Abstract
Adenosine belongs to the class of neuromodulators rather than neurotransmitters, since it is not stored in vesicles, nor released by exocytosis as a classical neurotransmitter. Moreover, it does not induce synaptic potentials but influences the release and the action of neurotransmitters. This mostly occurs through interactions with other G protein-coupled receptors as well as of receptors for neurotrophic factors, ion channels, ionotropic receptors, and neurotransmitter transporters. The actions of adenosine are operated by four different G protein-coupled membrane receptors (A1, A2A, A2B, A3), which activate several downstream signaling pathways, the main focus of the present review. Cross talk between adenosine receptors and receptors for neurotransmitters or other neuromodulators may result from interactions between common signaling cascades, as well as through receptor–receptor interactions, including receptor heteromerization. The key receptor in this synaptic interplay appears to be the A2A receptor, whereas A1 receptors mainly act as modulators of neurotransmitter release or by counteracting A2A receptor-mediated actions. We herein review some of the most recent data on the regulation of adenosine availability, as well as on the consequences of adenosine actions in synapses and the corresponding downstream signaling pathways. Moreover, we discuss how activation of adenosine receptors and regulation of extracellular adenosine levels is operated by combined mechanisms. It is highlighted that modulation of neuronal activity by adenosine involves a diversity of enzymes, receptors and signaling cascades that act in a concerted way to fine tune the activity of neurons and glia, including astrocyte-to-neuron signaling.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Extracellular formation of adenosine
- Nucleoside transporters
- Intracellular adenosine clearance
- Astrocyte control of extracellular adenosine levels
- Signaling pathways operated by adenosine receptors
- Neuromodulation
- Fine-tuning of neuronal activity
1 Introduction
Adenosine, due to the way it operates in the nervous system, belongs to the class of neuromodulators rather than neurotransmitters (for a review on these concepts see, for instance, Ribeiro and Sebastião 2010). So, intracellular adenosine is not stored in vesicles, nor released by exocytosis as a classical neurotransmitter. Instead, adenosine is released to the extracellular space through equilibrative nucleoside transporters that function bidirectionally according to the gradient across the cell membrane. Adenosine is also formed in the extracellular space through degradation of released ATP. Once in the extracellular space, adenosine activates membrane located G protein-coupled receptors (GPCR) and through these receptors affects neuronal functioning at different levels, including changes in the ability to release or respond to neurotransmitters or even gliotransmitters, but so far, no neurotransmitter-like actions for adenosine have been identified.
Adenosine also behaves as a retaliatory metabolite, influencing and reflecting cell energy state, as well as metabolic demand and nutrient supply. More than 25 years have elapsed since it was first proposed that adenosine efficiently connects synaptic activity, energy expenditure, and nucleic acid metabolism by acting as a sensor of the bioenergetic state of the cell (Newby et al. 1985). Moreover, intracellular adenosine directly regulates transmethylation reactions, including DNA methylation (Boison et al. 2002), which can lead to long-lasting epigenetic modifications. Other than at the nerve tissue level, the influence of adenosine upon cerebral blood flow enables it to further act as an energy balancing metabolite. Thus, when metabolism is increased, the elevated ATP catabolism will produce higher amounts of adenosine, which through the activation of A2AR, (Phillis 1989) will induce vasodilation, allowing an improvement of oxygen and nutrient delivery via the cerebral vasculature.
In the present work we discuss some of the most recent data on the regulation of adenosine availability and its effects through adenosine receptors and how their activation is regulated by extracellular adenosine levels. Downstream mechanisms of receptor activation and receptor cross talk are then reviewed on the basis of recently published data, highlighting the functional outcomes of the subtle ways adenosine fine-tunes neuronal activity.
2 Adenosine Formation
The main source of adenosine, in the central nervous system, is the dephosphorylation of 5′-AMP by 5′-nucleotidases (5′-NTs) (Meghji 1993). These enzymes dephosphorylate noncyclic nucleoside monophosphate to nucleosides and inorganic phosphate. So far, seven distinct nucleoside monophosphate phosphohydrolases or 5′-nucleotidases (EC 3.1.3.5 and EC 3.1.3.6) have been cloned. Five are localized in the cytosol; one is attached to the outer side of the plasma membrane and one in the mitochondrial matrix. Nucleotidases are responsible for both intracellular and extracellular synthesis of adenosine from the dephosphorylation of AMP. An alternative source of adenosine synthesis results from the hydrolysis of S-adenylhomocysteine (SAH), which is catalyzed by SAH hydrolase (SAHH) (Palmer and Abeles 1979) (see Fig. 7.1).
Adenosine metabolism. Adenosine can be synthesized intra- and extracellularly. Inside the cell adenosine is formed from AMP metabolism through endo-5′-nucleotidase (5′NT) or by the transmethylation reaction catalyzed by SAHH, which converts SAH into adenosine and homocysteine. At the extracellular space, adenosine derives from the metabolism of ATP/ADP/AMP, being the last reaction catalyzed by the ectonucleotidase (Ecto 5′NT). The release of adenosine through equilibrative nucleoside transporters is an alternative source of adenosine. Regarding clearance of extracellular adenosine, in some cases it can be converted into inosine by ecto-adenosine deaminase (ecto-ADA) in the extracellular space, but in most cases adenosine is taken up by the equilibrative nucleoside transporter into cells where adenosine can be phosphorylated to AMP by adenosine kinase (ADK) or deaminated to inosine by adenosine deaminase (ADA)
2.1 Extracellular Formation of Adenosine
Adenosine found in the extracellular space can be released via equilibrative nucleotide transporters or be synthesized locally via ATP catabolism, which involves several enzymes, including the enzymes of ectonucleoside triphosphate diphosphohydrolase (E-NTPDase) family, ectonucleotide pyrophosphatase/phosphodiesterase (E-NPP) family, ecto-5′-nucleotidase/CD73, and alkaline phosphatases (Yegutkin 2008). Through this cascade, adenine nucleotides are dephosphorylated into 5′-AMP, which is then dephosphorylated by ecto-5′-nucleotidase into adenosine. The entire catalytic pathway is complete in a few hundred milliseconds, and the rate-limiting step being the dephosphorylation of AMP into adenosine by ecto-5′-nucleotidase (Dunwiddie et al. 1997).
2.1.1 Ecto-5′-Nucleotidase
After the vesicular release of ATP (which is cosecreted with neurotransmitters or even released as a neurotransmitter), ATP is metabolized by a cascade of ectonucleotidases, including ecto-ATPase, ecto-ADPase (E-NTPDase family) and apyrase (E-NPP family) and finally ecto-5′-nucleotidase, producing adenosine (Ribeiro and Sebastião 1987; Richardson and Brown 1987; Zimmermann et al. 1986). Additionally cAMP can also be released into the extracellular space, by a probenecid-sensitive transporter (Rosenberg and Li 1995) in sufficient amounts to increase extracellular adenosine concentrations (Brundege et al. 1997; Dunwiddie et al. 1992).
In neuronal cells, ectonucleotidases are able to convert most adenine nucleotides (except cAMP) into adenosine in less than a second (Dunwiddie et al. 1997). Indeed, even stable ATP analogues can be converted into adenosine by ectonucleotidases (e.g., Cascalheira and Sebastião 1992; Cunha et al. 1998). Several ectonucleotidases, including alkaline phosphatase and nucleoside triphosphate diphosphohydrolase 2, are associated with subsets of progenitor cell populations in the mouse embryonic, postnatal, and adult neurogenic zones (Langer et al. 2007). Knockdown of tissue nonspecific alkaline phosphatase impairs neural stem cell proliferation and differentiation (Kermer et al. 2010), highlighting their relevance in neurogenesis, including adult neurogenesis (see Zimmermann 2011).
The ecto-5′-nucleotidase is a cell surface protein attached to the plasma membrane by a glycosyl phosphatidylinositol (GPI) anchor at its C terminal (Misumi et al. 1990). The hydrolysis of extracellular AMP is considered the main function of this enzyme, but 5′-nucleotidase is also involved in cell adhesion, as it also binds laminin and fibronectin (Mrhul et al. 1993; Olmo et al. 1992). Ecto-5′-nucleotidase acts also as a coreceptor in T cell activation (see Resta and Yamashita 1998).
Ecto-5′-nucleotidase is highly expressed in the brain, where it is mainly associated with glial cell membranes, namely, astrocytes, oligodendrocytes, and also microglia (Kreutzberg et al. 1978; Naidoo 1962; Schoen and Kreutzberg 1995). In fact, the predominant glial expression of ecto-5′-nucleotidase is related to an enhancement in the contribution of extracellular conversion of AMP into adenosine when astrocytes are cocultured with neurons (Zamzow et al. 2008a).
Regarding localization in neurons, some initial cytochemical studies associated ecto-5′-nucleotidase with the surface of migrating and immature nerve cells and with subsets of synapses during part of their regeneration period as well as during synapse remodeling and regeneration (Schoen et al. 1991, 1993). Later on, the ecto-5′-nucleotidase expression by mature neurons was also demonstrated in the cerebellum (Maienshein and Zimmermann 1996) and in hippocampal nerve terminals (Cunha et al. 2000). More recently, it has been demonstrated that ecto-5′-nucleotidase is expressed by nociceptive neurons in dorsal root ganglia and on terminals in substantia gelatinosa of spinal cord, where the conversion of AMP into adenosine promotes antinociception (Sowa et al. 2010).
Functionally, there is some evidence that ectonucleotidases are in close physical proximity with presynaptic adenosine receptors (Cunha et al. 1996; Dunwiddie and Masino 2001), so that recently formed adenosine becomes immediately available to the presynaptic receptors involved in modulation of neurotransmitter release. Topographical arrangement of membrane bound molecules involved in purinergic signaling may determine the type of receptor activated by adenosine, since there is evidence that adenosine formed from released ATP preferentially activates facilitatory receptors (Cunha et al. 1996).
