Abstract
The human preimplantation embryo undergoes significant changes in its physiology during in vitro development and has thus to be adequately supported. The goal of IVF laboratories is to preserve the developmental competence of the gametes and resulting embryos, and provide a safe environment during the entire IVF handling and culture procedures. During the past decade, most efforts have been focused on optimization of the culture system for oocytes and preimplantation-stage embryos, paying special attention to media and gas components that together may be considered key factors affecting the success of the assisted reproduction procedures.
This chapter will review the main aspects of oocyte and embryo culture, with a focus on current efforts to provide a more appropriate environment for embryo growth, particularly in the context of introducing new sophisticated platforms and engineered devices.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
During the pre-ovulatory period, the follicular environment nurtures growth and development of the oocyte in the dominant follicle to produce a meiotically competent oocyte capable of resuming meiosis and progressing through meiosis to metaphase II. The cumulus-corona cells that surround the oocyte are critically important in sustaining oocyte nutrition and maturation, and providing essential metabolites, hormones, and growth factors [1–4]. After exposure to the pre-ovulatory surge of luteinizing hormone, considerable changes in the organization of these surrounding layers occur. Corona cell processes retract from the oolemma, gap junctions throughout the cumulus complex are disrupted, and glycosaminoglycans, predominantly hyaluronic acid, are secreted causing considerable expansion of the cumulus corona mass. At the time of follicular rupture, this mass will protect the oocyte from exposure to the transitional chemical and physical conditions that it will encounter during its journey along the fallopian tube.
The single most important goal of the IVF laboratory is to create an environment for the gametes and resulting embryos that is focused on maximizing their safety and developmental competence. Safety and maintenance of the oocytes from the moment of follicular aspiration, through insemination and embryo growth is paramount to IVF success. Due to the extreme sensitivity of these cells, even to slight changes in environmental conditions, all IVF handling and culture procedures should minimize oocyte exposure to biophysical and chemical fluctuations.
Oocyte Collection
The Procedure
In stimulated IVF cycles, oocyte retrieval is usually performed 36 h after hCG administration by ultrasound-guided transvaginal aspiration. Several factors including variables such as pump vacuum, velocity, needle lumen size and length, follicular pressure and size, and collection technique may affect oocyte competence and should be monitored and recorded before and during the retrieval procedure (reviewed by Horne et al. [5]). During collection, a maximum vacuum pressure of about 120 mmHg is recommended to dampen the risk of damage to the oocytes [6]. Moreover, to minimize changes in temperature, the collection tubes should be kept in a tube warmer maintained at 37°C before being connected to the collection system.
The follicles are aspirated in a systematic manner with each tube containing aspirate passed off to the laboratory immediately after it is full. The adjoining laboratory examines the follicular fluid in sterile plastic dishes. Cumulus–corona–oocyte complexes (CCOC) are identified, rinsed in pre-equilibrated medium in order to remove any blood residue from follicular fluid, and the CCOCs are then transferred to dishes containing pre-equilibrated medium and incubated in a defined atmosphere (see Oxygen concentration during embryo culture below) at 37°C.
The cohort of oocytes collected after ovarian hyperstimulation represents a range of maturational stages that may have specific nutritional requirements. However, the standard IVF protocol is to use the same medium for all oocytes after collection unless some are specifically designated for in vitro maturation. The collection and holding media must contain glucose (in the range of 2.0–5.5 mmol/l) as the cumulus cells require this glycolytic substrate as an energy source. The electrolytic and the osmotic needs are met by most balanced salt solutions [7].
QA Considerations
Human oocytes are exquisitely sensitive to any environmental perturbations including both physical stresses, as well as chemical stresses such as temperature and pH fluctuations, and environmental air contamination. Temperature oscillations associated with handling oocytes outside the incubator may impair the oocyte microtubular system. Changes in spindle organization have been observed in mature human oocytes exposed to room temperature even for only few minutes [8–10]. Notwithstanding the ability of the meiotic spindle to reassemble when the temperature is reestablished, the risk of aneuploidy occurrence is potentially increased after a temperature-induced depolymerization [9].
Because of the extreme sensitivity of human oocytes, all the equipment in use (including Petri dishes and Pasteur pipettes) should be pre-warmed at 37°C (Fig. 6.1). In order to maintain a stable temperature in the dishes, the working areas (hood and microscope) and the thermo plates should be calibrated regularly. Daily, in the early morning, an external calibrated certified thermometer should be used to monitor the temperature of all the heating devices. The observed values should be reported and compared to the tolerance limits defined for each instrument. For a detailed discussion on quality management issues, see Chap. 15.
As mentioned by Mortimer et al. [11], a poorly recognized aspect of temperature maintenance is that most disposable plastic platforms are designed in a way that does not allow the medium to come into direct contact with the microscope stage. This air gap reduces the efficacy of heated stages, making it very difficult to stabilize the temperature when using standard IVF dishes.
One of the most important roles of the handling media is to prevent a pH shift.
Although there is agreement regarding the need to monitor pH during IVF culture, there seems to be a less consensus regarding the actual correct value. In 1998, Dale et al. [12] demonstrated that the baseline intracellular pH (pHi) of the human oocyte is 7.4 ± 0.1 in HCO −3 /CO2-buffered medium. Recently, a lower pH (approximately 7.30) was found to be the optimum for culturing up to the pronuclear stage [13]. Unlike cleavage-embryos that have mechanisms for pH regulation, human oocytes lack the ability to regulate their internal pH; a problem that is even more marked in the cumulus-corona-free oocyte. Therefore, excursions in the extracellular pH can easily translate into deleterious intracellular perturbations that can compromise subsequent embryogenesis. Only 2–3 h after fertilization does the oocyte begin to recover the exchanger activity and the consequent ability to regulate its pH. The extracellular pH is generated by dynamic equilibrium between the CO2 concentration in the incubator and the amount of bicarbonate in the media. For that reason, monitoring and stabilizing the extracellular pH is particularly challenging during the handling of oocytes and embryos outside the incubator.
Specific buffer systems are currently used in commercially available handling media for assisted reproduction treatment: 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES) and 3-(N-morpholino)-propanesulphonic acid (MOPS). Since concerns have been raised regarding potential detrimental effects of these organic buffers on gamete competence [14, 15], media containing bicarbonate/CO2 buffers are preferable, although they require controlled chambers to maintain a 5–7% CO2 atmosphere. However, it is essential to note that many of the adverse effects of these buffer systems are largely dependent on interactions with other compounds in the media, and are not due to toxicity of the buffers themselves [16]. Regardless of the buffer chosen, it is crucial to maintain an appropriate and constant temperature, since temperature itself may alter the buffering ability of these compounds.
