Abstract
Norrin is a secreted protein which is encoded by the NDP gene mutated in Norrie disease. In the eye, the major site of Norrin expression is the Müller glia. Norrin activates the canonical Wnt/β-catenin signaling pathway via specific binding to the frizzled (Fzd)4/low-density lipoprotein receptor-related protein (Lrp)5/6 receptor complex, and is part of an essential signaling system that controls the formation of retinal capillaries during development. Independent from its angiogenic function, Norrin has pronounced neuroprotective properties on retinal ganglion cells via activation of Wnt/β-Catenin signaling and subsequent induction of neurotrophic growth factors in Müller cells. In addition, there is evidence that the expression of Norrin in uterus and placenta is required for reproduction
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Norrin
- Frizzled 4
- Wnt/β-catenin signaling
- Angiogenesis
- Retinopathy of prematurity
- Neuroprotection
- Transgenic mice
- Norrie disease
1 Introduction
Almost 20 years ago, mutations in the NDP (Norrie disease pseudoglioma) gene were identified as causative for Norrie disease. For a considerable number of following years, the available data on the functional role(s) of Norrin, the product of NDP, remained largely incomplete. Experimental studies were complicated by the fact that it turned out to be difficult to generate bioactive recombinant Norrin in larger amounts. In addition, the cells that secrete Norrin remained ill-defined, as the amounts of available mRNA and protein appeared to be very low in most tissues of the body. In the more recent years, this scenario has completely changed, as the signaling pathways of Norrin have been largely identified. Moreover, studies in animal (mouse) models have yielded important insights into a fundamental role of Norrin for capillary formation in retina and inner ear. Current data strongly indicate that Norrin has an additional neuroprotective role for retinal neurons, which appears to be largely independent from its role on the growth of retinal capillaries (Fig. 86.1).
2 Norrie Disease
Norrie disease is a congenital X-linked recessive disease which is characterized by atrophic irides, corneal clouding, cataract, and retinal dysplasia with early vascular proliferation (pseudoglioma) followed by bulbar atrophy. In addition, affected patients show progressive sensorineural hearing loss, psychotic features, and in one third of the cases mental retardation (Warburg 1966; Berger 1998). By linkage analysis and positional cloning, mutations in NDP were identified as cause of Norrie disease (Berger et al. 1992; Meindl et al. 1992). In addition, the X-linked form of familial exudative vitreoretinopathy (FEVR) was found to be caused by missense mutations in Norrin (Chen et al. 1993; Fuchs et al. 1995; Meindl et al. 1995; Shastry et al. 1997a; Torrente et al. 1997; Shastry 1998), while autosomal-dominant familial exudative vitreoretinopathy was found to be caused by mutant frizzled-4, the ligand of Norrin (Robitaille et al. 2002). Mutations in NDP have also been identified in patients with Coat’s disease (Black et al. 1999) and retinopathy of prematurity (Shastry et al. 1997b).
3 Norrin
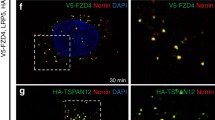
Norrin is a secreted small protein which is characterized by a carboxyl-terminal cysteine-rich domain, which has been suggested to facilitate formation of a homodimer containing a cyteine-knot motif (Meitinger et al. 1993). Similar motifs have been observed in growth factors of the TGF-β superfamily. Recent studies using a knock-in mouse model have found a wide spread expression of Norrin in astrocytes of both forebrain and midbrain, in Bergmann glia of the cerebellum, and in Müller glia of the retina (Ye et al. 2011). During development, expression of Norrin has been observed in regions of the hindbrain and throughout the dorsal and mid-dorsal regions of the neural tube (Ye et al. 2011). Norrin activates the canonical Wnt/β-catenin signaling pathway via specific binding to the frizzled (Fzd)4/low-density lipoprotein receptor-related protein (Lrp)5/6 receptor complex (Xu et al. 2004). Similar to the action of Wnt glycoproteins, binding of Norrin to the extracellular cysteine rich domain of Fzd4 promotes oligomerization of Fzd4 with Lrp5/6 coreceptors to inactivate the β-catenin destruction complex causing an accumulation of intracellular β-catenin. As additional component of the Fzd4/Lrp5/6 receptor complex, TSPAN12 has been identified that multimerizes Fzd4 to form receptor clusters, and specifically promotes Norrin, but not Wnt-mediated β-catenin signaling (Junge et al. 2009). Strikingly, mutations in Norrin and Fzd4 result in very similar morphological phenotypes, both in mice and men (Xu et al. 2004; Smallwood et al. 2007; Ye et al. 2009).