2.2 Intracellular Synthesis of Adenosine
For the net extracellular adenosine levels, intracellular synthesis of adenosine is at least as important as adenosine formation from breakdown of extracellular ATP (Lloyd and Fredholm 1995). Intracellular synthesis of adenosine occurs mainly by AMP dephosphorylation, which is catalyzed by cytosolic nucleotidases. The presence of cytosolic 5′-nucleotidase in the brain was firstly demonstrated in 1982 (Montero and Fes 1982). Although differential expression of 5′-nucleotidase among different brain areas has not been established so far, its ubiquitous role in the intracellular synthesis of adenosine is well known. Additionally, the cytosolic nucleotidases participate in substrate cycles that regulate the cellular levels of ribo- and deoxyribonucleoside monophosphates, regulating the intracellular pools of ribo- and deoxyribonucleotides (Reichard 1988) which are crucial for DNA/RNA synthesis.
Furthermore, adenosine produced inside the cell contributes to restoring ATP levels by decreasing ATP utilization and increasing oxygen and nutrients supply via blood flow (Newby 1984). Thus, adenosine is commonly considered to be a retaliatory metabolite, since adenosine produced during cytosolic ATP degradation behaves as a metabolic stress sign promoting retaliatory effects against the stress-causing conditions (Newby 1984).
Another source of adenosine is the transmethylation pathway, where adenosine results from the hydrolysis of S-adenosylhomocysteine (SAH) catalyzed by SAH hydrolase (SAHH, EC 3.3.1.1), which also produces l-homocysteine (Palmer and Abeles 1979; Schrader et al. 1981). This enzyme was firstly described in 1959, in rat liver. SAHH catalyzes a reversible reaction, that preferentially evolves towards S-adenosylhomocysteine synthesis (de la Haba and Cantoni 1959). In the heart, this pathway provides one-third of the total cardiac adenosine at normoxic conditions but generates undetectable levels under hypoxic conditions (Deussen et al. 1989). SAHH expression is widespread in the brain, with higher expression levels present in cortex and cerebellum. Inside the cell, SAHH displays a nuclear expression where it is involved in transmethylation mechanisms. In detail, different methyltransferases convert S-adenosylmethionine (SAM) into SAH, which is then metabolized into adenosine and l-homocysteine by SAHH. So, adenosine is an obligatory end product of SAM-dependent transmethylation reactions and because of that is able to inhibit methylation reactions. To avoid this inhibition, adenosine is phosphorylated into AMP by a long isoform of adenosine kinase (ADK), which was described as a nuclear ADK (Cui et al. 2009).
Under normal conditions SAHH has low impact upon neuronal excitability (Pak et al. 1994), suggestive of a minor role in the control of cytoplasmic levels of adenosine in neurons.
3 Nucleoside Transporters
The relevance of adenosine uptake by nucleoside transporters in terminating adenosine effects was first supported by different studies showing that the nucleoside transporter blockade produces vasodilation, potentiates the ability of adenosine to decrease locomotor activity (Crawley et al. 1983), depresses neuronal activity (Motley and Collins 1983), increases nociceptive thresholds (Yarbrough and McGuffin-Clineschmidt 1981), and exerts anticonvulsive effects (Dragunow and Goddard 1984). Therefore, nucleoside transporter inhibitors exacerbate the effects mediated by adenosine.
Nucleoside transporters can be divided in two main classes: the equilibrative (Na+-independent) nucleoside transporters (ENTs) and the concentrative (Na+-dependent) nucleoside transporters (CNTs) (Baldwin et al. 1999). Six isoforms of CNTs (CNT1–CNT6) and four isoforms of ENTs (ENT1–ENT4) have been cloned, to date. Equilibrative transporters mediate nucleoside transport in both directions, depending on the nucleoside concentration gradient across the membrane. The four transporters are widely distributed, and all of them are able to transport adenosine but they have different abilities to transport other nucleosides (Baldwin et al. 2004). The transport mediated by concentrative transporters is independent of nucleoside gradient and is coupled to sodium gradient. As intracellular concentrations of adenosine are kept low due to its conversion into AMP, and as catabolism of released nucleotides constitutes an additional and transporter-independent source of extracellular adenosine, the extracellular concentrations of adenosine are usually higher than the intracellular ones. Therefore, the usual direction of equilibrative adenosine transport is uptake into cells, rather than release. Indeed, adenosine uptake in the brain occurs primarily by facilitated diffusion via equilibrative transporters, although some of it (10–20 %) can be mediated by concentrative transporters (Geiger and Fyda 1991; Parkinson et al. 1994).
Equilibrative nucleoside transporters are crucial to regulate the levels of extracellular adenosine, being the main entity responsible for removing adenosine from the extracellular space. They are thus responsible for restraining, both spatially and temporally, adenosinergic modulation. Due to the equilibrative nature of the adenosine transporters in neuronal cells, changes in the activity of enzymes involved in ADO metabolism will modify the transporters’ activity. Accordingly, transporter inhibitors can either increase (Dunwiddie and Diao 1994; Phillis et al. 1989; Sanderson and Scholfield 1986) or decrease (Gu et al. 1995) extracellular adenosine levels, depending on the transmembrane adenosine gradient and consequently depending on transport direction, into or out of the cell. However, because the extracellular synthesis of adenosine from catabolism of nucleotides constitutes an alternative source of adenosine, which is not affected by transport blockade, the transporters inhibitors usually lead to an increase in the extracellular levels of the nucleoside.
The amount of adenosine released by nucleoside transporters is enhanced under some circumstances such as hypoxia or ischemia, when a massive increase in extracellular adenosine levels is observed, a process prevented by transporter blockade (Parkinson et al. 2002). At the synaptic level, however, the rise in extracellular levels of adenosine during hypoxia may increase rather than decrease upon inhibition of equilibrative nucleoside transporters (Frenguelli et al. 2007). Furthermore, recent evidence (Zhang et al. 2011) showed that neuronal nucleoside transporters contribute to the removal of extracellular adenosine from the synaptic space even during hypoxic or ischemic insults. Therefore, evidence now available allows suggesting that the control of extracellular adenosine levels may differ in different microdomains. As highlighted recently (Sebastião 2011), a deeper understanding of those microdomains as well as of the relative contribution of the different cell types (i.e., neurons vs. astrocytes) for the net production of adenosine is required to better predict the direction of the changes in adenosine levels after pharmacologic or genetic manipulation of adenosine transporters in pathological conditions.
Interestingly, adenosine release by nucleoside transporters is promoted by neurotransmitters. For example, glutamatergic agonists such as NMDA and kainate increase, in a dose-dependent manner, adenosine release (Carswell et al. 1997; Delaney et al. 1998). In fact, activation of NMDA receptors seems to promote release of adenosine itself instead of its precursor, ATP (Harvey and Lacey 1997; Manzoni et al. 1994). This may be part of a protective feedback loop since adenosine released through the transporters seems to preferentially activate adenosine A1 receptors (A1R; Cunha et al. 1996) and these are neuroprotective, namely, through inhibition of NMDA currents not only under normoxic (de Mendonça et al. 1995) but also under hypoxic (Sebastião et al. 2001) conditions.
4 Intracellular Adenosine Clearance
After being taken up through nucleoside transporters, adenosine is inactivated either by deamination through adenosine deaminase or by phosphorylation through adenosine kinase (Fig. 7.1). It is accepted that the pathway responsible for intracellular adenosine clearance is dependent on its concentration. As such, at low concentrations, adenosine is mainly inactivated by phosphorylation while at higher concentrations adenosine is predominantly deaminated by adenosine deaminase (Meghji and Newby 1990), in accordance with the affinity for adenosine and enzymatic capacity of those two enzymes.
4.1 Adenosine Kinase
ADK (EC 2.7.1.20) phosphorylates intracellular adenosine into AMP. Due to its low K m for adenosine, it is the main enzyme responsible for intracellular adenosine catabolism, at least, for low adenosine concentration. ADK is therefore a key target whenever manipulation of the neuromodulatory actions of adenosine is desirable. By phosphorylating adenosine into AMP, ADK has a double role for maintaining a homeostatic energy flux: (1) a direct ability to influence the cellular energy pool (AMP, ADP, and ATP) and (2) an influence upon intra- and extracellular levels of the homeostatic regulator, adenosine. The relevance of ADK for the homeostatic control (Boison et al. 2011) is supported by several lines of evidence, namely, (1) the release of higher amounts of adenosine by ADK-deficient fibroblasts in cultures, when compared to that released by ADA-deficient fibroblasts (Huber et al. 2001), (2) the ability of ADK inhibition to depress neuronal activity in hippocampal slices, in a way sensitive to A1R antagonists (Diógenes et al. 2004; Pak et al. 1994), and (3) the suppression of seizure activity caused by ADK inhibition in various models for epilepsy (Kowaluk and Jarvis 2000).
The immature brain is more vulnerable to seizure activity than the adult brain (Moshe 2000), an action probably related to the higher expression of ADK at early developmental stages (Studer et al. 2006). Interestingly, during maturation, there is a shift from neuronal to glial expression of ADK, suggestive of distinct functions of ADK and adenosine in immature and adult brain; thus, during neuronal development expression of ADK in neurons may provide a salvage pathway to utilize adenosine in nucleic acid synthesis, whereas in the mature brain predominant ADK expression in astrocytes contributes to maintenance of tonic adenosinergic inhibition in the central nervous system (Studer et al. 2006). Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis (Gouder et al. 2004). Furthermore, there is a prominent upregulation of ADK in astrocytes after induction of status epilepticus (SE) in animals as well as in humans with temporal lobe epilepsy (Aronica et al. 2011). Selective ADK downregulation in astrocytes almost completely abolishes spontaneous recurrent seizures in epileptic mice (Theofilas et al. 2011). Thus, ADK emerges as a key link in astrocyte-to-neuron communication, and its dysregulation after intense neuronal activity may contribute to epileptogenesis. Permanent changes in ADK expression in astrocytes will be reflected in decreases in ambient adenosine, leading to a further enhancement of neuronal activity and in such a way being part of a positive feedback loop to promote epileptogenesis. Accordingly, focal adenosine augmentation therapeutic strategies, mainly based in local manipulation of ADK activity, have been proposed as a useful strategy to control pharmacoresistant seizures (Boison et al. 2011).
The regulation of ambient adenosine levels by ADK might also have a key role in the susceptibility of brain tissue to ischemic injury (Lynch et al. 1998; Pignataro et al. 2007; Shen et al. 2011). Indeed, pharmacological inhibition of ADK in animal models is also an effective strategy to protect from stroke (Boison 2006; Kowaluk and Jarvis 2000).