Cumulus–Corona–Oocyte Complex Grading
At the end of the retrieval process, the maturational stage of the oocyte may be evaluated. Several scoring systems of the cumulus corona oocyte complex have been introduced to predict the nuclear maturity of the enclosed oocytes and identify the proper timing for insemination [17–24], although it is generally acknowledged that these assessment systems are not perfect.
Early studies from Rattanachaiyanont et al. [17], performed on oocytes collected for IVF treatment, reported no correlation between oocyte–corona–cumulus complex morphology and nuclear maturity, fertilization rate, and embryo cleavage. On the contrary, other authors reported that CCOC scoring was related to fertilization and pregnancy rates [22] as well as to blastocyst quality and development [23]. Lin and colleagues [23], proposed a grading system for CCOCs based on the morphology of the oocyte cytoplasm, cumulus mass, corona cells, and membrane granulosa cells, to select oocytes prior to insemination by conventional IVF. Five grades (Mature Group, Approximately Mature, Immature, Post-mature, and Atretic) were described. The authors reported higher fertilization rates for the oocytes belonging to the Mature Group compared to those belonging to the other groups. Moreover, the Immature Group was characterized by a higher incidence of poor morphology day 3 embryos as compared to the Mature Group.
It has also been suggested that the presence of either CCOC anomalies such as amorphous clumps, or blood clots, may be an index of a suboptimal follicular maturation [25, 26], and may impair the ability of the embryo to develop to the blastocyst stage. Oocytes from these CCOCs showed a significant alteration in the cytoplasmic texture probably related to the reduced fertilization rate obtained when those oocytes are used for insemination [26, 27]. Moreover, variations in temperature and pH as well as reactive oxygen species induced by the presence of blood clots have been suggested as responsible for the compromised competence of those oocytes [20].
Although cumulus-corona mass assessment is limited in terms of its ability to accurately predict oocyte maturity and competence, there is clearly some association between cumulus-corona disposition and meiotic status, with a fully expanded “sun burst” cumulus mass being associated with a mature oocyte (Fig. 6.2). Therefore, a careful assessment of CCOC morphology may be a useful tool to aid in oocyte selection when the oocytes are destined for standard insemination, rather than ICSI.
Insemination Procedures
Fertilization is achieved by conventional in vitro insemination procedure or ICSI according to the patients’ history and sperm parameters. Although both these procedures are well established, there is no universal agreement regarding the elapsed number of hours to perform them after oocyte retrieval. We observed that a pre-incubation period of 3 h after oocyte retrieval may improve the fertilization rate and embryo quality after ICSI [28]. Other studies have been published regarding the time of injection post-retrieval, but without reaching consistent conclusions [29–35]. Oocyte nuclear maturity can be easily assessed before ICSI by visualization of the first polar body, which is a characteristic of the mature, MII oocyte. However, nuclear and cytoplasmic maturation are acquired independently during oocyte maturation and both of them are required for normal fertilization [36]. Therefore oocyte preincubation prior to IVF or ICSI may induce cytoplasmic maturation that could eventually increase fertilization and also pregnancy rates. Balakier and colleagues [37] reported that human oocytes progressively develop the ability for full activation and normal development during the MII arrest stage. An improvement in fertilization rates was obtained when ICSI was carried out 6–8 h after the polar body extrusion. Presumably, the different optimum time intervals identified in the various studies reflect differences in patient populations and stimulation regimens used and, possibly, variations in culture systems.
Conventional insemination can be carried out using various platforms including multi-well dishes, microdrops, or tubes. Our current approach is to perform insemination in Nunc four-well dishes containing 600 μl of fertilization medium with an oil overlay. Up to three oocytes/well are inseminated with about 120,000–150,000 spermatozoa/mL. After 16–18 h of incubation, oocytes are then denuded to assess fertilization. Some studies have hypothesized a detrimental effect of prolonged oocyte exposure to spermatozoa in vitro due to the production of free oxygen radicals, present especially in high concentrations of spermatozoa [38–41]. Therefore, reducing exposure of oocytes to spermatozoa has been proposed to improve embryo viability, possibly due to decreasing potential damage from sperm metabolic waste products [42].
Embryo Culture
Media and Platforms
Efforts to improve culture conditions have resulted in a substantial breakthrough in the past 10 years, with widespread application of new approaches including the use of atmospheric oxygen (so-called low oxygen tension), elimination of toxic components, and the introduction of sequential and single medium systems to culture embryos to the blastocyst stage. As a result, culture to the blastocyst stage has become an achievable goal which, in turn, has facilitated selection of the most viable embryos. This approach allows for single blastocyst transfer with acceptably high pregnancy and birth rates, at least in selected good prognosis patients.
Embryo Culture Media Composition
In the last decade, knowledge acquired from several studies regarding embryo physiology and biochemistry has led to significant improvements in media formulations used for embryo culture, resulting in a remarkable increase in efficiency of human assisted reproduction all over the world [43].
Media for human embryo culture should contain the following basic components: pure water, common salts, plus sodium bicarbonate as a buffer, sodium salt of EDTA or another chelator, pyruvate and lactate, amino acids, macromolecules, and antibiotics. However, commercially available human embryo culture media use different concentrations of each component, and many include other constituents as well. There are marked differences even in concentrations of the simplest elements, such as potassium chloride and magnesium sulphate [44]. Similarly, the optimal osmolality for development of human embryos in culture has not yet been determined. Moreover, almost all media require supplementation with chemically undefined or partially defined factors as albumin or synthetic serum substitutes.
The composition of the majority of IVF media is based on one of three physiological salines: Earle’s balanced salt solution, Krebs-Ringer bicarbonate, and Tyrode’s solution [45–47]. So far, two major approaches have been used to determine the media composition and concentration of each compound. The first approach is the “empirical optimization” of components by bioassays—also known as “let the embryos choose” principle—established by Biggers and colleagues [48]. The common principles of this approach are: (1) that only a single medium is used to support development from the zygote to blastocyst stage; and (2) the concentration of constituents is defined according to bioassays using a systematic approach to measure the response of embryos to several combinations and concentrations of test components. The concentration and type of component selected for the medium is usually that which gives a maximum response. One of the limitations of the “empirical optimization” approach is the theoretical requirement of astronomic numbers of experiments and some compromises in the interpretation of the mathematical models to determine the most suitable medium composition [48, 49]. Nevertheless, KSOMAA, the optimum medium for mouse embryo culture, was developed using this approach (reviewed by [49]), and a slightly modified version of this formulation (Global medium) is a very effective medium for culturing human embryos to the blastocyst stage.
In contrast to the “let the embryos choose” approach, the “back to nature” approach uses the concentration of a substance that approximates the concentration to which the embryo is naturally exposed [50]. This approach was introduced by Leese [51]. The major problem with this principle is the extremely low amounts of the fluids in the oviduct and uterus available for assay, and the technical and ethical problems related to its collection and measurement. So far, investigations have been performed in vivo (by micropuncture, chronic, or acute in situ cannulation) or in vitro (by vascular and luminar perfusion). However, the composition of oviduct and uterine fluids likely differ from the in vivo microenvironment around the embryo [52].
