Abstract
In this chapter, we review the physiology of switchgrass from seed dormancy till the effects of water and nutrients stress on grown plants. These leftacteristics are presented and discussed mainly at the canopy and whole-plant level with emphasis on the agro-physiology of the species in view of the possible contribution of crop physiology to agricultural development. Switchgrass is noted for the variable degrees of seed dormancy regulated by endogenous and exogenous factors that determine the successful seedling establishment. Plant growth rates are determined by temperature while the reproductive phase is controlled mainly by photoperiod. There is also evidence that some physiological attributes, such as photosynthesis, transpiration, and water use efficiency differ between tetraploid, hexaploid, and octoploid ecotypes. But despite these differences, in general switchgrass combines important attributes of efficient use of nutrients and water with high yields thanks to its ability to acquire resources from extended soil volumes, especially at deep layers. Moreover at canopy level, resources capture and conservation are determined by morpho-physiological leftacteristics (C4 photosynthetic pathway, stomatal control of transpiration, high leaf area index, low light extinction coefficient) that enhance radiation use efficiency and reduce carbon losses. However, specific information on switchgrass physiology is still missing, in particular deeper understanding of physiological principles controlling the water and nutrients acquisition mechanisms and allocation under suboptimal growing conditions. The physiology of tillering and root respiration are also factors that need further investigation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
3.1 Introduction
Switchgrass (Panicum virgatum L.) is a perennial grass of temperate zones that has evolved into a forage crop and more recently into an energy crop. The species originates from the central plains of North America as a component of the tall grass prairie [1]. Basically switchgrass is a warm season, deep-rooted, photosensitive, C4-type metabolism species with a high adaptability to a wide geographical range and soil types. Considering habitat preferences and other plant leftacteristics, switchgrass is classified into upland and lowland ecotypes associated with latitudinal origin (northern and southern ecotypes) [2, 3]. In general switchgrass presents a high interspecific variability in physiological leftacteristics, with its own response adaptations to photoperiod, temperature, water logging or drought, and other stresses. These traits influence the carbon fixation efficiency and therefore crop production potential.
Surprisingly, a comprehensive review on switchgrass physiology as a crop species is lacking. In most of the cases, switchgrass physiology is covered along that of other forage grasses. Sanderson et al. [4], for example, provide a general overview of the morphological and physiological response to stress of forage grasses, but specific information on switchgrass is limited. Moreover, in a 10-year research program designed by the US Department of Energy to evaluate and develop switchgrass as an energy crop and that involved a large network of research sites, universities, laboratories, and US Department of Agriculture facilities, only two institutions were listed as interested in switchgrass physiology [5]. Hence, most of the information available on switchgrass physiology comes from previous studies that focused on its forage end use. Topics were wide ranging from seed dormancy physiology to the crop growth determinants. Across the world there is an increasing interest in switchgrass as a multipurpose crop species, but the establishment of a permanent switchgrass physiology program as an important part of agricultural research is needed.
In this chapter, we review the physiology of seed dormancy, seedling establishment, above- and belowground biomass development, resource use efficiency, and the effects of water and nutrient stress. These leftacteristics are presented and discussed mainly at the canopy and whole-plant level with emphasis on the agro-physiology of the species in view of the possible contribution of crop physiology to agricultural development. Organ and cellular levels are not presented here.
3.2 Seed Germination and Seedling Establishment
3.2.1 Physiology of Seed Dormancy
Switchgrass seeds, even within the same lot of seeds, have variable degrees of dormancy, which is an optimum strategy to survive in the wild and trough periods of environmental stress but at the same time is a major obstacle for its wide-spread cultivation as a forage or biomass crop [6, 7]. At harvest, more than 90% of the seeds of some cultivars could be dormant [6, 8]. Although dormancy declines naturally with time, the mechanisms of dormancy in switchgrass are not well understood. Several authors indicate that a combination of physical and physiological factors may be involved and, to a lesser extent, morphological factors too [9–13]. The seed coat is in part responsible for switchgrass dormancy. These outer layer coverings act as barriers for water and oxygen uptake, produce and encapsulate germination inhibitors, modify the light reaching to the embryos, and act as physical barriers that inhibit germination [10].
Since the embryos of switchgrass are fully developed at harvest time, it is suggested that after-ripening is not a major factor inducing dormancy break in switchgrass [9, 13]. The aforementioned authors reached such a conclusion based on their observations that after injuring, cutting, or completely removing the embryos, endosperms, and/or the seed coat, unspecified germination inhibitors were released, thus the germination percent increased significantly. Hence, primary dormancy in switchgrass may not be related to underdeveloped embryos but to dormancy mechanisms within the embryo and the required quiescence period of the seeds [6, 9, 13]. Under natural conditions this period could last months if not years [7, 13]. In order to accelerate the decay of dormancy, several artificial methods can be used. Mechanical and chemical scarification of switchgrass seeds, for example, resulted in 73% and 61% increased germination, respectively [10, 14]. Such a process may weaken the fiber tissue in the lemma, allowing more gas exchange and water uptake, and eliminate or weaken the physical barrier posed by the lemma and palea that impede the embryo expansion [10].
Impermeable membranes to oxygen but permeable to water in switchgrass seeds seem to be also responsible for dormancy. Under suboptimal temperatures, these membranes prevent the respiration of the stored energy within the seed and therefore delay or inhibit germination. However, by exposing seeds to cool temperatures and moisture (stratification), oxygen can be absorbed by the seeds and therefore dormancy reduced or broken. For example, Sanderson et al. [8] indicated that naturally (e.g., cool and wet conditions prevail in early springtime) and artificially stratified seeds germinated well and provided good stands and yields.
Moreover, Shen et al. [6] showed evidence that germination of Cave-in-Rock switchgrass seeds could be increased up to 80% within a 14-day stratification period at 5° C. However, dormancy break by stratification is not straightforward and unidirectional process, and it depends on undetermined factors within the seed and the surrounding environment. Shen et al. [6] indicated that stratified switchgrass seeds could become dormant again after the seeds are dried for mechanical planting. This tendency toward secondary dormancy was termed as reversibility. The authors suggested that this reversibility was linked to a physiological continuum that the seeds enter when the right environmental conditions occur for germination. In case such conditions do not occur or stop, the seed goes dormant again, which is called by some authors residual dormancy [12]. However, reversibility depends on the degree on which dormancy was removed. Shen et al. [6] suggested that a 42-day period of stratification would be enough to completely remove dormancy, but the length of such a period will depend on how well the seeds were after-ripened or aged, as stratification and after-ripening have additive effects. Moreover, residual dormancy is responsive to the modification of endogenous levels of nitric oxide (NO) and/or reactive oxygen species (ROS) [12]. In general, environmental stresses (e.g., drought, temperature, light, etc.) can cause mutations in the genes responsible for germination. Such mutations can be associated with the degree of seed maturation and with the biosynthesis of dormancy-regulator hormones [15, 16].
Toward seed maturity, the concentration of germination inhibitors, such as abscisic acids (ABA) in switchgrass, as in other dormant grass species, remains high in comparison to growth promoters, such as gibberellic acid (GA); therefore, dormancy prevails [17, 18]. The balance between ABA and GA seems to be affected by endogenous and micro-environmental factors; however, the mechanisms of such shifts are not fully understood [12]. These authors, however, indicated that NO and ROS are important reactive pathways to perceive ABA. Then these receptors and cognate proteins drive signaling cascades resulting in biological outputs [17, 18]. For example, exogenously applied ROS, as H2O2, inhibited the effects of ABA probably by overriding the ABA-dependent signals, resulting in enhanced switchgrass germination [17, 18]. Even though the exogenously applied NO was not able to overcome the ABA-dependent signals, probably because of a NO scavenger, Sarath et al. [11, 17, 18] indicated that high levels of endogenous NO are required for germination. Exogenously applied H2O2 may stimulate the production of endogenous NO in the aleurone layer, the main site for NO synthesis in switchgrass [17, 18], and therefore overcome the ABA inhibition of germination.
3.2.2 Seedling Establishment
Seedling establishment comprises the germination and emergence phase, and the adventitious root development phase [19]. A seed is considered germinated and emerged when the radicle protrudes from the seed coat and when the coleoptiles become visible; radicle extension precedes the coleoptile emergence [11, 12]. A rapid initial development of roots enables seedlings to acquire the necessary water and nutrients for growth. When the coleoptile emerges from the soil surface, subcoleoptile internode elongation stops, the coleoptile opens, and shoot growth and chlorophyll synthesis begins ([20]; Fig. 3.1). The speed and rate of germination and emergence are affected by environmental factors such as water, temperature, and light. In general, it is indicated that the base temperature for germination is between 8.1 and 10.3° C, and optimum is between 25 and 30° C, with maximum germination occurring after 72 h of imbibition [21, 22]. Maximum temperature for germination may be as high as 45° C [22], but all of these conditions seem to be cultivar dependent. Germination rate is affected by the degree of dormancy, imbibition rate, and respiration, which are temperature-dependent factors, while maximum seed germination and emergence is mainly affected by the degree of water uptake [22, 23].