4.2 Adenosine Deaminase
Adenosine deaminase (ADA, EC 3.5.4.4) catalyzes the hydrolytic deamination of adenosine into inosine. It has been known for several years that inhibition of ADA causes adenosine-like effects such as sedation (Major et al. 1981; Radulovacki et al. 1983), reduction of the infarct area in the hippocampus and decrease in neuronal degeneration in animal models of global forebrain ischemia or focal ischemia (Lin and Phillis 1992; Phillis and O’Regan 1989). Although ADA is expressed by both neurons and astrocytes (Haun et al. 1996; Nagy et al. 1984) it seems that it is in glial cells that this enzyme assumes a major role in the control of adenosine levels. This role is more relevant during stress conditions (like trauma or ischemia), when adenosine levels rise and astrocytes became reactive, probably playing an important role in adenosine conversion to inosine (Zamzow et al. 2008b). Inosine by itself can have a protective effect in stroke models (Shen et al. 2005). However, neuroprotection conferred by inhibitors of ADA during hypoxia or ischemia (Lin and Phillis 1992) mostly results from potentiation of the stress-induced increase in intracellular adenosine, which leads to enhanced adenosine release through transport reversal (Phillis and O’Regan 1989).
Although the enzyme localization is mainly cytosolic, there is evidence of the existence of an ectoenzyme, bound to the extracellular side of the membrane (Franco et al. 1998). The A1R may act as an anchoring protein for ecto-ADA, which through a nonenzymatic but allosteric interaction facilitates agonist and antagonist binding to A1R (Ciruela et al. 1996; Gracia et al. 2008; Ruíz et al. 2000; Saura et al. 1998). Like A1R, A2BR was also found to be anchored to ADA in lymphocytes and cultured cells. Similarly, binding of enzymatically active or inactive ADA to this receptor increases its affinity and signaling by a protein–protein interaction (Herrera et al. 2001).
5 Control of Extracellular Adenosine Levels by Astrocytes
In the brain, extracellular adenosine concentrations are normally kept in the range of 25–250 nM, therefore at concentrations that can tonically activate a substantial proportion of the high affinity A1R and A2AR (Dunwiddie and Masino 2001). A major player in the steady-state levels of adenosine is ADK, which has high affinity for adenosine and is mostly expressed in astrocytes (see above and Boison et al. 2010). ADA also predominates in astrocytes. Equilibrative nucleoside transporters are also expressed in astrocytes. Therefore, under physiological conditions astrocytes probably function as a major sink for adenosine, since its uptake is driven by the intracellular activity of ADK. It is also likely that under conditions that prompt increases in intracellular adenosine, such as hypoxia or ischemia, astrocytes provide a major source of adenosine, which is released by reversal of transport direction, but direct evidence for a predominant astrocytic origin of extracellular adenosine during hypoxic/ischemic insults is still lacking.
The levels of extracellular adenosine can be regulated by both A1R and A2R activity since A1R blockade increases extracellular levels of adenosine in cardiac fibroblasts (Andresen et al. 1999) and activation of A2R promotes adenosine transport in chromaffin cells (Delicado et al. 1990). Furthermore, it has been shown that A2AR activation at nerve endings enhances the activity of nucleoside transporters, leading to a decrease in the availability of adenosine to activate A1R under high frequency neuronal firing (Pinto-Duarte et al. 2005). Again, information is still lacking regarding the role of astrocytes in this process. In astrocytes, A1R and A2AR form tetramers constituted by two A1R and two A2AR molecules bound to Gi/0 and Gs proteins to regulate GABA transport in a deeply interactive and concerted way (Cristóvão-Ferreira et al. 2011). Whether these A1R-A2AR tetramers control adenosine transporters is also unknown.
6 Purines and Intracellular Signaling
ATP acts upon different classes of membrane receptors, the ionotropic P2X and the metabotropic P2Y (for reviews see, for instance, Illes and Ribeiro 2004; Ralevic and Burnstock 1998). Adenosine operates through activation of four distinct metabotropic receptors: A1R, A2AR, A2BR, and A3R. All these receptors are expressed in the brain, where adenosine is involved in a variety of physiological and pathological processes, namely, regulation of sleep–arousal cycle, neuroprotection, epilepsy, pain, fine control of movement, fine-tuning of neurotransmission (see Boison 2006; Dunwiddie and Masino 2001; Ribeiro 2005; Sebastião and Ribeiro 2009a).
7 Adenosine Receptors and Signaling Pathways
All four adenosine receptors have been cloned. Being GPCRs, adenosine receptors are formed by a single peptide chain, with seven alpha-helical transmembrane domains, an intracellular C-terminal, and an N-terminal facing the extracellular space. The N-terminal usually contains one or more glycosylation sites. The C-terminal contains phosphorylation and palmitoylation sites, which are involved in regulation of receptor desensitization and internalization (Perez and Karnik 2005).
Adenosine receptors are widely distributed throughout the body. In the brain they can be found pre-, post-, or nonsynaptically, in neurons as well as in glia. Their expression is not homogenous in the central nervous system (Fig. 7.2). Higher A1R expression levels are found in the cortex, hippocampus, cerebellum, thalamus, brain stem, and spinal cord (see Ribeiro et al. 2002 and references therein). Though at low density, A1Rs are also present in basal ganglia, both on dopaminergic nigrostriatal and glutamatergic corticostriatal terminals. Adenosine A2ARs are mostly expressed in the basal ganglia and olfactory bulb. However, it is possible to find mRNA encoding A2ARs or the A2AR protein in other brain regions where they are weakly expressed, namely, in the hippocampus and the cortex (see Sebastião and Ribeiro 1996). The A2BRs are mainly expressed in peripheral organs, being weakly expressed in the whole brain (Dixon et al. 1996). Finally, A3Rs have an intermediate expression level in the human cerebellum, and as A2BRs, they display a low expression in the entire brain (see Fredholm et al. 2001). The A1R and A2AR are high affinity receptors, with adenosine K d values of 70 and 150 nM, for A1R and A2AR respectively, which allow their tonic activation by basal adenosine levels. The A2BR and A3R are considered low affinity receptors, with adenosine affinity constant values around 5,100 and 6,500 nM, respectively (see Dunwiddie and Masino 2001).
Differential distribution of adenosine receptors (A1, A2A, A3) among the brain (adapted from Ribeiro et al. 2002). Higher expression corresponds to larger text size
Classically, A1R and A3R inhibit adenylate cyclase (AC) through coupling to Gi/0. A2AR and A2BR are coupled to Gs or Golf, promoting AC activity. The A2BR subtype is also coupled to Gq/11, through which it can activate phospholipase C (Ryzhov et al. 2006). The A3R can also couple to Gq/11, also activating phospholipase C (Fredholm et al. 2001). The increase of cAMP mediated by AC leads to activation of cAMP dependent protein kinase (PKA), which then phosphorylates different targets such as ionotropic receptors or neurotransmitter transporters. On the other hand, activation of phospholipase C converts phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). Then DAG activates protein kinase C (PKC), which phosphorylates different substrates, while IP3 triggers calcium release from intracellular stores. Then, elevation of cytosolic Ca2+ can stimulate a variety of signaling pathways, including a family of phosphatidyl serine-dependent serine/threonine-directed kinases collectively called protein kinase C (PKC), phospholipase A2 (PLA2), as well as Ca2+-dependent K+ channels, and nitric oxide synthase (NOS). IP3 can also promote calcium influx from extracellular sources if Ca2+ intracellular stores are depleted due to previous activation of IP3 receptors (see Ralevic and Burnstock 1998).
The activation of G proteins can also directly modify the activity of several enzymes and ion channels that directly or indirectly influence intracellular calcium levels. For example, A1R, via Gi/0 activation, leads to activation of several types of K+ channels and to blockade of N-, P-, and Q-type Ca2+ channels (see Fredholm et al. 2001). Direct evidence that A1R can inhibit calcium channels in nerve terminals under hypoxic conditions has been reported (Coelho et al. 2002). A3R are also able to modulate Ca2+ levels, through the inhibition of AC. Both A2AR and A2BR can also modify the levels of intracellular calcium (see Fredholm et al. 2001).
Aside from the involvement of AC/cAMP/PKA and PLC/IP3-DAG/PKC, other transduction pathways are associated with adenosine receptor activation, namely, the mitogen-activated protein kinase (MAPKs) (Schulte and Fredholm 2003). The MAPK family is constituted by three main groups: extracellular regulated kinases (ERK) such as ERK1 and ERK2, stress-activated protein kinases (SAPK) such as p38, and jun-N-terminal kinase (JNK). These kinases are usually activated by receptors with tyrosine-kinase activity (Seger and Krebs 1995), but GPCR can also signal through them (Gutkind 1998; Liebmann 2001; Marinissen and Gutkind 2001). In fact, all adenosine receptors can affect the MAPK pathway. This was first shown in COS-7 cells transiently transfected with A1R, leading to activation of ERK1/2 via Gi/0 (Faure et al. 1994). It was also early recognized that activation of A2AR can increase MAPK activity (Sexl et al. 1997). Interestingly, the signal pathway used by A2AR to activate MAPK can vary, depending on the cellular machinery available. Thus, in CHO cells, A2AR-mediated ERK1/ERK2 activation involves Gs-AC-cAMP-PKA-MEK1, while in HEK-293 cells, MAPK activation by A2AR involves PKC but not PKA, even though cAMP levels are found to be enhanced by Gs activity (Seidel et al. 1999). A2AR can also inhibit ERK phosphorylation (Hirano et al. 1996). The activation of A2BR can trigger the three main branches of MAPK family (ERK1/2, p38, and JNK) (see Fredholm et al. 2001). Finally, the A3R activate ERK1/2 in human fetal astrocytes (Neary et al. 1998). Also, the phosphorylation of ERK1/2 was also clearly demonstrated in CHO cells transfected with A3R (see Schulte and Fredholm 2000).