Numerous studies supporting the efficacy of both sequential and single media have been published [48, 49, 53–62], and the overall weight of the evidence indicates that probably neither system is superior to the other for growth of human embryos to the blastocyst stage. Indeed, both systems are used worldwide, with each having potential advantages and disadvantages (reviewed by Vajta et al. [63]). As a side note, since most of the data used for development of commercially available human media are derived from experiments performed on animal embryos, it is likely that a more intensive dialogue between human and domestic animal embryologists may eventually improve the composition of human media [64].
Oxygen Concentration During Embryo Culture
Until recently, human embryos have been cultured under atmospheric oxygen (20%), a procedure adapted from earlier somatic tissue culture protocols [65]. However, in the early 1990s three different research groups observed the beneficial effect of reduced oxygen concentration (5%) on embryo development in a protein-free medium without somatic cell support [66–68].
Supporting evidence of using reduced oxygen concentrations for human embryo culture is the low oxygen concentration measured within the oviduct and uterus of different mammalian species (2–8%) [69–71]. Moreover, by continuous assessment of embryo development, using time-lapse microscopy, the temporal effect of atmospheric oxygen on embryo development has been studied and the embryos response to either static or changing concentration of oxygen has been evaluated [72]. Authors have showed detrimental effects of atmospheric oxygen on mouse embryos during in vitro culture, as reflected by slower cleaving embryos and decreased cell numbers in cleavage-stage embryos, and poorer blastocyst development [72]. Compared with embryos cultured in 5% oxygen, embryos cultured in 20% oxygen were delayed at the first cleavage by 0.45 h (P < 0.05), at the second cleavage by 0.84 h (P < 0.01) and at the third cleavage by 3.19 h (P < 0.001). Importantly, these detrimental effects of atmospheric oxygen were irreversible, as switching the embryo to reduced oxygen concentration for the second 48 h of development (post-compaction) did not alleviate the developmental perturbations induced during the initial 48 h. A prospective, randomized study conducted by Waldenstrom et al. [73] on human embryos showed that blastocyst culture with low-oxygen (5%) versus high-oxygen (19%) concentration yielded a higher conversion rate to blastocyst and a marked improvement in birth rate. Recently, a meta-analysis has been accomplished to clarify whether or not the low O2 concentration significantly improves clinical outcomes compared to atmospheric O2 concentration [74]. When embryos were transferred on days 5 and 6, the meta-analysis showed a statistically significantly higher implantation rate in the group of embryos cultured at low oxygen tension as compared with those cultured in 20% oxygen (P = 0.006) [74].
The above published data suggest that, unless a future strong contra-indication is documented, low oxygen concentration should be a principle for culture of human embryos in all ART laboratories. This is one of the few questions where a definite answer is available and a worldwide consensus is currently being formed. However, a further decrease in oxygen concentration below 5% may have negative consequences. In fact, setting the oxygen concentration at 2%, although leading to increased blastocyst rates, may cause developmental abnormalities in ruminants [75]. On the other hand, there is no evidence that embryos need a continuous gas supply. A gas mixture volume of 50 ml in a closed system generously covers the requirements of 200 bovine embryos for 1 week, from the zygote to the blastocyst stage [76].
Embryo Culture Platforms
In sharp contrast to the widespread research regarding the design and utilization of optimum culture media, very little attention has been paid to devices used for embryo culture.
In routine IVF, gametes and embryos are cultured on inert surfaces such as glass or plastic polymers of varying configuration considered as “static” platforms, since they do not employ active means to agitate or stimulate embryo or media movement [77]. Usually, the media preparation consists of placing media in disposable polystyrene multiwell or Petri dishes, in 10–80 μl drops covered with oil with a subsequent overnight equilibration in the proper gas mixture at 37°C to stabilize the pH, temperature and achieve proper saturation with gasses. In most systems, the medium is changed on Day 3 (Day 0 being the day of oocyte retrieval), whether to a fresh drop of the same medium (the one-step system) or to a drop of a second growth medium (the sequential system). As discussed above, there is no consensus as to whether one of these systems affords an advantage over the other. As previously described [78], what is perhaps of greater importance is the fact that fundamental differences exist between these conventional culture systems and the oviduct. During the progression through the female tract, embryos are exposed to changing components of oviductal fluid. In addition, embryos in vivo develop in a dynamic environment and are subjected to changing gravitational positions. In contrast, in the in vitro environment, embryos are submerged in modified salt solutions, where autocrine factors are often diluted, and some of them diffuse into the oil layer; any change in composition of media occurs only once during the culture period, and not necessarily according to the proper metabolic needs of the embryo; no dynamic movements are ensured. Moreover, most dishes are not developed for embryological purposes, and the standard embryo culture system is based on monolayer culture methods developed more than 50 years ago for primary cultures of somatic cells and cell lines.
Fortunately, serious efforts are currently being focused on exploring the physical requirements of the embryo in the hope of optimizing embryo development in vitro. New culture platforms have been developed utilizing lower volumes of media with a limited surface area since it has been found in many animal models that co-culture of embryos in reduced volumes improves development, potentially through secretion of autocrine/paracrine factors [79–81]. However, this approach requires careful attention in media handling since shifts in pH and osmolality are more frequent.
In order to exploit the potential beneficial effects of increased embryo density, other platforms that combine the communal effect while allowing individual identification of embryos have been established. The Well of the Well or WOW system consists of small microwells produced on the bottom of a culture dish aiming to create a small microenvironment for individual embryos, while allowing them to share a larger common culture media reservoir above [80–82]. Improvement in the percentage of embryos developing into blastocysts can be achieved in WOWs compared to traditional cultures (56% vs. 37%, respectively), and promising pregnancy and birth rates have also been reported [82].
As an alternative to microwells, use of microchannels has been proposed for culturing embryos. The Glass Oviduct or GO system proposed by Thouas [81] is based on an open-ended 2 μl sterile capillary with 200 μm inner diameter. The GO system is an extremely simplified and static version of the microchannel system that allows embryos to be cultured vertically promoting increased cell contact.
Recently some special specialized surface-coated dishes have been proposed as intriguing means of potentially improving current in vitro embryo culture systems (reviewed by Swain and Smith [77]), however their application remains modest. Special surface treatments of dishes do not seem to have obvious benefits; therefore, further studies are required before drawing any conclusion regarding their true potential.
