Abstract
After introduction on the use of solid catalysts in wastewater treatment technologies, particularly advanced oxidation processes (AOPs), this review discussed the use of pillared clay (PILC) materials in three applications: (i) wet air catalytic oxidation (WACO), (ii) wet hydrogen peroxide catalytic oxidation (WHPCO) on Cu-PILC and Fe-PILC, and (iii) behavior of Ti-PILC and Fe-PILC in the photocatalytic or photo-Fenton conversion of pollutants. Literature data are critically analyzed to evidence the main direction to further investigate, in particularly with reference to the possible practical application of these technologies to treat industrial, municipal, or agro-food production wastewater.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
There is an increasing research and applicative interest in using advanced nanotech catalysts and materials for the purification/remediation of contaminated surface or groundwater and municipal or industrial wastewater [1–9]. Solid catalysts are used in promoting many water treatment and remediation technologies [6], such as in (i) various advanced oxidation processes (AOPs ) (photocatalytic treatment of wastewater, wet air catalytic oxidation (WACO), wet hydrogen peroxide catalytic oxidation (WHPCO), catalytic ozonation), (ii) selective reduction of pollutants in water (nitrate and nitrite catalytic reduction, catalytic hydrodehalogenation), (iii) catalytic permeable reactive barriers, and (iv) other less commonly used wastewater treatment technologies (supercritical water oxidation, sonochemical degradation, water treatment using non-thermal plasma or e-beam irradiation). The use of solid catalysts offers several potential advantages [6]:
-
operation in milder reaction conditions,
-
better engineering of the process,
-
possibility of selective conversion of specific target chemicals, and
-
combined removal by adsorption with catalytic regeneration in a separate step.
In addition, in photocatalytic technologies semiconductors such as titania are required to generate by photo-induced processes the active species (hydroxyl radicals) responsible for the degradation of the pollutants. The use of catalysts is also often indispensable to convert recalcitrant contaminants, such as endocrine-disrupting chemicals (EDCs): hormonally active agents, xenobiotics, pharmaceutically active compounds, effluent-derived microcontaminants, and persistent organic pollutants. EDCs are pollutants with estrogenic or androgenic activity at very low concentrations and are emerging as a major concern for water quality [10]. The concentration of these microcontaminants in water is continuously increasing, and great endeavors have been done on the removal of EDCs in wastewater, because conventional water treatment technologies are not effective in their elimination. AOP methods, in combination with solid catalysts, are the most effective technologies for the treatment of wastewaters containing pharmaceuticals, personal care products (PPCPs), and especially EDCs [11].
There are many other very relevant areas in which catalytic AOP methods are between the most promising technologies, although it should be commented that in water treatment technologies a combination of technologies (“technology train”) is typically necessary to realize the optimal compromise between cost and effectiveness. In particular, the integration between catalysis and membrane is a very relevant area to mention for the development of novel water treatment technologies and in general more sustainable processes [12]. Examples of areas of application of catalytic AOP methods are the following [5]:
-
Pulp Mill Effluents
-
Dyeing and Printing Wastewater
-
Oil Refinery Wastewater
-
Distillery Wastewater
-
Food Industries
-
Leather Industries
-
Municipal and Natural Wastes
In addition, an area of application which will fast increase in a near future will be the wastewater treatment from biorefineries. In comparison to oil-based refineries and chemical processes, the production of fuels and chemicals from biomass produces an order of magnitude higher volume of wastewater effluents with very large amounts of contaminants [13]. The development of novel technologies for the treatment of these large volume effluents is one of the conditions for the success of a bio-based economy.
7.1.1 Issues in Using Solid Catalysts in Wastewater Treatment
Notwithstanding the large potential field of application of catalytic AOP methods, the largest part of the studies is still often limited to model compounds such as phenols [2, 3], although phenol itself is a relevant industrial contaminant. However, the wastewater typically never contains a single contaminant and it is known that in the presence of complex mixtures completely different results and/or fast deactivation may be observed with respect to that observed using single model chemicals [6]. There are increasing examples in literature on catalytic AOP studies using realistic streams, even if this type of studies should be further incentivized.
The use of solid catalysts in AOP methods requires different characteristics of the solid with respect to those typically used in environmental catalysis applications [6]. Limited attempts have been made often to tailor solid catalysts for the water treatment applications, but more attention to this issue has been given recently. There are problems of
-
pore structure design (intraparticle diffusivity is usually an issue determining the catalytic performances),
-
leaching (often driven from the reaction products and thus depending on the progress of the reaction), and
-
stability (related, but not only depending on the possible leaching) and selectivity (typically, quite complex mixtures should be treated in practical applications).
As a consequence catalyst design for water treatment is different from that of most of other applications and even in the screening of the sample this aspect should be considered, as generally valid for the development of multiphase reaction processes [14]. New microstructured and nanostructured materials (fibers, nanotubes, multilayer composite materials) are available for a more effective catalyst design in water treatment technologies [14]. They offer also the possibility of a better reactor design. The efficient integration between catalyst and reactor design in wastewater treatment technologies is another of the critical factors to increase the use of solid catalysts in these technologies [6]. A further general issue in design of solid catalysts for AOP methods regards the fact that the effective oxidizing species are very reactive and short living, such as radical species (H·, HO·, HO2·, etc.). There is little knowledge on the catalyst design necessary to optimize performances in the presence of these very reactive species.
However, literature data on the catalytic AOP methods are often mainly phenomenological and based on the screening of different catalysts and analysis of the effect of the reaction conditions. Another limit in current literature, from the application point of view, regards the scarce data on the toxicological effects on humans and the environment of both used catalysts (in relation to possible leaching of some of the components – metal ions, particularly – and/or formation of suspended fine particles) and of the products of degradation of pollutants, in addition to the lack of use of more realistic streams. Oxidation of pollutants may give rise to intermediates which can be more toxic than the starting chemicals. It is thus necessary to put attention on the toxicological evaluation of the quality of treated wastewater, as well as the integration of the catalytic step with downstream technologies. For example, the catalytic technology is often a pre-treatment step before biological aerobic or anaerobic processes or to a membrane separation [15–17]. Quality of water required in these cases is different, as well as often the level of conversion necessary for an optimal integration with the downstream technology.
7.1.2 PILCs and Catalytic AOP Methods
This chapter will focus the discussion on the use of pillared clays (PILCs) in AOP methods for wastewater treatment. In general, layered materials offer many attractive characteristics for their use as catalysts [18]. Their structure consisting of stacked sheets represents an interesting opportunity for developing new materials with a tailored nanodesign, controlled accessibility to the sites and properties, tunable pore size and volume, and high surface area. The use of layered materials (layered perovskite, anionic clays, pillared clays) in catalytic reactions spans from new processes for environmental protection, including wastewater treatment, to petrochemistry and fine chemicals production and refinery/biorefinery as well [18]. More specifically, pillared clays (PILCs), due to their more precise tuning of the microporous structure, possibility of multifunctional design, and improved thermal stability find application in many acid-catalyzed reactions, as well as redox reactions (selective reduction of NOx, complete oxidation of volatile organic compounds (VOCs) or chlorofluorocarbons (CFCs), and wet catalytic oxidation of waste) after introduction of suitable transition metals into the pillars or exchangeable positions [18–21]. PILCs allow a fine control of the microstructure developed in it by adjusting the several parameters involved in the synthesis process [19].
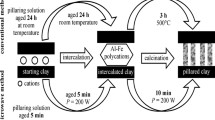
With respect to other layered catalytic materials, PILCs are characterized from the presence of oxide pillars between the layers. They are prepared by exchanging the charge-compensating cations present in the interlamellar space of the swelling clays by hydroxy-metal polycations. During calcination, the inserted polycations give rise to rigid, thermally stable oxide pillars, which limit the possibility of thermal collapse between the two-dimensional clay layers. The pillaring procedure of the clay thus allows to produce relatively stable layered materials with a tunable interlayer spacing by adjusting the several parameters involved in the synthesis process of alumina-pillared clays. The interlayer spacing typically ranges between 0.6 and 1.8 nm, e.g., situates in between microporous and mesoporous materials. For these reasons, they were originally developed to prepare thermally stable acid catalysts that are able to crack larger molecules than those addressed by microporous (zeolitic) materials [22, 23].
This structure shows interesting characteristics to develop advanced catalytic materials for AOP applications. The interlayer distance is influenced by the nature of the intercalant species, their length, and their degree of polymerization. They can be controlled during the synthesis by the pH, the temperature, and the time of aging [19]. The most widely used intercalating agents are polymeric or oligomeric cations produced by the base hydrolysis of Al, Zr, Ti, Cr, Fe, and Ga salts. It is thus possible:
-
To control the interlayer space and tailor the access to inner sites. This is important to allow access of relatively large molecules (often present in wastewater), but avoiding at the same time the humic substances that cause fouling of the active sites, e.g., to provide longer-term activity in the presence of real wastewater.
-
To introduce different transition metals into the pillars/or exchangeable positions, e.g., develop multifunctional catalysts (with Brönsted and Lewis acidity, as well as redox properties) to tailor the catalytic behavior in wet oxidation.
-
To control the hydrophilic character of the inner space between the layer, another important characteristic to control the catalytic performances in wet oxidation.
There are three main areas of use of PILCs in catalytic AOP methods. They are as follows:
-
Wet air catalytic oxidation (WACO ), e.g., the oxidation of organic compounds by oxygen at high temperatures (typically in the 150–250 °C temperature range) and under pressure (usually, in the 20–40 bar range) in autoclave reactors. It is a widely used technology, especially in the treatment of industrial wastewater or sludges. The use of catalysts allows operating at lower reaction temperatures, an important aspect not only to reduce operative costs, but also to mitigate corrosion problems related to the formation of acetic acid as the main by-product. Due to these corrosion problems, often special materials for the construction of the reactor (Ti, for example) are necessary, significantly increasing the fixed capital costs.
-
Wet hydrogen peroxide oxidation (WHPCO ; alternative names are catalytic wet peroxide oxidation – CWPO , and wet peroxidation or wet oxidation by hydrogen peroxide ) uses H2O2(hydrogen peroxide , HP) as oxidant instead of oxygen. It operates at significantly milder reaction conditions (temperature lower than 100 °C, atmospheric pressure) and do not require costly autoclave reactors, e.g., significant lower operative and fixed capital costs. However, HP is a more costly reactant than oxygen (air). It is based on the Fenton chemistry, where transition metals such as iron and copper are able to react with HP to generate hydroxyl radicals (HO·), which are the effective oxidizing agents. The original Fenton studies, and still many investigations and applications, are based on the use of homogeneous catalysts (Fe2+ and Cu+ salts) [24–26], but the use of solid catalysts containing iron or copper is increasing [27–33] (heterogeneous Fenton). UV radiation is also able to catalyze the formation of these hydroxyl radicals from HP by homolytic splitting and thus the combination of solid catalysts with UV radiation is used (photo-Fenton) [34–41].
-
Photocatalytic elimination of pollutants in water , using semiconductor nanoparticles (typically TiO2) immobilized within PILC layers or using titania-pillared clays (TiO2 PILCs), e.g., when the pillars of the clay are consisted from the semiconductor photo-active element [42–46].
