Abstract
Use of biochar fertilizer is potentially an attractive approach for soil amendment and carbon sequestration possibly at giga tons of carbon (GtC) scale. Cation exchange capacity (CEC) is an important parameter in retaining inorganic nutrients, such as K+ and NH +4 in soil. This experimental study showed that the CEC value of biochar is related to the biomass pyrolysis temperature. Biochar materials made from the pelletized peanut hulls at pyrolysis temperature of about 400C yield the best CEC value. As the pyrolysis temperature increases over 400C, the CEC value decreases. The biochar produced from the 400C pyrolysis possesses certain binding affinity for ammonium bicarbonate (NH4HCO3) probably because of the presence of more biochar surface functional groups. Addition of ammonium bicarbonate to biochar can help neutralize the pH of biochar material potentially beneficial for certain agricultural soil applications in relation to soil amendment and carbon sequestration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The world currently faces a systematic problem of increased CO2 emissions, decreased soil-carbon content, and global-climate change (global warming). The mean global atmospheric CO2 concentration has increased from 280 ppm in the 1,700 s to 380 ppm in 2005 at a progressively faster rate [1] because of: (1) CO2 emissions from fossil-fuel use; and (2) the CO2 flux from land-use change, including land clearing such as “slash and burn,” agriculture and intensive tillage. In certain areas, agriculture and intensive tillage have also caused a 30–50% decrease in soil organic carbon (SOC) since many soils were brought into cultivation more than 100 years ago [2]. To solve this massive global energy and environmental sustainability problem, it likely requires a comprehensive portfolio of R&D efforts with multiple energy technologies. Application of a modern smokeless biomass pyrolysis process for producing biofuels and biochar is possibly a significant approach for global carbon capture and sequestration [3]. This “carbon-negative” biomass-pyrolysis energy-production concept of applying biochar as a soil amendment and carbon sequestration agent was initiated in 2002 by two of us (Day and Lee) with a provisional US patent application followed by a PCT application [4]. Certain related studies, including biochar-related soil research, have also indicated the possibility of using biochar as a soil amendment for carbon sequestration [5–7]. According to our preliminary analysis, global use of biochar as soil amendment could potentially achieve carbon sequestration at giga tons of carbon (GtC) scale [8].
Globally, each year, there are about 6.6 Gt dry matter of biomass (3.3 GtC), such as crop stovers, that are appropriated but not used [9]. If this amount of biomass (3.3 Gt y−1) is processed through controlled pyrolysis assuming 50% conversion of biomass C to stable biochar C and 33% of the biomass energy to crude biofuels (syngas and biooils), it could produce biochar (1.65 GtC y−1) and crude biofuels (with heating value equivalent to that of 6,500 million barrels of crude oil). By storing 1.65 GtC y−1 of biochar (equivalent to 6 Gt of CO2) into soil and/or underground reservoirs alone, it could offset the world’s 8.67 GtC y−1 of fossil-fuel CO2 emissions by 19%, which is quite significant. According to a recent life-cycle assessment [10], for each ton of dry waste biomass utilized through biomass pyrolysis with biochar returned to soil, it could provide a net sequestration of about 800–900 kg of CO2 emissions (per ton of dry biomass). The life-cycle assessment also indicated that the biochar-producing biomass pyrolysis technology could be operated profitably if/when CO2 emission reductions are valued at or above about $60 ton−1 of CO2 equivalent emissions. Therefore, the envisioned photosynthetic biomass production and biofuel/biochar-producing biomass-pyrolysis approach should be considered as an option to mitigate the problem of global greenhouse-gas emissions.
Putting biochar into soil can potentially improve soil fertility and reduce fertilizer runoff to benefit the soil and water environment in agricultural/forest watersheds. However, the biochar C itself is not a crop nutrient except its ash contents which can serve as mineral nutrients for crop growth. It is probably better to apply biochar along with certain fertilizers, such as NH4HCO3 and/or urea, to achieve maximal environmental and agricultural benefits [11]. One of the options is to produce a biochar-NH4HCO3 (or biochar-urea) compound fertilizer that may make the biochar materials more suitable to stimulate plant growth and to maximally place the carbon of biochar and bicarbonate HCO −3 into soils [3, 12]. As illustrated in Fig. 1, the ammonia-carbonation-based scrubbing technology process [13] can provide an option to integrate biomass pyrolysis with major industrial combustion facilities, such as a coal-fired power plant to solidify major flue-gas CO2 emission and ppm levels of NO x and SO x emissions at the smokestacks into valuable fertilizers (mainly, NH4HCO3 with trace amount of other fertilizer species, such as NH4NO3 and (NH4)2SO4) with biochar particles to produce a biochar-NH4HCO3 and/or biochar-urea fertilizer which could not only benefit agriculture, but also sequester carbon into soils for protecting the global environment [3, 14]. Using biochar samples produced by Eprida from peanut hulls, we performed certain experimental studies of the biochar materials with an ammonia carbonation process and cation exchange capacity (CEC) assays. In this paper, we report certain experimental studies of designer biochar production from peanut hulls and test the biochar materials with an ammonia carbonation process in relation to the possible application of biochar for soil amendment and carbon sequestration.
