs (Luitjens et al. 2000; Mendez and Richter 2001). FOG-1 is found in germ cells that are committed to spermatogenesis, but not in spermatocytes and spermatids, indicating that high doses of FOG-1 are required for the onset on spermatogenesis (Lamont and Kimble 2007). A model for fog-1 action suggests that low doses of FOG-1, which are necessary for germ cell proliferation, are maintained in the distal region of the germline by the combined action of fbf and glp-1/Notch signaling. Moving proximally, the level of FOG-1 increases in germ cells destined to spermatogenesis (Thompson et al. 2005). The different germ cell decisions thus appear closely related and the amount of sperm produced is under the control of FOG-1 (Lamont and Kimble 2007). In addition to being post-transcriptionally regulated by FBF, fog-1 is also transcriptionally repressed by TRA-1 (Chen and Ellis 2000). Nevertheless, FOG-1 targets that function in spermatogenesis have not been identified to date.
Additional fog genes also direct spermatogenesis. fog-3 codes for a putative deadenylase and functions in parallel with fog-1 at the end of the sex determination cascade (Chen and Ellis 2000; Ellis and Kimble 1995). FOG-2 is located more upstream as it cooperates with GLD-1 for tra-2 mRNA-binding and repression for the onset of spermatogenesis in hermaphrodites (Table 3.1) (Jan et al. 1999; Schedl and Kimble 1988; Clifford et al. 2000).
3.6 Sex Determination and Germline Proliferation: Same Players, Similar Mechanisms, but Different Outcomes
The previous section pointed out an additional role of fog-1 in germ cell proliferation (Thompson et al. 2005). This applies to many other genes involved in germline sex determination (also see Hansen and Schedl 2012, Chap. 4). Proliferation of germ cells and maintenance of germline stem cells are primarily controlled by glp-1/Notch signaling in C. elegans (Austin and Kimble 1987; Morgan et al. 2010). glp-1(0) germlines produce only 8–16 spermatids, indicating that proliferating germ cells enter meiosis instead of expanding through mitotic divisions (Austin and Kimble 1987). Conversely, glp-1 gain-of-function germlines are tumorous: they produce neither spermatids nor oocytes but contain thousands of mitotic nuclei (Berry et al. 1997). Tumorous germlines are also found in gld-1(lf) gld-2(lf) double and glp-1(0); gld-1(lf) gld-2(lf) triple mutants indicating that glp-1 functions upstream and represses both gld-1 and gld-2 (Kadyk and Kimble 1998). gld-1 alleles cause a variety of mutant phenotypes, such as masculinization, feminization, and germline tumors (Francis et al. 1995b). Its intriguing genetics therefore indicate multiple functions. For example, the tumorous phenotype of gld-1 mutants is strongly enhanced in the absence of gld-2 (Kadyk and Kimble 1998). Moreover, gld-1 is also synthetically required for meiosis with gld-3, another sex determination gene (Kadyk and Kimble 1998; Hansen et al. 2004a,b; Eckmann et al. 2004). Although not as strong as in glp-1(gf) or gld-1 gld-2 mutants, germline tumors have also been observed as proximally proliferating nuclei in gld-3 nos-3 double mutants (Hansen et al. 2004b). Similarly, mog; gld-3 double mutants are also overproliferative, indicating an additional role of mog in the decision between mitosis and meiosis (Belfiore et al. 2004; Kasturi et al. 2010; Zanetti et al. 2011). As mentioned above, both fbf genes are required for robust germline proliferation, and genetically interact with fog-1 in this process. Therefore, germline proliferation and sex determination are tightly linked to each other. Both processes follow each other and share many common players.
As in sex determination, many regulatory events controlling mitosis or meiosis rely upon post-transcriptional mechanisms (Marin and Evans 2003; Crittenden et al. 2003; Ogura et al. 2003; Puoti et al. 2001; Clifford et al. 2000). Finally, since meiosis is different in oocytes and sperms, the sex of germ cells must be determined before meiosis has started. Even more dramatically, hermaphrodite and male germlines are sexually dimorphic in their distal region indicating that germ cells are sexually determined at a time when they still divide mitotically (Morgan et al. 2010). Nevertheless, their sexual fate remains labile since it can be redirected to oogenesis in fog, her-1, or fem mutants or to spermatogenesis in mog(0) or fem-3(gf) mutants (Barton et al. 1987; Chen and Ellis 2000; Graham and Kimble 1993).