It should be mentioned that PILCs are used often for the removal of pollutants in water by adsorption, either organic substances [47–52] or toxic heavy metal ions [53–56]. However, we will limit our discussion here to the use of PILCs as catalysts in water treatment technologies.
It should be also mentioned that there is an increasing R&D interest on organic-pillared clays, e.g., organic–inorganic nanocomposites [57]. Although the use of these hybrid clays as advanced materials for the removal of pollutants from industrial wastewaters have been proposed from long time [58], new methods have been proposed more recently [59–61] to prepare microporous clay mineral with organic–inorganic hybrid pillars having very high surface area (about 500 m2 g− 1) [60]. These organophilic-pillared clays (organoclays) may be prepared with different kinds of organic materials in the interlayers, and due to the better tuning the hydrophobic properties, these materials show enhanced selective adsorption toward some pollutants [62, 63]. Their applicability for catalytic AOP methods, particularly regarding stability, has to be verified, although they were proposed to prepare highly active titania-pillared clays for cleaning up the water polluted by the toxic organic compounds with poor polarity such the endocrine-disrupting chemicals [64].
7.2 Wet Air Catalytic Oxidation (WACO )
The number of publications on the use of PILCs in WACO applications is significantly lower than that of using PILC catalysts for wet oxidation using hydrogen peroxide (WHPCO). The motivation is related mainly to the fact that the reaction mechanism in WACO is different from that in WHPCO. In the latter, short-living hydroxyl radical species are generated which are highly reactive, as discussed later. In WACO, a combined redox and heterogeneous free radical mechanism is present.
Metal oxides are capable of initiating free radicals by activating reactant molecules directly and facilitates their decomposition into radicals, or by accelerating the decomposition into radicals, with the hydroperoxides either being present in the system or forming slowly by the first mechanism [65]. Oxygen may participate in a reaction either as an adsorbed species on the catalyst surface or as a part of the lattice oxygen presented in the metal oxides. The presence of catalysts creates an ionic environment that enhances heterolytic reactions. Both free radical (homolytic) and ionic (heterolytic) oxidation reaction mechanisms have been proposed for WACO [65].
The consequence of this difference in the reaction mechanism is that the amount of active catalyst in WACO should be higher than in WHPCO. Metal oxide should be present in PILCs essentially in the pillars to avoid a fast leaching, as discussed later. Therefore, the amount of metal oxide which can be introduced in PILCs to have a stable wet oxidation activity is limited. This amount is typically not enough for active WACO catalysts (e.g., other oxide-based catalysts have typically better performances), but can be good in the case of WHPCO. Although these aspects are not considered in discussing the catalytic behavior of PILC catalysts in wet oxidation, they should be taken into account to consider literature results and the applicability of this class of catalysts in water treatment technologies.
7.2.1 PILC-Based Catalysts for Wet Oxidation
The first report on the use of PILC catalyst in the wet air oxidation was of Guo and Al-Dahhan [66] who investigated the kinetics of conversion of phenol as model compound using Al–Fe-pillared clay in the form of extrudates (cylindrical, approximately 2 × 8 mm). Reaction temperatures were in the range of 90–150 °C and air pressure range was 0.8–2.5 MPa. The variables studied included reaction temperature, air pressure, solution pH, initial phenol concentration, and catalyst loading. The results were compared with those obtained using hydrogen peroxide oxidation [67]. The same catalyst has been used in both types of tests, e.g., an Al–Fe-PILC prepared by NTUA (National Technical University of Athens, Greece), starting from bentonite (Zenith–N). The main composition (wt%) was as follows: SiO2 52.50, Al2O3 27.56, Fe2O3 7.02. In addition, the kinetics in both wet oxidation with air or H2O2 was investigated [66, 67]. Therefore, it is one of the few cases in literature in which a good comparison between the performances in catalytic oxidation with wet air or H2O2 (WACO and WHPCO, respectively) of the same catalyst is possible. This PILC catalyst (indicated with the acronym FAZA (Al–Fe-PILC) ) based on bentonite (Zenith–N) was used also by other authors in wet oxidation with H2O2 [68–70]. Furthermore, it has been used in various other catalytic applications, such as alkylation [71] and conversion of various alcohols [72], and its characteristics extensively discussed [73].
Reported in Fig. 7.1 is the behavior of FAZA in phenol conversion under WACO and WHPCO conditions. The reaction conditions, apart from the reaction temperature, are comparable. It should be mentioned, however, that for WACO the further increase in the air partial pressure above 1.5 MPa has minimal effect on the rate of reaction (e.g., O2 concentration in solution maintains close to saturation during the batch reactor tests), while for WHPCO the increase in the H2O2 concentration above 0.3 M significantly improves the reaction rate. The results reported in Fig. 7.1 clearly indicate that FAZA is more active in WHPCO than in WACO. By extrapolating the results at the same temperature (70 °C), the rate in WHPCO is from 20 to 100 times higher than that of WACO, depending on H2O2 concentration. By comparing the reaction rates at 70 °C for WHPCO and 110 °C for WACO, the former is about 2 times higher than the latter. For an air pressure of 1.5 MPa and a reaction temperature of 110 °C, the oxygen concentration in water is about 0.2 M [74]. It increases by lowering the reaction temperature. The concentration of O2 and H2O2 in solution, in the tests in Fig. 7.1, is thus quite comparable. The different reactivity of the catalyst in WHPCO and WACO is thus related to a different rate of generation of active oxygen species able to give oxidative degradation of phenol.
Behavior of FAZA (Al–Fe-PILC) catalyst in WACO and WHPCO conversion of phenol. Experimental conditions: 500 ppm phenol in distilled water, 10 g/l catalyst, batch reactor – 600 ml, 1.5 MPa air (WACO) or 0.3 M H2O2 – continuous fed (WHPCO), temperature 110 °C (WACO) or 70 °C (WHPCO), natural pH. Adapted from Guo and Al-Dahhan [66, 67]
Guo and Al-Dahhan in their kinetic analysis of WACO and WHPCO of phenol [66, 67] concluded that in WACO the rate-controlling step was the surface reaction between adsorbed reactant species, while in WHPCO the reaction takes place to a significant extent both in the liquid phase and on the catalyst surface. However, the discrimination between the different reaction models was not based on a correct statistical methodology, such as the F-test. In addition, the rate equations were partially phenomenological, being a mixed between an LH (Langmuir–Hinshelwood) modeling and power law. The rate of phenol disappearance is the product of a term derived from LH approach (single or dual site surface mechanism) and the concentration of the catalyst elevated to a factor of 0.8–0.9. Therefore, reliable mechanistic indications from this type of kinetic approach cannot be derived.
In WHPCO the active oxidizing species (hydroxyl radicals or other reactive species) are generated by the reaction of H2O2 with the catalyst iron sites, according to the well-known Fenton reaction mechanism:
Side reactions are the following:
In wet air catalytic oxidation (WACO), the main reactions are described in Eqs. (7.5–7.12). Hydroxyl radicals are produced from the dissociation and oxidation of water according to Eqs. (7.5 and 7.6). Hydroperoxyl radicals are formed from the oxidation of water (Eq. 7.6) and the target compound RH (Eq. 7.10). Hydroxyl radicals are also produced from hydrogen peroxide (Eq. 7.8) and from the reaction of atomic oxygen with the target compound (Eq. 7.12). Hydrogen peroxide is produced by the recombination of hydroperoxyl radicals (Eq. 7.7) or by the reaction of hydroperoxyl radicals with the target compound (Eq. 7.11). Atomic oxygen is produced from the dissociation of oxygen (Eq. 7.9). Although the hydroperoxyl radical is less reactive than the hydroxyl radical (their relative oxidation power is 2.06 and 1.25, respectively, with respect to Cl2 put equal to 1), it plays an important role because of its relative abundance.
In addition, during catalytic wet oxidation, oxygen may participate in reaction either as an adsorbed species on the catalyst surface or as a part of the lattice oxygen present in metal oxides [65]. We may simplify the difference between WHPCO and WACO mechanisms in the case of Al–Fe-PILC catalyst (FAZA), indicating that in the former the rate of reaction depends on the redox reaction of H2O2 with iron to form the active radical species, while in WACO the rate depends on electrophilic character of the catalyst, e.g., its rate of generation of surface radical species. Although also this property depends on the presence of redox sites, the Fenton mechanism (Eqs. 7.1 and 7.2) is much more effective to close the cycle. Therefore, in WACO it is necessary to delocalize more effectively the charge than in WHPCO, e.g., (iron)oxide nanocrystals are necessary with respect to nearly isolated iron sites for the Fenton mechanism. The former may be instead negative for WHPCO, because it catalyzes the decomposition of hydrogen peroxide.
In conclusion, although apparently the same Fe-PILC catalyst is active in WACO and WHPCO reactions, the nature of the active sites in the two reactions is different and as a consequence the optimal characteristics and design of the catalyst to optimize the performances. The difference in the nature of the active sites and reaction mechanisms to activate H2O2 and O2 explains the different observed reactivity, which is instead typically attributed to only the latter aspect.
More recently, Najjar et al. [75] have investigated similar Fe–Al-pillared clays, but prepared from Wyoming montmorillonite, in gallic acid wet air oxidation in mild conditions (90 °C, 5 bar). Gallic acid is a phenolic compound used as model chemicals for the polyphenolic fractions present in olive oil milling wastewater (OMW). Good performances were observed, with over 90% gallic acid conversion, although for the samples showing the higher iron leaching (15%). These samples contain iron oxide hydroxide (FeHO2), which forms α-Fe2O3 (hematite) by calcination. This is in agreement with the previous comments on the need for small iron oxide particles to obtain more active Fe-PILC catalysts in WACO reaction, although evidences show that these particles are more prone to be leached during the catalytic reaction. The same catalysts were also studied in the catalytic photo-oxidation with H2O2 of tyrosol, another representative compound of the polyphenolic fraction in OMW [76]. In the presence of H2O2 and UV radiation (which favors the homolytic cleavage of hydrogen peroxide to two hydroxyl radicals) complete conversion of tyrosol may also be obtained at room temperature, with good results also in terms of toxicity of the effluent. They conclude that this methodology using (Al-Fe)-PILC heterogeneous catalysts is a promising technique for the pre-treatment of OMW before biological treatment, in order to enhance biodegradability and reduce biotoxicity of polyphenolic fraction.
No other studies have been reported on the wet air oxidation using pillared clays, apart from further aspects on the phenol oxidation by Guo and Al-Dahhan [77, 78], mainly in relation to reactor and catalyst engineering.
7.3 Wet Hydrogen Peroxide Catalytic Oxidation (WHPCO )
More studies have been dedicated to the use of PILC catalysts in wet oxidation using hydrogen peroxide. In this case, in addition to the use of model compounds, several studies have also reported the behavior using real or more complex solutions which allow evaluating the applicability of these catalysts and technology to practical cases. In few cases, also indications on the stability of the catalysts have been reported, because this is one of the critical issues. Indication on the biotoxicity of the treated effluents, another critical aspect, has been also reported in some cases. Therefore, although most of the studies have mainly an academic character, there are some indications on the applicability of the catalysts and wastewater treatment technology. Barrault et al. [79, 80] were between the first reporting the good performances of mixed (Al–Cu)-pillared clays prepared from a crude bentonite sample (H) (Tunisia deposit) in phenol oxidation with hydrogen peroxide (indicated as CWPO reaction; as noted before, often different acronyms are used for this reaction). They evidenced that the introduction of copper in pillaring position results in more effective catalysts, although the amount of copper which can be introduced was limited to less than 0.5 wt%. They also evidenced that copper introduced in this position results stable against leaching and the catalyst is reusable in successive batch reactor tests.