This figure presents a conceptual design as an option to remove CO2 emissions in industrial combustion facilities, such as a coal-fired power plant by flexible combinations of biomass pyrolysis and ammonia scrubbing. This CO2-solidifying technology produces valuable soil amendment fertilizer products, such as NH4HCO3-char, that could be placed into soil and subsoil terrains through intelligent agricultural practice for soil amendment and carbon sequestration
2 Experimental Materials and Methods
2.1 Biochar Materials Made with Temperature Variation
All of the charcoal samples were made in Omegalux LMF-3550 oven. A special box was constructed to allow preheated steam to flow through the charring material. The oven and steam flow settings for the charcoal production were: steam flow of 2.0 kg h−1, with oven and steam temperature settings of 365, 385, 408, and 435°C. The material was removed from the oven when the temperature of the charcoal reached a maximum and stabilized for 5 min. The temperatures that were reached are: peanut hulls-371, 402, 426, 442°C. The batch system has been described in greater detail previously [15]. All of the char samples were crushed in a roller crusher and sieved to a size fraction of −850 +420 μm.
2.2 Cation Exchange Capacity Assay Protocol
CEC analysis was performed using the following method: The ground char sample was thoroughly mixed and 2 g was placed in a 250-mL Erlenmeyer flask. Hundred milliliters of 0.5 N HCl was added, the flask was covered with parafilm and shaken vigorously periodically for 2 h. Sample was filtered using a glass fiber filter in a Buchner funnel, washing with 100 mL portions of H2O until wash shows no precipitate with AgNO3. Filtrate was discarded. Moist char was immediately transferred to a clean 250-mL Erlenmeyer flask and a total of 100 mL 0.5 N Ba(OAc)2 was added and a stopper placed on the flask. The mixture was shaken vigorously periodically for 1 h and was filtered, washing with three 100 mL portions H2O. The char was discarded, and the filtrate was titrated with 0.0714 N NaOH using phenolphthalein to first pink. The following equation was used to calculate the CEC value:
2.3 Designer Biochar Material
Previous research has shown that a 400°C pyrolysis process produced a preferred biochar for agriculture and ammonia adsorption [3]. A 400°C biochar sample was produced by Day using pyrolysis of pelletized peanut hulls at 400°C. Specifically, the cross-draft reactor was brought up to 400°C empty with the exhaust from natural gas burner. Then, pelletized peanut hulls (biomass) were slowly fed in maintaining that internal reactor temperature. Once the biochar discharge sensor reached 400°C, the rotary discharge valve released biochar, the burner was switched off and the system operated without any air or outside heat. This point coincides near the end of the exothermic zone for peanut shells. It also corresponds with the temperature range at which the resulting biochar had an increased fertilizer binding capacity as previously reported [3, 4]. The feed rate was controlled by the automated discharge of the biochar reaching 400°C and averaged 5–7 kg h−1. The exothermic reaction allowed continuous feed to the pyrolysis reactor; however, the feed rate was 20% of what occurs when combustion of part of the pyrolysis vapors was used to augment the natural exothermic reaction.