3.7 The Role of Maternal RNAs, New Regulatory Mechanisms
In C. elegans, some of the genes that control sexual fate maternally supply mRNA and/or protein through the oocyte for zygotic sex determination (Ahringer et al. 1992; Doniach 1986; Doniach and Hodgkin 1984; Graham et al. 1993; Rosenquist and Kimble 1988). In some cases, maternal mRNAs are translated upon fertilization and permit protein synthesis before zygotic transcription sets in. A new role for maternal RNA has now been proposed for fem-1, which is required for spermatogenesis and male development. Heterozygous fem-1(0)/+ progeny can be obtained by mating a wild-type male with a feminized fem-1 null mutant. Because fem-1 null alleles are recessive, such progenies are expected to be phenotypically wild type (Doniach and Hodgkin 1984). Andrew Spence’s group has found that progeny descended from females that produce no fem-1 RNA, and therefore are totally lacking maternal fem-1 RNA, are unable to use the paternal wild-type copy of fem-1. As a consequence, their germlines are feminized even if one wild-type copy of fem-1 is available (Johnson and Spence 2011). Intriguingly, the open reading frame of the fem-1 RNA does not need to be intact, indicating that the maternal effect is caused by the fem-1 RNA and not its protein product. Moreover, the effect is heritable thus suggesting that epigenetic control licenses the expression of zygotic fem-1 (Johnson and Spence 2011). This mechanism uses sense RNA and is independent of rde-1, indicating that it is distinct from RNA interference (Tabara et al. 1999; Fire et al. 1998). Similarly to what has been proposed for RNA interference and epigenetic control, we notice again that the germline makes great efforts to avoid expressing foreign gene products (Kelly and Fire 1998).
3.8 Summary Paragraph
Sex determination has been studied in numerous species. Among these, the nematode C. elegans has a prominent role because it led to numerous genes that function in various aspects of gamete formation. Here we describe most of these genes and put them into their regulatory and functional contexts.
References
Ahringer J, Kimble J (1991) Control of the sperm-oocyte switch in Caenorhabditis elegans hermaphrodites by the fem-3 3’ untranslated region. Nature 49:346–348
Ahringer J, Rosenquist TA, Lawson DN, Kimble J (1992) The Caenorhabditis elegans sex determining gene fem-3 is regulated post-transcriptionally. EMBO J 11:2303–2310
Austin J, Kimble J (1987) glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell 51:589–599
Bachorik JL, Kimble J (2005) Redundant control of the Caenorhabditis elegans sperm/oocyte switch by PUF-8 and FBF-1, two distinct PUF RNA-binding proteins. Proc Natl Acad Sci USA 102:10893–10897
Baker BS, Wolfner MF (1988) A molecular analysis of doublesex, a bifunctional gene that controls both male and female sexual differentiation in Drosophila melanogaster. Genes Dev 2:477–489
Barton MK, Kimble J (1990) fog-1, a regulatory gene required for specification of spermatogenesis in the germ line of Caenorhabditis elegans. Genetics 125:29–39
Barton MK, Schedl TB, Kimble J (1987) Gain-of-function mutations of fem-3, a sex-determination gene in Caenorhabditis elegans. Genetics 115:107–119
Belfiore M, Mathies LD, Pugnale P, Moulder G, Barstead R, Kimble J, Puoti A (2002) The MEP-1 zinc-finger protein acts with MOG DEAH box proteins to control gene expression via the fem-3 3 ‘ untranslated region in Caenorhabditis elegans. RNA 8:725–739
Belfiore M, Pugnale P, Saudan Z, Puoti A (2004) Roles of the C. elegans cyclophilin-like protein MOG-6 in MEP-1 binding and germline fates. Development 131:2935–2945
Bell LR, Horabin JI, Schedl P, Cline TW (1991) Positive Autoregulation of Sex-Lethal by Alternative Splicing Maintains the Female Determined State in Drosophila. Cell 65:229–239
Berry LW, Westlund B, Schedl T (1997) Germ-line tumor formation caused by activation of glp-1 a Caenorhabditis elegans member of the Notch family of receptors. Development 124(4):925–936
Bridges CB (1916) Non-disjunction as proof of the chromosome theory of heredity (concluded). Genetics 1:107–163
Carmi I, Kopczynski JB, Meyer BJ (1998) The nuclear hormone receptor SEX-1 is an X-chromosome signal that determines nematode sex. Nature 396:168–173