Barrault et al. [68, 69, 81] were also between the first reporting the excellent behavior of mixed (Al–Fe)-pillared clays (FAZA ) in WHPCO conversion of phenol [69]. In mild reaction conditions, about 80% of the initial amount of phenol was transformed into CO2, at 70 °C, in 2 h under atmospheric pressure. They evidenced that the reaction is heterogeneous and that the TOC (total organic carbon) abatement obtained with the FAZA catalyst is much higher than that observed with homogeneous iron species in the same reaction conditions. The little amounts of leached iron (less than 1 ppm) give a negligible contribution to the phenol oxidation. The catalyst leaching remains very low and catalyst performance is stable, even after three cycles of reaction.
Some years later, Barrault et al. [32] discussed the nature of the iron species active in the reaction. The characterization by electron spin resonance (ESR) of the iron species contained in the Al-Fe-pillared clay (FAZA) has shown that three different iron species were present:
-
Isolated iron species in highly distorted octahedral symmetry, located in the layer of the clay, probably in substitution of Al atoms.
-
Iron species belonging to oxide clusters.
-
Isolated iron species in octahedral symmetry, present only in the catalyst pillared by AlFe mixed complexes, probably belonging to the pillars as extra-framework species or by substituting the Al atoms of the pillars.
The latter species are those active in the total phenol oxidation by hydrogen peroxide. These species are also rather stable against leaching. The authors also reported long-time (350 h) catalytic experiments in a continuous flow reactor which showed the high stability of the Al-Fe-pillared clay. After these tests, the total amount of leached iron was less than 5 wt% of the initial value. This long-term result, in addition to demonstrate the suitability of the catalyst for application, evidences that a heterogeneous mechanism occurs, although it does not allow to discriminate whether it is a truly heterogeneous mechanism or the solid acts as catalyst to activate only H2O2.
7.3.1 Heterogeneous Versus Homogeneous Reactions
The question of heterogeneous versus homogeneous reaction in phenol oxidation by hydrogen peroxide in the presence of Fe-PILC catalysts was discussed specifically few years later by the same authors [82]. Both heterogeneous and homogeneous catalytic systems behave similarly. In both cases, the highest phenol conversion and TOC abatement being obtained at a pH value close to 3.7. However, the heterogeneous catalytic system results were less sensitive to the pH and more efficient in TOC abatement than the homogeneous one. The measurement of OH· production by an ESR spin trapping technique using 5,5-dimethylpyrroline-N-oxide (DMPO) as trapping agent showed that the OH· production followed the same profile in function of the pH value than the catalytic activity in phenol oxidation. This indicates that the main active species are hydroxyl (OH·) and/or hydroperoxyl (HO2·) radicals generated by hydrogen peroxide reaction with iron species in both homogeneous and heterogeneous systems. The main difference between homogeneous and heterogeneous systems is not the formation of different active oxygen from hydrogen peroxide, but the ability of the heterogeneous catalyst to adsorb on to its surface the phenol and/or the reaction intermediate products, favoring then their reaction with oxygen species formed by hydrogen peroxide activation.
These indication are in line with those reported by Perathoner and Centi [29] discussing the WHPCO of organic waste in agro-food and industrial streams. They also indicated that the adsorption of reactants on the solid and the different properties of iron or copper ions (or species) anchored on solid matrices (with respect to the same ions in solution) pointed out a difference between homogeneous and solid Fenton-type catalysts, notwithstanding the analogies in the behavior. The limited information in literature on the radical mechanisms in the presence of solid Fenton-type catalysts were also evidenced, as well as the little knowledge on the mass/heat diffusion aspects inside microporous materials during these fast radical-type reactions as and when hydroxyl radicals are generated.
Another aspect evidenced, which usually was neglected in literature, is the oxygen formation during the reactions:
as well as that derived from the direct H2O2 decomposition to water and oxygen influences the catalytic behavior. In fact, it competes with hydrogen peroxide to reoxidize the reduced Fe2+ or Cu+ species which are those which generate hydroxyl radicals on reaction with H2O2 (Eq. 7.1). In addition, a gas cap may form on the catalyst surface, especially in micropores, such as in PILC. The formation of this gas cap depends on the hydrophobic character of the catalyst, but may significantly affect the intraparticle mass diffusion in addition to quenching the reduced iron or copper species.
The issue of homogeneous versus heterogeneous catalytic reactions to eliminate organics from wastewater using H2O2 was discussed in detail by Perathoner et al. [83] with specific reference to Cu2+-pillared clays. Homogeneous (Cu2+ ions) and heterogeneous (Cu2+-PILC ) Fenton-like catalysts were compared in the conversion of p-coumaric acid, another model compound of polyphenolic fractions of OMW. The performances of the two classes of catalysts are similar for an analogous amount of copper, but there are some relevant differences in terms of (i) the presence of an induction time, (ii) the turnover frequency, (iii) the efficiency in the use of H2O2, (iv) the initial attack of p-coumaric acid (hydroxylation on the aromatic ring or oxidative attack on the double bond of the lateral chain), and (v) the effect of dissolved oxygen on the removal of total organic carbon (TOC) [83].
Scheme 7.1 shows an overview of the possible reaction pathways in the Fenton and Fenton-like mechanisms [83]. We indicate here Fenton mechanism that starting from Cu+ and/or Fe2+ ions, while Fenton-like mechanism that starting from Cu2+ and/or Fe3+ ions which reduces to the active Fenton species by reaction with organics. For solid heterogeneous catalysts, Fenton-like mechanism is prevailing. Scheme 7.1 is reported for copper ions, but it is well equivalent for the Fe2+/Fe3+ couple. Besides the “classic” redox cyclic mechanism involving Cu+ and Cu2+ species (indicated with A in Scheme 7.1), an additional pathway with formation of a copper hydroperoxo complex intermediate which transforms then to cupryl ion generating an hydroxyl radical is possible (indicated with B in Scheme 7.1). The two pathways are characterized by a different turnover frequency as well as efficiency in H2O2 use. Starting from Cu2+ ions (Fenton-like catalysts), the presence of the pathway A or B depends on the energetic stability of the hydroperoxo complex, which in turn depends on the ligand effect of the substituents.
Reaction mechanism for the generation of radical oxygen species by interaction with H2O2, with copper ions in solution or on the solid catalyst. Adapted from Perathoner et al. [83]
For the Fenton-like reagents (Cu2+, Fe3+), initially no O–O bond breaking takes place, but instead a metal hydroperoxo intermediate is formed as the first step via hydrolysis:
This intermediate might be able to react with organic substrates or break up into smaller active species in the second step. The metal hydroperoxo may homolyze either at the Fe–O bond generating M(n − 1)+ and producing the reactive HOO· radical or at the O–O bond producing the M=O species (example ferryl species) and an HO· radical:
Alternatively, O–O bond heterolysis could take place, producing the highly reactive M(n+1)+ species:
Static density functional theory (DFT) calculations on the hydrated Fenton-like reagent in vacuo, [FeIII(H2O)5(H2O2)]3+, and ab initio molecular dynamics (AIMD) simulations of the Fenton-like reagent in aqueous solution, Fe3+/H2O2(aq) [84], showed that the first reaction step consists of the donation of the α-proton of hydrogen peroxide to the solvent shortly after the hydrogen peroxide coordinates to iron(III) (Eq. 7.15), and the second step involves probably the O–O bond homolysis producing the ferryl ion and a hydroxyl radical. A strong solvent effect was noted indicating that in the presence of ligands, particularly those inducing a low-spin iron(III)hydroperoxo intermediate, the pathway of transformation can be different.
This suggests that in solid Fenton catalysts such as Fe-PILC a significant change in the pathway of transformation is expected. The formation of the ferryl intermediate, e.g., [FeIVO]2+, was also suggested to be a key step in the reaction mechanism over Fe-ZSM-5 Fenton-type solid catalysts [85], but forming by oxidation of a Fe(III)–OH intermediate, not from the peroxo [FeIIIOOH]2+ species. It is likely that this ferryl (or analogous cupryl) species plays a critical role also in Fe-PILC or Cu-PILC catalyst. Therefore, Fenton and Fenton-like chemistry which is generally believed to proceed via similar mechanisms instead probably follows different reaction pathways, intermediates, and reactive species.
In Cu-PILC , the preferable pathway involves thus the formation of the Cu2+ hydroperoxo complex (pathway B in Scheme 7.1). Further transformation gives rise to the formation of hydroxyl radicals and cupryl ions. The latter may oxidize organic molecule thereby reducing to Cu+ ions. The change from aquo to organic ligands in the copper complex may change the stability of the hydroperoxo complex. It is therefore reasonable to suggest that in the homogeneous catalyst the initial Fenton mechanism involves the hydroperoxo complex (route B), while in the progress of the reaction the mechanism changes to the “Cu+ ⇆ Cu2+” cyclic one (route A) as a consequence of the change in the formation of products of reactions (oxalic acid, for example) which substitute water molecules in coordinating the copper ions. This explains the presence of an induction time and the related change in the turnover frequency.
Theoretical studies [84, 86–88] have shown that the Fe(II)–aquo complex dissociates fast after coordination of H2O2 to a hydroxo group coordinated to iron and an HO· radical which attacks a solvent water molecule that was hydrogen bonded to the hydrogen peroxide. In fact, the generated hydroxyl radical has a short life-time which restricts its effective range of action to a few nanometers. When the organic substrate concentration is high there is a good chance that the hydroxyl radical may react with the organic molecule in the neighborhood of the iron complex where the hydrogen peroxide is coordinated. Therefore, the HO· radical contributes to the oxidation reactions, because the radical transfer could find a path along an H bond attached to an organic substrate molecule if the latter is close enough. Otherwise, the radical passage can go via a few solvent water molecules and end up with hydrogen abstraction from one of the water ligands of the same iron complex, resulting in the formation of a dihydroxo complex:
The iron(IV)dihydroxo complex is in equilibrium with its conjugate base:
After few picoseconds, one of the water ligands leaves the first solvation shell, but the formed complex [(H2O)3FeIIO(OH)]+ undergoes no more spontaneous chemical changes in the next picoseconds [88]. In other words, if the hydroxyl radical formed by the Fe(II)–aquo complex and H2O2 does not encounter an organic molecule in the neighborhood, the process leads to a quenching of the hydroxyl radical. However, if the pentaaqua iron(II)hydrogen peroxide complex would be in the neighborhood of a hexaaqua iron(II) complex, the HO· radical passage might be, via two or three solvent water molecules, to a water ligand of the hexaaqua iron(II), with two mono-hydroxo pentaaqua iron(III) complexes as the result. Their further transformation generates new radical species. For higher iron concentrations, there is thus an additional pathway of reaction of the short-living hydroxyl radicals. The hydroxyl radical reaction with a neighboring iron complex extends its area of action. Therefore increases the probability that the hydroxyl radical reacts with an organic molecule and the efficiency of the use of H2O2. It is known, on the other hand, that optimal iron/H2O2 and iron/organic ratios exist to maximize the Fenton and Fenton-like processes, in agreement with the above mechanistic interpretation.