To conclude, MAPK activation by adenosine receptors is quite similar to that prompted by other GPCR (Gutkind 1998; Luttrell et al. 1999; Sugden and Clerk 1998). Interestingly, ERK1/2 phosphorylation is promoted either by receptors coupled to Gs (A2A/A2B) or to Gi/0 (A1/A3) proteins. The MAPK-mediated effects of adenosine receptors mainly impact modulation of DNA synthesis, cellular differentiation, proliferation, and apoptosis (see Schulte and Fredholm 2003).
Adenosine receptor activity is regulated by its expression at the membrane level which results from a balance between endocytosis and exocytosis rates. An enhancement of the endocytosis rate, which restrains the intensity and duration of the signal, is often preceded by receptor phosphorylation and uncoupling from G proteins, a well-known process of receptor desensitization. Adenosine receptor subtypes desensitize differently. A1R are slowly phosphorylated and internalized, a time frame of several hours being needed to complete the process. A2AR and A2BR desensitize in a faster way, with downregulation kinetics less than 1 h. The A3R have the fastest desensitization profile, a process often occurring within minutes (Klaasse et al. 2008).
8 Implications for Modulation of Neuronal Function
The variety of downstream pathways operated by adenosine receptors highly supports the pluripotential of this nucleoside to interfere with a multiplicity of intracellular functions essential to regulate neuronal activity either directly or indirectly via interaction with several neurotransmitters and/or neuromodulators. These interactions can occur within the same cell, in some cases involving receptor heteromerization, or be a result of transcellular communication. Being a small and easily diffusible molecule, adenosine easily acts in a paracrine way, affecting cells away from the release point. Its role as a trans-synaptic modulator, involving neuron-to-astrocyte communication at tripartite synapses is now well accepted (Fields and Burnstock 2006; Hamilton and Attwell 2010; Perea et al. 2009).
Within the same cell there are many possibilities of cross talk between transduction pathways that have several kinases and other key molecules in common (see Fig. 7.3). GPCRs can interact at the G protein level, by sharing βγ-subunits or common α-subunits, affecting the activation kinetics of other GPCRs. This also applies to A1R and A2AR, and related mechanisms are most probably involved in the ability of adenosine receptors to interact with other receptors for neurotransmitters or neuromodulators (Sebastião and Ribeiro 2000).
Schematic representation of the different signaling pathways associated with adenosine receptors. Adenosine receptors are GPCRs. A1R and A3R couple to Gi/0, inhibiting AC, which will reduce cAMP levels and consequently decrease PKA activity. A2AR and A2BR are coupled to Gs, promoting AC activity and consequently PKA activity. A3R and A2BR can also couple to Gq/11, enhancing PLC activity. PLC catalyzes PIP2 into DAG and IP3. DAG will directly activate PKC, while IP3 will increase intracellular Ca2+ levels. Furthermore, all adenosine receptors can activate the MAPK pathway
A1R and A2AR can form heteromeric complexes (Ciruela et al. 2006). As clearly shown in astrocytes, A1R-A2AR heteromers appear as heteromers of homomers with a minimal structure consisting of an A1R-A1R-A2AR-A2AR complex (Cristóvão-Ferreira et al. 2011). The heterotetramer makes it possible to accommodate the two different G proteins, and the A1R-A2AR heteromer in astrocytes is clearly coupled to Gi/0 and Gs proteins (Cristóvão-Ferreira et al. 2011). Importantly, the blockade of a single partner in the A1R/A2AR/Gi/0/Gs complex leads to adjustments in the functioning of the whole unit (Cristóvão-Ferreira et al. 2011).
Heteromerization between adenosine receptors and receptors of other neurotransmitters/neuromodulators also occurs, being the A2AR-D2R heteromer the first to be recognized (Hillion et al. 2002). Through this heteromer, adenosine restrains D2R-mediated effects. The relevance of dopaminergic signaling and dysfunction in several pathologies turns A2AR-D2R heteromers into promising therapeutic targets (Altamura et al. 2005; Ferré et al. 1997). A close interaction between A1R and D1R was also described (Ginés et al. 2000). Once again, adenosine, through the activation of A1R, inhibits D1R-mediated effects. In detail, A1R activation leads to uncoupling of D1R from AC (Ginés et al. 2000), reinforcing the A1R-induced inhibition of D1R, which is mediated by Gi/0 activation (Ferré et al. 1994, 1998).
Adenosine A2AR can also heteromerize with cannabinoid CB1R in the striatum (Carriba et al. 2007), and this has putative implications for pharmacotherapy drug addiction (Ferré et al. 2010). Psychomotor stimulation by A2AR antagonists also depends upon A2AR-CB1R cross talk (Lerner et al. 2010). A1R also interact with CB1R receptor-mediated actions in the hippocampus, a process with implications for CB1R induced memory impairment, which is exacerbated by chronic caffeine consumption (Sousa et al. 2011).
The predominant neuromodulatory action of adenosine, inhibition of neurotransmitter release, is controlled by A1R and relates to presynaptic inhibition of calcium responses (Fredholm et al. 1990; Fossier et al. 1999; Ribeiro 1978). However, A2AR can also modulate extracellular transmitter levels and they do so either by enhancing release and/or by influencing uptake. Indeed, A2AR activation in the hippocampus facilitates GABA release (Cunha and Ribeiro 2000) and GABA uptake into presynaptic terminals (Cristóvão-Ferreira et al. 2009) and astrocytes (Cristóvão-Ferreira et al. 2011). In the striatum, where A2AR expression is higher, the activation of these receptors leads to an inhibition of GABA uptake (Gonzalez et al. 2006). Glutamate release from hippocampal (Lopes et al. 2002) and cortical (Marchi et al. 2002) synaptosomes is also enhanced by A2AR activation. The same occurs with acetylcholine release from hippocampal nerve terminals (Cunha et al. 1995).
During excitotoxic conditions, such as hypoxia, the A1R-mediated presynaptic inhibition of calcium responses (Coelho et al. 2002), together with inhibition of NMDA responses (Sebastião et al. 2001) confers protection against synaptic damage. In contrast, A2AR may facilitate ionotropic receptor activation, as it is the case of their ability to enhance AMPA receptor-mediated responses at the postsynaptic and extrasynaptic level, affecting the reserve of the GluR1-containing AMPA receptors at the extrasynaptic pool, priming them for synaptic insertion and for reinforcement of synaptic strength (Dias et al. 2012). This action is sustained even after brief A2AR activation, involves cyclic AMP and PKA activation and leads to enhancement of long-term potentiation (LTP) in Schaffer collateral-CA1 synapses of the hippocampus (Dias et al. 2012). LTP facilitation by A2AR is also evident in aged animals (Costenla et al. 2011). Interestingly, A2AR blockade in vivo impairs conditional learning as well as potentiation of CA1 hippocampal potentials recorded concomitantly in freely moving animals (Fontinha et al. 2009). A2AR localized postsynaptically at synapses between mossy fibers and CA3 pyramidal cells are essential for a form of long-term potentiation (LTP) induced by short bursts of mossy fiber stimulation, which requires the activation of NMDA and metabotropic glutamate receptors (mGluR5) to increase cytoplasmic Ca2+ levels (Rebola et al. 2008).
A2AR seem to be devoted to interacting with other metabotropic receptors, not only of the GPCR family (Sebastião and Ribeiro 2000) but also with tropomyosin-related kinase (Trk) receptors (Sebastião and Ribeiro 2009b). A2AR are able to transactivate TrkB receptors in the absence of the neurotrophin (Lee and Chao 2001). This transactivation requires long-term incubation with A2AR agonist and requires receptor internalization (Rajagopal et al. 2004). Furthermore, adenosine A2AR activation is also a crucial step for the functioning of neurotrophic receptors at synapses, through a mechanism most probably different from TrkB transactivation, and which involves translocation of TrkB molecules to lipid rafts (Assaife-Lopes et al. 2010). The A2AR-mediated gating and/or facilitation of the actions of neurotrophins, has been shown for the facilitatory actions of brain derived neurotrophic factor (BDNF) on synaptic transmission (Diógenes et al. 2004; Tebano et al. 2008) and on plasticity (Fontinha et al. 2008) at the CA1 area of the hippocampus. The actions of BDNF are blocked by either A2AR blockade or inhibition of Trk phosphorylation, but a Trk phosphorylation inhibitor does not prevent A2AR-mediated facilitation of synaptic transmission (Pousinha et al. 2006, indicating that A2AR operate upstream of TrkB activation. Synaptic actions of other neurotrophic factors, such as glial derived neurotrophic factor (GDNF) are also under influence of A2AR in the striatum (Gomes et al. 2006, 2009). Adenosine A2AR and BDNF TrkB receptors can coexist in the same nerve ending since the facilitatory action of A2AR upon TrkB-mediated BDNF action is also visible at the neuromuscular junction (Pousinha et al. 2006), a single nerve ending synapse model. Colocalization of A2AR and Ret, a component of the GDNF receptor complex, has also been shown in single axon terminals in the striatum (Gomes et al. 2009).
The ability of BDNF to facilitate synaptic transmission and synaptic plasticity is dependent on the age of the animals (Diógenes et al. 2007, 2011) and this may be related to the degree of activation of adenosine A2AR by endogenous adenosine at different ages. Thus, to trigger a BDNF facilitatory action at synapses of infant animals it is necessary to increase the extracellular levels of adenosine, either by inhibiting ADK or by a brief depolarization (Diógenes et al. 2004; Pousinha et al. 2006) or even by high frequency neuronal firing (Fontinha et al. 2008). These adenosine-triggered BDNF actions are lost by blocking adenosine A2AR with selective antagonists. In adult animals, BDNF per se can facilitate synaptic transmission, but this effect is also fully lost with blockade of adenosine A2AR (Diógenes et al. 2007) or in A2AR knockout mice (Tebano et al. 2008). Interestingly, the enhanced hippocampal synaptic plasticity in aged animals can be related not only to a higher influence of adenosine A2AR (Costenla et al. 2011) but also to an enhanced BDNF TrkB-mediated facilitatory tonus also dependent from cross talk with A2AR (Diógenes et al. 2011). Nicotinic alpha7 cholinergic currents in GABAergic hippocampal neurons are inhibited by BDNF, and this also requires coactivation of adenosine A2AR (Fernandes et al. 2008). Inhibition of GABA transporters (GAT) by BDNF at nerve terminals does not fully depend upon coactivation of A2AR, since it is not abolished by A2AR blockade, though this inhibitory BDNF action can be exacerbated by A2AR coactivation (Vaz et al. 2008). Interestingly, in astrocytes BDNF facilitates GABA transport, and this facilitation is fully dependent upon A2AR activation (Vaz et al. 2011). Contrasting with A2AR which promote the actions of neurotrophic factors, A1R inhibit neurite outgrowth of cultured dorsal root ganglion neurons, both in the absence and in the presence of NGF (Thevananther et al. 2001).