In order to implement a radically new embryo culture system, the possibility to employ dynamic culture platforms has been investigated, including those specifically engineered to stimulate controlled media flow/movement. Macroscopically, the usual microfluidic device consists of the following parts: a glass microscopic slide base; a plastic (for example polydimethylsiloxane) layer with the channels and valves; and connections to mechanical or pneumatic pumps. Sporadic and isolated attempts in the past decade to improve culture systems have demonstrated that microfluids are suitable to perform almost all steps of human IVF varying from selection of motile spermatozoa, oocyte cumulus removal, in vitro fertilization by insemination and embryo culture [83–85]. Initial steps to assemble the isolated steps into a production line have also been successfully performed. Accordingly, there is a chance to make complex procedures completely automated including the whole human IVF laboratory process [83]. The only unproven step that would need to be adopted into the microfluidic system is ICSI; however, this procedure may ultimately be replaced with alternative solutions, or performed in a semi-automated way [86].
Embryo Grading and Selection
Static Morphology Evaluation
Embryo morphological grading remains the standard method for evaluation and selection because of its simplicity and cost-effectiveness, and due to the failure of recent “-omics” technologies (metabolomics, transcriptomics, and proteomics) to improve selection (reviewed by [87]). Different morphological criteria for cleavage embryo assessment have been described through the years and a variety of characteristics have been proposed as indicative of embryo viability: early cleavage [88–108], cleavage rate [95–98], blastomere size [96, 97, 99], presence of multinucleation [99–104], extent of fragmentation [96, 97, 105–109] and distribution of fragments [107, 108].
One of the most critical factors in the evaluation of cleavage-stage embryos is the strict timing for the assessment. For standardization, a European consensus was reached to perform the 2-day and 3-day evaluation respectively at 44 ± 1 and 68 ± 1 h post insemination [110]. Early cleavage in two daughter cells has been associated with higher development and pregnancy and implantation rates [88, 90–93], indicating its use as a valuable additional embryo selection criterion.
A great number of embryo morphology scoring systems have been proposed [111–115]. However, at present the lack of standardization (in the nomenclature used as well as the number of characteristics considered and the calculated threshold values) is an obstacle for an easy and unequivocal interpretation of the different results. Therefore, two consensus groups have proposed standardized systems for staging the embryos, one group from the Society for Assisted Reproductive Technology/American Society for Reproductive Medicine (SART/ASRM) [116], the other from Alpha [110]. In addition, both groups proposed the simple categories of “good,” “fair,” and “poor” as related to embryo quality [110, 116], and the Alpha group further defined these categories with regards to blastomere number, degree of fragmentation, extent of blastomere asymmetry, and presence of multinucleation (Fig. 6.3) [110].
Day 3 embryos of varying quality: (a) Good quality, characterized by mild fragmentation (<10%), stage-specific cell size and absence of multinucleation; (b) Fair quality, characterized by the presence of moderate fragmentation (10–25%), stage-specific cell size for the majority of blastomeres and no evidence of multinucleation; (c) Poor quality, characterized by severe fragmentation (>25%), cell size not stage-specific and evidence of multinucleation
It has been also suggested that the capacity of the embryo to reach the blastocyst stage could have additional prognostic value in identifying the best embryo(s). Indeed, increases in pregnancy and implantation rates have been reported after both fresh and cryopreserved blastocyst transfers [117–121]. However data are still controversial, since some authors found comparable results after cleavage embryo transfers [113, 122, 123]. Moreover, a large number of embryos fail to develop to the blastocyst stage in extended culture and it is not possible to know which of these embryos would have implanted if they had been replaced earlier [113, 124].
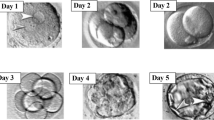
Since blastocyst development is dynamic, grading should be evaluated 112–114 h post insemination when a defined inner cell mass, a blastocoel cavity, and a ring of evenly spaced and sized trophectoderm cells should be observed [110].
Different scoring systems for blastocysts have been described [125–127] however, the most commonly used is that described by Gardner et al. [125]. The authors defined an alphanumeric scoring system on the basis of degree of blastocyst expansion and hatching status, the development of the inner cell mass and the development of the trophectoderm (Figs. 6.4 and 6.5).
Blastocyst grading according to Gardner and Schoolcraft. Blastocysts are classified by a numerical score from 1 to 6 on the basis of their degree of expansion and hatching status: (1) an early blastocyst with a blastocoel that is less than half of the volume of the embryo; (2) a blastocyst with a blastocoel that is half of or greater than half of the volume of the embryo; (3) a full blastocyst with a blastocoel completely filling the embryo; (4) an expanded blastocyst with a blastocoel volume larger than that of the early embryo, with a thinning zona. For fully developed blastocysts, the development of the inner cell mass is assessed: (A) tightly packed, many cells; (B) loosely grouped, several cells; (C) very few cells. The trophectoderm is assessed according to the number and appearance of trophectoderm cells: (A) many cells forming a cohesive epithelium; (B) few cells forming a loose epithelium; (C) very few large cells. Adapted from Sakkas and Gardner, Textbook of Assisted Reproductive Techniques: Laboratory and Clinical Perspectives, Second Ed.
Time-Lapse Imaging
One major limitation to classic morphology grading is the static evaluation of the embryos at one, or at the most, a few discrete time points. Continual monitoring by means of time-lapse cinematography allows noninvasive, dynamic imaging of embryo morphological changes and permits correlations to be made among morphokinetics, further development and clinical fate. Indeed, timing of different embryonic developmental events post-insemination has been proposed as an additional criterion in embryo selection [128–130]. A recent study revealed that an optimal time range (time window) exists for every early cell division, supporting the hypothesis that viable embryos undergo tightly regulated cellular events [130].
Various combinations of different morphological criteria, from the oocyte, to the cleavage-stage embryo, to the blastocyst have been evaluated, and many have proven to have predictive power for selecting developmentally competent embryos (reviewed by [87]). Indeed, morphological grading remains the first-line method for evaluation and selection of embryos in clinical IVF.
Noninvasive Media Profiling
Improving knowledge about gamete and embryo physiology should allow the identification of novel markers of embryo quality that may be useful as additional selection criteria. In this regard, targeted approaches that measure specific components in the culture medium (such as amino acids [131]), as well as the application of recent “-omics” technologies [132, 133] hold promise. Among these new methods, the employment of a noninvasive screening technology using near infrared spectroscopy to analyze the metabolomic profiling of embryo culture medium has been proposed as one of the most promising selection methods. However, besides the initial benefits reported in retrospective studies [132, 134–139], recent prospective randomized studies have shown that the evaluation of metabolomic profiles by near infrared spectroscopy does not improve implantation rates [140, 141].