Inside the layer of Cu-PILC or Fe-PILC catalyst, a similar mechanism exists, but further complicated from the presence of radical quenching mechanisms, due to the interaction with the solid or possible enhancement effects, related to the specific microenvironment. This short discussion evidences the high complexity of the issue of discussing homogeneous versus heterogeneous reactions in wet oxidation with hydrogen peroxide using PILC-based solid catalysts. We may note, however, that some indications on the reactivity are consistent with above indications on the reaction mechanism. For example, the observation [83] that a reduction of the TOF is observed together with an increase in the efficiency in H2O2 use increases the copper concentration in Cu-PILC catalysts. In fact, increasing copper concentration decreases the H2O2/Cu2+ ratio, but increases the probability to have two neighboring copper complexes and thus the possibility of limiting the quenching of the generated hydroxyl radicals.
7.3.2 Mechanism of Organic Transformation
An aspect which is often not well discussed regards the mechanism of organic transformation in WHPCO using PILC-based catalysts. The mechanism is highly depending on the type of organic, but let us to exemplify the problematic using as an example the reaction mechanism in the conversion of p-coumaric acid [83]. This model compound exemplifies the problematic in the conversion of polyphenolic compounds in OMW and in general of aromatic substances in wastewater.
The reaction network of p-coumaric acid degradation is summarized in Scheme 7.2 [83]. There are two main pathways for the conversion of p-coumaric acid: (i) hydroxylation of the aromatic ring resulting in the formation of caffeic acid (CA) and (ii) epoxidation of the double bond of the lateral chain with the intermediate formation of the corresponding diol which is then quickly converted to 4-hydroxybenzaldehyde (4HB) with the elimination of oxalic acid. There are various possible pathways of subsequent degradation. Scheme 7.2 reports a pathway which appears to be the most probable.
Reaction mechanism in the conversion of p-coumaric acid. The compounds in the bracket were supposed as reaction intermediate, but not identified analytically. Adapted from Perathoner et al. [83]
After caffeic acid formation it is possible to have the attack on the double bond of the lateral chain to form the 3,4-dihydroxybenzaldehyde which can also be formed by hydroxylation of the aromatic ring of 4HB. Oxidation of the 3,4-dihydroxybenzaldehyde gives 3,4-dihydroxybenzoic acid and further conversion of this compound gives a sequence of intermediate products, such as triacids, which are difficult to identify analytically. Finally, the degradation of these intermediates gives oxalic acid as stable product recalcitrant to further conversion and transformation to CO2.
According to this reaction scheme, two different oxidizing attacks are possible on p-coumaric acid, the epoxidation on the double bond of the lateral chain which forms 4HB as first identified product and the hydroxylation of the aromatic ring which gives CA.
There is a different trend with respect to the time on stream of the CA/4HB ratio for the homogeneous and heterogeneous cases. In the latter CA is mainly formed almost immediately, while in the homogeneous case the CA takes some time (around 15 min) to reach its maximum formation. This suggests an evolution in the nature of the active sites in the homogeneous case which agrees with the previous discussion about the mechanism responsible for an induction time in p-coumaric acid conversion.
The attack on the double bond of the lateral chain first followed by the hydroxylation of the aromatic ring (pathway A in Scheme 7.2) is due to the route involving the hydroperoxo complex formation, while the pathway B (first hydroxylation of the aromatic ring and then attack on the lateral chain) involves the “Cu+ ⇆ Cu2+” cyclic mechanism. The first mechanism (via hydroperoxo complex) initially dominates the conversion in the homogeneous case, but then there is a progressive change to the “Cu+⇆ Cu2+” cyclic mechanism. This explains an experimentally observed initial increase in the CA/4HB ratio in the homogeneous case after the induction time [83]. The ligand effect of the oxide in the case of the Cu-PILC, instead, inhibits the pathway via the hydroperoxo complex. Therefore, the “Cu+ ⇆ Cu2+” cyclic mechanism is already the dominant route and CA/4HB ratio is already high at the beginning. The ratio then decreases due to the conversion of the caffeic acid itself.
This discussion further evidences the differences in the reaction pathways in the generation of the radical oxygen species between homogeneous and heterogeneous Fenton reaction on solid PILC-based catalysts.
7.3.3 A Survey of Literature Data on the Use of PILC-Based Materials
Detailed studies on the WHPCO reaction mechanism of PILC-based catalysts are lacking and most of the studies are limited to the analysis of the performances of PILC catalysts in the conversion of different substrates. Chirchi and Ghorbel [89] have investigated the conversion of 4-nitrophenol by H2O2 with iron(III)-exchanged and pillared montmorillonite as catalysts. The aspects investigated were the influence of different types of catalysts, their amounts, and the H2O2 concentration. Ramaswamy et al. [90] have investigated phenol conversion using alumina-pillared montmorillonite (A1-PILC) on which copper–tetra decachlorophthalocyanine (Cu–Cl14Pc) complex was immobilized. Catrinescu et al. [91] have investigated the effect of different reaction parameters (pH, temperature, catalyst concentration) as well as catalyst stability in the WHPCO of phenol solutions. They used Fe-exchanged Al-pillared synthetic beidellite. Delaminated Fe-exchanged Ti-pillared interlayered montmorillonites (Fe–Ti-PILC) were instead used by Mei et al. [92] again in the WHPCO of phenol solutions. Carriazo et al. [93, 94] used instead a Colombian bentonite pillared with mixed polyhydroxocationic solutions of Al–Fe or Al–Ce–Fe again in the WHPCO of phenol solutions. They observed that the incorporation of Ce in the solids showed a favorable effect in the pillaring of the materials, allowing the increase of the basal spacing and enhancing the catalytic activity of the solids. Catalysts based on pillared clays with Al, Zr, and Al–Fe and Zr–Fe (starting from commercial bentonite) have been investigated also in WHPCO of phenol by Molina et al. [95]. The Al–Fe-pillared clay showed a higher activity than the Zr–Fe one for phenol oxidation, because the second was more active for H2O2 decomposition. Luo et al. [96] also investigated recently clay pillared with Fe–Al for Fenton oxidation of phenol by hydrogen peroxide. As original results cited that (i) the pillaring process altered the basal space of clay, (ii) the catalytic activity is related to the accessible iron species, (iii) the Fenton reaction exhibited an induction period, and (iv) the rate of the oxidation process depends on the concentrations of phenol and H2O2, the amount of catalyst, pH, and temperatures. As commented above, these catalysts (Fe–Al-pillared clays) and almost all the findings were already well established in previous literature. Recently, Timofeeva et al. [97] also investigated Fe,Al-pillared clays (obtained from Mukhortala (Buryatia) montmorillonites) in phenol oxidation, although extending the investigation to also monoazo dye acid chrome dark-blue (ACDB). Most of the results were again analogous to those previously reported. The effect of OH/(Fe + Al) ratio, aging time, and calcination temperature was examined. The state of iron atoms can be controlled by Al/Fe and OH/(Fe + Al) ratios, aging period of Fe,Al-pillaring solution, and calcination temperature. The increase in Al/Fe ratio and calcination temperature from 400 to 500 °C decreases in the formation of oligomeric iron species. The increase in aging time decreases iron leaching and increases the reaction rate, due to the formation of isolated iron species. The same authors [98] also investigated the use of mixed Fe,Cu,Al clays. The introduction of copper ions results in an increase in the reaction rate, according to what discussed above.
This brief survey of literature results evidences that notwithstanding the continuous and also recent publications on the use of PILC catalysts in WHPCO reaction limited new information are available to produce better catalysts and/or develop on a commercial scale a process based on these catalysts. Different starting clays were used as raw materials, but without a rational choice. In addition to phenol, and the cited results on 4-nitrophenol [89] and p-coumaric acid [83], few other molecules were tested in WHPCO using PILC catalysts. Tabet et al. [99] investigated Fe-pillared montmorillonite for the removal of cinnamic acid in water. The influences of the cinnamic acid, catalyst and H2O2 concentrations, and pH on the removal rate of cinnamic acid have been studied. The results show that the efficiency of Fe-pillared montmorillonite is higher than that of the Fe ions in the homogeneous phase, and less sensitive to pH, in agreement with the results for other organic pollutants, as discussed above. Herney-Ramirez et al. [100, 101] analyzed the degradation and mineralization of Orange II solutions using catalysts based on pillared saponite impregnated with different iron salts. In the best conditions and after 4 h of oxidation, 99% of dye degradation with 91% of total organic carbon (TOC) reduction (at 70 °C), using only ca. 90 mg of clay catalyst per liter of solution was observed; 96% of dye removal with 82% of mineralization was also reached at 30 °C. The amount of iron released into the final solution was lower than 1 ppm. Copper-supported-pillared clay catalysts were investigated in the conversion of tyrosol in batch and continuous reactors [102, 103]. Stable performances were observed. Around 84 and 53% substrate and TOC conversion, respectively, were obtained for 120-h reaction. Total elimination of TOC is difficult since oxalic acid, as the main intermediate, is resistant to WHPCO. The same authors [104] also investigated the catalytic wet hydrogen peroxide oxidation of p-coumaric acid over Al–Fe-pillared clay. It was found that the distribution of the intermediate compounds was strongly dependent on the pH of the solution.
Few results have been reported in WHPCO using PILC catalysts for treating real wastewater streams. One of the first results has been reported by Kim et al. [105, 106] who investigated the WHPCO of real dyehouse effluents in both batch reactor and continuous flow pilot plant-scale reactor by using Cu/Al2O3 and Al–Cu-PILC catalysts. The removal of TOC and color was strongly related to the consumption of H2O2. Copper components in the catalysts, especially in the Al–Cu-PILCs, showed successful activity toward complete removal of TOC and color. In addition the Al–Cu-PILC catalysts were extremely stable against copper leaching.
Copper-based and iron-based pillared clays (Cu-PILC, Fe-PILC) have been studied and compared in the WHPCO of both model phenolic compounds (p-coumaric and p-hydroxybenzoic acids) and real olive oil milling wastewater (OMW) by Caudo et al. [107]. These two catalysts show comparable performances in all these reactions, although they show some differences in the rates of the various steps of reaction. In particular, Cu-PILC shows a lower formation of oxalic acid (main reaction intermediate) with respect to Fe-PILC. Both catalysts show no leaching of the transition metal differently from other copper-based catalysts prepared by wetness impregnation on oxides (alumina, zirconia) or ion-exchange of clays (bentonite) or zeolite ZSM-5. No relationship was observed between copper reducibility in the catalyst and the performance in WHPCO, as well as between the rate of copper leaching and catalytic behavior. Cu-PILC shows a comparable activity to dissolved Cu2+ions, although the turnover number is lower assuming that all copper ions in Cu-PILC are active. Cu-PILC shows a high resistance to leaching and a good catalytic performance, which was attributed to the presence of copper essentially in the pillars of the clay. A high efficiency in H2O2 use in the first hour of reaction with the participation of dissolved O2 in solution was also shown. For longer reaction times, however, the efficiency of H2O2 use considerably decreases.