A2AR, due to their ability to enhance excitotoxic phenomena, including glutamate release (Lopes et al. 2002; Marchi et al. 2002), are mostly regarded as promoters of neuronal death. However, in some cases, such as cultured retinal neurones, A2AR have been shown to protect neurones against glutamate-induced excitotoxicity (Ferreira and Paes-de-Carvalho 2001). Whether this is due to the ability of A2AR to facilitate actions of neurotrophic factors, as it has been shown to occur in relation to A2AR-mediated neuroprotection of motor neurones (Wiese et al. 2007), is not yet known. The pathophysiological implications of the cross talk between A2AR and receptors for neurotrophic factors have been discussed in detail elsewhere (Sebastião and Ribeiro 2009c).
Adenosine receptor activation may also induce release of neurotrophic factors. Thus, the expression and/or release of NGF are enhanced by activation of A2AR in microglia (Heese et al. 1997) and by activation of A1R in astrocytes (Ciccarelli et al. 1999). Adenosine A2BR in astrocytes are also able to enhance GDNF expression (Yamagata et al. 2007). In the whole hippocampus, A2AR are required for normal BDNF levels (Tebano et al. 2008). Interestingly, in a mouse model of Huntington’s disease, A2AR are also required to keep striatal BDNF levels close to those obtained in wild-type mice (Potenza et al. 2007).
Finally, interactions among purinergic, growth factor, and cytokine signaling regulate neuronal and glial maturation as well as development. In neuronal-dependent glial maturation both ATP and adenosine purinoceptors are involved (see, for instance, Fields and Burnstock 2006). The extracellular adenosine levels during high frequency neuronal firing are sufficient to stimulate adenosine receptors in oligodendrocyte ancestor cells inhibiting their proliferation and stimulating their differentiation into myelinating oligodendrocytes (Stevens et al. 2002). In premyelinating Schwann cells, A2AR activate phosphorylation of extracellular signal-regulated kinases (ERKs), namely, ERK1/2, and inhibit Schwann cell proliferation without arresting differentiation (Stevens et al. 2004). Contrasting with A2AR, which usually promote the actions of neurotrophic factors, adenosine A1R inhibit neurite outgrowth of cultured dorsal root ganglion neurons, both in the absence and in the presence of NGF (Thevananther et al. 2001).
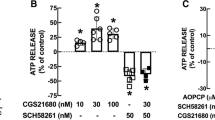
Besides influencing the activity of other neuromodulatory receptors, adenosine A2AR also affect the activity of equilibrative transporters, as in the case of adenosine transporters (Pinto-Duarte et al. 2005). Thus, activation of A2AR with the selective agonist CGS 21680 facilitates adenosine uptake and enhances release of adenosine, which points to a direct effect of A2AR on nucleoside transporters, rather than an indirect action resulting from a modification of the adenosine gradient of concentrations across the plasma membrane (i.e., a metabolic effect). Furthermore, high frequency neuronal firing activates A2AR and concomitantly enhances nucleoside transporters. The main consequence of this A2AR-mediated enhancement of nucleoside transporters is a marked reduction of the tonic activation of inhibitory A1R upon high-frequency firing. This action of A2AR on the activity of the adenosine transporters constitutes a clear demonstration that a neuromodulatory receptor is able to control the extracellular levels of its endogenous ligand and, hence, to influence its ability to control neurotransmitter release (Pinto-Duarte et al. 2005).
A2ARs facilitate GAT-1 mediated GABA transport into nerve terminals, an effect that is mediated by AC activation, which restrains the inhibition of GAT-1 by PKC (Cristóvão-Ferreira et al. 2009). Transport facilitation by A2ARs is due to an increase in the membrane expression of GAT-1 molecules, reflected in increased maximum transport velocity (Cristóvão-Ferreira et al. 2009). This A2AR-mediated facilitation of GABA transport into nerve endings, if coupled to an increase in the release of GABA (Cunha and Ribeiro 2000), may contribute to faster neurotransmitter recycling, leading to an enhancement of phasic GABAergic signaling.
A2ARs also facilitate GABA transport into astrocytes, by enhancing GAT-1 and GAT-3 mediated transport, an action under tight control of A1R, due to formation of A1R-A2AR heteromers (Cristóvão-Ferreira et al. 2011). While the A1 protomer of the heteromer inhibits GABA transport, the A2A protomer enhances it, the shift from inhibition to enhancement of GABA uptake probably occurring at low micromolar concentrations of extracellular adenosine (Cristóvão-Ferreira et al. 2011). These concentrations are easily attained at a tripartite synapse, where astrocytes and neurons release considerable amounts of ATP. The higher the release of ATP (as may occur at high neuronal firing rates in reciprocal neuron-to-astrocyte communication) the higher the expected concentration of extracellular adenosine. It is therefore likely that sustained neuronal firing promotes activation of the A2AR protomer of the A1R-A2AR heteromer leading to facilitation of GABA uptake. Activation of GABA uptake by astrocytes will lead to a decrease in ambient GABA and a subsequent depression of tonic GABAergic inhibition resulting in enhanced excitatory tonus. Conversely, at submicromolar adenosine concentrations, there is a preferential activation of the A1 protomer of the heteromer and so, GABA uptake by astrocytes would be inhibited. Consequently, tonic inhibition by GABA would be enhanced. Thus, through an adenosine action upon A1R-A2AR heteromers, astrocytes might behave as dual amplifiers, facilitating excitation of intense astrocytic-to-neuronal signaling and increasing inhibition at low neuronal firing rates. This switch in neural activity requires a highly efficient control to avoid sudden state transitions, and this seems to be the main advantage of heteromerization of A1R and A2AR in astrocytes. Indeed, overstimulation of just one of the receptor protomers leads to internalization of the whole functional unit (Cristóvão-Ferreira et al. 2011), therefore allowing a double brake in the system and avoiding an abrupt inhibitory signaling and a sudden switch from excitation to inhibition as a consequence of desensitization of only the excitatory protomer.
9 Concluding Remarks
Operating on multiple downstream signaling pathways, adenosine receptors influence the activity of other GPCRs as well as of receptors for neurotrophic factors, ion channels, ionotropic receptors, and neurotransmitter transporters. Modifications of extracellular adenosine levels, due to changes in its metabolic pathways, lead to alterations in the degree of activation of adenosine receptors, which will impact their ability to enhance or restrain the action of other neurotransmitters or neuromodulators. The cross talk between adenosine receptors and other membrane receptors results in part from intracellular cascade processes occurring between common transducing systems and through protein phosphorylation processes that involve PKC or PKA (Fig. 7.3). The key receptor in this synaptic interplay appears to be the A2AR, whereas the A1R can counteract A2AR mediated actions.
In summary, synaptic transmission is under tight control of endogenous extracellular adenosine, which through pre- and postsynaptic actions interplays with other synaptic molecules involved in neurotransmission as well as with membrane proteins (receptors, and transporters) essential for transmission to harmonically influence neuronal activity.