Concluding Remarks
Applications of new culture approaches including use of atmospheric oxygen level and the introduction of single-step and sequential media have resulted in several significant breakthroughs in the clinical IVF laboratory. The ability to improve preservation of embryonic developmental potential has increased the likelihood of obtaining a consistent number of embryos reaching the blastocyst stage. With these improvements, application of single blastocyst transfer should continue to increase, at least in good prognosis patients. This, in turn, will continue to decrease the incidence of multiple pregnancies while preserving the overall efficiency of the treatment.
Notwithstanding the demonstrated benefits of innovative and sophisticated platforms, the wide-spread use of these technologies is currently limited due to the costs of these devices and design pitfalls that can make them more labor intensive to utilize. In a futuristic view, a complex, automated system may be established to perform all steps that lead to embryo production [77]. The system could also be enhanced with a time lapse imaging system to allow noninvasive detailed analysis of embryo development and with various sensors to measure, for instance, embryo-derived biomarkers (metabolomics) or gene expression profiles (transcriptomics). The enormous amount of information derived from morphological (including phase-contrast) images and time-lapse videos together with biochemical parameters may prove invaluable for determining the optimal time for embryo transfer, for selecting the best embryo(s) for transfer, and for comparison of various versions of culture methods and parameters.
Although improvements in the IVF culture system have resulted in significantly improved clinical outcomes, one of the major limitations in IVF laboratory technology still relates to identification of the most viable embryo(s) to transfer. Currently, selection of the best embryo(s) is based on static assessment of morphological features, but most of the embryos transferred fail to implant. In the future, implementation of current culture systems with time lapse cinematography and “-omics” technology may improve the identification of novel markers of embryo quality to provide additional selection criteria. Furthermore, preimplantation genetic screening may help in the determination of embryonic “health” through screening the genetic constitution of the embryo (see Chap. 8). However, this technology is still far from being routinely used in IVF clinics and further investigations are needed to ensure the reliability and sensitivities of these methods.
References
Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oöcyte and zygote. Proc Natl Acad Sci USA. 1967;58(2):560–7.
Donahue RP, Stern S. Follicular cell support of oocyte maturation: production of pyruvate in vitro. J Reprod Fertil. 1968;17(2):395–8.
Brower PT, Schultz RM. Intercellular communication between granulosa cells and mouse oocytes: existence and possible nutritional role during oocyte growth. Dev Biol. 1982;90(1):144–53.
Haghighat N, Van Winkle LJ. Developmental change in follicular cell-enhanced amino acid uptake into mouse oocytes that depends on intact gap junctions and transport system Gly. J Exp Zool. 1990;253(1):71–82.
Horne R, Bishop CJ, Reeves G, et al. Aspiration of oocytes for in-vitro fertilization. Hum Reprod Update. 1996;2:77–85.
Edwards RG, Steptoe PC, Fowler RE, Baillie J. Observations on preovulatory human ovarian follicles and their aspirates. Br J Obstet Gynaecol. 1980;87(9):69–79.
Pool TB, Ord VA. Oocyte treatment: from retrieval to insemination. In: Gardner DK, Weissman A, Howles CM, Shoham Z, editors. Textbook of assisted reproductive techniques. 2nd ed. Philadelphia, PA: Taylor & Francis; 2004. p. 107–14.
Sathananthan AH, Trounson A, Freemann L, Brady T. The effects of cooling human oocytes. Hum Reprod. 1988;3:968–77.
Pickering SJ, Braude PR, Johnson MH, Cant A, Currie J. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertil Steril. 1990;54:102–8.
Almeida PA, Bolton VN. The effect of temperature fluctuations on the cytoskeletal organisation and chromosomal constitution of the human oocyte. Zygote. 1995;3:357–65.
Mortimer D, Mortimer ST. Quality and risk management in the IVF laboratory. Cambridge: Cambridge University Press; 2005.
Dale B, Menezo Y, Cohen J, DiMatteo L, Wilding M. Intracellular pH regulation in the human oocyte. Hum Reprod. 1998;13(4):964–70.
Hentemann M, Mousavi K, Bertheussen K. Differential pH in embryo culture. Fertil Steril. 2011;95:1291–4.
Iwasaki T, Kimura E, Totsukawa K. Studies on a chemically defined medium for in vitro culture of in vitro matured and fertilized porcine oocytes. Theriogenology. 1999;51:709–20.
Morgia F, Torti M, Montigiani M, Piscitelli C, Giallonardo A, Schimberni M, Giannini P. Sbracia Use of a medium buffered with N-hydroxyethylpiperazine-N-ethanesulfonate (HEPES) in intracytoplasmic sperm injection procedures is detrimental to the outcome of in vitro fertilization. Fertil Steril. 2006;85(5):1415–9.
Swain JE. Optimizing the culture environment in the IVF laboratory: impact of pH and buffer capacity on gamete and embryo quality. Reprod Biomed Online. 2010;21(1):6–16.
Rattanachaiyanont M, Leader A, Léveillé MC. Lack of correlation between oocyte-corona-cumulus complex morphology and nuclear maturity of oocytes collected in stimulated cycles for intracytoplasmic sperm injection. Fertil Steril. 1999;71(5):937–40.
Veeck LL. The morphologic estimation of mature oocytes and their preparation for insemination. In: Jones Jr HW, Jones GS, et al., editors. In-vitro fertilization—Norfolk. Baltimore, MD: Williams and Wilkins; 1986. p. 81.
Wolf DP. Oocyte quality and fertilization. In: Wolf DP, editor. In-vitro fertilization and embryo transfer. New York: Plenum; 1988. p. 129–38.
Daya S, Kohut J, Gunby J, et al. Influence of blood clots in the cumulus complex on oocyte fertilization and cleavage. Hum Reprod. 1990;5:744–6.
Veeck LL. The morphological assessment of human oocytes and early conception. In: Keel BA, Webster BW, editors. Handbook of the laboratory diagnosis and treatment of infertility. Boca Raton, FL: CRC; 1990. p. 353–69.
Ng ST, Chang TH, Wu TC. Prediction of the rates of fertilization, cleavage, and pregnancy success by cumulus-coronal morphology in an in vitro fertilization program. Fertil Steril. 1999;72:412–7.
Lin YC, Chang SY, Lan KC, et al. Human oocyte maturity in vivo determines the outcome of blastocyst development in vitro. J Assist Reprod Genet. 2003;20:506–12.
Balaban B. Urman B Effect of oocyte morphology on embryo development and implantation. Reprod Biomed Online. 2006;12:608–15.
Motta PM, Nottola SA, Pereda J, et al. Ultrastructure of human cumulus oophorus: a transmission electron microscopic study on oviductal oocytes and fertilized eggs. Hum Reprod. 1995;10:2361–7.
Ebner T, Moser M, Shebl O, Sommergruber M, Yaman C, Tews G. Blood clots in the cumulus-oocyte complex predict poor oocyte quality and post-fertilization development. Reprod Biomed Online. 2008;16(6):801–7.