The behavior of Cu-PILC in the pre-treatment of OMW was compared with that of Cu-zeolites (MFI structure) by Giordano et al. [108]. Both the catalysts showed a high conversion in the oxidation of polyphenols and were able to drastically reduce the chemical oxygen demand, the biochemical oxygen demand, and the non-biodegradability of the olive oil mill wastewaters. However, the Cu-PILC shows better stability. More recently, the performances of Cu-PILC for agro-food wastewater purification with H2O2 were discussed more in depth [109]. These catalysts were studied in the treatment of various real wastewaters from agro-food production: (i) deriving from citrus juice production, (ii) extracted concentrated polyphenolics fraction from olive oil milling (OMW), and (iii) OMW derived from three different sources. In the latter cases, tests were made both in a lab-scale reactor and in a larger volume (about 10 l) reactor. The results showed that Cu-PILC might be used to treat real wastewater from agro-food production, and not only simple model chemicals as typically made in the literature. In all cases, using a semi-batch slurry-type reactor with a continuous feed of H2O2, the behavior both in TOC (total organic carbon) and in polyphenols abatement may be described using pseudo-first-order reaction rates. Using real wastewater the rate constants are one to two orders of magnitude lower than using model molecules, and a decrease in the ratio between rate constant of phenols conversion and rate constant of TOC abatement is observed. However, this ratio maintains over 1 in all cases. A typical value is around 2, but the composition of wastewater and reaction conditions influence this ratio. Scaling-up to a larger volume semi-continuous slurry-type reactor causes a further lowering of one order of magnitude in the rate constants of TOC and polyphenols depletion, due to fouling of the catalyst related to the preferential coupling of the organic radicals and deposition over the catalyst with respect to their further degradation by hydroxyl radicals generated from H2O2 activation on the copper ions of the catalyst. The use of a different reactor to overcome this problem was suggested.
The application of WHPCO for the treatment of complex effluents from electronic industry was also discussed by Perathoner and Centi in reviewing the use of iron-based or copper-based solid catalysts for the treatment of industrial waste aqueous streams [29]. To note that in this case, homogeneous Fenton catalysts were completely inactive due to the harsh reaction conditions (many complexing chemicals present in the feed), while relatively good performances were obtained using the solid catalysts.
In conclusion, this short survey on the use of PILC-based catalysts for WHPCO reaction has evidenced that these materials show interesting and stable performances. They can be used for practical applications, although data on their practical use are limited. Most of the literature data focused on the use of only model compounds. In addition, often data for a more rational design are limited and published results were mainly related to the analysis of the effect of the reaction conditions and/or catalyst composition, sometimes also rediscovering older results. This section has evidenced that it is necessary for a much more in depth understanding of the reaction mechanism and in particular the catalytic chemistry inside the microporous clay layer. The generation of short-living and highly reactive radical species (hydroxyl radicals, for example) changes significantly the picture with respect to conventional liquid–solid catalytic chemistry. It is thus necessary to put attention on these aspects, as well as on other critical aspects, such as the hydrophobic characteristics of the inner layered structure and the formation of gas caps inside the layers.
7.4 Photocatalytic Behavior of Ti-PILC and Fe-PILC
7.4.1 Ti-PILC
The first attempt to prepare photocatalysts based on titania-pillared clays (Ti-PILCs) has been reported by Ding et al. [42]. They used a sol–gel method and different drying methods (air drying, air drying after ethanol extraction, and supercritical drying), because they have significant effect on the photocatalytic efficiency for the oxidation of phenol in water. Ti-PILC obtained by supercritical drying has the highest external and micropore surface area, largest amount and smallest crystallite size of anatase, and exhibited the highest photocatalytic activity. It should be noted, however, that these materials are not truly titania-pillared clays, but should be better described as layered materials with deposited TiO2 nanocrystals. In parallel to the work of Ding et al. [42], Ooka et al. [110] reported a study on the synthesis of pillared montmorillonite with crystallized TiO2. The TiO2 pillars in pillared montmorillonite were crystallized to anatase by hydrothermal treatment, while retaining the porosity of the pillared clay. The size of the crystallized TiO2 pillars and the average pore diameter could be controlled in the range ca. 0.4–0.9 nm by changing the treatment conditions. These materials were studied in the photocatalytic degradation of trichloroethylene in water. The hydrothermal crystallization enhances the catalytic activity both per unit weight of TiO2 and per exposed unit surface area of TiO2. The catalytic activity of the TiO2 pillars was also enhanced by quantum-size effects: as the TiO2 pillar size decreased, the pillared montmorillonites exhibited higher catalytic activity and showed larger blueshifts in their UV absorption spectra. The same group [111] also reported that titania-pillared clay could adsorb dibutyl phthalate (DBP), one of the endocrine disruptors, and degrade it photocatalytically.
These initial studies started a relevant activity for the preparation of Ti-PILC-based photocatalysts. The main motivations are related to the possibility of having highly dispersed nanosized TiO2 pillars which acting as photo-active centers in a microenvironment (the inner layer of the clay) could control the access of pollutants and prevent deactivation by fouling. Worth to note is the possibility to control the hydrophobic character of the PILC and use this property to control the selective behavior in the photocatalysis. For example, Shimuzu et al. [112] investigated the selective photo-oxidation of benzene and cyclohexane using TiO2-pillared clays (mica, montmorillonite, and saponite). In an aqueous environment, TiO2-pillared clays showed higher selectivity to oxygenates than TiO2 (Degussa P25). The product distribution among oxygenates also depends on the type of the clay host. For the cyclohexane photo-oxidation, TiO2-pillared clay showed much higher selectivity than TiO2, because of the hydrophobic nature of pillared clay.
Awate and Suzuki [113] investigated the use of titania-pillared montmorillonite clay prepared by two different routes (ion-exchange method and hydrothermal treatment after ion-exchange) in the photocatalytic oxidation of two dyes (Methylene Blue and Victoria Pure Blue). The Ti-PILC prepared by post-hydrothermal route has shown enhanced adsorption capacity and photocatalytic oxidation. Different other groups were active in the photocatalytic degradation of dyes and other pollutants using TiO2-pillared clays: (i) 2,4-dichlorophenol and Orange II [114], (ii) benzene and cyclohexane [115], phthalate esters (di-n-butyl phthalate and dimethyl phthalate) [44], 4-chlorophenol [115], Methyl Orange [116], decabromodiphenyl ether (BDE 209) [117], dimethyl phthalate ester, as model of endocrine disruptor [118], the dye acidic fuchsine [119], phenol [120, 121], 4BS dye [122], textile azo dye (Solophenyl Red 3BL) [123, 124], and azoic dyes (Congo Red) [125, 126]. A larger variety of substrate was thus investigated using Ti-PILC photocatalysts with respect to the previously discussed case of WHPCO, although the use of many different substrates is typical of photocatalytic studies. Most of the studies were also in this case limited to the analysis of the effect of the reaction conditions and catalyst characteristics. Nevertheless, some clear direction could be evidenced.
Several authors put attention on the issue of hydrophobic characteristics of PILC to control the reactivity. Ooka et al. [44] investigated four kinds of TiO2-pillared clays prepared from different raw clays (montmorillonite, saponite, fluorine hectorite, and fluorine mica) and put in relation their surface hydrophobicities with the performances in adsorption-photocatalytic degradation of phthalate esters. The surface hydrophobicity of TiO2-pillared clays largely varies with the host clay: it increases in the order saponite < fluorine hectorite < montmorillonite < fluorine mica. The order of performance in adsorption and successive photocatalytic degradation of phthalate ester was consistent with that of surface hydrophobicity. Using the host clay that enhances surface hydrophobicity of the TiO2-pillared clay, such as fluorine mica, it is possible to obtain highly active photocatalysts for photodegradation of organic compounds in water. The performances could be increased by improving TiO2 nanoparticle crystallinity using a treatment with aqueous hydrogen peroxide at room temperature [127]. Inorganic/organic-pillared clays (Ti/cetyltrimethylammonium bromide-pillared clays) could be prepared for some scope of controlling hydrophobic character and enhance photocatalytic activity in the removal of non-polar organics in water [128].
Other authors [129] have investigated the use of cetyltrimethylammonium bromide (CTAB) to modify the characteristics of TiO2-pillared montmorillonite photocatalysts. The “growth” of the hydrated Ti-polycations within clay interlayers could be regulated and controlled by the surfactant CTAB. An et al. [117] also prepared TiO2-immobilized hydrophobic montmorillonite photocatalysts via ion-exchange reaction between sodium montmorillonite with cation surfactant, cetyltrimethylammonium bromide (CTAB). These materials were designed for the oxidation of persistent organic pollutants in water, by combining the pre-adsorption and concentrated effects for aqueous microorganic pollutants with the photocatalytic destruction of organic pollutants. Ding et al. [118] also prepared TiO2-pillared montmorillonites by introducing Ti4+ into a layer of montmorillonite modified with or without cetyltrimethylammonium bromide. However, in studying the photocatalytic degradation of dimethyl phthalate ester they observed that the hydrophobic photocatalyst was slightly less active than the hydrophilic one. The same authors [130] analyzed the role of acid (acetic acid and hydrochloric acid) in catalyzing hydrolysis of titanium hydrate sols during the preparation of hydrophobic montmorillonite clay treated with organic cationic surfactant (hexadecyltrimethylammonium bromide). Hydrophobic TiO2-pillared clay prepared with acetic acid as the acid hydrolysis catalysts possesses higher photocatalytic activity than that with hydrochloric acid. Organoclays having titania nanocrystals in interlayer hydrophobic field [64], prepared by intercalating the cetyltrimethylammonium cations to the montmorillonite layer and introducing Ti by cation-exchange, were also shown to exhibit higher photocatalytic activity for BPA (bisphenol A) degradation, due to their hydrophobic character.
Therefore, many studies focused on the role of hydrophobic character of inner microenvironment in PILC materials to have a preferential concentration of non-polar pollutants close to the active photocatalyst, usually TiO2 nanoparticles either present as pillars or as simple deposits inside the layers. The latter situation, however, gives rise to less stable photocatalysts, even if this aspect was typically not well investigated.
Some authors claimed a novel architecture for the Ti-PILC photocatalysts, although the novelty is questionable. Yang et al. [120], for example, discussed a new photocatalyst design to increase the content of anatase nanocrystals and improve the accessibility of these crystals to reactants (UV photons, organic contaminant molecules, and oxygen molecules), in order to enhance the photocatalytic performance. However, the proposed new synthesis strategy involves reaction of clay suspensions with TiOSO4, which leads to formation of anatase nanocrystals attaching to leached clay layers through Ti–O–Si bonds. They also claimed to have discovered that the crystal size, the pore size, and the specific surface area of the catalysts can be tailored by manipulating the acidity, the ratio of Ti/clay, and the hydrothermal temperature of the synthesis systems. Although there are some differences with respect to the conventional approach of pillared intercalated layered clays, the resulting materials are not largely different from those earlier reported and discussed above. The photocatalytic performances in the conversion of phenol also do not appear rather spectacular, with about 50% conversion in 4–5 h of a highly diluted (25 ppm) phenol solution.