Since the seeds of switchgrass are very small (Table 3.1), the amount of water required for germination is also very small, especially at the hydration phase. The water requirements will increase as the seedling develops and its juvenile root system (composed of the primary root, seminal roots, and subcoleoptile internode roots) starts growing and functioning [24]. Radicle protrusion coincides with radicle emergence, which is leftacterized by short duration. Some authors consider it to be a negligible part of a switchgrass seedling [25]. However, it plays a fundamental role in the early establishment phase of the young seedling, a role that has not yet been clearly defined [24]. Proper soil moisture is essential at this stage as the establishment of a viable seedling is not yet ensured. However, information on switchgrass water requirements for germination and seedling establishment is scarce. The few data available indicate that under drought stress (−0.3 MPa) only 2.5% of all sowed seeds emerged, representing 5% survival of the germinable seeds [26]. Hence, these results suggest that the lower limit of moisture availability for switchgrass germination might be around −0.3 MPa, regardless of the sowing depth and soil texture.
Adequate soil surface moisture is also essential for the formation of adventitious roots. The long-term survival of the seedling will be determined by the development of robust adventitious roots as they will become the major root system of the seedlings ([1, 19]; Fig. 3.2). In any case, Smart and Moser [27] suggested that few long adventitious roots that reach moist subsurface soil layers are enough for the successful early establishment of seedlings. Moreover, Newman and Moser [19] showed that in switchgrass the number of adventitious roots increased rapidly between 4 and 8 weeks after planting following 4 or more days of consecutive rain. However, during the first 4 weeks after planting, there were few adventitious roots even under adequate soil surface moisture conditions. In fact, switchgrass starts to develop adventitious roots by the third-leaf stage [24]. After this period, water flow may be preferential to the growing shoot, as it is suggested to happen in blue grama [28]. Xu et al. [29] found that switchgrass seedlings (fifth- to sixth-leaf stage) exposed to continuous soil dehydration increase by 11% their allocation of carbohydrates to the roots. Such a change in carbon partitioning may be a useful strategy for the seedling survival.
Adventitious roots develop in clusters from the coleoptilar node or seedling crown ([19]; Fig. 3.2). Since the crown node is pushed to the soil surface by the elongating subcoleoptile internode until a certain light level is sensed, the location of the seedling crown and that of the adventitious roots will be close to the soil surface regardless the sowing depth [19, 20]. At greater soil depths, moisture and temperature conditions are more favorable for the successful development and functioning of the adventitious roots, which in turn will secure the seedling establishment and survival. On the other hand, if the seedling’s crown is at or close to the soil surface, its exposition to faster soil desiccation may be abortive or limiting for the development of adventitious roots, especially in drier environments [1, 19]. In general, switchgrass has excessive crown node elevation, which makes its successful establishment difficult. However, Elbersen et al. [20] demonstrated that populations with low crown placement can be selected and that this trait is heritable. Such genotypes have shorter subcoleoptile internodes which facilitate water flow toward the coleoptile and transpiring leaves and therefore accelerate emergence and establishment. Thus, these genotypes are better able to withstand drought conditions before adventitious roots are developed [30]. In fact, in field trials with alternating wet and dry periods, it was shown that the selected genotype for low crown placement had greater seedling germination and emergence rates [30].
Selection for seed size can also improve switchgrass seedling establishment. Several authors indicated that larger seeds accelerated the germination, emergence, growth rates, and development of adventitious roots, but all these advantages associated with seed size were no longer evident at later growth stages, even when soil moisture was suboptimal [1, 27, 31, 32].
3.3 Canopy and Root Development
3.3.1 Phenological Stages
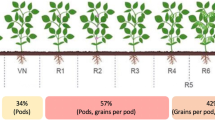
Based on habitat, morphological leftacteristics, and ploidy level, switchgrass has been classified into two ecotypes: lowland (mainly grown in lower and wetter environments) and upland (mainly grown in mesic environments). Each ecotype is further subdivided according to its geographical origin into southern and northern ecotypes ([33, 34]; Table 3.1). Casler et al. [2] indicated that latitude is the largest determinant of switchgrass productivity and survival. Lowland ecotypes are usually tetraploid (2n = 4x = 36 chromosomes), while the upland types range from tetraploid to hexaploid (2n = 6x = 54 chromosomes) to octoploid (2n = 8x = 72 chromosomes) [35–38]. This large genetic diversity result in morphologically and physiologically different plants. In general, the lowland ecotypes are taller, with thicker stems, longer bluish-green leaves, have larger panicles, and produce higher yields but have lower initial growth rates than upland ecotypes [1, 39]. When lowland/southern ecotypes are moved to northern latitudes, they produce higher biomass yields because they remain vegetative for a longer period of time with a longer photosynthetically active period, but they may not reach maturity and form seeds. In fact, in order to survive the winter, switchgrass should acquire adequate dormancy and translocate storage carbohydrates to the roots, but due to the extended photoperiod, southern ecotypes may start this process too late when moved to northern latitudes, and their survival may be compromised. Moreover, the harvested biomass from immature plants can have high moisture and ash contents due to the partial translocation of nutrients to the rhizomes, which is unfavorable for energy purposes. On the other hand, upland/northern cultivars moved southward have lower biomass yields and are more susceptible to diseases [2, 33, 40, 41] because they are exposed to shorter-than-normal days during the summer, which leads to early flowering. So far, hybridization attempts between these two ecotypes have been unsuccessful [42], and therefore at the moment, the functional advantages of one ecotype cannot be introduced into the other. In order to study the growth and development of switchgrass as function of environmental and management variables, descriptive indices of its phenological stages have been developed (Table 3.2). The general method used for the study of perennial grasses proposed by Moore et al. [43] and specifically adapted by Sanderson [34] for switchgrass indicates five main phenological stages: (a) emergence, (b) vegetative/leaf development (c) stem elongation, (d) reproductive/floral development, and (e) seed development and ripening. Each stage is sub-divided into sub-stages, which are identified by numerical codes that go from 0.5 to 35. These codes provide a descriptive attribute of the physiological and ecological status of the plant and enable researchers to quantitatively assess the successive growth stages and statistically determine deviations from the normal growth. Under natural prairie conditions, seed shattering and complete dormancy could also be considered as additional growth stages during the life cycle of switchgrass [40]. In switchgrass as in other perennial grasses, the vegetative growth stages are discrete, so leaf growth continues even when the stem elongation stage has started [44]. Moreover, the floral development starts when there are still some leaf primordia and unemerged leaves on the apex [45].
3.3.2 Growth Analysis
The physiological development of switchgrass is typical of that of perennial grasses and follows the general growth sequence indicated above (Table 3.2). Al-though the duration of each stage is cultivar dependent, the vegetative growth of switchgrass (including leaf development and internode elongation) is strongly influenced by temperature [46]. Sanderson and Wolf [44], for example, indicated that when Cave-in-Rock (northern/upland) and Alamo (southern/lowland) cultivars that are grown close to their place of origin require 200 and 430 growing degree-days (GDD) above a base temperature of 10° C to complete the leaf elongation stage and 378 and 1,020 GDD for the stem elongation phase. On the other hand, the reproductive phase of switchgrass is controlled mainly by the day length (photoperiod), regardless of temperature and moisture availability [41], suggesting a facultative short-day response, though the influence of other factors cannot be excluded completely. The effect of photoperiod extension was studied by Van Esbroeck et al. [41] on two switchgrass cultivars; when Cave-in-Rock was subjected to long days (16 hr), panicle emergence was delayed by 18 days and the time for panicle exsertion was increased by 243% as compared to the control treatment (12-hr photoperiod). Such delay was associated with an increase in the phyllochron (the time between the appearance of two successive leaves) and in leaf size, while the number of leaves was not altered. In the case of the Alamo cultivar, the number of leaves and their size decreased. In both cases, however, the duration of the panicle exsertion was extended. Then, an increase in the duration of the panicle development could maximize seed production, but its effects on biomass accumulation remain unclear. On the other hand, early flowering results in fewer leaves, reduced photosynthetic capacity, and lower yields.
In switchgrass, the leaf appearance rate (LAR) is somehow also related to photoperiod. The LAR decreases when days are long and increases when days are short. The faster LAR is associated with a short period between floral initiation and floral emergence [41, 45], thus reducing the vegetative period and potential biomass yield. In early maturing cultivars from northern latitudes, the phyllochron was almost double than that of southern late cultivars, suggesting an important role of latitude in controlling maturity time [45]. In general the lamina extension rate (LER) range from 0.20 to 0.30 cm GDD−1, with the longest leaves located near the middle of the canopy up to the seventh leaf. Even though leaf growth continues until the leaf collar has emerged [47], when panicle development begins (around 1,000–1,200 GDD) LERs decline and shorter leaves are formed on the top [45], probably due to the increased sink force of the emerging panicles. Among several switchgrass cultivars, the final number of leaves on spring-emerged tillers range from 9 to 11, while from summer-emerged tillers the range was from 6 to 8 [45]. The same authors indicated that leaf formation in spring tillers, and thus biomass accumulation, continues until environmental conditions induce floral development. Flowering in switchgrass is induced by decreases in day length following the summer solstice. However, the photoperiod requirements of the diverse cultivars change depending on the latitude of origin of each ecotype.