Casper AL, Van Doren M (2009) The establishment of sexual identity in the Drosophila germline. Development 136:3821–3830
Chagnovich D, Lehmann R (2001) Poly(A)-independent regulation of maternal hunchback translation in the Drosophila embryo. Proc Natl Acad Sci USA 98:11359–11364
Chen P, Ellis RE (2000) TRA-1A regulates transcription of fog-3, which controls germ cell fate in C. elegans. Development 127:3119–3129
Chin-Sang ID, Spence AM (1996) Caenorhabditis elegans sex-determining protein FEM-2 is a protein phosphatase that promotes male development and interacts directly with FEM-3. Genes Dev 10:2314–2325
Chu DS, Dawes HE, Lieb JD, Chan RC, Kuo AF, Meyer BJ (2002) A molecular link between gene-specific and chromosome-wide transcriptional repression. Genes Dev 16:796–805
Ciosk R, DePalma M, Priess JR (2004) ATX-2, the C. elegans ortholog of ataxin 2, functions in translational regulation in the germline. Development 131:4831–4841
Clifford R, Lee MH, Nayak S, Ohmachi M, Giorgini F, Schedl T (2000) FOG-2, a novel F-box containing protein, associates with the GLD-1 RNA binding protein and directs male sex determination in the C. elegans hermaphrodite germline. Development 127:5265–5276
Cline TW (1978) Two closely linked mutations in Drosophila melanogaster that are lethal to opposite sexes and interact with daughterless. Genetics 90:683–698
Cline TW (1984) Autoregulatory functioning of a Drosophila gene-product that establishes and maintains the sexually determined state. Genetics 107:231–277
Coschigano KT, Wensink PC (1993) Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev 7:42–54
Crittenden SL, Bernstein DS, Bachorik JL, Thompson BE, Gallegos M, Petcherski AG, Moulder G, Barstead R, Wickens M, Kimble J (2002) A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature 417:660–663
Crittenden SL, Eckmann CR, Wang L, Bernstein DS, Wickens M, Kimble J (2003) Regulation of the mitosis/meiosis decision in the Caenorhabditis elegans germline. Philos Trans R Soc Lond B Biol Sci 358:1359–1362
Dawes HE, Berlin DS, Lapidus DM, Nusbaum C, Davis TL, Meyer BJ (1999) Dosage compensation proteins targeted to X chromosomes by a determinant of hermaphrodite fate. Science 284:1800–1804
DeLong L, Plenefisch JD, Klein RD, Meyer BJ (1993) Feedback control of sex determination by dosage compensation revealed through Caenorhabditis elegans sdc-3 mutations. Genetics 133:875–896
Doniach T (1986) Activity of the sex-determining gene tra-2 is modulated to allow spermatogenesis in the C. elegans hermaphrodite. Genetics 114:53–76
Doniach T, Hodgkin J (1984) A sex-determining gene, fem-1, required for both male and hermaphrodite development in Caenorhabditis elegans. Dev Biol 106:223–235
Donovan PJ, Stott D, Cairns LA, Heasman J, Wylie CC (1986) Migratory and postmigratory mouse primordial germ cells behave differently in culture. Cell 44:831–838
Eckmann CR, Kraemer B, Wickens M, Kimble J (2002) GLD-3, a bicaudal-C homolog that inhibits FBF to control germline sex determination in C. elegans. Dev Cell 3:697–710
Eckmann CR, Crittenden SL, Suh N, Kimble J (2004) GLD-3 and control of the mitosis/meiosis decision in the germline of Caenorhabditis elegans. Genetics 168:147–160
Ellis RE (2008) Sex determination in the Caenorhabditis elegans germ line. Curr Top Dev Biol 83:41–64
Ellis R (2010) The sperm/oocyte decision, a C. elegans perspective. In: Verlhac M-H, Villeneuve A (eds) Oogenesis: the universal process. Wiley, New York
Ellis RE, Kimble J (1994) Control of germ cell differentiation in Caenorhabditis elegans. Ciba Found Symp 182:179–188
Ellis RE, Kimble J (1995) The fog-3 gene and regulation of cell fate in the germ line of Caenorhabditis elegans. Genetics 139:561–577
Ellis R, Schedl T (2007) Sex determination in the germ line. WormBook: 1–13
Ewen KA, Koopman P (2010) Mouse germ cell development: from specification to sex determination. Mol Cell Endocrinol 323:76–93
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Ford CE, Jones KW, Polani PE, De Almeida JC, Briggs JH (1959) A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner’s syndrome). Lancet 1:711–713
Francis R, Barton MK, Kimble J, Schedl T (1995a) gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics 139:579–606