Xuzhuang et al. [121] also claimed for a new composite structure with superior photocatalytic activity for phenol decomposition, by reaction between TiOSO4 and a synthetic layered clay laponite. The authors claimed a superior photocatalytic activity due to the unique structure of the composite, in which anatase nanocrystals were attached on leached laponite fragments. These photocatalysts take about 2 h to convert 25 ppm phenol using UV light source. Although this result is better than that of the “traditional” pillared clay used as reference, it is still a rather poor result with respect to “conventional” photocatalysts in the same reaction, taking also into account that only phenol conversion and not TOC removal was reported.
The “superior” photocatalytic activity derives from a hydrothermal treatment which partially destroys the clay (making thus more accessible) and especially make more crystalline the anatase TiO2 nanocrystals. This aspect is that probably determining the higher performances, together with the fact that the TiO2 content in the most active samples is very high (about 60 wt%). The destruction of the characteristic pillaring structure eliminates the possibility of controlling the microenvironment and thus to have a selective photocatalysis, as discussed above. On the other hand, in terms of absolute photocatalytic performances these “novel” materials appear rather poor with respect to well-optimized TiO2 photocatalysts. It is thus difficult to support the authors claim that the knowledge acquired in their study is useful for designing photocatalysts with high efficiency.
We have discussed more in depth this specific example, although similar remarks may be made to also other published papers, to evidence that also for the use of Ti-PILC catalysts most of the studies have limited practical objectives. Being well established the fields of photocatalysis using semiconductor materials, the questions to address while studying Ti-PILC photocatalysts are the following:
-
What kind of advantages (in terms of performances per unit weight or catalyst cost, considering the low cost of TiO2) is offered from using Ti-PILC materials?
-
There are specific characteristics which can make unique these materials.
In terms of absolute photocatalytic properties, Ti-PILC does not compete with well-optimized TiO2 (note that the often used Degussa P-25 is not always the best, depending on the substrate to convert), in terms of activity per unit weight and unit cost. The reasons are related to lower amount of TiO2 (with respect to pure TiO2), the difficulty in obtaining well-crystallized nanocrystals, and the quantum-size effect, when small TiO2 nanocrystals are present, e.g., a blueshift in the absorption edge. This is a shift in the opposite direction to what necessary to have activity with visible light. The main problem in using photocatalytic methodologies for the elimination of pollutants is the need to operate with highly diluted solutions (few ppm), which greatly limit their possible application in practical cases. For this reason, the use of solar light is essentially required in photocatalytic waste treatment, as the use of UV lamps is not being feasible. Therefore, the issue is to analyze how to use the PILC structure to promote visible light activity. The other possible approach is to use the hydrophobic microenvironment of PILC to absorb the non-polar pollutants and then make periodic photocatalytic regeneration, e.g., to not use in a continuous illumination mode. Some authors have used the latter route, as discussed above. This would imply, however, to preserve the layered structure and optimize the inner hydrophobic properties.
Few authors have instead investigated how to use the PILC structure to promote visible light activity. Zhang et al. [122] used nitrogen and sulfur co-doped TiO2-pillared montmorillonite for the degradation of 4BS dye under visible light irradiation (λ > 400 nm). The absorption edge of the doped samples shows a redshift as compared to that of pure TiO2. Their photocatalytic activity is comparable to that of Degussa P25 under visible light irradiation, although it is forgotten that dyes adsorbing in the visible region are self-sensitizer. The use of dyes adsorbing in the visible region is not a suitable test to analyze the photocatalytic properties of materials in the visible region. In addition, it is well known that the issue is the complete conversion of the dye and not its partial conversion, e.g., to evaluate TOC and not dye conversion, due to this self-sensitizer effect. Yang et al. [119] also investigated the use of TiO2-pillared montmorillonite composite photocatalyst for the degradation reaction of acidic fuchsine by sunlight. Similar remarks to those evidenced above could also be made in this case.
In conclusion, several papers have been published on the use of Ti-PILC as advanced photocatalysts, and they were utilized in the conversion of many pollutants, although essentially in academic studies. Their practical applicability is still questionable and suitable data do not exist to forecast their use. On the other hand, it should be remarked that not enough effort has been made in literature to evaluate the specific features of these catalysts in relation to practical use and to optimize their design in relation to these characteristics.
7.4.2 Fe-PILC for Photo-Fenton
From the practical point of view, more interesting prospects exist on the use of Fe-PILC for the photo-Fenton reaction, e.g., the combination of use of H2O2 and light. With respect to photocatalytic oxidation, it is necessary to use a more expensive reactant (H2O2), but the higher reaction rates and the possibility to address wastewater containing higher concentrations of pollutants (the typical case) make this technology preferable. Many reviews have discussed the advantages and limits of this technology and their applicability in the treatment of hard degradable wastewater from dye, phenols, explosive, pesticide, paper-making, and some other industries [34, 41, 131–137].
Sum et al. [138] were the first in reporting the behavior of pillared laponite clay-based Fe nanocomposites in the photo-Fenton (H2O2 + UV radiation) mineralization of azo-dye Acid Black l (AB1); 100% mineralization could be achieved, while only trace amounts of leached ferric ions were detected. Liu et al. [139] applied Fe-pillared bentonite in the photo-Fenton degradation of an organic azo-dye (Orange II). They also evidenced that the rate of the heterogeneous photo-Fenton process is much faster than that of homogeneous photo-Fenton process. The catalyst can also be easily recovered, regenerated, and re-used. Bobu et al. [140, 141] used iron-pillared laponite for the photodegradation of two important phenyl urea herbicides: Monuron and Isoproturon. However, the mineralization degree of both herbicides was only around 10–20%. The same authors applied this catalyst in the photo-assisted Fenton mineralization of ciprofloxacin (CFX), a broad-spectrum antibiotic used in human and veterinary medicine. The effects of reaction parameters such as H2O2 concentration, catalyst loading, and initial pH of the solution on the mineralization of CFX were investigated. It was found that at the optimal reaction conditions (60 mM H2O2, 1.0 g l− 1 catalyst, initial solution pH 3.0) complete CFX degradation and over 57% total organic carbon (TOC) removal of CFX can be achieved after 30 min of reaction. Also these authors observed very limited leaching of iron and stable performances.
De León et al. [142] investigated the photo-Fenton conversion of Methylene Blue using Fe-PILC catalysts with two different-sized particle fractions (<250 μm and within the range of 250–450 μm, respectively). Both catalysts showed activity in the discoloration of aqueous dye solution, but the differences in catalyst performance were correlated with textural parameters.
All these tests were made using H2O2 and UV light, but recently the possibility of using visible light was reported. Chen et al. [143] reported the decolorization and mineralization of reactive brilliant orange X-GN under visible light irradiation (λ ≥ 420 nm) by using iron-pillared montmorillonite(Fe-Mt/H2O2 as the heterogeneous photo-Fenton reagent. The characterization of these materials suggested that small-sized hydrolyzed iron successfully intercalates into the interlayer spaces of the clay via pillaring. The catalytic results showed that at a reaction temperature of 30 °C, pH 3.0, 4.9 mmol/l H2O2, and 0.6 g/l catalyst dosage, 98.6% discoloration and 52.9% TOC removal of X-GN were achieved under visible irradiation after 140 min of treatment. Furthermore, the maximum concentration of dissolved iron ions was 1.26% of the total iron content in the Fe-Mt catalyst after photocatalysis.
Therefore, although results on Fe-PILC catalysts for photo-Fenton conversion are still limited, the performances and preliminary stability indications are promising. However, it is necessary to pass from batch to continuous reactor tests, evaluate the performances using real waste solutions, and develop/test the performances in the form of thin supported films, because from a practical point of view the use of slurry-type photoreactors is not possible.
7.5 Conclusions
The use of solid catalysts for wastewater treatment, particularly by AOP method, is a fast growing area, from both the scientific and applicative perspectives. Pillared clays, particularly containing Fe, Cu, and Ti as active elements, show some interesting perspectives, although most of the reported publications still give a too limited attention on the critical aspects for application. Also from the scientific perspective, often results are essentially phenomenological, e.g., the effect of the reaction conditions and catalyst composition/characteristics, more than focused at giving a more scientific approach toward the understanding of the critical issues governing the reactivity, kinetics, and reaction mechanism. Due to the formation of short-living and highly reactive oxidizing species, such as the hydroxyl radical, the catalytic chemistry is quite different from that present in “conventional” liquid–solid catalytic reactions. This aspect, and the relevance for the design of improved catalysts, has received limited attention in literature.
There are limited, but relevant differences between the use of these heterogeneous catalysts and homogeneous catalysts, as discussed more specifically for wet oxidation with hydrogen peroxide. These differences and the implications in terms of reaction mechanism should be considered for a better catalyst design.
From the practical perspective, the use of PILC-based catalysts is promising using H2O2 as the reactant (WHPCO or photo-Fenton) on iron-pillared or copper-pillared clays. These materials combine good activity with stable performances, although data on long-term stability in continuous flow reactors are quite limited. The problem of controlling the hydrophobic microenvironment within the layers of the pillared clays is an important issue to improve the performances, but was mainly investigated for the Ti-PILC materials for the photocatalytic oxidation of pollutants. The applicability of the latter reaction is questionable, and in general the limited attention given in literature to consider the key questions for their use and design has to be remarked. The use of PILC catalysts in wet oxidation with air also shows limited perspectives from the application point of view.