2.4 Test of Biochar Material with Ammonia Carbonation
The designer biochar material made from the pelletized peanut hulls at 400°C was tested with a gas-phase ammonia carbonation process which is thermodynamically favored with the standard-free energy change ( ΔG 0) of −18.05 kJ mol−1:
In the experiments, about 500 g of the biochar material were packed into the reactor as illustrated in Fig. 2 to test whether the char material has binding affinity for the NH4HCO3 formed through the gas-phase ammonia-scrubbing CO2-solidifying process which is reported in ref. [13]. Briefly, a compressed gas containing 15 vol.% of CO2 in N2 (primary standard, supplied by Air Liquid) was taken as a synthetic flue gas. The synthetic flue gas was continuously fed into the reactor through a side port at the top of the reactor with a controlled constant flow rate at about 350 mL min−1 while the tail gas flowing out of the reactor through its bottom port. To mimic the moisture condition of a real-world flue gas, which commonly contains a nearly saturated amount of water vapor, the synthetic flue gas was humidified by bubbling it through a water tank kept at 40°C in an isothermal water bath (model RMS, Lauda) before it flowed into the reactor. The concentration of CO2 in the tail gas that exited from the bottom of the reactor was monitored in-line with a digital gas analyzer (model 866, Honeywell) and recorded by DATAQ instruments. Prior to the detection of CO2 concentration, the tail gas first went through a dilute aqueous sulfuric acid solution (1 N) for the adsorption of residual NH3 and through an ice bath for the condensation of moisture. The compressed gas containing 15% (vol) of CO2 in N2 (primary standard, supplied by Air Liquid) and the pure N2 (ultrahigh purity, supplied by Air Liquid) were used to calibrate the CO2 detector prior to each experiment, using the same procedure as that used during the experiment. The NH3 gas was generated by evaporation from anhydrous liquid NH3 (purity ≥99.95%) that was purchased from Sigma-Aldrich. The flow rates of NH3 gas and flue gas were controlled by calibrated mass flow controllers (MKS Instruments, Inc.). When the initial air in the reactor was removed by the constant flow of the humid flue gas for more than 15 min and when the concentration of CO2 in the outlet gas reached a steady value, ammonia gas was then introduced through a central port located at the top of the glass tube reactor. Thus, all reactants were introduced from the top of the reactor. The ratio of NH3 flow rate to the flue-gas flow rate was adjusted to about 0.12 where the steady-state CO2 removal efficiency was about 44% for the biochar ammonia carbonation test.
3 Results and Discussions
3.1 Cation Exchange Capacity of Biochar Materials
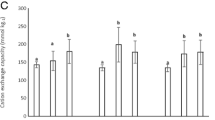
The value of CEC was measured for the biochar materials made from the pelletized peanut hulls at pyrolysis temperature of 371, 402, 426, and 442°C. As shown in Fig. 3, the measurements demonstrated that the CEC value of the biochar materials is dependent on the pyrolysis temperature. Pyrolysis temperature of 402°C yielded the highest CEC value (18.2 meq/100 g) while the pyrolysis temperatures of 371, 426, and 442°C resulted in CEC values of 17.1, 16.1, and 13.9 meq/100 g, respectively. This experimental result indicated that it is important to control pyrolysis temperature for higher CEC value of biochar product. The optimal pyrolysis temperature for high-CEC biochar production is likely at around 400°C.
Biochar cation exchange capacity (CEC) vs. pyrolysis temperature. Char samples were created at four temperatures in a range from 370 to 450°C and were analyzed for CEC. A peak value was obtained for the char sample created at 402°C with CEC values decreasing as pyrolysis temperature increases from this point
3.2 Biochar-NH4HCO3 Experimental Results
In this part of the study, we tested the compatibility between NH4HCO3 and the designer biochar material that was produced at 400°C from peanut hulls. Since the CO2-solidifying-NH4HCO3 production process occurs in a gas phase, the char particles could potentially serve as nucleation site for the formation of solid NH4HCO3 crystals thus enhancing the CO2-removal chemical engineering technology. The experimental objective was to explore the possibility of whether a compatible NH4HCO3-char fertilizer product can be created to enhance storage of carbons (both HCO −3 and biochar) into soil and subsoil earth layers and also benefit the agricultural industry. The experimental result demonstrated that the biochar material indeed has certain binding affinity for the NH4HCO3 formed in the gas-phase process. In the experiments, when ammonia carbonation was initiated by introducing ammonia gas along with the moisturized synthetic flue gas into the reactor (with about 500 g of designed biochar material), the formation of ammonium salts (solid products) appeared immediately as a fog and white material that condensed on the biochar materials. As shown in Fig. 4, the char grains after treated with the ammonia-scrubbing CO2-solidifying process became significantly whiter than the control biochar sample because of the deposition of NH4HCO3 onto the surfaces of the char grains by the process treatment.
This observation is consistent with the results of our pH measurements for the treated char, untreated char (control), and NH4HCO3-char mixture that was produced by mixing equal weight (50%/50% by weight) of NH4HCO3 and char. In the pH experiment, 1 g for each of these samples (the treated char, untreated char, and NH4HCO3-char mixture) was dissolved into 10 mL of distilled water. Then, pH values of the solutions were measured by a standard Beckman pH electrode system. As presented in Table 1, the pH measurement clearly demonstrated that the addition of NH4HCO3 can significantly neutralize the alkaline pH of the char material. The pH value of the untreated char material was 9.85 while that of the NH4HCO3-char mixture (50%/50% by weight) was 7.89. This is expected since the bicarbonate HCO −3 of NH4HCO3 can act as a pH buffer in neutralizing certain alkaline ash components in the char material. The gas-phase ammonia-carbonation treatment (by the NH3-CO2-solidifying process) reduced the pH value of the biochar from 9.85 to 8.76. According to the pH change in the treated char, the NH3 scrubbing-CO2-solidifying process resulted in deposition of NH4HCO3 onto the char grains by roughly about 40% of the char weight.