Beaty et al. [48] indicated that switchgrass tillers behave as true biennale tillers; that is, the first-year tillers remain as rhizomes buds. Then in the coming spring, when temperatures are adequate, a flush of tillers emerges (Fig. 3.2). The physiological mechanisms responsible for tillering initiation in switchgrass have not been fully studied. Perhaps, as is suggested for other perennial grasses, the antagonistic actions of hormones such as auxin produced in the apical meristems, and cytokinin and strigolactones produced in the roots, together with resource availability (e.g., nutrients, water) and photosensitivity to red and far red light play an important and decisive role in the growth of axillary meristems [49], but specific information on such mechanisms is still lacking. Lower internodes begin to elongate after some leaves have been produced and continue until the inflorescence has emerged [48, 50], at which stage the carbohydrate reserves in the stem bases are the lowest [51]. Elongation rates can range from 1.4 to 2.8 cm d−1 depending on the cultivar and environmental conditions, with the more southern-origin ecotypes having greater growth rates [2, 46]. Upland ecotypes can reach 1.5–2 m in height, while lowland ecotypes are 3–4 m tall [52]. In general, tiller density during the vegetative growth stage is high but declines with advancement towards the following growth stages [53]. The final tiller density is, however, highly variable in number and physiological stage, depending on cultivar and environmental conditions. For example, 3-year-old stands of Cave-in-Rock and Dacotah cultivars, grown between 43°N (Arlington, WI) and 44°N (Brookings, SD) in the USA had tiller densities of 677 and 1,355 tillers m−2, respectively, while the reproductive tiller fraction, averaged across cultivars, was 81 and 8% at Arlington and Brookings [54].
The tillering capacity is an important component of switchgrass plasticity and its ability to respond to environmental stimuli in time and space. The general issues concerning the physiology of tillering in crop plants may apply to switchgrass, but specific information is not available. For example, in dense grass canopies, tiller elongation rate is stimulated and apical dominance is enhanced due to the low red–far red light ratio and low blue light perceived by the phytochromes [55]. However, information on this subject is still lacking in the case of switchgrass.
In addition to the genotype, photoperiod, and temperature, other factors such as plant density, irradiance, water, and nutrition may influence tiller initiation. Muir et al. [56] indicated that nitrogen fertilization and row spacing have a direct effect on the number of productive tillers, which play an important role in increased productivity. However, the same authors indicated increased tiller mass rather than an increased number of tillers is the main mechanism by which biomass yield is increased. Moreover, as in other grasses, new switchgrass tillers may obtain water from the mother plant until functional adventitious roots are developed; otherwise, the new tiller may die [57].
3.3.3 Root Growth and Function
Initial root growth of switchgrass is very rapid, especially during the first 3 weeks after sowing, and then slows down gradually but remains the major carbohydrate sink at least for the first 15 weeks after sowing [58]. The root-to-shoot ratio of 3-week-old seedlings was 5.5, while at 15 weeks of age the ratio was reduced to 2.0 [58]. Such a large allocation of carbohydrates and rapid initial root growth rate are fundamental to the successful establishment of switchgrass. The C4 physiology of switchgrass may allow a large and well-structured root system to develop that would ensure more active and efficient acquisition of soil resources and increase the nutrient storage capacity. Unfortunately there is no information on the root growth patterns and carbon partitioning of older plants, especially at what growth stage the tillers would become the major carbon sink. Actually most of the information available is for plants that have already reached maturity (4 or more years old), but in general mature plants follow a similar pattern to the one described above. The whole function of the plant is then determined by the canopy architecture and carbohydrate allocation [5]. For example, Garten et al. [59] indicated that the root-to-shoot ratio of a 4-year-old switchgrass stand averaged over four cultivars changed from 5.8 at the beginning of the growing season (April) to 0.76 at mid season (July) and to 0.77 at the end of the season (October). In contrast to the initial growth stages of switchgrass seedlings where most of the seed reserves are allocated to the development of a vigorous root system, in the case of mature plants a well-developed root system is already present where most of the nutrient reserves, mainly N and nonstructural carbohydrates, are accumulated and therefore able to sustain a rapid growth of the aboveground parts of the plant in the following season. In mature plants, starch is the primary and most dynamic nonstructural carbohydrate stored in the roots. Sucrose is secondary in importance [60].
The dynamic root growth throughout a growing season is heavily influenced by the annual harvest/clipping practices of the aboveground biomass, but typically the root system of switchgrass continues to grow even until advanced autumn, probably due to the continuous production of rhizomes. In fact, the highest root biomass production occurs at the end of the growing season (from midsummer to autumn; [59]), while aboveground biomass production is maximized from spring to midsummer. In central Iowa, the largest mass of fine (0–2 mm diameter) and probably small roots too (2–5 mm diameter) were found between August and October at 6- and 7-year-old plantations, while the lowest root mass was found in May [61]. Similarly, Xu et al. [62] found uninterrupted growth of root mass throughout the season (from April to November) and throughout the whole soil profile (from 0 to 1.50 m depth) in a 5-year-old switchgrass plantation in Northwest China. These observations suggest that an increased root system of switchgrass is not limited to shallower layers and that root growth terminates well beyond the flowering stage.
Roots of mature switchgrass plants can reach more than 3 m in depth [63], but the bulk of the roots is commonly found in the upper 1 m of the soil profile [61, 63, 64]. In a few of the studies available that measured the root length density (RLD) of switchgrass, Monti and Zatta [64] reported a RLD of 311 cm cm−3 and that only 35% of the root mass was located in the top 0.35 m of the soil, while at lower layers, up to 1.2 m, roots were more uniformly distributed. In the study of Ma et al. [63], however, more than 68% of the roots were found in the top 0.15 m of the soil. Similarly, Bolinder et al. [65] indicated that at least 78% of the roots were located in the top 0.15 m of the soil at the peak standing crop growth stage. Garten and Wullschleger [66] indicated that up to 94% of coarse root mass (>2 mm diameter) was located in the upper 0.4 m of soil. These results differ from those of Monti and Zatta [64] possibly due to the different soil types and cultivars used in each study.
Soil respiration at a switchgrass plantation, as with any other species, can be related to live roots that directly contribute to soil respiration and dead roots and exudates that provide energy and nutrients for microbial respiration. However, there is limited information on switchgrass root respiration and turnover rates. Tufekcioglu et al. [67] and Frank et al. [68] reported similar soil respiration patterns throughout the growing season of several switchgrass cultivars; they concluded that soil respiration increased rapidly from winter to midsummer and then decreased rapidly toward the autumn. Such seasonal changes were highly related to temperature changes and, to a lower degree, to soil moisture changes. Moreover, Tufekcioglu et al. [67] indicated that the annual soil respiration rates were strongly related to the production fine roots (<2 mm) and soil organic carbon, suggesting that the large resources allocated to produce an extensive fine root system not only increase the resource capture capacity of the plant but also the soil carbon inputs through root turnover. Frank et al. [68] indicated that about half of the carbohydrates captured in plant biomass during a growing season is lost through soil respiration. Garten and Wullschleger [69] estimated the range of turnover to be between 2.4 and 4.3 years for particulate organic matter and between 26 and 40 years for more recalcitrant mineral-associated organic matter.
Water uptake capacity and efficiency of switchgrass roots seem to be directly related with RLD but independent of root distribution along the soil profile ([64]; Fig. 3.3). Thus water may passively move into switchgrass roots in response to water potential gradients, rather than actively pumping solutes in order to create an osmotic gradient, in the cell-to-cell pathway as the wetting front moves downwards. A general feature of drought-resistant crops is a deep root system which facilitates access to deep-moist soil layers. Eggemeyer et al. [70], using the stable isotopes of hydrogen and oxygen, determined the water sources of switchgrass in the Sandhills grasslands of Nebraska. In agreement with the results shown in Fig. 3.3, Eggemeyer et al. [70] found that throughout the growing season switchgrass mainly extracts water from the upper 0.5 m of the soil profile. During the dry period (August), they found that water uptake increased at deeper layers but the amount acquired was insignificant in relation to the total amount of water used by the crop. The reason for the limited contribution was not discussed, but reduced hydraulic conductivity due to lignification of deep roots may be excluded since Garten et al. [59] indicated that lignin concentration in deep coarse and fine live switchgrass roots was lower than that in shallower layers. Hence, the amount of available water at deep layers might be the limiting factor.
In general, switchgrass has low nutrient amendment requirements compared to annual cereals, mainly because a pool of nutrients is recycled/conserved annually. The long-lived rhizomes (up to 10 years) may act as sites of nitrogen and carbon storage [71]. Several authors indicated that nutrients and nonstructural carbohydrates are translocated from the canopy to the crown/root system at the end of each growing season (after anthesis but before killing frost) and vice versa during resprouting [60, 72–74]. This is corroborated by Reynolds et al. [75], who found a higher nitrogen concentration in aboveground biomass of diverse switchgrass genotypes when harvested in mid season than when harvested during the fall. Moreover, in the fall harvest more nitrogen was present in the aboveground biomass in a double harvest system than in a single harvest system, probably because in the double harvest system the re-grown tillers were at a younger stage, and translocation of nutrients to roots was interrupted because they did not reach full senescence [75]. In a single harvest system, Garten et al. [59] indicated that nitrogen reserves in the root system declined to 1.4 g N m−2 due to acropetal translocation during the period of fast growth of the canopy. On the other hand, about 50% of the nitrogen fixed in the aboveground biomass was translocated to the roots by the time the plants had become dormant [59]. Lemus et al. [72] estimated that the total amount of nitrogen remobilized from roots to shoots and vice versa may range from 40 to 100 kg N ha−1. Griffin and Jung [76] reported that phosphorus levels in switchgrass and big bluestem decreased with maturity. They found that stem tissue phosphorus content declined from an average of 0.24–0.14% with increasing maturity. Smith and Greenfield [77] reported the highest phosphorus accumulation in the inflorescence. Radiotis et al. [78] reported 0.12% phosphorus in switchgrass tissues at the reproductive stage, and it declined to 0.04% when it was left to overwinter. Seasonal recycling of phosphorus, potassium, and other nutrients follow more or less a similar pattern to nitrogen [5, 73], but instead of translocation, the main recycling mechanisms may be related to leaching from senesced/dead leaves within the switchgrass extensive rooting zone. In either case, nutrients are returned to the soil during the winter, helping to maintaining soil fertility and reducing fertilizer requirements.