References
Altamura AC, Bassetti R, Cattaneo E, Vismara S (2005) Some biological correlates of drug resistance in schizophrenia: a multidimensional approach. World J Biol Psychiatry 6(suppl 2):23–30
Andresen BT, Gillespie DG, Mi Z, Dubey RK, Jackson EK (1999) Role of adenosine A(1) receptors in modulating extracellular adenosine levels. J Pharmacol Exp Ther 291:76–80
Aronica E, Zurolo E, Iyer A, de Groot M, Anink J, Carbonell C, van Vliet EA, Baayen JC, Boison D, Gorter JA (2011) Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy. Epilepsia 52:1645–1655
Assaife-Lopes N, Sousa VC, Pereira DB, Ribeiro JA, Chao MV, Sebastião AM (2010) Activation of adenosine A2A receptors induces TrkB translocation and increases BDNF-mediated phospho-TrkB localization in lipid rafts: implications for neuromodulation. J Neurosci 30:8468–8480
Baldwin SA, Mackey JR, Cass CE, Young JD (1999) Nucleoside transporters: molecular biology and implications for therapeutic development. Mol Med Today 5:216–224
Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD (2004) The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. Eur J Physiol 447:735–743
Boison D (2006) Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends Pharmacol Sci 27:652–658
Boison D, Scheurer L, Zumsteg V, Rulicke T, Litynski P, Fowler B, Brandner S, Mohler H (2002) Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci U S A 99:6985–6990
Boison D, Chen JF, Fredholm BB (2010) Adenosine signaling and function in glial cells. Cell Death Differ 17:1071–1082
Boison D, Masino SA, Geiger JD (2011) Homeostatic bioenergetic network regulation—a novel concept to avoid pharmacoresistance in epilepsy. Expert Opin Drug Discov 6:713–724
Brundege JM, Diao L, Proctor WR, Dunwiddie TV (1997) The role of cyclic AMP as a precursor of extracellular adenosine in the rat hippocampus. Neuropharmacology 36:1201–1210
Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, Müller C, Woods AS, Hope BT, Ciruela F, Casadó V, Canela EI, Lluis C, Goldberg SR, Moratalla R, Franco R, Ferré S (2007) Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology 32:2249–2259
Carswell HV, Graham DI, Stone TW (1997) Kainate-evoked release of adenosine from the hippocampus of the anaesthetised rat: possible involvement of free radicals. J Neurochem 68:240–247
Cascalheira JF, Sebastião AM (1992) Adenine nucleotide analogues, including gamma-phosphate-substituted analogues, are metabolised extracellularly in innervated frog sartorius muscle. Eur J Pharmacol 222:49–59
Ciccarelli R, Di Iorio P, Bruno V, Battaglia G, D’Alimonte I, D’Onofrio M, Nicoletti F, Caciagli F (1999) Activation of A(1) adenosine or mGlu3 metabotropic glutamate receptors enhances the release of nerve growth factor and S-100beta protein from cultured astrocytes. Glia 27:275–281
Ciruela F, Saura C, CanelaEI MJ, Lluis C, Franco R (1996) Adenosine deaminase affects ligand-induced signaling by interacting with cell surface adenosine receptors. FEBS Lett 380:219–223
Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortés A, Canela EI, López-Giménez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R (2006) Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci 26:2080–2087
Coelho JE, Faas GC, de Mendonça A, Ribeiro JA, Saggau P (2002) Effects of hypoxia on calcium signaling in excitatory presynaptic terminals of rat hippocampal slices. Society for Neuroscience, Orlando, Fl
Costenla AR, Diógenes MJ, Canas PM, Rodrigues RJ, Nogueira C, Maroco J, Agostinho PM, Ribeiro JA, Cunha RA, de Mendonça A (2011) Enhanced role of adenosine A(2A) receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur J Neurosci 34:12–21
Crawley JN, Patel J, Marangos PJ (1983) Adenosine uptake inhibitors potentiate the sedative effects of adenosine. Neurosci Lett 36:169–174
Cristóvão-Ferreira S, Vaz SH, Ribeiro JA, Sebastião AM (2009) Adenosine A2A receptors enhance GABA transport into nerve terminals by restraining PKC inhibition of GAT-1. J Neurochem 10:336–347
Cristóvão-Ferreira S, Navarro G, Brugarolas M, Pérez-Capote K, Vaz SH, Fattorini G, Conti F, Lluis C, Ribeiro JA, McCormick PJ, Casadó V, Franco R, Sebastião AM (2011) Adenosine A1R-A2AR heteromers modulate GAT-1- and GAT-3-mediated GABA uptake by astrocytes. J Neurosci 31:15629–15639
Cui XA, Singh B, Park J, Gupta RS (2009) Subcellular localization of adenosine kinase in mammalian cells: the long isoform of AdK is localized in the nucleus. Biochem Biophys Res Commun 388:46–50
Cunha RA, Ribeiro JA (2000) Purinergic modulation of [(3)H]GABA release from rat hippocampal nerve terminals. Neuropharmacology 39:1156–1167
Cunha RA, Johansson B, Fredholm BB, Ribeiro JA, Sebastião AM (1995) Adenosine A2A receptors stimulate acetylcholine release from nerve terminals of the rat hippocampus. Neurosci Lett 196:41–44
Cunha RA, Correia-de-Sá P, Sebastião AM, Ribeiro JA (1996) Preferential activation of excitatory adenosine receptors at rat hippocampal and neuromuscular synapses by adenosine formed from released adenine nucleotides. Br J Pharmacol 119:253–260
Cunha RA, Sebastião AM, Ribeiro JA (1998) Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ecto-nucleotidases into adenosine and channeling to adenosine A1 receptors. J Neurosci 18:1987–1995
Cunha RA, Brendel P, Zimmermann H, Ribeiro JA (2000) Immunologically distinct isoforms of ecto-5′-nucleotidase in nerve terminals of different areas of rat hippocampus. J Neurochem 74:334–338
de la Haba G, Cantoni GL (1959) The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem 234:603–608
de Mendonça A, Sebastião AM, Ribeiro JA (1995) Inhibition of NMDA receptor-mediated currents in isolated rat hippocampal neurones by adenosine A1 receptor activation. Neuroreport 6:1097–1100
Delaney SM, Shepel PN, Geiger JD (1998) Levels of endogenous adenosine in rat striatum. I. Regulation by ionotropic glutamate receptors, nitric oxide and free radicals. J Pharmacol Exp Ther 285:561–567
Delicado EG, Rodrigues A, Sen RP, Sebastiao AM, Ribeiro JA, Miras-Portugal MT (1990) Effect of 5′-(N-ethylcarboxamido)adenosine on adenosine transport in cultured chromaffin cells. J Neurochem 54:1941–1946
Deussen A, Lloyd HG, Schrader J (1989) Contribution of S-adenosylhomocysteine to cardiac adenosine formation. J Mol Cell Cardiol 21:773–782
Dias RB, Ribeiro JA, Sebastião AM (2012) Enhancement of AMPA currents and GluR1 membrane expression through PKA-coupled adenosine A(2A) receptors. Hippocampus 22:276–291
Diógenes MJ, Fernandes CC, Sebastião AM, Ribeiro JA (2004) Activation of adenosine A2A receptor facilitates brain-derived neurotrophic factor modulation of synaptic transmission in hippocampal slices. J Neurosci 24:2905–2913
Diógenes MJ, Assaife-Lopes N, Pinto-Duarte A, Ribeiro JA, Sebastião AM (2007) Influence of age on BDNF modulation of hippocampal synaptic transmission: interplay with adenosine A2A receptors. Hippocampus 17:577–585
Diógenes MJ, Costenla AR, Lopes LV, Jerónimo-Santos A, Sousa VC, Fontinha BM, Ribeiro JA, Sebastião AM (2011) Enhancement of LTP in aged rats is dependent on endogenous BDNF. Neuropsychopharmacology 36:1823–1836
Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC (1996) Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol 118:1461–1468
Dragunow M, Goddard GV (1984) Adenosine modulation of amygdala kindling. Exp Neurol 84:654–665
Dunwiddie TV, Diao L (1994) Extracellular adenosine concentrations in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. J Pharmacol Exp Ther 268:537–545
Dunwiddie TV, Masino SA (2001) The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24:31–55
Dunwiddie TV, Taylor M, Heginbotham LR, Proctor WR (1992) Long-term increases in excitability in the CA1 region of rat hippocampus induced by beta-adrenergic stimulation: possible mediation by cAMP. J Neurosci 12:506–517
Dunwiddie TV, Diao L, Proctor WR (1997) Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci 17:7673–7682
Faure M, Voyno-Yasenetskaya TA, Bourne HR (1994) cAMP and beta gamma subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J Biol Chem 269:7851–7854
Fernandes CC, Pinto-Duarte A, Ribeiro JA, Sebastião AM (2008) Postsynaptic action of brain-derived neurotrophic factor attenuates alpha7 nicotinic acetylcholine receptor-mediated responses in hippocampal interneurons. J Neurosci 28:5611–5618
Ferré S, Popoli P, Giménez-Llort L, Finnman U-B, Martínez E, Scotti de Carolis A, Fuxe K (1994) Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. Neuroreport 6:73–76
Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K (1997) Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci 20:482–487
Ferré S, Torvinen M, Antoniou K, Irenius E, Civelli O, Arenas E, Fredholm BB, Fuxe K (1998) Adenosine A1 receptor-mediated modulation of dopamine D1 receptors in stably cotransfected fibroblast cells. J Biol Chem 273:4718–4724
Ferré S, Lluís C, Justinova Z, Quiroz C, Orru M, Navarro G, Canela EI, Franco R, Goldberg SR (2010) Adenosine-cannabinoid receptor interactions. Implications for striatal function. Br J Pharmacol 160:443–453
Ferreira JM, Paes-de-Carvalho R (2001) Long-term activation of adenosine A(2a) receptors blocks glutamate excitotoxicity in cultures of avian retinal neurons. Brain Res 900:169–176
Fields RD, Burnstock G (2006) Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci 7:423–436
Fontinha BM, Diógenes MJ, Ribeiro JA, Sebastião AM (2008) Enhancement of long-term potentiation by brain-derived neurotrophic factor requires adenosine A2A receptor activation by endogenous adenosine. Neuropharmacology 54:924–933
Fontinha BM, Delgado-García JM, Madroñal N, Ribeiro JA, Sebastião AM, Gruart A (2009) Adenosine A(2A) receptor modulation of hippocampal CA3-CA1 synapse plasticity during associative learning in behaving mice. Neuropsychopharmacology 34:1865–1874
Fossier P, Tauc L, Baux G (1999) Calcium transients and neurotransmitter release at an identified synapse. Trends Neurosci 22:161–166
Franco R, Valenzuela A, Lluis C, Blanco J (1998) Enzymatic and extraenzymatic role of ecto-adenosine deaminase in lymphocytes. Immunol Rev 161:27–42