These results demonstrated that the char material has certain binding affinity for NH4HCO3 and the pH value of the char material can be improved by the addition of NH4HCO3. The binding affinity for NH4HCO3 could be explained by the presence of biochar surface acids groups, such as carboxyl groups, that are known to form preferably at the biomass pyrolysis temperature of around 400°C [4]. The results also indicate that biochar could probably be used as an adsorbing filtration material along with the NH3 scrubbing-CO2-solidifying process to clean certain industrial flue gases and, at the same time, produce a valuable soil amendment and/or, perhaps, “organic slow-release” biochar fertilizer (such as biochar-NH4HCO3) that can potentially enhance sequestration of carbon into soil and subsoil earth layers, reduce NO −3 run-off, and stimulate photosynthetic fixation of CO2 from the atmosphere [12].
These experimental results may have practical implications for biochar soil applications. For examples, because of their alkaline ash contents, the pH of biochar material can sometimes be as high as about 10, which would be unfavorable for use in alkaline soils (pH above 8) such as those in the western part of the United States because the addition of an alkaline material could make the alkaline soil pH worse for plant growth. Since NH4HCO3 can act as a pH buffer, co-application of biochar and NH4HCO3 as a mixture or compound fertilizer is likely to improve biochar fertilizer pH. As shown in Table 1, mixing (50/50 by weight) with NH4HCO3 can neutralize the pH of biochar material from 9.85 to 7.89, which could make the biochar fertilizer pH more favorable to use in many soils, including (but not limited to) the alkaline soils. On the other hand, biochar can effectively adsorb ammonia (NH3) and other nutrients to minimize fertilizer nutrient loss. This type of chemisorption properties is typical of biochar since the biomass pyrolysis thermochemical process involves the fracture of many chemical bonds initially present in the biomass feedstock. The product biochar carbon does not go through a fluid state during the pyrolysis; consequently many of these bonds are left “dangling” [16]. As described by Antal and Gronli [16], these dangling bonds are believed to give rise to some of the chemisorption properties of biochar. In addition, certain polar functional groups, such as hydroxyl (−OH) and carboxyl (−COOH) groups of the biochar materials, may give rise to the property of CEC, which is important also in helping retain nutrients, such as ammonium and potassium ions (NH +4 and K+) in soil. Therefore, co-application of biochar and NH4HCO3 (or urea) can probably maximize the beneficial effects. Furthermore, as illustrated in Fig. 5, the bicarbonate HCO −3 of NH4HCO3 (or urea) that could be used in this manner may stay in the alkaline soils, since its HCO −3 could neutralize certain alkaline earth minerals, such as [Ca(OH)]+ and/or Ca2+, to form stable carbonated mineral products such as CaCO3 that can serve as a permanent sequestration of the carbon in soil and/or subsoil earth layers. Therefore, co-use of NH4HCO3 and char materials together could allow continued formation of carbonated mineral products, such as CaCO3 and/or MgCO3, to sequester maximal amount of carbons into the soil and subsoil terrains while still maintaining good soil properties for plant growth. In addition, use of the carbon-based nitrogen fertilizer could also provide an option to help solve the environmental problem of nitrate (NO −3 ) run off from the current use of ammonium nitrate (NH4NO3) as a fertilizer in the United States. However, more research and development efforts are still needed to test this option.
Note, ammonium bicarbonate (NH4HCO3) has a quite limited stability; it can decompose at a temperature above a range of 36–60°C. For best result, therefore, a biochar-NH4HCO3 fertilizer should be applied immediately into/underneath soil or stored in a tightly sealed container (e.g., plastic bag) at temperatures below 27°C to minimize decomposition. If the stability of ammonium bicarbonate (NH4HCO3) is a problem, the alternative is to use biochar with urea which is much more stable. In addition, biochar material alone can also be used in soil application as well.