3.3.4 Crop Modeling
Field trials for the herbaceous energy crop switchgrass are beginning to provide valuable insights into the climatic, genetic, soil, and management practices that govern the production of biomass for this species [79–81]. Bioenergy crop models are a useful tool for summarizing information gained through field studies and for understanding the potential supply, resource utilization, and environmental impacts associated with the large-scale expansion of bioenergy crops [82].
Bioenergy models for many first- and second-generation energy crops, including switchgrass, can be broadly classified into empirical and mechanistic models. Empirical models are developed using statistical methods that establish relationships between biomass yield and biophysical and agronomic variables. Wullschleger et al. [83] developed an empirical biomass yield model for switchgrass using 39 field trials conducted across the United States. A nonlinear parametric model was used to determine the relationship between biomass yields, with physical, climatic, and management variables such as precipitation, temperature, nitrogen fertilization, and ecotype (lowland and upland cultivars). Results showed that lowland cultivars produced 1.5 times more biomass than did upland cultivars. Temperature, precipitation, and nitrogen application during the season showed a significant and positive effect on biomass yield. Jager et al. [80] further explored the determinants of biomass yield in switchgrass cultivars by developing an ecotype-specific empirical model based on a slightly expanded dataset from that used by Wullschleger et al. [83]. The results showed that the responses to several biophysical and management variables were different for lowland and upland ecotypes. The impact of growing season temperature and precipitation on the production of biomass was greater for lowland than for upland ecotypes. The minimum winter temperature showed a positive response on biomass yield of both ecotypes but was highly significant only for biomass yield of upland ecotypes. Applied nitrogen also showed a positive and significant response on biomass yield of lowland ecotypes. A significant and positive response to soil moisture was found for upland but not for lowland ecotypes.
In contrast to empirical models, mechanistic models provide details of under-lying physiological and morphological processes and their interactions on crop yield. Two models in particular are widely used in switchgrass, ALMANAC and EPIC. ALMANAC was developed to understand crop growth and yield across varied environments by accounting for competition from other crops/weeds and abiotic stresses [84], while EPIC was developed to account for the environmental impact of production practices along with biomass yield estimation [85, 86]. EPIC, which simulates switchgrass growth and development, is a process-based model capable of simulating a wide array of ecosystem processes including plant growth, crop yield, water and nutrient balances and soil erosion. ALMANAC is related to EPIC in many ways; ALMANAC uses biophysical subroutines and process descriptions from EPIC with additional details for plant growth processes and is capable of simulating several crops including switchgrass [87]. Inputs for both models are consistent with field measurements gathered for field crops including leaf area index, radiation use efficiency, carbon gain and allocation, and phenological stages of development. Thus, crop models for switchgrass are relatively easy to parameterize and interpret. Biomass produced under a range of scenarios can be derived using EPIC or ALMANAC at individual sites [88, 89] or larger spatial scales more suitable for regional analyses [82, 90].
Although mechanistic models require more extensive parameterization, they are widely used to forecast yields at spatial scales from local to regional level and, in some instances, to relate the production of biomass to other environmental consequences including soil erosion and water quality. This information is essential for developing a technically feasible, environmentally sound, and economically viable supply of bioenergy. Models that allow users to obtain reliable estimates of bioenergy crop production provide an essential framework to understand site-specific information that in turn can facilitate pragmatic decisions regarding the suitability of certain regions where bioenergy crops can be sustainably produced and any environmental benefits or consequences associated with that production.
3.4 Resource Use Efficiency
3.4.1 Radiation Use Efficiency
Radiation use efficiency (RUE) can be defined as the biomass production per unit light interception. Monteith [91] was the first to provide a strong and convincing theoretical foundation for this parameter by demonstrating experimentally a robust relationship between light interception and stress-free biomass production for several agricultural crops. Since then, RUE has been considered a crop-specific parameter and a widely used efficiency measure for comparing plant productivity across different crops and management practices.
Field experiment-based RUE measurements for switchgrass are very limited, and any available data covers only North America. In general, RUE is higher for switchgrass as compared to other traditional cultivated crops. A mean RUE value of 4.7 g MJ−1 intercepted photosynthetically active radiation (IPAR) was reported for Alamo switchgrass and 3.7 g MJ−1 IPAR was reported for maize in Texas [92]. This is not surprising because switchgrass possesses ideal qualities that support high RUE, such as C4 photosynthesis, a high leaf area index, and a low light extinction coefficient [93]. In general octoploid switchgrass cultivars have higher leaf gas exchange rates than tetraploid ones (Table 3.3), and this is attributed to the greater activity of RuBP carboxylase, PEP carboxylase, and NAD-malic enzymes and concentration of biochemical constituents and smaller cell size [94]. However, Wullschleger et al. [38] indicated that, more than ploidy level, photosynthetic rates in either type are determined by seasonal changes and/or water stress.
RUE measurements vary widely across switchgrass cultivars, growing locations, growing seasons, and management practices. Mean RUE value for Alamo switchgrass ranged from 3.04 g MJ−1 IPAR for the high plains of Texas to 5.05 g MJ−1 for Missouri [93]. The same switchgrass cultivar had different RUE values for two different growth periods: 3.2 g MJ−1 for 1995–1997 and 4.4 g MJ−1 IPAR for 2008–2010 [93, 95]. Madakadze et al. [96] reported that RUE values vary across different upland switchgrass cultivars in Canada: 1.98 g MJ−1 IPAR was reported for the cultivar Sunburst to 2.38 g MJ−1 IPAR for the cultivar Cave-in-Rock. Heaton et al. [97] showed that under North America conditions (IL), RUE for Cave-in-Rock can be as low as 1.2 g MJ−1 IPAR. Kiniry et al. [93] conducted a comparative study of different cultivars for RUE measurements in Missouri. Results of this study revealed that mean RUE for the cultivar Cave-in-Rock was 3.17 g MJ−1 IPAR, which is below the mean for the lowland cultivar Alamo (4.3 g MJ−1 IPAR) but noticeably higher than the earlier report for Cave-in-Rock from IL by Heaton et al. [79]. Management practices also play a major role for different RUE values for the same switchgrass cultivar. Under irrigated conditions, Alamo exhibited higher RUE as compared to the water deficit condition, and the relative reduction in RUE under a water deficit environment was greatest for fields with higher plant density than lower plant density [93, 95].
Modeling based on RUE is considered to be the most preferred approach for crop growth modeling because of the sheer simplicity in factors needed and its straightforward implementation [98]. Two commonly applied crop simulation models for switchgrass such as EPIC and ALMANAC are based on a RUE approach for biomass simulation. In ALMANAC, a RUE value of 4.7 g MJ−1 IPAR was used for simulating switchgrass biomass at several sites across the United States [87–89].
3.4.2 Water Use Efficiency
Switchgrass is often described as a drought-tolerant, warm season perennial due to its deep roots and C4 metabolism. Physiologically, C4 plants are known to efficiently use water through stomatal control of transpiration. As indicated before, root systems of perennials, especially C4 grasses, can access water stored deep within the soil profile and thus extract more water at depth than annual crops [99]. Although this rooting leftacteristic could translate to higher rates of water use for switchgrass, few studies report estimates of either water use or water use efficiency (WUE) for this emerging bioenergy feedstock. Early investigations by Stout et al. [100] and Stout [101] measured WUE for switchgrass cultivated in the northeastern United States. In their studies, WUE for the cultivar Cave-in-Rock was 25.0 kg ha−1 mm−1 for summer-growth switchgrass. More recently, Hickman et al. [102] compared rates of evapotranspiration, water use, and WUE for switchgrass, maize, and miscanthus grown in Illinois. Rates of water use exceeded 750 mm whereas WUE was 9.7 kg ha−1 mm−1 for the cultivar Cave-in-Rock grown in Illinois.
While information on water use and WUE is sparse, the database compiled by Wullschleger et al. [83] can be used to derive estimates of WUE for a large number of lowland and upland cultivars of switchgrass. Here annual biomass production can be analyzed along with growing season precipitation to obtain a reasonable estimate of WUE. Across the entire database, which includes almost 1,200 observations of biomass and precipitation for 25 upland and 14 lowland cultivars, WUE averaged 21.6 with a range from 2.3 to 103.1 kg ha−1 mm−1 (Fig. 3.4). Sixty-eight percent of the observations fell within the range of 10 to 30 kg ha−1 mm−1 interval. Separation of the data revealed that WUE was, on average, higher for lowland than for upland cultivars (Fig. 3.5). WUE for lowland cultivars averaged 25.6 (Fig. 3.5a), whereas WUE for upland cultivars averaged 16.2 kg ha−1 mm−1 (Fig. 3.5b).
3.4.3 Nutrient Use Efficiency
Nitrogen use efficiency (NUE) is dependent on many factors including soil nitrogen availability, uptake and assimilation, and carbon–nitrogen flux and is one of the major limiting factors in increasing crop productivity.