4 Angiogenic Properties of Norrin
Ndpy/− mutant mice with a targeted disruption of Ndp have been generated (Berger et al. 1996). The animals show complete absence of intraretinal capillaries and persistence of the hyaloid vasculature (Richter et al. 1998; Rehm et al. 2002; Luhmann et al. 2005b; Ohlmann et al. 2005). Comparable findings have been reported for the vessels within the stria vascularis of the cochlea (Rehm et al. 2002). βB1-crystallin-norrin transgenic mice with ocular overexpression of ectopic norrin under control of the lens-specific βB1-crystallin promoter have been developed and in mixed βB1-crystallin-norrin/Ndpy/− mice, the vascular phenotype is completely rescued (Ohlmann et al. 2005). Recombinant Norrin induces proliferation, migration, and tube formation in retinal microvascular endothelial cells, effects that involve Wnt/β-catenin signaling and induction of Sox17 and angiopoietin-2 (Ye et al. 2009; Ohlmann et al. 2010). Overall, there is considerable evidence that Norrin, Fzd-4, and Lrp5 constitute an essential signaling system that controls the formation of retinal capillaries during development (Ye et al., 2009). Norrin is not only required during the development of retinal capillaries, but substantially protects against vascular damage induced by high oxygen such as observed in retinopathy of prematurity (Ohlmann et al. 2010). In transgenic mice overexpressing norrin, vascular loss following oxygen exposure was significantly smaller as compared to wild-type littermates. In addition, the anatomical correct regrowth of vessels was significantly increased, while pathological neovascularization was suppressed.
5 Neuroprotective Properties of Norrin
Norrin-deficient mice show an early specific loss of retinal ganglion cells (RGC) suggesting a neuroprotective role of Norrin (Ohlmann et al. 2005). To analyze such a putative neuroprotective role of Norrin in more detail, RGC survival in mouse eyes was studied following damage after N-methyl-D-aspartate (NMDA) injection into the vitreous body (Seitz et al. 2010). After injection of NMDA, the numbers of optic nerve axons and of perikarya of surviving RGC were significantly higher in NMDA/Norrin injected eyes as compared to NMDA treated eyes, an effect that could be blocked by adding dickkopf (DKK)-1, an inhibitor of the Wnt/β-catenin signaling pathway. Comparable results were obtained by TUNEL labeling. Treatment of eyes with combined Norrin/NMDA activated Wnt/β-catenin signaling and increased the retinal expression of leukemia inducible factor and endothelin-2, as well as that of neurotrophic growth factors such as fibroblast growth factor-2, brain-derived neurotrophic factor, lens epithelium-derived growth factor and ciliary neurotrophic factor. A similar activation of Wnt/β-catenin signaling and an increased expression of neurotrophic factors were observed in cultured Müller cells after treatment with norrin, effects that again could be blocked by adding DKK-1. In addition, Norrin and conditioned cell culture medium of norrin-treated Müller cells increased survival of differentiated RGC-5 cells. Overall, norrin appears to have pronounced neurotrophic properties on retinal neurons with the distinct potential to decrease the damaging effects of NMDA-induced RGC loss. Norrin and the molecules involved in its signaling pathway appear to be promising targets to develop strategies that increase RGC survival not only in experimental animal models, but also in patients with RGC damage following ischemia or glaucoma. It is tempting to speculate that Norrin-induced activation of combined leukemia inducible factor, endothelin-2, fibroblast growth factor-2 and/or Wnt/β-catenin signaling could also be neuroprotective for other types of retinal neurons.
6 The Role of Norrin in Reproduction and Development
Expression of Norrin has been observed in decidua and uteri of wild-type mice and in human placentae indicating a role for Norrin in reproduction. Indeed, there is impaired female fertility due to distinct reduction of decidualization in female Ndp −/− mice. Deciduae are smaller and do not reach as deep into the endometrium as in wild-type mice. Fibrocytes of the deeper endometrial zone do not show transition to large roundish decidua cells, and are separated from each other by wide intercellular spaces (Luhmann et al. 2005a). Overall, Norrin appears to act on reproduction in two ways: by impairing vascularization of deciduae and by inducing malformation of endometrial fibrocytes.