Francis R, Maine E, Schedl T (1995b) Analysis of the multiple roles of gld-1 in germline development: interactions with the sex determination cascade and the glp-1 signaling pathway. Genetics 139:607–630
Gallegos M, Ahringer J, Crittenden S, Kimble J (1998) Repression by the 3’ UTR of fem-3, a sex-determining gene, relies on a ubiquitous mog-dependent control in Caenorhabditis elegans. EMBO J 17:6337–6347
Gonzalez AN, Lu H, Erickson JW (2008) A shared enhancer controls a temporal switch between promoters during Drosophila primary sex determination. Proc Natl Acad Sci USA 105:18436–18441
Goodwin EB, Okkema PG, Evans TC, Kimble J (1993) Translational regulation of tra-2 by its 3’ untranslated region controls sexual identity in C. elegans. Cell 75:329–339
Graham PL, Kimble J (1993) The Mog-1 gene is required for the switch from spermatogenesis to oogenesis in Caenorhabditis Elegans. Genetics 133:919–931
Graham PL, Schedl T, Kimble J (1993) More Mog genes that influence the switch from spermatogenesis to oogenesis in the hermaphrodite germ-line of Caenorhabditis elegans. Dev Genet 14:471–484
Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R (1990) A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346:245–250
Hamaoka BY, Dann CE 3rd, Geisbrecht BV, Leahy DJ (2004) Crystal structure of Caenorhabditis elegans HER-1 and characterization of the interaction between HER-1 and TRA-2A. Proc Natl Acad Sci USA 101:11673–11678
Hansen D, Schedl T (2012) Stem cell proliferation versus meiotic fate decision in C. elegans. Advances in Experimental Medicine and Biology 757:71–99. (Chap. 4, this volume) Springer, New York
Hansen D, Hubbard EJ, Schedl T (2004a) Multi-pathway control of the proliferation versus meiotic development decision in the Caenorhabditis elegans germline. Dev Biol 268:342–357
Hansen D, Wilson-Berry L, Dang T, Schedl T (2004b) Control of the proliferation versus meiotic development decision in the C. elegans germline through regulation of GLD-1 protein accumulation. Development 131:93–104
Hargitai B, Kutnyanszky V, Blauwkamp TA, Stetak A, Csankovszki G, Takacs-Vellai K, Vellai T (2009) xol-1, the master sex-switch gene in C. elegans, is a transcriptional target of the terminal sex-determining factor TRA-1. Development 136:3881–3887
Harley VR, Goodfellow PN (1994) The biochemical role of Sry in sex determination. Mol Reprod Dev 39:184–193
Hodgkin J (1980) More sex-determination mutants of Caenorhabditis elegans. Genetics 96:649–664
Hodgkin J (1986) Sex determination in the nematode C. elegans: analysis of tra-3 suppressors and characterization of fem genes. Genetics 114:15–52
Hodgkin J (1987) A genetic analysis of the sex-determining gene, tra-1, in the nematode Caenorhabditis elegans. Genes Dev 1:731–745
Hodgkin JA, Brenner S (1977) Mutations causing transformation of sexual phenotype in the nematode Caenorhabditis elegans. Genetics 86:275–287
Hodgkin J, Zellan JD, Albertson DG (1994) Identification of a candidate primary sex determination locus, fox-1, on the X chromosome of Caenorhabditis elegans. Development 120:3681–3689
Hoshijima K, Inoue K, Higuchi I, Sakamoto H, Shimura Y (1991) Control of doublesex alternative splicing by transformer and transformer-2 in Drosophila. Science 252:833–836
Hsu V, Zobel CL, Lambie EJ, Schedl T, Kornfeld K (2002) Caenorhabditis elegans lin-45 raf is essential for larval viability, fertility and the induction of vulval cell fates. Genetics 160:481–492
Jacobs PA, Strong JA (1959) A case of human intersexuality having a possible XXY sex-determining mechanism. Nature 183:302–303
Jager S, Schwartz HT, Horvitz HR, Conradt B (2004) The Caenorhabditis elegans F-box protein SEL-10 promotes female development and may target FEM-1 and FEM-3 for degradation by the proteasome. Proc Natl Acad Sci USA 101:12549–12554
Jan E, Motzny CK, Graves LE, Goodwin EB (1999) The STAR protein, GLD-1, is a translational regulator of sexual identity in Caenorhabditis elegans. EMBO J 18:258–269
Johnson CL, Spence AM (2011) Epigenetic licensing of germline gene expression by maternal RNA in C. elegans. Science 333:1311–1314