Although NUE can be calculated in a number of ways [103], a simple yet useful measure is biomass yield per unit of nitrogen applied to the soil. More specifically, NUE can be calculated from a series of nitrogen addition plots where annual biomass yield is determined for a range of soil nitrogen additions, including a control where no supplemental soil nitrogen is applied: NUE = (yield of Nx–yield at N0)/kg of nitrogen applied; where Nx = N rate > 0, and N0 = no N applied. Although the definition of NUE is simple, and although the response of biomass yield to applied nitrogen has been repeatedly studied for switchgrass [1], NUE for bioenergy crops including switchgrass has not been well quantified (Table 3.4). While annuals depend more on acquired nutrients for growth, perennial crops, as indicated before, may derive benefits through traits such as remobilization of carbon and nitrogen reserves in the spring that can then support growth from overwintering rhizomes or roots. Thus, perennial plants have a higher NUE than annual crops [104]. In addition, switchgrass has a higher NUE than traditional annual crops in part due to differences in harvest time and management, which allow higher rates of translocation of nitrogen to storage organs like stems and roots. Based on field trials conducted at various locations, Staley et al. [105] and Lemus et al. [72] have reported the most thorough analysis of NUE for switchgrass to date. In those studies, these authors examined the NUE, nitrogen concentration, total nitrogen uptake, and apparent nitrogen recovery for switchgrass fertilized with 0, 90, 180, or 270 kg nitrogen per hectare. Field data collected over the years revealed a diminishing return or inefficiency in NUE with higher rates of nitrogen (Table 3.4). Averaged across all treatments in the study of Lemus et al. [72], there was a yield advantage with nitrogen fertilization of about 9 kg of biomass per kg of applied nitrogen per year. These findings suggest that applying ≤90 kg nitrogen per hectare per year would provide good yields for switchgrass produced with two cuttings. In a subsequent study by these same investigators [73], it was shown that nitrogen removal exceeded the amounts of nitrogen applied in both one- and two-cut management, suggesting that nitrogen was being supplied via mineralization or other processes. Others have obtained similar results, leading Parrish and Fike [1] to conclude that switchgrass is quite efficient and inherently thrifty in its use of applied nitrogen with a capacity to obtain nitrogen from sources that other crops cannot tap. Studies are just now beginning to examine the potential shifts in microbial community composition beneath bioenergy crops and the potential exists for unknown associations of microbes that facilitate nitrogen acquisition and uptake for energy crops like switchgrass and miscanthus [106].
The high NUE of switchgrass is also in part attributed to its deep root system and its symbiotic associations with mycorrhiza. Huang et al. [107] indicated that about 22% of the total nitrogen required by the crop could be supplied by deep roots (deeper than 1.2 m). Moreover, the capacity of the root system to recover/use deep nitrogen sources changes with the season, with the maximum recovery occurring just before/during anthesis; afterwards a significant reduction was registered due to shoot senescence. In general switchgrass can uptake between 1.49 and 2.63 kg nitrogen ha−1 d−1, depending on soil nitrogen levels and nitrogen fertilization [108].
3.5 Yield Gap
Evaluations of existing commercial varieties and new cultivars have served to identify the most productive ones and some management practices that would improve productivity. Yield improvements of up to 50% were already obtained with the identification of the most suitable varieties to determined agroecological zones [90]. However, within a production system, switchgrass is usually thought of as being suitable for or allocated to marginal areas where soil resources such as water, nutrients, etc., are available at minimum levels. Such stresses reduce the crop’s growth and potential yield; therefore, increased scientific knowledge on how stresses limit the productivity of switchgrass is needed in order to move forward and further improve the productivity and sustainability of switchgrass.
3.5.1 Limitation of Productivity by Water and Salinity
Under drought conditions, switchgrass yield losses can be limited by a combination of drought avoidance and tolerance mechanisms, as with most perennial grasses [4]. Such mechanisms include the development of deep roots, a high leaf area index, pubescent and waxy leaves, changes in leaf orientation, leaf senescence, and osmotic adjustment. However, in upland and lowland ecotypes, some acting mechanisms may not be the same. For example, an interesting difference that might contribute to the reduction of transpiration losses in upland cultivars and upland x lowland switchgrass reciprocal hybrids is the presence of different amounts of pubescent hairs on the leaf blades [3, 109]. Leaf blades in lowland cultivars are more bluish and with waxy covers [95]. Adaxial leaf rolling is another defense mechanism in both ecotypes that reduces the leaf surface area (stomata) exposed to sunlight and therefore reduces the radiation load on the leaves [110, 111]. This may allow a reduction in the water stress level while remaining photosynthetically active.
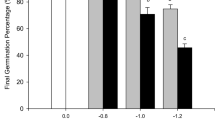
Barney et al. [26] indicated that biomass production, plant height, number of tillers, leaf area, and specific leaf area were significantly reduced (up to 80%) by severe drought and extreme drought (−4.0 and −11.0 MPa) in both upland and lowland ecotypes. In general, upland ecotypes are considered to be more drought tolerant [112], but more conclusive evidence is needed as Stroup et al. [113] did not find any significant reduction in biomass production of both ecotypes when the drought stress was less severe (−1 MPa). However, upland ecotypes were somewhat less affected than lowland ecotypes under such stress level, indicating the generic capacity of switchgrass to tolerate drought. In response to drought, both ecotypes increase the proportion of leaves with respect to the total dry matter produced [113], probably due to shorter tillers. On the other hand, even though lowland ecotypes outperformed upland ecotypes under waterlogged conditions, the reduced tiller numbers and length, leaf area, and biomass of both ecotypes were not that large, suggesting the wetland facultative properties of switchgrass [26, 114].
Several studies indicate that photosynthetic rates and leaf water potential of switchgrass are reduced to varying degrees depending on the water stress level. For example, Sanderson and Reed [115] indicated that at a soil water tension lower than −45 kPa, photosynthesis and xylem water potential are reduced by 10% and 48%, respectively. However, transpiration efficiency of diverse switchgrass cultivars was not affected by drought probably because the leaf components, mainly proteins, and enhanced transpiration efficiency [116]. Knapp [117] indicated that the photosynthetic activity of switchgrass was virtually stopped during the driest period of the season, but after a substantial rainfall, photosynthetic rates recovered to about pre-drought period values. The decreased photosynthesis in switchgrass is accompanied with a decrease in stomatal conductance and significant osmotic adjustment. The capacity of switchgrass to adjust osmotically reflects its capacity to recover from drought. Apart from that, drought-induced reductions in photosynthesis are associated with shoot nitrogen retranslocation to the roots as a probable mechanism to ensure the availability of resources for growth and survival after drought [118]. Even though variations in photosynthetic rates and ploidy levels were identified, it is not yet clear how these differences affect productivity [38].
In switchgrass, physiological growth stages are delayed by drought stress at the primary and regrowth stages [113]. Depending on the stand age and growth stage of the drought occurrence, yields would be variably affected. Sanderson and Reed [115] suggested that switchgrass is more sensitive to water stress at the seeding year than when the plants are already fully established. Moreover, in a dry year, the typical 30–37% of the total biomass concentrated in the roots can be increased up to 60–73% [95] as a response mechanism to limited water availability. Then the increased root growth may ensure a better plant water status and improved nutrient acquisition.
In general, salt stress reduces seed germination, stand establishment, and yield of perennial grasses, such as switchgrass, to varying degrees [4]. Information on the salt tolerance of switchgrass ecotypes, however, is almost nonexistent. Most of the limited available information is focused on germination and seedlings establishment but not on mature plants, except for a study (as far as we know) that found lowland Alamo to have moderate tolerance to salinity [119]. Aboveground biomass yield of switchgrass was only 29% of the control when NaCl was applied in a 2.65 M solution for 5 weeks in pots [120]. Dkhili and Anderson [121] tested the effects of soil salinity (1.1, 6.5, 9.8, and 14.9 dS/m) in combination with different amounts saline irrigation water (0, 4, and 8 dS/m) on pathfinder switchgrass seedlings growth. Their results showed that switchgrass seedlings cannot survive soil salinity levels of 14.9 dS/m or irrigation water with an electric conductivity of 8 dS/m. Moreover, even slight soil salinity levels (6.5 dS/m) delayed emergence, decreased percentage of emergence, reduced seedling height, and reduced dry matter production of aboveground and belowground organs. However, the interactions between saline irrigation water and soil salinity decreased the salt effects as the amount of irrigation increased. Similarly, Kim et al. [122] indicated that the growth and development of Cave-in Rock switchgrass started to show the effects of salinity and ion imbalances in plant tissues at around an electric conductivity of 5 dS/m.
Although switchgrass increases the size of its stomata and develops salt glands to excrete salt excess, these response mechanisms to salinity seem to not function well as large amounts of sodium accumulate in the roots and shoots, even when exposed to moderate salinity levels [122].
3.5.2 Limitation of Productivity by Nutrients
Plant nutrients are essential for the growth and development of the different plant parts and for their correct functioning. Nitrogen is mostly involved in enzymatic processes and in proteins. Biomass productivity of upland and lowland switchgrass ecotypes is mainly determined by nitrogen availability rather than by water [113]. The same authors indicated that at low levels of nitrogen (10 kg ha−1) the plants were so small that their water requirement never reached stressful levels even though their water supply was limited. Similarly, Sanderson and Reed [115] indicated that well-watered nitrogen-deficient plants developed a higher soil water tension than droughted high-nitrogen-fertilized plants, probably because the smaller transpiring canopy of nitrogen-deficient plants. Other studies, however, indicate that shortage of water may be the most important limiting factor for switchgrass growth in semiarid regions [123, 124] probably because nitrogen mobility decreases substantially as soil moisture decrease. In any case, Stout et al. [123] showed evidence that when precipitation was evenly distributed, nitrogen level accounted for 80% of the variation in yield and water use efficiency.