Fredholm BB, Dunér-Engström M, Fastbom J, Hu PS, van der Ploeg I (1990) Role of G proteins, cyclic AMP, and ion channels in the inhibition of transmitter release by adenosine. Ann N Y Acad Sci 604:276–288
Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552
Frenguelli BG, Wigmore G, Llaudet E, Dale N (2007) Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem 101:1400–1413
Geiger JD, Fyda DM (1991) Adenosine transport in nervous tissues. In: Stone TW (ed) Adenosine in the nervous system. Academic, London, pp 1–23
Ginés S, Hillion J, Torvinen M, Le Crom S, Casadó V, Canela EI, Rondin S, Lew JY, Watson S, Zolii M, Agnatii LF, Vernie P, Lluis C, Ferré S, Fuxe K, Franco R (2000) Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci U S A 97:8606–8611
Gomes CA, Vaz SH, Ribeiro JA, Sebastião AM (2006) Glial cell line-derived neurotrophic factor (GDNF) enhances dopamine release from striatal nerve endings in an adenosine A2A receptor-dependent manner. Brain Res 1113:129–136
Gomes CA, Simões PF, Canas PM, Quiroz C, Sebastião AM, Ferré S, Cunha RA, Ribeiro JA (2009) GDNF control of the glutamatergic cortico-striatal pathway requires tonic activation of adenosine A receptors. J Neurochem 108:1208–1219
Gonzalez B, Paz F, Florán L, Aceves J, Erlij D, Florán B (2006) Adenosine A2A receptor stimulation decreases GAT-1-mediated GABA uptake in the globus pallidus of the rat. Neuropharmacology 51:154–159
Gouder N, Scheurer L, Fritschy JM, Boison D (2004) Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci 24:692–701
Gracia E, Cortés A, Meana JJ, García-Sevilla J, Herhsfield MS, Canela EI, Mallol J, Lluís C, Franco R, Casadó V (2008) Human adenosine deaminase as an allosteric modulator of human A(1) adenosine receptor: abolishment of negative cooperativity for [H](R)-pia binding to the caudate nucleus. J Neurochem 107:161–170
Gu JG, Foga IO, Parkinson FE, Geiger JD (1995) Involvement of bidirectional adenosine transporters in the release of L-[3H]adenosine from rat brain synaptosomal preparations. J Neurochem 64:2105–2107
Gutkind JS (1998) The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem 273:1839–1842
Hamilton NB, Attwell D (2010) Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11:227–238
Harvey J, Lacey MG (1997) A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci 17:5271–5280
Haun SE, Segeleon JE, Trapp VL, Clotz MA, Horrocks LA (1996) Inosine mediates the protective effect of adenosine in rat astrocyte cultures subjected to combined glucose-oxygen deprivation. J Neurochem 67:2051–2059
Heese K, Fiebich BL, Bauer J, Otten U (1997) Nerve growth factor (NGF) expression in rat microglia is induced by adenosine A2a-receptors. Neurosci Lett 231:83–86
Herrera C, Casadó V, Ciruela F, Schofield P, Mallol J, Lluís C, Franco R (2001) Adenosine A2B receptors behave as an alternative anchoring protein for cell surface adenosine deaminase in lymphocytes and cultured cells. Mol Pharmacol 59:127–134
Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, Canela EI, Zoli M, Agnati LF, Ibanez CF, Lluis C, Franco R, Ferre S, Fuxe K (2002) Coaggregation, cointernalization and codesensitization of adenosine A2A eceptors and dopamine D2 receptors. J Biol Chem 277:18091–18097
Hirano D, Aoki Y, Ogasawara H, Kodama H, Waga I, Sakanaka C, Shimizu T, Nakamura M (1996) Functional coupling of adenosine A2a receptor to inhibition of the mitogen-activated protein kinase cascade in Chinese hamster ovary cells. Biochem J 316:81–86
Huber A, Padrun V, Déglon N, Aebischer P, Möhler H, Boison D (2001) Grafts of adenosine-releasing cells suppress seizures in kindling epilepsy. Proc Natl Acad Sci U S A 98:7611–7616
Illes P, Ribeiro JA (2004) Neuronal P2 receptors of the central nervous system. Curr Top Med Chem 4:831–838
Kermer V, Ritter M, Albuquerque B, Leib C, Stanke M, Zimmermann H (2010) Knockdown of tissue nonspecific alkaline phosphatase impairs neural stem cell proliferation and differentiation. Neurosci Lett 485:208–211
Klaasse EC, Ijzerman AP, de Grip WJ, Beukers MW (2008) Internalization and desensitization of adenosine receptors. Purinergic Signal 4:21–37
Kowaluk EA, Jarvis MF (2000) Therapeutic potential of adenosine kinase inhibitors. Expert Opin Investig Drugs 9:551–564
Kreutzberg GW, Barron KD, Schubert P (1978) Cytochemical localization of 5′-nucleotidasein glial plasma membranes. Brain Res 158:247–257
Langer D, Ikehara Y, Takebayashi H, Hawkes R, Zimmermann H (2007) The ectonucleotidases alkaline phosphatase and nucleoside triphosphate diphosphohydrolase 2 are associated with subsets of progenitor cell populations in the mouse embryonic, postnatal and adult neurogenic zones. Neuroscience 150:863–879
Lee FS, Chao MV (2001) Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci U S A 98:3555–3560
Lerner TN, Horne EA, Stella N, Kreitzer AC (2010) Endocannabinoid signaling mediates psychomotor activation by adenosine A2A antagonists. J Neurosci 30:2160–2164
Liebmann C (2001) Regulation of MAP kinase activity by peptide receptor signaling pathway: paradigms of multiplicity. Cell Signal 13:777–785
Lin Y, Phillis JW (1992) Deoxycoformycin and oxypurinol: protection against focal ischemic brain injury in the rat. Brain Res 571:272–280
Lloyd HG, Fredholm BB (1995) Involvement of adenosine deaminase and adenosine kinase in regulating extracellular adenosine concentration in rat hippocampal slices. Neurochem Int 26:387–389
Lopes LV, Cunha RA, Kull B, Fredholm BB, Ribeiro JA (2002) Adenosine A(2A) receptor facilitation of hippocampal synaptic transmission is dependent on tonic A(1) receptor inhibition. Neuroscience 112:319–329
Luttrell LM, Daaka Y, Lefkowitz RJ (1999) Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol 11:177–183
Lynch JJ 3rd, Alexander KM, Jarvis MF, Kowaluk EA (1998) Inhibition of adenosine kinase during oxygen-glucose deprivation in rat cortical neuronal cultures. Neurosci Lett 252:207–210
Maienshein V, Zimmermann H (1996) Immunocytochemical localization of ecto-5′-nucleotidase in cultures of cerebellar granule cells. Neuroscience 70:429–438
Major PP, Agarwal RP, Kufe DW (1981) Clinical pharmacology of deoxycoformycin. Blood 58:91–96
Manzoni OJ, Manabe T, Nicoll RA (1994) Release of adenosine by activation of NMDA receptors in the hippocampus. Science 265:2098–2101
Marchi M, Raiteri L, Risso F, Vallarino A, Bonfanti A, Monopoli A, Ongini E, Raiteri M (2002) Effects of adenosine A1 and A2A receptor activation on the evoked release of glutamate from rat cerebrocortical synaptosomes. Br J Pharmacol 136:434–440
Marinissen MJ, Gutkind JS (2001) G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci 22:368–376
Meghji P (1993) Storage, release, uptake and inactivation of purines. Drug Dev Res 28:214–219
Meghji P, Newby AC (1990) Sites of adenosine formation, action and inactivation in the brain. Neurochem Int 16:227–232
Misumi Y, Ogata S, Ohkubo K, Hirose S, Ikehara Y (1990) Primary structure of human placental 5′-nucleotidase and identification of the glycolipid anchor in the mature form. Eur J Biochem 191:563–569
Montero JM, Fes JB (1982) Purification and characterization of bovine brain 5′-nucleotidase. J Neurochem 39:982–989
Moshe SL (2000) Seizures early in life. Neurology 55(suppl 1):S15–S20
Motley SJ, Collins GG (1983) Endogenous adenosine inhibits excitatory transmission in the rat olfactory cortex slice. Neuropharmacology 22:1081–1086
Mrhul B, Aubery M, Mannherz HG, Codogno P (1993) Dual mechanism of laminin modulation of ecto-5′-nucleotidase activity. J Cell Biochem 52:266–274
Nagy JI, LaBella LA, Buss M, Daddona PE (1984) Immunohistochemistry of adenosine deaminase: implications for adenosine neurotransmission. Science 224:166–168
Naidoo D (1962) The activity of 5′-nucleotidase determined histochemically in the developing rat brain. J Histochem Cytochem 10:421–434
Neary JT, McCarthy M, Kang Y, Zuniga S (1998) Mitogenic signaling from P1 and P2 purinergic receptors to mitogen-activated protein kinase in human fetal astrocyte cultures. Neurosci Lett 242:159–162
Newby AC (1984) Adenosine and the concept of “retaliatory metabolites”. Trends Biochem Sci 9:42–44
Newby AC, Worku Y, Holmquist CA (1985) Adenosine formation. Evidence for a direct biochemical link with energy metabolism. Adv Myocardiol 6:273–284
Olmo N, Turnay J, Risse G, Deutzmann R, vonder Mark K, Lizarbe A (1992) Modulation of 5′-nucleotidase activity in plasma membranes and intact cells by the extracellular matrix proteins laminin and fibronectin. Biochem J 282:181–188
Pak MA, Haas HL, Decking UK, Schrader J (1994) Inhibition of adenosine kinase increases endogenous adenosine and depresses neuronal activity in hippocampal slices. Neuropharmacology 33:1049–1053
Palmer JL, Abeles RH (1979) The mechanism of action of S-adenosylhomocysteinase. J Biol Chem 254:1217–1226
Parkinson FE, Rudolphi KA, Fredholm BB (1994) Propentofylline: a nucleoside transport inhibitor with neuroprotective effects in cerebral ischemia. Gen Pharmacol 25:1053–1058
Parkinson FE, Sinclair CJ, Othman T, Haughey NJ, Geiger JD (2002) Differences between rat primary cortical neurons and astrocytes in purine release evoked by ischemic conditions. Neuropharmacology 43:836–846
Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431
Perez DM, Karnik SS (2005) Multiple signaling states of G-protein-coupled receptors. Pharmacol Rev 57:147–161
Phillis JW (1989) Adenosine in the control of the cerebral circulation. Cerebrovasc Brain Metab Rev 1:26–54
Phillis JW, O’Regan MH (1989) Deoxycoformycin antagonizes ischemia-induced neuronal degeneration. Brain Res Bull 22:537–540
Phillis JW, O’Regan MH, Walter GA (1989) Effects of two nucleoside transport inhibitors, dipyridamole and soluflazine, on purine release from the rat cerebral cortex. Brain Res 481:309–316
Pignataro G, Simon RP, Boison D (2007) Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J Cereb Blood Flow Metab 27:1–5
Pinto-Duarte A, Coelho JE, Cunha RA, Ribeiro JA, Sebastião AM (2005) Adenosine A2A receptors control the extracellular levels of adenosine through modulation of nucleoside transporters activity in the rat hippocampus. J Neurochem 93:595–604