Jones AR, Francis R, Schedl T (1996) GLD-1, a cytoplasmic protein essential for oocyte differentiation, shows stage- and sex-specific expression during Caenorhabditis elegans germline development. Dev Biol 180:165–183
Jungkamp AC, Stoeckius M, Mecenas D, Grun D, Mastrobuoni G, Kempa S, Rajewsky N (2011) In vivo and transcriptome-wide identification of RNA binding protein target sites. Mol Cell 44:828–840
Kadyk LC, Kimble J (1998) Genetic regulation of entry into meiosis in Caenorhabditis elegans. Development 125:1803–1813
Karashima T, Sugimoto A, Yamamoto M (2000) Caenorhabditis elegans homologue of the human azoospermia factor DAZ is required for oogenesis but not for spermatogenesis. Development 127:1069–1079
Kashimada K, Koopman P (2010) Sry: the master switch in mammalian sex determination. Development 137:3921–3930
Kasturi P, Zanetti S, Passannante M, Saudan Z, Muller F, Puoti A (2010) The C. elegans sex determination protein MOG-3 functions in meiosis and binds to the CSL co-repressor CIR-1. Dev Biol 344:593–602
Kawano T, Kataoka N, Dreyfuss G, Sakamoto H (2004) Ce-Y14 and MAG-1, components of the exon-exon junction complex, are required for embryogenesis and germline sexual switching in Caenorhabditis elegans. Mech Dev 121:27–35
Kelly WG, Fire A (1998) Chromatin silencing and the maintenance of a functional germline in Caenorhabditis elegans. Development 125:2451–2456
Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P (1996) A male-specific role for SOX9 in vertebrate sex determination. Development 122:2813–2822
Kerins JA, Hanazawa M, Dorsett M, Schedl T (2010) PRP-17 and the pre-mRNA splicing pathway are preferentially required for the proliferation versus meiotic development decision and germline sex determination in Caenorhabditis elegans. Dev Dyn 239:1555–1572
Kershner AM, Kimble J (2010) Genome-wide analysis of mRNA targets for Caenorhabditis elegans FBF, a conserved stem cell regulator. Proc Natl Acad Sci USA 107:3936–3941
Kim KW, Nykamp K, Suh N, Bachorik JL, Wang L, Kimble J (2009) Antagonism between GLD-2 binding partners controls gamete sex. Dev Cell 16:723–733
Kim KW, Wilson TL, Kimble J (2010) GLD-2/RNP-8 cytoplasmic poly(A) polymerase is a broad-spectrum regulator of the oogenesis program. Proc Natl Acad Sci USA 107:17445–17450
Kimble J, Crittenden SL (2007) Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol 23:405–433
Kimble J, Edgar L, Hirsh D (1984) Specification of male development in Caenorhabditis elegans: the fem genes. Dev Biol 105:234–239
Koebernick K, Pieler T (2002) Gli-type zinc finger proteins as bipotential transducers of Hedgehog signaling. Differentiation 70:69–76
Konishi T, Uodome N, Sugimoto A (2008) The Caenorhabditis elegans DDX-23, a homolog of yeast splicing factor PRP28, is required for the sperm-oocyte switch and differentiation of various cell types. Dev Dyn 237:2367–2377
Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovellbadge R (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351:117–121
Kraemer B, Crittenden S, Gallegos M, Moulder G, Barstead R, Kimble J, Wickens M (1999) NANOS-3 and FBF proteins physically interact to control the sperm-oocyte switch in Caenorhabditis elegans. Curr Biol 9:1009–1018
Kuwabara PE (1996) A novel regulatory mutation in the C. elegans sex determination gene tra-2 defines a candidate ligand/receptor interaction site. Development 122:2089–2098
Lamont LB, Kimble J (2007) Developmental expression of FOG-1/CPEB protein and its control in the Caenorhabditis elegans hermaphrodite germ line. Dev Dyn 236:871–879
Lee MH, Schedl T (2001) Identification of in vivo mRNA targets of GLD-1, a maxi-KH motif containing protein required for C. elegans germ cell development. Genes Dev 15:2408–2420
Lee MH, Schedl T (2004) Translation repression by GLD-1 protects its mRNA targets from nonsense-mediated mRNA decay in C. elegans. Genes Dev 18:1047–1059
Lee MH, Schedl T (2006) RNA-binding proteins. WormBook: 1–13
Lee MH, Ohmachi M, Arur S, Nayak S, Francis R, Church D, Lambie E, Schedl T (2007) Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germline development. Genetics 177:2039–2062
Lieb JD, de Solorzano CO, Rodriguez EG, Jones A, Angelo M, Lockett S, Meyer BJ (2000) The Caenorhabditis elegans dosage compensation machinery is recruited to X chromosome DNA attached to an autosome. Genetics 156:1603–1621