Nitrogen-deficient plants are chlorotic, with lower photosynthetic rates, lower growth rates, and lower aboveground and belowground resources acquisition capacity [4, 115, 113]. Suplick et al. [125] reported that the LAR and LER in switchgrass respond to increasing nitrogen fertilization in a quadratic fashion, with the highest rates around 164 kg nitrogen ha−1. The lower LAR and LER rates at lower nitrogen levels than the aforementioned threshold could be attributed to reduced cell division rather than reduced cell elongation [126]. Because nitrogen deposition in the growing zone (cell division zone) of elongating leaves is reduced at low nitrogen levels, LER and therefore yield are reduced due to lower number of cells produced [127]. Moreover, Suplick et al. [125] found that LER was highly correlated with the total dry biomass production. However, Stroup et al. [113] suggested that the reduced partitioning of carbohydrates to stems and sheaths, commonly associated with nitrogen limitation, is the main reason for reduced yields at low levels of nitrogen. On the other hand, high nitrogen fertilization rates result in increased weight and number of tillers [113].
Apart from storing nitrogen in the roots for regrowth the following spring, perennial grasses such as switchgrass remobilize nitrogen to the roots when availability of external sources decline [128]. However, this nitrogen reserve could be completely depleted if additional nitrogen sources are not made available. Although no signs of deficiency were reported, Lemus et al. [72] demonstrated that in the course of 3 years the internal root-stored nitrogen reserves accumulated during the first year decreased from 1.05 to 0.50% in the following years in the absence of fertilization following crop establishment. They also speculated that 0.50% may be the lower limit before inadequate nitrogen levels start to affect productivity.
Currently there is not much information on the effects of phosphorus deficiency on switchgrass productivity. Brejda [129] and Muir et al. [56], among others, reported little or no yield response of switchgrass to phosphorus fertilization, while Parrish and Fike [1] indicated that switchgrass is inherently thrifty in the use of applied phosphorus. Phosphorus provides plants with, among other things, a means of using the energy harnessed by photosynthesis to drive its metabolism [130]. According to Mills and Jones [131], concentrations between 0.8 and 1.7 g kg−1 of phosphorus are sufficient for optimal switchgrass growth. In general, phosphorus is a relatively immobile element in the soil, and the majority of the phosphorus absorbed is via diffusion; therefore, roots have to grow toward where the pool of phosphorus is located or other factors, such as mycorrhizae and exudation of hydroxyl ions and organic acids [132], have to intervene to make it available for the plant. Research indicates that switchgrass phosphorus uptake increased by 37 times when mycorrhizae were present [133]. The higher phosphorus uptake may be related to the enlarged root surface absorption by the symbiotic association with mycorrhizae.
Understanding the basic processes of crop resource capture (nutrients, water, etc.) and allocation have been invaluable tools for designing and evaluating agronomic management practices to improve crop resistance to stress. However, there are still many unrevealed agro-physiological leftacteristics at canopy and root level that may contribute to improve switchgrass resource use efficiency and to enrich its agronomic outcome. The establishment of a permanent switchgrass physiology program as an important part of agricultural research is urgently needed in ordered to incorporate the already acquired knowledge and further develop the scientific understanding of physiological mechanisms underpinning the control of growth and plant resources use.
References
Parrish DJ, Fike JH (2005) The biology and agronomy of switchgrass for biofuels. Crit Rev Plant Sci 24:423–459
Casler MD, Vogel KP, Taliaferro CM, Wynia RL (2004) Latitudinal adaptation of switchgrass populations. Crop Sci 44:293–303
Hultquist SJ, Vogel KP, Lee DJ, Arumuganathan K, Kaeppler S (1996) Chloroplast DNA and nuclear DNA content variations among cultivars of switchgrass, Panicum virgatum L. Crop Sci 36:1049–1052
Sanderson MA, Stair DW, Hussey MA (1997) Physiological and morphological responses of perennial forages to stress. Adv Agron 59:171–224
McLaughlin SB, Kszos LA (2005) Development of switchgrass (Panicum virgatum) as a bio-energy feedstock in the United States. Biomass Bioenerg 28:515–535
Shen ZX, Parrish DJ, Wolf DD, Welbaum GE (2001) Stratification in switchgrass seeds is re-versed and hastened by drying. Crop Sci 41:1546–1551
Zarnstorff ME, Keys RD, Chamblee DS (1994) Growth regulator and seed storage effects on switchgrass germination. Agron J 86:667–672
Sanderson MA, Reed RL, McLaughlin SB et al (1996) Switchgrass as a sustainable bioenergy crop. Bioresour Technol 56:83–93
Duclos DV (2009) Investigating seed dormancy in switchgrass. American society for horticultural science annual conference. St. Louis, Missouri, 25–28 July 2009
Jensen NK, Boe A (1991) Germination of mechanically scarified neoteric switchgrass seed. J Range Manage 44:299–301
Sarath G, Bethke PC, Jones R, Baird LM, Hou G, Mitchell RB (2006) Nitric oxide accelerates seed germination in warm-season grasses. Planta 223:1154–1164
Sarath G, Mitchell RB (2008) Aged switchgrass seed lot’s response to dormancy-breaking chemicals. Seed Technol 30:7–16
Zhang J, Maun MA (1989) Seed dormancy of Panicum virgatum L on the shoreline sand dunes of lake Erie. Am Midl Nat 122:77–87
Haynes JG, Pill WG, Evans TA (1997) Seed treatments improve the germination and seedling emergence of switchgrass (Panicum virgatum L). Hort Sci 32:1222–1226
Bentsink L, Koornneef M (2008) Seed dormancy and germination. American society of plant biologists. The arabidopsis Book. Wageningen University, The Netherlands
Parcy F, Valon C, Kohara A, Misera S, Giraudat J (1997) The ABSCISIC ACID-INSENSITIVE3, FUSCA3, and LEAFY COTOLEDON1 loci act in concert to control multiple aspects of arabidopsis seed development. Plant Cell 9:1265–1277
Sarath G, Hou G, Baird LM, Mitchell RB (2007) Reactive oxygen species, ABA and nitric ox-ide interactions on the germination of warm-season C4-grasses. Planta 226:697–708
Sarath G, Hou G, Baird LM, Mitchell RB (2007) ABA, ROS and NO are key players during switchgrass seed germination. Plant Signal Behav 2:492–493
Newman PR, Moser LE (1988) Grass seedling emergence, morphology, and establishment as affected by planting depth. Agron J 80:383–387
Elbersen HW, Ocumpaugh WR, Hussey MA, Sanderson MA, Tischler CR (1998) Crown node elevation of switchgrass and kleingrass under low light. Crop Sci 38:712–716
Hsu FH, Nelson CJ, Matches AG (1985) Temperature effects on germination of perennial warm-season for-age grasses. Crop Sci 25:215–220
Seepaul R, Macoon B, Reddy KR, Baldwin B (2011) Switchgrass (Panicum virgatum L) intraspecific variation and thermotolerance classification using in vitro seed germination assay. Am J Plant Sci 2:134–147
Vassey TL, George JR, Mullen RE (1985) Early-, mid-, and late-spring establishment of switchgrass at several seeding rates. Agron J 77:253–257
Newman PR, Moser LE (1988) Seedling root development and morphology of cool-season and warm-season forage grasses. Crop Sci 28:148–151
Hsu FH, Nelson CJ, Matches AG (1985) Temperature effects on seedling development of perennial warm-season forage grasses. Crop Sci 25:249–255
Barney JN, Mann JJ, Kyser GB, Blumwald E, Van Deynze A, DiTomaso JM (2009) Tolerance of switchgrass to extreme soil moisture stress: ecological implications. Plant Sci 177:724–732
Smart AJ, Moser LE (1999) Switchgrass seedling development as affected by seed size. Agron J 91:335–338
Wilson AM, Hyder DN, Briske DD (1976) Drought resistance leftacteristics of blue grama seedlings. Agron J 68:479–484
Xu B, Li F, Shan L, Ma Y, Ichizen N, Huang J (2006) Gas exchange, biomass partition, and water relationships of three grass seedlings under water stress. Weed Biol Manag 6:79–88
Elbersen HW, Ocumpaugh WR, Hussey MA, Sanderson MA, Tischler CR (1999) Field evaluation of switchgrass seedlings divergently selected for crown node placement. Crop Sci 39:475–479
Aiken GE, Springer TL (1995) Seed size distribution, germination, and emergence of 6 switch-grass cultivars. J Range Manage 48:455–458