Potenza RL, Tebano MT, Martire A, Domenici MR, Pepponi R, Armida M, Pèzzola A, Minghetti L, Popoli P (2007) Adenosine A(2A) receptors modulate BDNF both in normal conditions and in experimental models of Huntington’s disease. Purinergic Signal 3:333–338
Pousinha PA, Diogenes MJ, Ribeiro JA, Sebastião AM (2006) Triggering of BDNF facilitatory action on neuromuscular transmission by adenosine A2A receptors. Neurosci Lett 404:143–147
Radulovacki M, Virus RM, Djuricic-Nedelson M, Green RD (1983) Hypnotic effects of deoxycorformycin in rats. Brain Res 271:392–395
Rajagopal R, Chen ZY, Lee FS, Chao MV (2004) Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci 24:6650–6658
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Rebola N, Lujan R, Cunha RA, Mulle C (2008) Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 57:121–134
Reichard P (1988) Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem 57:349–374
Resta R, YamashitaY TLF (1998) Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev 161:95–109
Ribeiro JA (1978) ATP; related nucleotides and adenosine on neurotransmission. Life Sci 22:1373–1380
Ribeiro JA (2005) What can adenosine neuromodulation do for neuroprotection? Curr Drug Targets CNS Neurol Disord 4:325–329
Ribeiro JA, Sebastião AM (1987) On the role, inactivation and origin of endogenous adenosine at the frog neuromuscular junction. J Physiol 384:571–585
Ribeiro JA, Sebastião AM (2010) Modulation and metamodulation of synapses by adenosine. Acta Physiol 199:161–169
Ribeiro JA, Sebastião AM, de Mendonça A (2002) Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol 68:377–392
Richardson PJ, Brown SJ (1987) ATP release from affinity-purified cholinergic nerve terminals. J Neurochem 48:622–630
Rosenberg PA, Li Y (1995) Adenylyl cyclase activation underlies intracellular cyclic AMP accumulation, cyclic AMP transport, and extracellular adenosine accumulation evoked by beta-adrenergic receptor stimulation in mixed cultures of neurons and astrocytes derived from rat cerebral cortex. Brain Res 692:227–232
Ruíz MA, Escriche M, Lluís C, Franco R, Martín M, Andrés A, Ros M (2000) Adenosine A(1) receptor in cultured neurons from rat cerebral cortex: colocalization with adenosine deaminase. J Neurochem 75:656–664
Ryzhov S, Goldstein AE, Biaggioni I, Feoktistov I (2006) Cross-talk between G(s)- and G(q)-coupled pathways in regulation of interleukin-4 by A(2B) adenosine receptors in human mast cells. Mol Pharmacol 70:727–735
Sanderson G, Scholfield CN (1986) Effects of adenosine uptake blockers and adenosine on evoked potentials of guinea-pig olfactory cortex. Pflugers Arch 406:25–30
Saura CA, Mallol J, Canela EL, Lluis C, Franco R (1998) Adenosine deaminase and A1 adenosine receptors internalize together following agonist-induced receptor desensitization. J Biol Chem 273:17610–17617
Schoen SW, Kreutzberg GW (1995) Evidence that 5′-nucleotidaseis associated with malleable synapses. An enzyme cytochemical investigation of the olfactory bulb of adult rats. Neuroscience 65:37–50
Schoen SW, Graeber MB, Toth L, Kreutzberg GW (1991) Synaptic 5′-nucleotidase is transient and indicative of climbing fiber plasticity during the postnatal development of rat cerebellum. Brain Res Dev Brain Res 61:125–138
Schoen SW, Kreutzberg GW, Singer W (1993) Cytochemical redistribution of 5′-nucleotidase in the developing cat visual cortex. Eur J Neurosci 5:210–222
Schrader J, Schütz W, Bardenheuer H (1981) Role of S-adenosylhomocysteine hydrolase in adenosine metabolism in mammalian heart. Biochem J 196:65–70
Schulte G, Fredholm BB (2000) Human adenosine A(1), A(2A), A(2B), and A(3) receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol Pharmacol 58:477–842
Schulte G, Fredholm BB (2003) Signaling from adenosine receptors to mitogen-activated protein kinases. Cell Signal 15:813–827
Sebastião AM (2011) Neuronal ENT1 takes up synaptic adenosine even under hypoxia/ischemia. J Neurochem 118:1–3
Sebastião AM, Ribeiro JA (1996) Adenosine A2 receptor-mediated excitatory actions on the nervous system. Prog Neurobiol 48:167–189
Sebastião AM, Ribeiro JA (2000) Fine-tuning neuromodulation by adenosine. Trends Pharmacol Sci 21:341–346
Sebastião AM, Ribeiro JA (2009a) Adenosine receptors and the central nervous system. Handb Exp Pharmacol 193:471–534
Sebastião AM, Ribeiro JA (2009b) Tuning and fine-tuning synapses with adenosine. Curr Neuropharmacol 7:180–194
Sebastião AM, Ribeiro JA (2009c) Triggering neurotrophic factor actions through adenosine A2A receptor activation: implications for neuroprotection. Br J Pharmacol 158:15–22
Sebastião AM, de Mendonca A, Moreira T, Ribeiro JA (2001) Activation of synaptic NMDA receptors by action potential-dependent release of transmitter during hypoxia impairs recovery of synaptic transmission on reoxygenation. J Neurosci 21:8564–8571
Seger R, Krebs EG (1995) The MAPK signaling cascade. FASEB J 9:726–735
Seidel MG, Klinger M, Freissmuth M, Höller C (1999) Activation of mitogen-activated protein kinase by the A(2A)-adenosine receptor via a rap1-dependent and via a p21(ras)-dependent pathway. J Biol Chem 274:25833–25841
Sexl V, Mancusi G, Höller C, Gloria-Maercker E, Schütz W, Freissmuth M (1997) Stimulation of the mitogen-activated protein kinase via the A2A-adenosine receptor in primary human endothelial cells. J Biol Chem 272:5792–5799
Shen H, Chen GJ, Harvey BK, Bickford PC, Wang Y (2005) Inosine reduces ischemic brain injury in rats. Stroke 36:654–659
Shen HY, Lusardi TA, Williams-Karnesky RL, Lan JQ, Poulsen DJ, Boison D (2011) Adenosine kinase determines the degree of brain injury after ischemic stroke in mice. J Cereb Blood Flow Metab 31:1648–1659
Sousa VC, Assaife-Lopes N, Ribeiro JA, Pratt JA, Brett RR, Sebastião AM (2011) Regulation of hippocampal cannabinoid CB1 receptor actions by adenosine A1 receptors and chronic caffeine administration: implications for the effects of Δ9-tetrahydrocannabinol on spatial memory. Neuropsychopharmacology 36:472–487
Sowa NA, Taylor-Blake B, Zylka MJ (2010) Ecto-5′-nucleotidase (CD73) inhibits nociception by hydrolyzing AMP to adenosine in nociceptive circuits. J Neurosci 30:2235–2244
Stevens B, Porta S, Haak LL, Gallo V, Fields RD (2002) Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36:855–868
Stevens B, Ishibashi T, Chen JF, Fields RD (2004) Adenosine: an activity-dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron Glia Biol 1:23–34
Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, Fritschy JM, Boison D (2006) Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience 142:125–137
Sugden PH, Clerk A (1998) Regulation of mitogen-activated protein kinase cascades in the heart. Adv Enzyme Regul 38:87–98
Tebano MT, Martire A, Potenza RL, Grò C, Pepponi R, Armida M, Domenici MR, Schwarzschild MA, Chen JF, Popoli P (2008) Adenosine A(2A) receptors are required for normal BDNF levels and BDNF-induced potentiation of synaptic transmission in the mouse hippocampus. J Neurochem 104:279–286
Theofilas P, Brar S, Stewart KA, Shen HY, Sandau US, Poulsen D, Boison D (2011) Adenosine kinase as a target for therapeutic antisense strategies in epilepsy. Epilepsia 52:589–601
Thevananther S, Rivera A, Rivkees SA (2001) A1 adenosine receptor activation inhibits neurite process formation by Rho kinase-mediated pathways. Neuroreport 12:3057–3063
Vaz SH, Cristóvão-Ferreira S, Ribeiro JA, Sebastião AM (2008) Brain-derived neurotrophic factor inhibits GABA uptake by the rat hippocampal nerve terminals. Brain Res 1219:19–25
Vaz SH, Jørgensen TN, Cristóvão-Ferreira S, Duflot S, Ribeiro JA, Gether U, Sebastião AM (2011) Brain-derived neurotrophic factor (BDNF) enhances GABA transport by modulating the trafficking of GABA transporter-1 (GAT-1) from the plasma membrane of rat cortical astrocytes. J Biol Chem 286:40464–40476
Wiese S, Jablonka S, Holtmann B, Orel N, Rajagopal R, Chao MV, Sendtner M (2007) Adenosine receptor A2A-R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proc Natl Acad Sci U S A 104:17210–17215
Yamagata K, Hakata K, Maeda A, Mochizuki C, Matsufuji H, Chino M, Yamori Y (2007) Adenosine induces expression of glial cell line-derived neurotrophic factor (GDNF) in primary rat astrocytes. Neurosci Res 59:467–474
Yarbrough GG, McGuffin-Clineschmidt JC (1981) In vivo behavioral assessment of central nervous system purinergic receptors. Eur J Pharmacol 76:137–144
Yegutkin GG (2008) Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783:673–694
Zamzow CR, Xiong W, Parkinson FE (2008a) Astrocytes affect the profile of purines released from cultured cortical neurons. J Neurosci Res 86:2641–2649
Zamzow CR, Xiong W, Parkinson FE (2008b) Adenosine produced by neurons is metabolized to hypoxanthine by astrocytes. J Neurosci Res 86:3447–3455
Zhang D, Xiong W, Albensi BC, Parkinson FE (2011) Expression of human equilibrative nucleoside transporter 1 in mouse neurons regulates adenosine levels in physiological and hypoxic-ischemic conditions. J Neurochem 118:4–11
Zimmermann H (2011) Purinergic signaling in neural development. Semin Cell Dev Biol 22:194–204
Zimmermann H, Grondal EJM, Keller F (1986) Hydrolysis of ATP and formation of adenosine at the surface of cholinergic nerve endings. In: Kreutzberg GW, Reddington M, Zimmermann H (eds) Cellular biology of ectoenzymes. Springer, Berlin, pp 35–48
Acknowledgments
The work in the authors’ laboratory is supported by research grants from Faculdade de Medicina, Fundação para a Ciência e Tecnologia (FCT), Gulbenkian Foundation, and the European Union (COST B30).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Sebastião, A.M., Cristóvão-Ferreira, S., Ribeiro, J.A. (2013). Downstream Pathways of Adenosine. In: Masino, S., Boison, D. (eds) Adenosine. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-3903-5_7
Download citation
DOI: https://doi.org/10.1007/978-1-4614-3903-5_7
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-3902-8
Online ISBN: 978-1-4614-3903-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)