Kahraman S, Yakin K, Donmez E, et al. Relationship between granular cytoplasm of oocytes and pregnancy outcome following intracytoplasmic sperm injection. Hum Reprod. 2000;15:2390–3.
Rienzi L, Ubaldi F, Anniballo R, et al. Preincubation of human oocytes may improve fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1998;13:1014–9.
Yanagida K, Yazawa H, Katayose H, et al. Influence of oocyte preincubation time on fertilization after intracytoplasmic sperm injection. Hum Reprod. 1998;13:2223–6.
Van de Velde H, de Vos A, Joris H, et al. Effect of timing of oocyte denudation and micro-injection on survival, fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1998;13:3160–4.
Jacobs M, Stolwijk AM, Wetzels AM. The effect of insemination/injection time on the results of IVF and ICSI. Hum Reprod. 2001;16:1708–13.
Ho JY, Chen MJ, Yi YC, et al. The effect of preincubation period of oocytes on nuclear maturity, fertilization rate, embryo quality, and pregnancy outcome in IVF and ICSI. J Assist Reprod Genet. 2003;9:358–64.
Isiklar A, Mercan R, Balaban B, et al. Impact of oocyte pre-incubation time on fertilization, embryo quality and pregnancy rate after intracytoplasmic sperm injection. Reprod Biomed Online. 2004;6:682–6.
Dozortsev D, Nagy P, Abdelmassih S, et al. The optimal time for intracytoplasmic sperm injection in the human is from 37 to 41 hours after administration of human chorionic gonadotropin. Fertil Steril. 2004;6:1492–6.
Falcone P, Gambera L, Pisoni M, et al. Correlation between oocyte preincubation time and pregnancy rate after intracytoplasmic sperm injection, Gynecol Endocrinol. 2008;6:295–9.
Eppig JJ, Schultz RM, O’Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol. 1994;164:1–9.
Balakier H, Sojecki A, Motamedi G, Librach C. Time dependent capability of human oocytes for activation and pronuclear formation during metaphase II arrest. Hum Reprod. 2004;19:982–7.
Aitken JR, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 1987;81:459–69.
Dumoulin JCM, Bras M, Land JA, Pieters M, Enginsu ME, et al. Effect of the number of inseminated spermatozoa on subsequent human and mouse embryonic development in vitro. Hum Reprod. 1992;7:1010–3.
Parinaud J, Labal B, Mieusset R, Rkhoilley G, Vieitez G. Influence of sperm parameters on embryo quality. Fertil Steril. 1993;60:888–92.
Aitken JR. A free radical theory of male infertility. Reprod Fertil Dev. 1994;6:19–24.
Gianaroli L, Fiorentino A, Magli MC, et al. Prolonged sperm–oocyte exposure and high sperm concentration affect human embryo viability and pregnancy rate. Hum Reprod. 1996;11:2507–11.
Quinn P. The development and impact of culture media for assisted reproductive technologies. Fertil Steril. 2004;81:27–9.
Gardner DK, Lane M. Culture of the mammalian preimplantation embryo. In: Gardner DK, Lane M, Watson AJ, editors. A laboratory guide to the mammalian embryo. Oxford, NY: Oxford University Press; 2004. p. 41–61.
Earle WR. Production of malignancy in vitro. IV. The mouse fibroblast cultures and changes in living cells. J Natl Cancer Inst. 1943;4:165–212.
Krebs HA, Henseleit K. Untersuchungen uÈ ber die Harnstoffbildung im Tierkorper. Z Physiol Chem. 1932;210:33–66.
Tyrode MV. The mode of action of some purgative salts. Arch Int Pharmacodyn. 1910;20:205–23.
Biggers JD, McGinnis LK. Evidence that glucose is not always an inhibitor of mouse preimplantation development in vitro. Hum Reprod. 2001;16:153–63.
Summers MC, Biggers JD. Chemically defined media and the culture of mammalian preimplantation embryos: historical perspective and current issues. Hum Reprod Update. 2003;9(6):557–82.
Gardner DK, Lane M. Development of viable mammalian embryos in vitro: evolution of sequential media. In: Cibelli J, Lanza RP, Campbell KHS, West MD, editors. Principles of cloning. NY: Academic; 2002. p. 187–213.
Leese HJ. Human embryo culture: back to nature. J Assist Reprod Genet. 1998;15:466–8.
Leese HJ, Tay JI, Reischl J, et al. Formation of fallopian tubal fluid: role of a neglected epithelium. Reproduction. 2001;121:339–46.
Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3:367–82.
Gardner DK, Lane M. Blastocyst transfer. Clin Obstet Gynaecol. 2003;46:231–8.
Gardner DK. Dissection of culture media for embryos: the most important and less important components and characteristics. Reprod Fertil Dev. 2008;20:9–18.
Gardner DK, Lane M. Embryo culture systems. In: Gardner DK, editor. In vitro fertilization: a practical approach. New York: Informa Healthcare; 2007. p. 221–82.
Lane M, Gardner DK. Embryo culture medium: which is the best? Best Pract Res Clin Obstet Gynaecol. 2007;21:83–100.
Pool TB. An update on embryo culture for human assisted reproductive technology: media, performance, and safety seminars. Semin Reprod Med. 2002;23:309–18.
Pool TB. Recent advances in the production of viable human embryos in vitro. Reprod Biomed Online. 2005;4:294–302.
Biggers JD, McGinnis LK, Lawitts JA. One-step versus two-step culture of mouse preimplantation embryos: is there a difference? Hum Reprod. 2005;20:3376–84.
Biggers JD, Summers MC. Choosing a culture medium: making informed choices. Fertil Steril. 2008;90:473–83.
Sepu´lveda S, Garcia J, Arriaga E, et al. In vitro development and pregnancy outcomes for human embryos cultured in either a single medium or in a sequential media system. Fertil Steril. 2008;91:1765–70.
Vajta G, Rienzi L, Cobo A, Yovich J. Embryo culture: can we perform better than nature? Reprod Biomed Online. 2010;20:453–69. Review.
Bavister BD. How animal embryo research led to the first documented human IVF. Reprod Biomed Online. 2002;4 Suppl 1:24–9.
Edwards RG. Test-tube babies. Nature. 1981;293:253–6.
Nagao Y, Saeki K, Hoshi M, et al. Effects of oxygen concentration and oviductal epithelial tissue on the development of in vitro matured and fertilized bovine oocytes cultured in protein-free medium. Theriogenology. 1994;41:681–7.
Trounson A, Pushett D, Maclellan LJ, et al. Current status of IVM/IVF and embryo culture in humans and farm animals. Theriogenology. 1994;41:57–66.
Voelkel SA, Hu YX. Effect of gas atmosphere on the development of one-cell bovine embryos in two culture systems. Theriogenology. 1992;37:1117–31.
Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993;99:673–9.
Maas DH, Storey BT, Mastroianni Jr L. Oxygen tension in the oviduct of the rhesus monkey (Macaca mulatta). Fertil Steril. 1976;27:1312–7.
Mastroianni Jr L, Jones R. Oxygen Tension within the Rabbit Fallopian Tube. J Reprod Fertil. 1965;9:99–102.
Wale PL, Gardner DK. Time-lapse analysis of mouse embryo development. Reprod Biomed Online. 2010;21(3):402–10.
Waldenstrom U, Engstrom AB, Hellberg D, Nilsson S. Low-oxygen compared with high-oxygen atmosphere in blastocyst culture, a prospective randomized study. Fertil Steril. 2009;91(6):2461–5.
Gomes Sobrinho DB, Oliveira JB, Petersen GC, et al. IVF/ICSI outcomes after culture of human embryos at low oxygen tension: a meta-analysis. Reprod Biol Endocrinol. 2011;9:143.
Thompson JG, Peterson AJ. Bovine embryo culture in vitro: new developments and post-transfer consequences. Hum Reprod. 2000;15 Suppl 5:59–67.
Vajta G, Holm P, Greve T, et al. The submarine incubation system, a new tool for in vitro embryo culture. A technique report. Theriogenology. 1997;48:1379–85.
Swain JE, Smith GD. Advances in embryo culture platforms: novel approaches to improve preimplantation embryo development through modifications of the microenvironment. Hum Reprod Update. 2011;17(4):541–57.
Rienzi L, Vajta G, Ubaldi F. New culture devices in ART. Placenta. 2011;32 Suppl 3:S248–51.
Lane M, Gardner DK. Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum Reprod. 1992;7:558–62.
Vajta G, Peura TT, Holm P, et al. New method for culture of zona-included and zone-free embryos: the Well of the Well (WOW) system. Mol Reprod Dev. 2000;55:256–64.
Thouas GA, Jones GM, Trounson AO. The ‘GO’ system–a novel method of microculture for in vitro development of mouse zygotes to the blastocyst stage. Reproduction. 2003;126:161–9.
Vajta G, Korösi T, Du Y, et al. The Well-of-the-Well system: an efficient approach to improve embryo development. Reprod Biomed Online. 2008;17:73–81.
Beebe DJ, Wheeler M, Zeringue H, et al. Microfluidic technology for assisted reproduction. Theriogenology. 2002;57:125–35.
Smith GD, Takayama S. Gamete and embryo isolation and culture with microfluidics. Theriogenology. 2007;68S:S190–5.
Thompson JG. Culture without the Petri dish. Theriogenology. 2007;67:16–20.
Wang W, Liu X, Gelinas D, Ciruna B, Sun Y. A fully automated robotic system for microinjection of zebrafish embryos. PLoS One. 2007;2(9):e862.
Machtinger R, Racowsky C. The bioinformatics of embryo development and assessment. RBMOnline (In Press).
Shoukir Y, Campana A, Farley T, et al. Early cleavage of in-vitro fertilized human embryos to the 2-cell stage: a novel indicator of embryo quality and viability. Hum Reprod. 1997;12:1531–6.
Sakkas D, Shoukir Y, Chardonnens D, et al. Early cleavage of human embryos to the two-cell stage after intacytoplasmic sperm injection as an indicator of embryo viability. Hum Reprod. 1998;13:182–7.
Sakkas D, Percival G, D’Arcy Y, et al. Assessment of early cleaving in vitro fertilized human embryos at the 2-cell stage before transfer improves embryo selection. Fertil Steril. 2001;76:1150–6.
Salumets A, Hyden-Granskog C, Makinen S, et al. Early cleavage predicts the viability of human embryos in elective single embryo transfer procedures. Hum Reprod. 2003;18:821–5.
Neuber E, Rinaudo P, Trimarchi JR, et al. Sequential assessment of individually cultured human embryos as an indicator of subsequent good quality blastocyst development. Hum Reprod. 2003;18:1307–12.
Van Montfoort AP, Dumoulin JC, Kester AD, et al. Early cleavage is a valuable addition to existing embryo selection parameters: a study using single embryo transfers. Hum Reprod. 2004;19:2103–8.
Guerif E, Le Gouge A, Giraudeau B, et al. Limited value of morphological assessment at day 1 and 2 to predict blastocyst development potential: a prospective study based on 4042 embryos. Hum Reprod. 2007;22:1973–81.
Lewin A, Schenker JG, Safran A, et al. Embryo growth rate in vitro as an indicator of embryo quality in IVF cycles. J Assist Reprod Genet. 1994;11:500–3.
Giorgetti C, Terriou P, Auquier P, et al. Embryo score to predict implantation after in-vitro fertilization: based on 957 single embryo transfers. Hum Reprod. 1995;10:2427–31.
Ziebe S, Petersen K, Lindberg S, et al. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12:1545–9.
Desai NN, Goldstein J, Rowland DY, et al. Morphological evaluation of human embryos and derivation of an embryo qyality scoring system specific for day 3 embryos: a preliminary study. Hum Reprod. 2000;15:2190–6.
Handarson T, Hanson C, Sjögren A, et al. Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: indications for aneuploidy and multinucleation. Hum Reprod. 2001;16:313–8.
Kligman I, Benavida C, Alikani M, et al. The presence of multinucleated blastomeres in human embryos is correlated with chromosomal abnormalities. Hum Reprod. 1996;11:1492–8.
Jackson KV, Ginsburg ES, Hornstein MD, et al. Multinucleation in normally fertilized embryos is associated with an accelerated ovulation induction response and lower implantation and pregnancy rates in in vitro fertilization-embryo transfer cycles. Fertil Steril. 1998;70:60–6.
Palmstierna M, Murkes D, Csemiczky G, et al. Zona pellucida thickness variation and occurrence of visible mononucleated blastomers in preembryos are associated with a high pregnancy rate in IVF treatment. J Assist Reprod Genet. 1998;15:70–5.
Pelinck MJ, De Vos M, Dekens M, et al. Embryos cultured in vitro with multinucleated blastomeres have poor implantation potential in human in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod. 1998;13:960–3.
Van Royen E, Mangelschots K, Vercruyssen M, et al. Multinucleation in cleavage stage embryos. Hum Reprod. 2003;18:1062–9.
Staessen C, Camus M, Bollen N, et al. The relationship between embryo quality and the occurrence of multiple pregnancies. Fertil Steril. 1992;57:626–30.
Roseboom TJ, Vermeiden JP, Schoute E, et al. The probability of pregnancy after embryo transfer is affected by the age of the patient, cause of infertility, number of embryos transferred and the average morphology score, as revealed by multiple logistic regression analysis. Hum Reprod. 1995;10:3035–41.