Luitjens C, Gallegos M, Kraemer B, Kimble J, Wickens M (2000) CPEB proteins control two key steps in spermatogenesis in C. elegans. Genes Dev 14:2596–2609
Lum DH, Kuwabara PE, Zarkower D, Spence AM (2000) Direct protein-protein interaction between the intracellular domain of TRA-2 and the transcription factor TRA-1A modulates feminizing activity in C. elegans. Genes Dev 14:3153–3165
Madl JE, Herman RK (1979) Polyploids and sex determination in Caenorhabditis elegans. Genetics 93:393–402
Maine EM, Hansen D, Springer D, Vought VE (2004) Caenorhabditis elegans atx-2 promotes germline proliferation and the oocyte fate. Genetics 168:817–830
Mantina P, MacDonald L, Kulaga A, Zhao L, Hansen D (2009) A mutation in teg-4 which encodes a protein homologous to the SAP130 pre-mRNA splicing factor, disrupts the balance between proliferation and differentiation in the C. elegans germ line. Mech Dev 126:417–429
Marin VA, Evans TC (2003) Translational repression of a C. elegans Notch mRNA by the STAR/KH domain protein GLD-1. Development 130:2623–2632
Mason DA, Rabinowitz JS, Portman DS (2008) dmd-3 a doublesex-related gene regulated by tra-1, governs sex-specific morphogenesis in C. elegans. Development 135:2373–2382
Mattaj IW, Habets WJ, van Venrooij WJ (1986) Monospecific antibodies reveal details of U2 snRNP structure and interaction between U1 and U2 snRNPs. EMBO J 5:997–1002
McLaren A (1984) Meiosis and differentiation of mouse germ cells. Symp Soc Exp Biol 38:7–23
Mendez R, Richter JD (2001) Translational control by CPEB: a means to the end. Nat Rev Mol Cell Biol 2:521–529
Merritt C, Rasoloson D, Ko D, Seydoux G (2008) 3’UTRs are the primary regulators of gene expression in the C. elegans germline. Curr Biol 18:1476–1482
Meyer BJ (2005) X-Chromosome dosage compensation. WormBook: 1–14
Meyer BJ, Casson LP (1986) Caenorhabditis elegans compensates for the difference in X chromosome dosage between the sexes by regulating transcript levels. Cell 47:871–881
Miller LM, Plenefisch JD, Casson LP, Meyer BJ (1988) xol-1: a gene that controls the male modes of both sex determination and X chromosome dosage compensation in C. elegans. Cell 55:167–183
Morgan DE, Crittenden SL, Kimble J (2010) The C. elegans adult male germline: stem cells and sexual dimorphism. Dev Biol 346:204–214
Murata Y, Wharton RP (1995) Binding of pumilio to maternal hunchback mRNA is required for posterior patterning in Drosophila embryos. Cell 80:747–756
Nagengast AA, Salz HK (2001) The Drosophila U2 snRNP protein U2A’ has an essential function that is SNF/U2B” independent. Nucleic Acids Res 29:3841–3847
Nigon V (1952) Experimental modifications of the sex ratio in a pseudogamous nematode. C R Hebd Seances Acad Sci 234:2568–2570
Nousch M, Eckmann CR (2012) Translational control in the C. elegans germ line. Advances in Experimental Medicine and Biology 757:205–247. (Chap. 8, this volume) Springer, New York
Nusbaum C, Meyer BJ (1989) The Caenorhabditis elegans gene sdc-2 controls sex determination and dosage compensation in XX animals. Genetics 122:579–593
Nykamp K, Lee MH, Kimble J (2008) C. elegans La-related protein, LARP-1, localizes to germline P bodies and attenuates Ras-MAPK signaling during oogenesis. RNA 14:1378–1389
Ogura K, Kishimoto N, Mitani S, Gengyo-Ando K, Kohara Y (2003) Translational control of maternal glp-1 mRNA by POS-1 and its interacting protein SPN-4 in Caenorhabditis elegans. Development 130:2495–2503
Oliver B, Perrimon N, Mahowald AP (1988) Genetic-evidence that the Sans-Fille locus is involved in Drosophila sex determination. Genetics 120:159–171
Ostrer H (2000) Sexual differentiation. Semin Reprod Med 18:41–49
Otori M, Karashima T, Yamamoto M (2006) The Caenorhabditis elegans homologue of deleted in azoospermia is involved in the sperm/oocyte switch. Mol Biol Cell 17:3147–3155
Perry MD, Li W, Trent C, Robertson B, Fire A, Hageman JM, Wood WB (1993) Molecular characterization of the her-1 gene suggests a direct role in cell signaling during Caenorhabditis elegans sex determination. Genes Dev 7:216–228
Perry MD, Trent C, Robertson B, Chamblin C, Wood WB (1994) Sequenced alleles of the Caenorhabditis elegans sex-determining gene her-1 include a novel class of conditional promoter mutations. Genetics 138:317–327