Kneebone WR, Cremer CL (1955) The relationship of seed size to seedling vigor in some native grass species. Agron J 47:472–477
Casler MD (2005) Ecotypic variation among switchgrass populations from the northern USA. Crop Sci 45:388–398
Sanderson MA (1992) Morphological development of switchgrass and kleingrass. Agron J 84:415–419
Gunter LE, Tuskan GA, Wullschleger SD (1996) Diversity among populations of switchgrass based on RAPD markers. Crop Sci 36:1017–1022
Hopkins AA, Taliaferro CM, Murphy CD, Christian D (1996) Chromosome number and nuclear DNA content of several switchgrass populations. Crop Sci 36:1192–1195
Martinez-Reyna JM, Vogel KP (2002) Incompatibility systems in switchgrass. Crop Sci 42:1800–1805
Wullschleger SD, Gunter LE, Garten CT (1996) Genetic diversity and long-term stability of yield in the bioenergy crop switchgrass. Five-year summary report to ORNL biofuels feed-stock development program, Oak Ridge, Tennessee
Fike JH, Parrish DJ, Wolf DD, Balasko JA, Green JT Jr, Rasnake M, Reynolds JH (2006) Switchgrass production for the upper southeastern USA: influence of cultivar and cutting frequency on biomass yields. Biomass Bioenerg 30:207–213
Olson WW (1984) Phenology of selected varieties of warm season native grasses. In: Proceedings of the 9th North American prairie conference. 29 July 1984–1 Aug 1984, Moorhead, Minnesota
Van Esbroeck GA, Hussey MA, Sanderson MA (2003) Variation between Alamo and cave-in-rock switchgrass in response to photoperiod extension. Crop Sci 43:639–643
Taliaferro CM, Hopkins AA (1997) Breeding and selecting of new Switchgrass varieties for in-creased biomass production. Five year summary report. Oak Ridge National Laboratory, Oak Ridge
Moore KJ, Moser LE, Vogel KP, Waller SS, Johnson BE, Pederson JF (1991) Describing and quantifying growth stages of perennial forage grasses. Agron J 83:1073–1077
Sanderson MA, Wolf DD (1995) Morphological development of switchgrass in diverse environments. Agron J 87:908–915
Van Esbroeck GA, Hussey MA, Sanderson MA (1997) Leaf appearance rate and final leaf number of switchgrass cultivars. Crop Sci 37:864–870
Madakadze I, Coulman BE, Stewart K, Peterson P, Samson R, Smith DL (1998) Phenology and tiller leftacteristics of big bluestem and switchgrass cultivars in a short growing season area. Agron J 90:489–495
Redfearn DD, Moore KJ, Vogel KP, Waller SS, Mitchell RB (1997) Canopy architecture and morphology of switchgrass populations differing in forage yield. Agron J 89:262–269
Beaty ER, Engel JKL, Powell JD (1978) Tiller development and growth in switchgrass. J Range Manage 31:361–365
Murphy JS, Briske DD (1992) Regulation of tillering by apical dominance: chronology, inter-pretive value, and current perspectives. J Range Manage 45:419–429
George JR, Reigh GS (1987) Spring growth and tiller leftacteristics of switchgrass. Can J Plant Sci 672:167–174
Smith D (1975) Trends of nonstructural carbohydrates in the stem bases of switchgrass. J Range Manage 28:389–391
Moser LE, Vogel KP (1995) Switchgrass, bigbluestem and Indiangrass. In: Barnes RF, Miller DA, Nelson CJ (eds) Forages, an introduction to grassland agriculture, vol 1. Iowa State University Press, Ames
Mitchell RB, Moser LE, Moore KJ, Redfearn DD (1998) Tiller demographics and leaf area index of four perennial pasture grasses. Agron J 90:47–53
Boe A, Casler MD (2005) Hierarchical analysis of switchgrass morphology. Crop Sci 45:2465–2472
Schacht WH, Smart AJ, Anderson BE, Moser LE, Rasby R (1998) Growth responses of warm- season tallgrasses to dormantseason management. J Range Manage 51:442–446
Muir JP, Sanderson MA, Ocumpaugh WR, Jones RM, Reed RL (2001) Biomass production of ‘Alamo’ switchgrass in response to nitrogen, phosphorus, and row spacing. Agron J 93:896–901
Carman JG, Briske DD (1982) Root initiation and leaf elongation of dependent little bluestem tillers following defoliation. Agron J 74:432–435
Dalrymple RL, Dwyer DD (1967) Root and shoot growth of five range grasses. J Range Manage 20:141–145
Garten CT Jr, Smith JL, Tyler DD et al (2010) Intra-annual changes in biomass, carbon, and nitrogen dynamics at 4-year old switchgrass field trials in west Tennessee, USA. Agr Ecosyst Environ 136:177–184
Vogel KP (2004) Switchgrass. In: Sollenberger LE, Moser L, Burson B (eds) Warm-season (C4) grasses. Agron monogr 45. ASA, CSSA, SSSA, Madison, WI, USA
Tufekcioglu A, Raich JW, Isenhart TM, Schultz RC (1999) Fine root dynamics, coarse root biomass, root distribution, and soil respiration in a multispecies riparian buffer in Central Iowa, USA. Agroforest Syst 44:163–174
Xu B, Li F, Shan L (2010) Seasonal root biomass and distribution of switchgrass and milk vetch intercropping under 2:1 row replacement in a semiarid region in northwest China. Commun Soil Sci Plan 41:1959–1973
Ma Z, Wood CW, Bransby DI (2000) Impacts of soil management on root leftacteristics of switchgrass. Biomass Bioenerg 18:105–112
Monti A, Zatta A (2009) Root distribution and soil moisture retrieval in perennial and annual energy crops in Northern Italy. Agr Ecosyst Environ 132:252–259
Bolinder MA, Angers DA, Bélanger G, Michaud R , Laverdière MR (2002) Root biomass and shoot to root ratios of perennial forage crops in eastern Canada. Can J Plant Sci 82:731–737
Garten CT Jr, Wullschleger SD (1999) Soil carbon inventories under a bioenergy crop (Switchgrass): measurement limitations. J Environ Qual 28:1359–1365
Tufekcioglu A, Raich JW, Isenhart TM, Schultz RC (2001) Soil respiration within riparian buffers and adjacent crop fields. Plant Soil 229:117–124
Frank AB, Berdahl JD, Hanson JD, Liebig MA, Johnson HA (2004) Biomass and carbon parti-tioning in switchgrass. Crop Sci 44:1391–1396
Garten CT Jr, Wullschleger SD (2000) Soil carbon dynamics beneath switchgrass as indicated by stable isotope analysis. J Environ Qual 29:1–9
Eggemeyer KD, Awada T, Harvey FE, Wedin DA, Zhou X, Zanner CW (2008) Seasonal changes in depth of water uptake for encroaching trees juniperus virginiana and pinus ponderosa and two dominant C4 grasses in a semiarid grassland. Tree Physiol 29:157–169
Tufekcioglu A, Raich JW, Isenhart TM, Schultz RC (2003) Biomass, carbon and nitrogen dy-namics of multi-species riparian buffers within an agricultural watershed in Iowa, USA. Agroforest Syst 57:187–198
Lemus R, Parrish DJ, Abaye O (2008) Nitrogen-use dynamics in switchgrass grown for bio-mass. Bioenerg Res 1:153–162
Lemus R, Parrish DJ, Wolf DD (2009) Nutrient uptake by “Alamo” switchgrass used as an energy crop. Bioenerg Res 2:37–50
Zegada-Lizarazu W, Elbersen W, Cosentino SL, Zatta A, Alexopoulou E, Monti A (2010) Agronomic aspects of future energy crops in Europe. Biofuel Bioprod Bior 4:674–691
Reynolds JH, Walker CL, Kirchner MJ (2000) Nitrogen removal in switchgrass biomass under two harvest systems. Biomass Bioenerg 19:281–286
Griffin JL, Jung GA (1983) Leaf and stem forage quality of big bluestem and switchgrass. Agron J 75:723–726
Smith D, Greenfield SB (1979) Distribution of chemical constituents among shoot parts of timothy and switchgrass at anthesis. J Plant Nutr 1:81–99
Radiotis T, Li J, Goel K, Eisner R (1999) Fiber leftacteristics, pupability, and bleachability of switchgrass. Tappi J 82:100–105
Heaton E, Voigt T, Long SP (2004) A quantitative review of comparing the yields of two candidate C4 perennial biomass crops in relation to nitrogen, temperature and water. Biomass Bioenerg 27:21–30
Jager HI, Baskaran LM, Brandt CC, Davis EB, Gunderson CA, Wullschleger SD (2010) Empirical geographic modeling of switchgrass yields in the United States. GCB Bioenergy 2:248–257
Wang D, Le Bauer DS, Dietze MC (2010) A quantitative review comparing the yield of switchgrass in monocultures and mixtures in relation to climate and management factors. GCB Bioenerg 2:16–25
Zhang X, Izaurralde RC, Manowitz D, West TO, Post WM, Thomson AM, Bandura VP, Nichols J, Williams JR (2010) An integrative modeling framework to evaluate the productivity and sustainability of biofuels crop production systems. GCB Bioenergy 2:258–277
Wullschleger SD, Davis EB, Borsuk ME, Gunderson CA, Lynd LR (2010) Biomass production for the herbaceous bioenergy crop switchgrass: database description and determinants of yield. Agron J 102:1158–1168
Kiniry JR, Williams JR, Gassman PW, Debaeke P (1992) A general, process-oriented model for two competing plant species. Trans ASAE 35:801–810