References
Theron J, Walker JA, Cloete TE (2008) Nanotechnology and water treatment: applications and emerging opportunities. Crit Rev Microbiol 34:43–69
Busca G, Berardinelli S, Resini C, Arrighi L (2008) Technologies for the removal of phenol from fluid streams: a short review of recent developments. J Hazard Mater 160:265–288
Liotta LF, Gruttadauria M, Di Carlo G, Perrini G, Librando V (2009) Heterogeneous catalytic degradation of phenolic substrates: catalysts activity. J Hazard Mater 162:588–606
Kwon S, Fan M, Cooper AT, Yang H (2008) Photocatalytic applications of micro- and nano-TiO2 in environmental engineering. Crit Rev Environ Sci Tech 38:197–226
Bhargava SK, Tardio J, Prasad J, Föger K, Akolekar DB, Grocott SC (2006) Wet oxidation and catalytic wet oxidation. Ind Eng Chem Res 45:1221–1258
Centi G, Perathoner S (2005) Use of solid catalysts in promoting water treatment and remediation technologies. In: Spivey JJ (ed) Catalysis, vol 18. Royal Society of Chemistry Publishing, Cambridge, UK, pp 46–71
Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment II: hybrid methods. Adv Environ Res 8:553–597
Oliviero L, Barbier J Jr, Duprez D (2003) Wet air oxidation of nitrogen-containing organic compounds and ammonia in aqueous media. Appl Catal B: Environ 40:163–184
Imamura S (1999) Catalytic and noncatalytic wet oxidation. Ind Eng Chem Res 38:1743–1753
Liu Z-h, Kanjo Y, Mizutani S (2009) Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment – physical means, biodegradation, and chemical advanced oxidation: a review. Sci Total Environ 407:731–748
Esplugas S, Bila DM, Krause LGT, Dezotti M (2007) Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J Hazard Mater 149:631–642
Rios GM, Belleville MP, Paolucci-Jeanjean D, Sanchez J (2009) Membrane technologies at the service of sustainable development through process intensification. In: Centi G, Trifiró F, Perathoner S, Cavani F (eds) Sustainable industrial processes, Chapter 4. Wiley, Weinheim, Germany, pp 257–278
Centi G, van Santen RA (2007) Catalysis for renewables. Wiley, Weinheim, Germany
Centi G, Perathoner S (2003) Novel catalyst design for multiphase reactions. Catal Today 79–80:3–13
Centi G, Perathoner S (1999) Recycle rinse water: problems and opportunities. Catal Today 53:11–21
Centi G, Gotti M, Perathoner S, Pinna F (2000) Rinse water purification using solid regenerable catalytic adsorbents. Catal Today 55:51–60
Marco A, Esplugas S, Saum G (1997) How and why combine chemical and biological processes for wastewater treatment. 3Water Sci Technol 35:321–327
Centi G, Perathoner S (2008) Catalysis by layered materials: a review. Micropor Mesopor Mater 107:3–15
Gil A, Korili SA, Vicente MA (2008) Recent advances in the control and characterization of the porous structure of pillared clay catalysts. Catal Rev Sci Eng 50(2):153–221
Ding Z, Kloprogge JT, Frost RL, Lu GQ, Zhu HY (2001) Porous clays and pillared clays-based catalysts. Part 2: a review of the catalytic and molecular sieve applications. J Porous Mater 8:273–293
Cheng S (1999) From layer compounds to catalytic materials. Catal Today 49:303–312
Pinnavaia TJ (1983) Intercalated clay catalysts. Science 220:365–371
Figueras F (1988) Pillared clays as catalysts. Catal Rev Sci Eng 30:457–499
Suty H, De Traversay C, Cost M (2004) Applications of advanced oxidation processes: present and future. Water Sci Technol 49:227–233
Vandevivere PC, Bianchi R, Verstraete W (1998) Treatment and reuse of wastewater from the textile wet-processing industry: review of emerging technologies. J Chem Tech Biotechnol 72:289–302
Neyens E, Baeyens J, Weemaes M, De Heyder B (2003) Pilot-scale peroxidation (H2O2) of sewage sludge. J Hazard Mater 98:91–106
Centi G, Perathoner S, Romeo G (2001) Fe/MFI as a new heterogeneous Fenton-type catalyst in the treatment of wastewater from agroindustrial processes. Stud Surf Sci Catal 135:5156–5163
Centi G, Perathoner S, Torre T, Verduna MG (2000) Catalytic wet oxidation with H2O2 of carboxylic acids on homogeneous and heterogeneous Fenton-type catalysts. Catal Today 55:61–69
Perathoner S, Centi G (2005) Wet hydrogen peroxide catalytic oxidation (WHPCO) of organic waste in agro-food and industrial streams. Top Catal 33:207–224
Chakinala AG, Gogate PR, Burgess AE, Bremner DH (2009) Industrial wastewater treatment using hydrodynamic cavitation and heterogeneous advanced Fenton processing. Chem Eng J 152:498–502
Li D, Qu J (2009) The progress of catalytic technologies in water purification: a review. J Environ Sci 21:713–719
Guélou E, Barrault J, Fournier J, Tatibouët J-M (2003) Active iron species in the catalytic wet peroxide oxidation of phenol over pillared clays containing iron. Appl Catal B: Environ 44:1–8
Carriazo JG, Guelou E, Barrault J, Tatibouët J-M, Moreno S (2003) Catalytic wet peroxide oxidation of phenol over Al–Cu or Al–Fe modified clays. Appl Clay Sci 22:303–308
Sirtori C, Zapata A, Oller I, Gernjak W, Agüera A, Malato S (2009) Solar photo-Fenton as finishing step for biological treatment of a pharmaceutical wastewater. Environ Sci Technol 43:1185–1191
Andreozzi R, Canterino M, Di Somma I, Lo Giudice R, Marotta R, Pinto G, Pollio A (2008) Effect of combined physico-chemical processes on the phytotoxicity of olive mill wastewaters. Water Res 42:1684–1692
Kanmani S, Raja A (2008) Pilot plant treatment of distillery wastewater by solar photo-Fenton process. Indian J Environ Prot 28:218–226
Pérez M, Torrades F, Domènech X, Peral J (2002) Fenton and photo-Fenton oxidation of textile effluents. Water Res 36:2703–2710
Bauer R, Fallmann H (1997) The photo-Fenton oxidation – a cheap and efficient wastewater treatment method. Res Chem Intermediates 23:341–354
Kiwi J, Pulgarin C, Peringer P (1994) Effect of Fenton and photo-Fenton reactions on the degradation and biodegradability of 2 and 4-nitrophenols in water treatment. Appl Catal B Environ 3:335–350
Jain DM (2005) Photo Fenton degradation for environmental application. J Ind Pollut Control 21:181–194
Malato S, Fernández–Ibáñez P, Maldonado MI, Blanco J, Gernjak W (2009) Decontamination and disinfection of water by solar photocatalysis: recent overview trends. Catal Today 147:1–59
Ding Z, Zhu HY, Lu GQ, Greenfield PF (1999) Photocatalytic properties of titania pillared clays by different drying methods. J Colloid Interface Sci 209:193–199
Li J, Chen C, Zhao J, Zhu H, Ding Z (2002) Photodegradation of dye pollutants on TiO2 pillared bentonites under UV light irradiation. Sci China B 45:445–448
Ooka C, Yoshida H, Suzuki K, Hattori T (2004) Highly hydrophobic TiO2 pillared clay for photocatalytic degradation of organic compounds in water. Micropor Mesopor Mater 67:143–150
Romero A, Dorado F, Asencio I, García PB, Valverde JL (2006) Ti-pillared clays: synthesis and general characterization. Clays Clay Miner 54:737–747
Damardji B, Khalaf H, Duclaux L, David B (2009) Preparation of TiO2-pillared montmorillonite as photocatalyst. Part II. Photocatalytic degradation of a textile azo dye. Appl Clay Sci 45:98–104
Danis TG, Albanis TA, Petrakis DE, Pomonis PJ (1998) Removal of chlorinated phenols from aqueous solutions by adsorption on alumina pillared clays and mesoporous alumina aluminum phosphates. Water Res 32:295–302
Wibulswas R, White D, Rautiu R (1998) Removal of humic substances from water by alumina-based pillared clays. Environ Technol 19:627–632
Tahani A, Karroua M, El Farissi M, Levitz P, Van Damme H, Bergaya F, Margulies L (1999) Adsorption of phenol and its chlorine derivatives on PILCS and organo-PILCS. J Chimie Physique et de Physico Chimie Biol 96:464–469
Konstantinou IK, Albanis TA, Petrakis DE, Pomonis PJ (2000) Removal of herbicides from aqueous solutions by adsorption on Al-pillared clays, Fe–Al pillared clays and mesoporous alumina aluminum phosphates. Water Res 34:3123–3136
Bouras O, Houari M, Khalaf H (2001) Using of surfactant modified Fe-pillared bentonite for the removal of pentachlorophenol from aqueous stream. Environ Technol 22(1):69–74
Zeng XQ, Liu WP (2005) Adsorption of direct green B on mixed hydroxyl-Fe–Al pillared montmorillonite with large basal spacing. J Environ Sci 17:159–162
Ortiz-Polo A, Richards-Uribe RM, Otazo-Sánchez EM, Prieto-García F, Hernández-Ávila J, Acevedo-Sandoval O, Gordillo-Martínez A (2008) New organo-inorganic materials for water contaminants remediation. In: Barbé C, Laine RM, Sanchez C, Schubert U (eds) Organic/Inorganic hybrid materials-2007, Mater Res Soc Symp Proc, Volume 1007. Warrendale, PA, pp.1007-S04-38.
Guerra DL, Airoldi C, Lemos VP, Angélica RS (2008) Adsorptive, thermodynamic and kinetic performances of Al/Ti and Al/Zr-pillared clays from the Brazilian Amazon region for zinc cation removal. J Hazard Mater 155:230–242
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interface Sci 140:114–131
Karamanis D, Assimakopoulos PA (2007) Efficiency of aluminum-pillared montmorillonite on the removal of cesium and copper from aqueous solutions. Water Res 41:1897–1906
Zhu JX, He HP, Guo JG, Yang D (2002) Recent advances in research on organic pillared montmorillonite. Bull Mineral Petrol Geochem 21:234–237
Srinivasan KR, Fogler HS (1990) Use of inorgano-organo-clays in the removal of priority pollutants from industrial wastewaters: structural aspect. Clays Clay Miner 38:277–286
Georgakilas V, Gournis D, Petridis D (2001) Organoclay derivatives in the synthesis of macrocycles. Ang Chemie Int Ed 40:4286–4288
Nakatsuji M, Ishii R, Wang Z-M, Ooi K (2004) Preparation of porous clay minerals with organic–inorganic hybrid pillars using solvent-extraction route. J Colloid Interface Sci 272:158–166
Bergaya F, Mandalia T, Amigouët P (2005) A brief survey on CLAYPEN and nanocomposites based on unmodified PE and organo-pillared clays. Colloid Polym Sci 283:773–782
Zhou Q, He HP, Zhu JX, Shen W, Frost RL, Yuan P (2008) Mechanism of p-nitrophenol adsorption from aqueous solution by HDTMA+-pillared montmorillonite – implications for water purification. J Hazard Mater 154:1025–1032
López-Cortés C, Osorio-Revilla G, Gallardo-Velázquez T, Arellano-Cárdenas S (2008) Adsorption of vapor-phase VOCs (benzene and toluene) on modified clays and its relation with surface properties. Can J Chem 86:305–311
Sasai R, Hotta Y, Itoh H (2008) Preparation of organoclay having titania nano-crystals in interlayer hydrophobic field and its characterization. J Ceram Soc Jpn (Nippon Seramikkusu Kyokai Gakujutsu Ronbunshi) 116:205–211