References
Berger W (1998) Molecular dissection of Norrie disease. Acta Anat (Basel) 162:95–100
Berger W, van de PD, Bachner D et al (1996) An animal model for Norrie disease (ND): gene targeting of the mouse ND gene. Hum Mol Genet 5:51–59
Berger W, van de PD, Warburg M et al (1992) Mutations in the candidate gene for Norrie disease. Hum Mol Genet 1:461–465
Black GC, Perveen R, Bonshek R et al (1999) Coats’ disease of the retina (unilateral retinal telangiectasis) caused by somatic mutation in the NDP gene: a role for norrin in retinal angiogenesis. Hum Mol Genet 8:2031–2035
Chen ZY, Battinelli EM, Fielder A et al (1993) A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat Genet 5:180–183
Fuchs S, Kellner U, Wedemann H et al (1995) Missense mutation (Arg121Trp) in the Norrie disease gene associated with x-linked exudative vitreoretinopathy. Hum Mutat 6:257–259
Junge HJ, Yang S, Burton JB et al (2009) TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell 139:299–311
Luhmann UF, Meunier D, Shi W et al (2005a) Fetal loss in homozygous mutant Norrie disease mice: a new role of Norrin in reproduction. Genesis 42:253–262
Luhmann UF, Lin J, Acar N et al (2005b) Role of the Norrie disease pseudoglioma gene in sprouting angiogenesis during development of the retinal vasculature. Invest Ophthalmol Vis Sci 46:3372–3382
Meindl A, Lorenz B, Achatz H et al (1995) Missense mutations in the NDP gene in patients with a less severe course of Norrie disease. Hum Mol Genet 4:489–490
Meindl A, Berger W, Meitinger T et al (1992) Norrie disease is caused by mutations in an extracellular protein resembling C-terminal globular domain of mucins. Nat Genet 2:139–143
Meitinger T, Meindl A, Bork P et al (1993) Molecular modelling of the Norrie disease protein predicts a cystine knot growth factor tertiary structure. Nat Genet 5:376–380
Ohlmann A, Seitz R, Braunger B et al (2010) Norrin promotes vascular regrowth after oxygen-induced retinal vessel loss and suppresses retinopathy in mice. J Neurosci 30:183–193
Ohlmann A, Scholz M, Goldwich A et al (2005) Ectopic norrin induces growth of ocular capillaries and restores normal retinal angiogenesis in Norrie disease mutant mice. J Neurosci 25:1701–1710
Rehm HL, Zhang DS, Brown MC et al (2002) Vascular defects and sensorineural deafness in a mouse model of Norrie disease. J Neurosci 22:4286–4292
Richter M, Gottanka J, May CA et al (1998) Retinal vasculature changes in Norrie disease mice. Invest Ophthalmol Vis Sci 39:2450–2457
Robitaille J, MacDonald ML, Kaykas A et al (2002) Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet 32:326–330
Seitz R, Hackl S, Seibuchner T et al (2010) Norrin mediates neuroprotective effects on retinal ganglion cells via activation of the Wnt/beta-Catenin signaling pathway and the induction of neuroprotective growth factors in Müller cells. J Neurosci 30:5998–6010
Shastry BS (1998) Identification of a recurrent missense mutation in the Norrie disease gene associated with a simplex case of exudative vitreoretinopathy. Biochem Biophys Res Commun 246:35–38
Shastry BS, Hejtmancik JF, Trese MT (1997a) Identification of novel missense mutations in the Norrie disease gene associated with one X-linked and four sporadic cases of familial exudative vitreoretinopathy. Hum Mutat 9:396–401
Shastry BS, Pendergast SD, Hartzer MK et al (1997b) Identification of missense mutations in the Norrie disease gene associated with advanced retinopathy of prematurity. Arch Ophthalmol 115:651–655
Smallwood PM, Williams J, Xu Q et al (2007) Mutational analysis of Norrin-Frizzled4 recognition. J Biol Chem 282:4057–4068
Torrente I, Mangino M, Gennarelli M et al (1997) Two new missense mutations (A105T and C110G) in the norrin gene in two Italian families with Norrie disease and familial exudative vitreoretinopathy. Am J Med Genet 72:242–244
Warburg M (1966) Norrie disease: a congenital oculo-acoustico-cerebral degeneration. Acta Ophthalmol 89(suppl.):1–147
Xu Q, Wang Y, Dabdoub A et al (2004) Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116:883–895
Ye X, Smallwood P, Nathans J (2011) Expression of the Norrie disease gene (Ndp) in developing and adult mouse eye, ear, and brain. Gene Expr Patterns 11:151–155
Ye X, Wang Y, Cahill H et al (2009) Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 139:285–298
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media, LLC
About this paper
Cite this paper
Braunger, B.M., Tamm, E.R. (2012). The Different Functions of Norrin. In: LaVail, M., Ash, J., Anderson, R., Hollyfield, J., Grimm, C. (eds) Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology, vol 723. Springer, Boston, MA. https://doi.org/10.1007/978-1-4614-0631-0_86
Download citation
DOI: https://doi.org/10.1007/978-1-4614-0631-0_86
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4614-0630-3
Online ISBN: 978-1-4614-0631-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)