4 Conclusions
The CEC value of biochar is related to the biomass pyrolysis temperature. Biochar materials made from peanut hulls at pyrolysis temperature of about 400°C yield the highest CEC value. As the pyrolysis temperature increases over 400°C, the CEC value decreases. The biochar materials appear to have certain binding affinity for ammonium bicarbonate (NH4HCO3) formed through a gas-phase ammonia carbonation reaction. The binding affinity for NH4HCO3 could be explained by the presence of biochar surface functional groups, such as carboxyl groups, that are known to form preferably at a pyrolysis temperature of around 400°C. Addition of ammonium bicarbonate to biochar can help neutralize biochar pH. Use of biochar fertilizer is potentially an attractive approach for soil amendment and carbon sequestration.
References
Raupach MR, Marland G, Ciais P, Quéré CL, Canadell JG, Klepper G, Field CB (2007) Global and regional drivers of accelerating CO2 emissions. Proc Natl Acad Sci U S A 104(24): 10288–10293
Schlesinger WH (1985) Changes in soil carbon storage and associated properties with disturbance and recovery. In: Trabalha JR et al (eds) The changing carbon cycle: a global analysis. Springer, New York, NY, pp 194–220
Day D, Evans RJ, Lee JW, Reicosky D (2005) Economical CO2, SOx, and NOx capture from fossil-fuel utilization with combined renewable hydrogen production and large-scale carbon sequestration. Energy 30:2558–2579
Day DM, Lee JW (2004) The production and use of a soil amendment made by the combined production of hydrogen, sequestered carbon and utilizing off gases containing carbon dioxide. PCT Int Appl, WO 2004037747 A2, 58
Gundale MJ, DeLuca TH (2007) Charcoal effects on soil solution chemistry and growth of Koeleria macrantha in the ponderosa pine/Douglas fir ecosystem. Biol Fertil Soils 43:303–311
Solomon D, Lehmann J, Thies J, Schafer T, Liang B, Kinyangi J, Neves E, Petersen J, Luizao F, Skjemstad J (2007) Molecular signature and sources of biochemical recalcitrance of organic C in Amazonian dark earths. Geochim Cosmochim Acta 71:2285–2298
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems—a review. Mitig Adapt Strat Glob Chang 11:403–427
Lee JW, Hawkins B, Day DM, Reicosky DC (2010) Sustainability: the capacity of smokeless biomass pyrolysis for energy production, global carbon capture and sequestration. Energy Environ Sci 3(11):1609–1812
Krausmann F, Erb K, Gingrich S, Lauk C, Haberl H (2008) Global patterns of socioeconomic biomass flows in the year 2000: a comprehensive assessment of supply, consumption and constraints. Ecol Econ 65:471–487
Roberts K, Gloy BA, Joseph S, Scott NR, Lehmann J (2010) Life cycle assessment of biochar systems: estimating the energetic, economic, and climate change potential. Environ Sci Technol 44:827–833
Asai H, Samson BK, Stephan HM, Songyikhangsuthor K, Homma K, Kiyono Y, Inoue Y, Shiraiwa T, Horie T (2009) Biochar amendment techniques for upland rice production in Northern Laos 1. Soil physical properties, leaf SPAD and grain yield. Field Crop Res 111:81–84
Lee JW, Li R (2003) Integration of fossil energy systems with CO2 sequestration through NH4HCO3 production. Energ Convers Manage 44(9):1535–1546
Li X, Hagaman E, Tsouris C, Lee JW (2003) Removal of carbon dioxide from flue gas by ammonia carbonation in the gas phase. Energy Fuel 17:69–74
Lee JW, Li R (2002) Method for reducing CO2, CO, NOx, and SOx emissions. United States Patent No. 6,447,437 B1
Das KC, Singh K, Adolphson R, Hawkins B, Oglesby R, Lakly D, Day D (2009) Steam pyrolysis and catalytic steam reforming for hydrogen and biochar production. Appl Eng Agric 26(1):137–146
Antal MJ, Gronli M (2003) The art, science, and technology of charcoal production. Ind Eng Chem Res 42(8):1619–1640
Acknowledgment
The authors wish to thank Mac Post and Joe Katz for stimulating discussions. This research was supported in parts by Oak Ridge National Laboratory Director’s Seed Money Project Funds and by the US Department of Energy (DOE) Office of Science Young Scientist Award and the US Presidential Early Career Award for Scientists and Engineers (to J.W. Lee). Oak Ridge National Laboratory is managed by UT-Battelle, LLC, for DOE under contract No. DE-AC05-00OR22725.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Lee, J.W., Hawkins, B., Li, X., Day, D.M. (2013). Biochar Fertilizer for Soil Amendment and Carbon Sequestration. In: Lee, J. (eds) Advanced Biofuels and Bioproducts. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-3348-4_6
Download citation
DOI: https://doi.org/10.1007/978-1-4614-3348-4_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-3347-7
Online ISBN: 978-1-4614-3348-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)