Alikani M, Cohen J, Tomkin G, et al. Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil Steril. 1999;71:836–42.
Antczak M, Van Blerkom J. Temporal and spatial aspects of fragmentation in early human embryos: possible effects on developmental competence and association with the differential elimination of regulatory proteins from polarized domains. Hum Reprod. 1999;14:429–47.
Van Royen E, Mangelschots K, De Neuborg D, et al. Characterization of a top quality embryo, a step towards single-embryo transfer. Hum Reprod. 1999;14:2345–9.
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83.
Veeck LL. Preembryo grading and degree of cytoplasmic fragmentation. In: Veeck LL, editor. An Atlas of Human Gametes and Conceptuses. New York: Parthenon Publishing Group; 1999. p. 46–50.
Fisch JD, Rodriguez H, Ross R, et al. The Graduated Embryo Score (GES) predicts blastocyst formation and pregnancy rate from cleavage-stage embryos. Hum Reprod. 2001;16:1970–5.
Rienzi L, Ubaldi F, Iacobelli M, et al. Significance of morphological attributes of the early embryo. Reprod Biomed Online. 2005;10:669–81.
Cutting R, Morrol D, Roberts SA, et al. Elective single embryo transfer: guidelines for practice British Fertility Society and Association of Clinical Embryologists. Hum Fertil. 2008;11:131–46.
Stensen MH, Tanbo T, Storeng R, et al. Routine morphological scoring systems in assisted reproduction treatment fail to reflect age-related impairment of oocyte and embryo quality. Reprod Biomed Online. 2010;21:118–25.
Racowsky C, Vernon M, Mayer J, et al. Standardization of grading embryo morphology. Fertil Steril. 2010;94(3):1152–3.
Gardner DK, Lane M, Stevens J, et al. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8.
Schoolcraft WB, Gardner DK. Blastocyst culture and transfer increases the efficiency of oocyte donation. Fertil Steril. 2000;74:482–6.
Langley MT, Marek DM, Gardner DK, et al. Extended embryo culture in human assisted reproduction treatments. Hum Reprod. 2001;16:902–8.
Schwarzler P, Zech H, Auer M, et al. Pregnancy outcome after blastocyst transfer as compared to early cleavage stage embryo transfer. Hum Reprod. 2004;19:2097–102.
Blake DA, Farquhar CM, Johnson N, et al. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst Rev. 2007; CD002118
Coskun S, Hollanders J, Al-Hassan S, et al. Day 5 versus day 3 embryo transfer: a controlled randomized trial. Hum Reprod. 2000;15:1947–52.
Huisman GJ, Fauser BC, Eijkemans MJ, et al. Implantation rates after in vitro fertilization and transfer of a maximum of two embryos that have undergone three to five days of culture. Fertil Steril. 2000;73:117–22.
Alper M, Brinsden P, Fischer R, et al. To blastocyst or not to blastocyst? That is the question. Hum Reprod. 2001;16:617–9.
Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: fertility and genetics beyond. London: Parthenon Publishing; 1999. p. 378–88.
Veeck LL, Zaninovic N. Grading criteria for human blastocysts. An atlas of human blastocysts. New York: Parthenon Publishing; 2003. p. 118.
Stephenson EL, Braude PR, Mason C. International community consensus standard for reporting derivation of human embryonic stem cell lines. Regen Med. 2007;2:349–62.
Lemmen JG, Agerholm I, Ziebe S. Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICSI-fertilized oocytes. Reprod Biomed Online. 2008;17:385–91.
Wong CC, Loewke KE, Bossert NL, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28:1115–21.
Meseguer M, Herrero J, Tejera A, et al. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658–71.
Brison DR, Houghton FD, Falconer D, Roberts SA, Hawkhead J, Humpherson PG, Lieberman BA, Leese HJ. Identification of viable embryos in IVF by non-invasive measurement of amino acid turnover. Hum Reprod. 2004;19:2319–24.
Seli E, Sakkas D, Scott R, Kwok SC, Rosendahl SM, Burns DH. Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil Steril. 2007;88:1350–7.
Katz-Jaffe MG, McReynolds S, Gardner DK, Schoolcraft WB. The role of proteomics in defining the human embryonic secretome. Mol Hum Reprod. 2009;15:271–7.
Seli E, Vergouw CG, Morita H, Botros LL, Roos P, Lambalk CB, Yamashita N, Kato O, Sakkas D. Noninvasive metabolomic profiling as an adjunct to morphology for noninvasive embryo assessment in women undergoing single embryo transfer. Fertil Steril. 2010;94:535–42.
Seli E, Bruce C, Botros LL, Henson M, Roos P, Judge K, Hardarson T, Ahlström A, Harrison P, Henman M, et al. Receiver operating characteristic (ROC) analysis of day 5 morphology grading and metabolomics viability Score on predicting implantation outcome. J Assist Reprod Genet. 2011;28:137–44.
Nagy ZP, Sakkas D, Behr B. Non-invasive assessment of embryo viability by metabolomic profiling of culture media (‘metabolomics’). Reprod Biomed Online. 2008;17:502–7.
Nagy ZP, Jones-Colon S, Roos P, Botros L, Greco E, Dasig J, Behr B. Metabolomic assessment of oocyte viability. Reprod Biomed Online. 2009;18:219–25.
Vergouw CG, Botros LL, Roos P, Lens JW, Schats R, Hompes PGA, Burns DH, Lambalk CB. Metabolomic profiling by near infrared spectroscopy as a tool to assess embryo viability: a novel, non-invasive method for embryo selection. Hum Reprod. 2008;23:1499–504.
Ahlström A, Wikland M, Rogberg L, Barnett JS, Tucker M, Hardarson T. Cross-validation and predictive value of near-infrared spectroscopy algorithms for day-5 blastocyst transfer. Reprod Biomed Online. 2011. doi:10.1016/j.rbmo.2011.01.009.
Hardarson T, Ahlstrom A, Rogberg L, et al. Non-invasive metabolomic profiling of Day 2 and 5 embryo culture medium: a prospective randomized trial. Hum Reprod. 2012;27(1):89–96.
Vergouw CG, Kieslinger DC, Kostelijik EH, et al. Day 3 embryo selection by metabolomic profiling of culture medium with near-infrared spectroscopy as an adjunct to morphology: a randomized controlled trial. Hum Reprod. 2012;27:2304–11.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media New York
About this chapter
Cite this chapter
Maggiulli, R., Ubaldi, F., Rienzi, L.F. (2012). Oocyte Insemination and Culture. In: Ginsburg, E., Racowsky, C. (eds) In Vitro Fertilization. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-9848-4_6
Download citation
DOI: https://doi.org/10.1007/978-1-4419-9848-4_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-9847-7
Online ISBN: 978-1-4419-9848-4
eBook Packages: MedicineMedicine (R0)