Izaurralde RC, Williams JR, McGill WB, Rosenberg NJ, Jakas MCQ (2006) Simulating soil C dynamics with EPIC: model description and testing against long-term data. Ecol Model 192:362–384
Williams JR, Jones CA, Kiniry JR, Spanel DA (1989) The EPIC crop growth model. Trans ASAE 32:497–511
Kiniry JR, Sanderson MA, Williams JR et al (1996) Simulating Alamo switchgrass with the ALMANAC model. Agron J 88:602–606
Kiniry JR, Cassida KA, Hussey MA et al (2005) Switchgrass simulation by the ALMANAC model at diverse sites in the southern US. Biomass Bioenerg 29:419–425
Kiniry JR, Schmer MR, Vogel KP, Mitchell RB (2008) Switchgrass biomass simulations at diverse sites in the northern great plains of the US. Bioenerg Res 1:259–264
McLaughlin SB, Kiniry JR, Taliaferro CM, De La Torre Ugarte D (2006) Projecting yield and utilization potential of switchgrass as an energy crop. Adv Agron 90:267–297
Monteith JL (1977) Climate and the efficiency of crop production in Britain. Phil Trans R Soc Lond B 281:277–294
Kiniry JR, Bean B, Xie Y, Chen P (2004) Maize yield potential: critical processes and simulation modeling in a high-yielding environment. Agric Syst 82:45–56
Kiniry JR, Johnson MVV, Bruckerhoff SB, Kaiser JU, Cordsiemon RL, Harmel RD (2011) Clash of the titans: comparing productivity via radiation use efficiency for two grass giants of the biofuel field. Bioenerg Res. doi:10.1007/s12155-011-9116.8
Warner DA, Ku MSB, Edwards GE (1987) Photosynthesis, leaf anatomy, and cellular constituents in the polyploid C4 grass Panicum virgatum. Plant Phys 84:461–466
Kiniry JR, Tischler CR, van Esbroeck GA (1999) Radiation use efficiency and leaf CO2 exchange for diverse C4 grasses. Biomass Bioenerg 17:95–112
Madakadze IC, Stewart K, Peterson PR, Coulman BE, Samson R, Smith DL (1998) Light interception, use-efficiency, and energy yield of switchgrass (Panicum virgatum L) grown in a short season area. Biomass Bioenerg 15:475–482
Heaton EA, Dohleman FC, Long SP (2008) Meeting US biofuel goals with less land: the potential of miscanthus. Glob Change Biol 14:1–15
Sinclair TR, Muchow RC (1999) Radiation use efficiencyRadiation use efficiency. Adv Agron 65:215–265
Hall RL (2003) Grasses for energy production hydrological guidelines. B/CR/00783/Guidelines/Grassesurn 03/882. Department of Trade and Industry, New and Renewable Energy Programme—Centre for Ecology and Hydrology, London, UK
Stout WL, Jung GA, Shaffer JA (1988) Effects of soils and nitrogen on water-use efficiency of tall fescue and switchgrass under humid conditions. Soil Sci Soc Am J 52:429–434
Stout WL (1992) Water-use efficiency of grasses as affected by soil, nitrogen, and temperature. Soil Sci Soc Am J 56:897–902
Hickman GC, Vanloocke A, Dohleman FG, Bernacchi CJ (2010) A comparison of canopy evapotranspiration for maize and two perennial grasses identified as potential bioenergy crops. Glob Change Biol Bioenerg 2:157–168
Good AG, Shrawat AK, Muench DG (2004) Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci 9:597–605
Jorgensen U, Schelde K (2001) Energy crop water and nutrient use efficiency. SRC IEA Bioenergy Task 17, International Energy Agency, Tjele, Denmark
Staley TE, Stout WL, Jung GA (1991) Nitrogen use by tall fescue and switchgrass on acidic soils of varying water holding capacity. Agron J 83:732–738
Ma Y, An Y, Shui J, Sun Z (2011) Adaptability evaluation of switchgrass (Panicum virgatum L.) cultivars on the loess plateau of China. Plant Sci 181:638–643
Huang Y, Rickerl DH, Kephart KD (1996) Recovery of deep-point injected soil nitrogen-15 by switchgrass, alfalfa, ineffective alfalfa, and corn. J Environ Qual 25:1394–1400
Stout WL, Jung GA (1995) Biomass and nitrogen accumulation in switchgrass: effects of soil and environment. Agron J 87:663–669
Martinez-Reyna JM, Vogel KP, Caha C, Lee DJ (2001) Meiotic stability, chloroplast DNA polymorphisms, and morphological traits of upland x lowland switchgrass reciprocal hybrids. Crop Sci 41:1579–1583
Awada T, Moser LE, Schacht WH, Reece PE (2002) Stomatal variability of native warm-season grasses from the Nebraska Sandhills. Can J Plant Sci 82:349–355
Zhang Y, Zalapa J, Jakubowski AR, Price DL et al (2011) Natural hybrids and gene flow between upland and lowland switchgrass. Crop Sci 51:1–16
Nickell GL (1973) The physiological ecology of upland and lowland Panicum virgatum. Ph.D Dissertation, University of Oklahoma
Stroup JA, Sanderson MA, Muir JP, McFarland MJ, Reed RL (2003) Comparison of growth and performance in upland and lowland switchgrass types to water and nitrogen stress. Bioresour Technol 86:65–72
Porter CL (1966) An analysis of variation between upland and lowland switchgrass, Panicum virgatum L, in central Oklahoma. Ecology 47:980–992
Sanderson MA, Reed RL (2000) Switchgrass growth and development: water, nitrogen, and plant density effects. J Range Manage 53:221–227
Byrd GT, May PA II (2000) Physiological comparisons of switchgrass cultivars differing in transpiration efficiency. Crop Sci 40:1271–1277
Knapp AK (1985) Effect of fire and drought on the ecophysiology of andropogon gerardii and Panicum virgatum in a tallgrass prairie. Ecology 66:1309–1320
Heckathorn SA, Delucia EH (1994) Drought-induced nitrogen retranslocation in perennial C4 grasses of tallgrass prairie. Ecology 75:1877–1886
Alderson J, Sharp WC (1995) Grass varieties in the United States. CRC Press, Boca Raton
Greub LJ, Drolsom PN, Rohweder DA (1983) Salt tolerance of grasses and legumes for roadside use. Agron J 77:76–80
Dkhili M, Anderson B (1990) Salt effects on seedling growth of switchgrass and big bluestem. Proceedings of the twelfth North American prairie conference. Cedar Falls, Iowa, 5–9 Aug 1990
Kim S, Rayburn AL, Voigt T, Parrish A, Lee DK (2011) Salinity effects on germination and plant growth of prairie cordgrass and switchgrass. Bioenerg Res. doi:10.1007/s12155-011-9145-3
Stout WL, Jung GA, Shaffer JA (1998) Effects of soil and nitrogen on water use efficiency of tall fescue and switchgrass under humid conditions. Soil Sci Soc Am J 52:429–434
Thomason WE, Raun WR, Johnson GV et al (2004) Switchgrass response to harvest frequency and time and rate of applied nitrogen. J Plant Nutr 27:1199–1226
Suplick MR, Read JC, Matuson MA, Johnson JP (2002) Switchgrass leaf appearance and lamina extension rates in response to fertilizer nitrogen. J Plant Nutr 25:2115–2127
MacAdam JW, Volenec JJ, Nelson CJ (1989) Effects of nitrogen on mesophyll cell division and epidermal cell elongation in tall fescue leaf blades. Plant Physiol 89:549–556
Gastal F, Nelson CJ (1994) Nitrogen use within the growing leaf blade of tall fescue. Plant Physiol 105:191–197
Engels C, Marschner H (1995) Plant uptake and utilization of nitrogen. In: Bacon PE (ed) Nitrogen fertilization in the environment. Dekker, New York
Brejda JJ (2000) Fertilization of native warm-season grasses. In: Anderson BE, Moore KJ (eds) CSSA special pub no. 30. Native warm-season grasses: research trends and issues, Crop Science Society of America, Madison
Lemus R (2004) Switchgrass as an energy crop: fertilization, cultivar, and cutting management. PhD Dissertation, Virginia Polytechnic Institute and State University, Blacksburg, Virginia
Mills HA, Jones JB Jr (1996) Plant analysis handbook II. Micro Macro Publishing, Athens
Marschner H (1995) Nutrition of higher plants, 2nd edn. Academic Press, San Diego
Brejda JJ, Moser LE, Vogel KP (1998) Evaluation of switchgrass rhizosphere microflora for enhancing seedling yield and nutrient uptake. Agron J 90:753–758
Wullschleger SD, Sanderson MA, McLaughlin SB, Biradar DP, Rayburn AL (1996) Photosynthetic rates and ploidy levels among populations of switchgrass. Crop Sci 36:306–312
Acknowledgments
Support for Stan D. Wullschleger and S. Surendran Nair was provided by the U.S. Department of Energy, Office of Science, Biological and Environmental Research. Oak Ridge National Laboratory is managed by UT-Battelle, LLC for the U.S. Department of Energy under contract DE-05-00OR22725.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag London
About this chapter
Cite this chapter
Zegada-Lizarazu, W., Wullschleger, S.D., Surendran Nair, S., Monti, A. (2012). Crop Physiology. In: Monti, A. (eds) Switchgrass. Green Energy and Technology. Springer, London. https://doi.org/10.1007/978-1-4471-2903-5_3
Download citation
DOI: https://doi.org/10.1007/978-1-4471-2903-5_3
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-2902-8
Online ISBN: 978-1-4471-2903-5
eBook Packages: EngineeringEngineering (R0)