Powell JR, Jow MM, Meyer BJ (2005) The T-box transcription factor SEA-1 is an autosomal element of the X:A signal that determines C. elegans sex. Dev Cell 9:339–349
Puoti A, Kimble J (1999) The Caenorhabditis elegans sex determination gene mog-1 encodes a member of the DEAH-box protein family. Mol Cell Biol 19:2189–2197
Puoti A, Kimble J (2000) The hermaphrodite sperm/oocyte switch requires the Caenorhabditis elegans homologs of PRP2 and PRP22. Proc Natl Acad Sci USA 97:3276–327681
Puoti A, Pugnale P, Belfiore M, Schlappi AC, Saudan Z (2001) RNA and sex determination in Caenorhabditis elegans. Post-transcriptional regulation of the sex-determining tra-2 and fem-3 mRNAs in the Caenorhabditis elegans hermaphrodite. EMBO Rep 2:899–904
Raymond CS, Shamu CE, Shen MM, Seifert KJ, Hirsch B, Hodgkin J, Zarkower D (1998) Evidence for evolutionary conservation of sex-determining genes. Nature 391:691–695
Rhind NR, Miller LM, Kopczynski JB, Meyer BJ (1995) xol-1 acts as an early switch in the C. elegans male/hermaphrodite decision. Cell 80:71–82
Rosenquist TA, Kimble J (1988) Molecular cloning and transcript analysis of fem-3, a sex-determination gene in Caenorhabditis elegans. Genes Dev 2:606–616
Rybarska A, Harterink M, Jedamzik B, Kupinski AP, Schmid M, Eckmann CR (2009) GLS-1, a novel P granule component, modulates a network of conserved RNA regulators to influence germ cell fate decisions. PLoS Genet 5:e1000494
Ryder SP, Frater LA, Abramovitz DL, Goodwin EB, Williamson JR (2004) RNA target specificity of the STAR/GSG domain post-transcriptional regulatory protein GLD-1. Nat Struct Mol Biol 11:20–28
Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA (1996) Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87:1079–1089
Salz HK, Erickson JW (2010) Sex determination in Drosophila: the view from the top. Fly 4:60–70
Schedl T (1997) Developmental genetics of the germ line. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR (eds) C. elegans Il. Cold Spring Harbor Laboratory Press, New York
Schedl T, Kimble J (1988) fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics 119:43–61
Schedl T, Graham PL, Barton MK, Kimble J (1989) Analysis of the role of tra-1 in germline sex determination in the nematode Caenorhabditis elegans. Genetics 123:755–769
Scherly D, Boelens W, Dathan NA, van Venrooij WJ, Mattaj IW (1990) Major determinants of the specificity of interaction between small nuclear ribonucleoproteins U1A and U2B’’ and their cognate RNAs. Nature 345:502–506
Schvarzstein M, Spence AM (2006) The C. elegans sex-determining GLI protein TRA-1A is regulated by sex-specific proteolysis. Dev Cell 11:733–740
Shimada M, Kanematsu K, Tanaka K, Yokosawa H, Kawahara H (2006) Proteasomal ubiquitin receptor RPN-10 controls sex determination in Caenorhabditis elegans. Mol Biol Cell 17:5356–5371
Sillekens PT, Beijer RP, Habets WJ, van Verooij WJ (1989) Molecular cloning of the cDNA for the human U2 snRNA-specific A’ protein. Nucleic Acids Res 17:1893–1906
Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN (1990) A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346:240–244
Skipper M, Milne CA, Hodgkin J (1999) Genetic and molecular analysis of fox-1, a numerator element involved in Caenorhabditis elegans primary sex determination. Genetics 151:617–631
Slanchev K, Stebler J, de la Cueva-Mendez G, Raz E (2005) Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc Natl Acad Sci USA 102:4074–4079
Sokol SB, Kuwabara PE (2000) Proteolysis in Caenorhabditis elegans sex determination: cleavage of TRA-2A by TRA-3. Genes Dev 14:901–906
Sonoda J, Wharton RP (1999) Recruitment of Nanos to hunchback mRNA by Pumilio. Genes Dev 13:2704–2712
Starostina NG, Lim JM, Schvarzstein M, Wells L, Spence AM, Kipreos ET (2007) A CUL-2 ubiquitin ligase containing three FEM proteins degrades TRA-1 to regulate C. elegans sex determination. Dev Cell 13:127–139
Steinmann-Zwicky M (1988) Sex determination in Drosophila - the X-chromosomal gene Liz is required for Sxl activity. EMBO J 7:3889–3898
Suh N, Crittenden SL, Goldstrohm A, Hook B, Thompson B, Wickens M, Kimble J (2009) FBF and its dual control of gld-1 expression in the Caenorhabditis elegans germline. Genetics 181:1249–1260
Tabara H, Sarkissian M, Kelly WG, Fleenor J, Grishok A, Timmons L, Fire A, Mello CC (1999) The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell 99:123–132
Tamashiro DA, Alarcon VB, Marikawa Y (2008) Ectopic expression of mouse Sry interferes with Wnt/beta-catenin signaling in mouse embryonal carcinoma cell lines. Biochim Biophys Acta 1780:1395–1402
Thompson BE, Bernstein DS, Bachorik JL, Petcherski AG, Wickens M, Kimble J (2005) Dose-dependent control of proliferation and sperm specification by FOG-1/CPEB. Development 132:3471–3481
Trent C, Purnell B, Gavinski S, Hageman J, Chamblin C, Wood WB (1991) Sex-specific transcriptional regulation of the C. elegans sex-determining gene her-1. Mech Dev 34:43–55
Unhavaithaya Y, Shin TH, Miliaras N, Lee J, Oyama T, Mello CC (2002) MEP-1 and a homolog of the NURD complex component Mi-2 act together to maintain germline-soma distinctions in C. elegans. Cell 111:991–1002
Vaiman D, Pailhoux E (2000) Mammalian sex reversal and intersexuality: deciphering the sex-determination cascade. Trends Genet 16:488–494
Villeneuve AM, Meyer BJ (1987) sdc-1: a link between sex determination and dosage compensation in C. elegans. Cell 48:25–37
Wang S, Kimble J (2001) The TRA-1 transcription factor binds TRA-2 to regulate sexual fates in Caenorhabditis elegans. EMBO J 20:1363–1372
Wang L, Eckmann CR, Kadyk LC, Wickens M, Kimble J (2002) A regulatory cytoplasmic poly(A) polymerase in Caenorhabditis elegans. Nature 419:312–316
Ward S, Roberts TM, Strome S, Pavalko FM, Hogan E (1986) Monoclonal antibodies that recognize a polypeptide antigenic determinant shared by multiple Caenorhabditis elegans sperm-specific proteins. J Cell Biol 102:1778–1786
Wawersik M, Milutinovich A, Casper AL, Matunis E, Williams B, Van Doren M (2005) Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature 436:563–567
Wharton RP, Sonoda J, Lee T, Patterson M, Murata Y (1998) The Pumilio RNA-binding domain is also a translational regulator. Mol Cell 1:863–872
Wolff JR, Zarkower D (2008) Somatic sexual differentiation in Caenorhabditis elegans. Curr Top Dev Biol 83:1–39
Wreden C, Verrotti AC, Schisa JA, Lieberfarb ME, Strickland S (1997) Nanos and pumilio establish embryonic polarity in Drosophila by promoting posterior deadenylation of hunchback mRNA. Development 124:3015–3023
Yonker SA, Meyer BJ (2003) Recruitment of C. elegans dosage compensation proteins for gene-specific versus chromosome-wide repression. Development 130:6519–6532
Zamore PD, Williamson JR, Lehmann R (1997) The Pumilio protein binds RNA through a conserved domain that defines a new class of RNA-binding proteins. RNA 3:1421–1433
Zanetti S, Meola M, Bochud A, Puoti A (2011) Role of the C. elegans U2 snRNP protein MOG-2 in sex determination, meiosis, and splice site selection. Dev Biol 354:232–241
Zanetti S, Grinschgl S, Meola M, Belfiore M, Rey S, Bianchi P, Puoti A (2012) The sperm-oocyte switch in the C. elegans hermaphrodite is controlled through abundance of the fem-3 mRNA. RNA 18:1385–1394
Zanin E, Pacquelet A, Scheckel C, Ciosk R, Gotta M (2010) LARP-1 promotes oogenesis by repressing fem-3 in the C. elegans germline. J Cell Sci 123:2717–2724
Zarkower D (2006) Somatic sex determination. WormBook: 1–12
Zarkower D, Hodgkin J (1992) Molecular analysis of the C. elegans sex-determining gene tra-1: a gene encoding two zinc finger proteins. Cell 70:237–249
Zhang B, Gallegos M, Puoti A, Durkin E, Fields S, Kimble J, Wickens MP (1997) A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature 390:477–484
Acknowledgments
We thank Tim Schedl and Nivedita Awasthi for comments on the manuscript. This work was partially supported by the Novartis Foundation for Medical and Biological Research, grant 10B34.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Zanetti, S., Puoti, A. (2013). Sex Determination in the Caenorhabditis elegans Germline. In: Schedl, T. (eds) Germ Cell Development in C. elegans. Advances in Experimental Medicine and Biology, vol 757. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4015-4_3
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4015-4_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4014-7
Online ISBN: 978-1-4614-4015-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)