Matatov-Meytal YI, Sheintuch M (1998) Catalytic abatement of water pollutants. Ind Eng Chem Res 37:309–326
Guo J, Al–Dahhan M (2003) Kinetics of wet air oxidation of phenol over a novel catalyst. Ind Eng Chem Res 22:5473–5481
Guo J, Al-Dahhan M (2003) Catalytic wet oxidation of phenol by hydrogen peroxide over pillared clay catalyst. Ind Eng Chem Res 42:2450–2460
Barrault J, Tatibouët J-M, Papayannakos N (2000) Catalytic wet peroxide oxidation of phenol over pillared clays containing iron or copper species. Comptes Rendus - Series IIC - Chem 3:777–783
Barrault J, Abdellaoui M, Bouchoule C, Majesté A, Tatibouët J-M, Louloudi A, Papayannakos N, Gangas NH (2000) Catalytic wet peroxide oxidation over mixed (Al–Fe) pillared clays. Appl Catal B: Environ 27:L225–L230
Guélou E, Barrault J, Fournier J, Tatibouët J-M (2003) Active iron species in the catalytic wet peroxide oxidation of phenol over pillared clays containing iron. Appl Catal B: Environ 44:1–8
De Stefanis A, Perez G, Tomlinson AAG, Bergström C (2001) Alkylation process. WO 2001–055061 assigned to Optatech Corp, Finland
Raimondo M, De Stefanis A, Perez G, Tomlinson AAG (1998) PLS vs. zeolites as sorbents and catalysts. 5. Evidence for Brønsted/Lewis acid crossover and high acidity in conversions of C1-3 alcohols in some alumina-pillared smectite clays. Appl Catal A: Gen 171:85–97
Tomlinson AAG (1998) Characterization of pillared layered structures. J Porous Mater 5:259–274
Tukac V, Hanika J (1998) Catalytic wet oxidation of substituted phenols in the trickle bed reactor. J Chem Tech Biotechnol 71:262–266
Ksontinia N, Najjar W, Ghorbel A (2008) Al–Fe pillared clays: synthesis, characterization and catalytic wet air oxidation activity. J Phys Chem Solids 69:1112–1115
Najjar W, Azabou S, Sayadi S, Ghorbel A (2007) Catalytic wet peroxide photo-oxidation of phenolic olive oil mill wastewater contaminants. Part I. Reactivity of tyrosol over (Al–Fe) PILC. Appl Catal B: Environ 74:11–18
Guo J, Al-Dahhan M (2005) Catalytic wet air oxidation of phenol in concurrent downflow and upflow packed-bed reactors over pillared clay catalyst. Chem Eng Sci 60:735–746
Guo J, Al-Dahhan M (2006) Activity and stability of iron-containing pillared clay catalysts for wet air oxidation of phenol. Appl Catal A: Gen 299:175–184
Barrault J, Bouchoule C, Echachoui K, Frini-Srasra N, Trabelsi M, Bergaya F (1998) Catalytic wet peroxide oxidation (CWPO) of phenol over mixed (AlCu)-pillared clays. Appl Catal B: Environ 15:269–274
Abdellaoui M, Barrault J, Bouchoule C, Srasra NF, Bergaya F (1999) Catalytic wet peroxide oxidation of phenol over mixed [Al–Cu]-pillared clays. J Chimie Phys et de Physico Chimie Biol 96:419–429
Barrault J, Bouchoule C, Tatibouët JM, Abdellaoui M, Majesté A, Louloudi I, Papayannakos N, Gangas NH (2000) Catalytic wet peroxide oxidation over mixed (Al–Fe) pillared clays. Stud Surf Sci Catal 130A:749–754
Tatibouët JM, Guélou E, Fournier J (2005) Catalytic oxidation of phenol by hydrogen peroxide over a pillared clay containing iron. Active species and pH effect. Top Catal 33:1–4
Caudo S, Centi G, Genovese C, Perathoner S (2006) Homogeneous versus heterogeneous catalytic reactions to eliminate organics from waste water using H2O2. Top Catal 40:207–219
Ensing B, Buda F, Baerends EJ (2003) Fenton-like chemistry in water: oxidation catalysis by Fe(III) and H2O2. J Phys Chem A 107:5722–5731
Kuznetsova EV, Savinov EN, Vostrikova LA, Parmon VN (2004) Heterogeneous catalysis in the Fenton-type system FeZSM-5/H2O2. Appl Catal B: Environ 51:165–170
Ensing B, Buda F, Blöchl PE, Baerends EJ (2002) A Car–Parrinello study of the formation of oxidizing intermediates from Fenton’s reagent in aqueous solution. Phys Chem Chem Phys 4:3619–3627
Ensing B, Buda F, Blöchl P, Baerends EJ (2001) Chemical involvement of solvent water molecules in elementary steps of the Fenton oxidation reaction. Ang Chemie 113:2977–2979
Ensing B (2003) Chemistry in water. First principles computer simulations. PhD Thesis, Vrije Universiteit Amsterdam, The Netherlands
Chirchi L, Ghorbel A (2002) Use of various Fe-modified montmorillonite samples for 4-nitrophenol degradation by H2O2. Appl Clay Sci 21:271–276
Ramaswamy V, Krishnan MS, Ramaswamy AV (2002) Immobilization and characterization of copper chlorophthalocyanine on alumina-pillared montmorillonite. J Mol Catal A: Chem 181:81–89
Catrinescu C, Teodosiu C, Macoveanu M, Miehe–Brendlé JL, Dred R (2003) Catalytic wet peroxide oxidation of phenol over Fe-exchanged pillared beidellite. Water Res 37:1154–1160
Mei JG, Yu SM, Cheng J (2004) Heterogeneous catalytic wet peroxide oxidation of phenol over delaminated Fe–Ti–PILC employing microwave irradiation. Catal Comm 5:437–440
Carriazo J, Guélou E, Barrault J, Tatibouët JM, Molina R, Moreno S (2005) Catalytic wet peroxide oxidation of phenol by pillared clays containing Al–Ce–Fe. Water Res 39:3891–3899
Carriazo JG, Molina R, Moreno S (2008) A study on Al and Al–Ce–Fe pillaring species and their catalytic potential as they are supported on a bentonite. Appl Catal A: Gen 334:168–172
Molina CB, Casas JA, Zazo JA, Rodríguez JJ (2006) A comparison of Al–Fe and Zr–Fe pillared clays for catalytic wet peroxide oxidation. Chem Eng J 118:29–35
Luo M, Bowden D, Brimblecombe P (2009) Catalytic property of Fe–Al pillared clay for Fenton oxidation of phenol by H2O2. Appl Catal B: Environ 85:201–206
Timofeeva MN, Khankhasaeva ST, Chesalov YA, Tsybulya SV, Panchenko VN, Dashinamzhilova ET (2009) Synthesis of Fe,Al-pillared clays starting from the Al,Fe-polymeric precursor: effect of synthesis parameters on textural and catalytic properties. Appl Catal B: Environ 88:127–134
Timofeeva MN, Khankhasaeva ST, Talsi EP, Panchenko VN, Golovin AV, Dashinamzhilova ET, Tsybulya SV (2009) The effect of Fe/Cu ratio in the synthesis of mixed Fe,Cu,Al-clays used as catalysts in phenol peroxide oxidation. Appl Catal B: Environ 90:618–627
Tabet D, Saidi M, Houari M, Pichat P, Khalaf H (2006) Fe-pillared clay as a Fenton-type heterogeneous catalyst for cinnamic acid degradation. J Env Manage 80:342–346
Herney-Ramirez J, Costa CA, Madeira LM, Mata G, Vicente MA, Rojas-Cervantes ML, López-Peinado AJ, Martín-Aranda RM (2007) Fenton-like oxidation of Orange II solutions using heterogeneous catalysts based on saponite clay. Appl Catal B: Environ 71:44–56
Herney-Ramirez J, Lampinen M, Vicente MA, Costa CA, Madeira LM (2008) Experimental design to optimize the oxidation of orange II dye solution using a clay-based Fenton-like catalyst. Ind Eng Chem Res 47:284–294
Achma RB, Ghorbel A, Dafinov A, Medina F (2008) Stability of copper supported pillared clay catalysts during oxidation of model pollutant tyrosol in batch and continuous reactors. Stud Surf Sci Catal 174B:1355–1358
Achma RB, Ghorbel A, Dafinov A, Medina F (2008) Copper-supported pillared clay catalysts for the wet hydrogen peroxide catalytic oxidation of model pollutant tyrosol. Appl Catal A: Gen 349:20–28
Najjar W, Ghorbel A, Perathoner S, Centi G (2008) Oxidation intermediates and reaction pathways of wet hydrogen peroxide oxidation of p-coumaric acid over (Al–Fe)PILC catalyst. Stud Surf Sci Catal 174B:1063–1068
Kim SC, Kim DS, Oh SS, Lee DK, Yang YK (2002) Catalytic wet oxidation of dyehouse effluents with Cu/Al2O3 and Cu–Al pillared clay. Stud Surf Sci Catal 145:355–358
Kim SC, Lee DK (2004) Preparation of Al–Cu pillared clay catalysts for the catalytic wet oxidation of reactive dyes. Catal Today 97:153–158
Caudo S, Centi G, Genovese C, Perathoner S (2007) Copper- and iron-pillared clay catalysts for the WHPCO of model and real wastewater streams from olive oil milling production. Appl Catal B: Environ 70:437–446
Giordano G, Perathoner S, Centi G, De Rosa S, Granato T, Katovic A, Siciliano A, Tagarelli A,Tripicchio F (2007) Wet hydrogen peroxide catalytic oxidation of olive oil mill wastewaters using Cu-zeolite and Cu-pillared clay catalysts. Catal Today 124:240–246
Caudo S, Genovese C, Perathoner S, Centi G (2008) Copper-pillared clays (Cu-PILC) for agro-food wastewater purification with H2O2. Micropor Mesopor Mater 107:46–57
Ooka C, Akita S, Ohashi Y, Horiuchi T, Suzuki K, Komai SI, Yoshida H, Hattori T (1999) Crystallization of hydrothermally treated TiO2 pillars in pillared montmorillonite for improvement of the photocatalytic activity. J Mater Chem 9:2943–2952
Yoshida H, Kawase T, Miyashita Y, Murata C, Ooka C, Hattori T (1999) Effect of hydrothermal treatment of titania-pillared montmorillonite for photocatalytic degradation of dibutyl phthalate in water. Chem Lett 8:715–716
Shimizu KI, Kaneko T, Fujishima T, Kodama T, Yoshida H, Kitayama Y (2002) Selective oxidation of liquid hydrocarbons over photoirradiated TiO2 pillared clays. Appl Catal A: Gen 225:185–191
Awate SV, Suzuki K (2001) Enhanced adsorption capacity and photo-catalytic oxidative activity of dyes in aqueous medium by hydrothermally treated titania pillared clay. Adsorption 7:319–326
Li J, Chen C, Zhao J, Zhu H, Ding Z (2002) Photodegradation of dye pollutants on TiO2 pillared bentonites under UV light irradiation. Sci China B 45:445–448
Pichat P, Khalaf H, Tabet D, Houari M, Saidi M (2005) Ti-montmorillonite as photocatalyst to remove 4-chlorophenol in water and methanol in air. Environ Chem Lett 2:191–194
Liu S, Yang JH, Choy JH (2006) Microporous SiO2–TiO2 nanosols pillared montmorillonite for photocatalytic decomposition of methyl orange. J Photochem Photobiol A: Chem 179:75–80
An T, Chen J, Li G, Ding X, Sheng G, Fu J, Mai B, O’Shea KE (2008) Characterization and the photocatalytic activity of TiO2 immobilized hydrophobic montmorillonite photocatalysts. Degradation of decabromodiphenyl ether (BDE 209). Catal Today 139:69–76
Ding X, An T, Guiying L, Chen J, Sheng G, Jiamo F, Zhao J (2008) Photocatalytic degradation of dimethyl phthalate ester using novel hydrophobic TiO2 pillared montmorillonite photocatalyst. Res Chem Intermediates 34:67–83
Yang W, Cheng LY, Xue ZL, Guo H, Gao JZ (2008) Preparation of TiO2 pillared montmorillonite photocatalyst and its photocatalytic activity to degradation reaction of acidic fuchsine by sunlight. Spectrosc Spectral Anal (Guang Pu Xue Yu Guang Pu Fen Xi) 28:1122–1125
Yang X, Zhu H, Liu J, Gao X, Martens WN, Frost RL, Shen Y, Yuan Z (2008) A mesoporous structure for efficient photocatalysts: anatase nanocrystals attached to leached clay layers. Micropor Mesopor Mater 112:32–44
Xuzhuang Y, Yang D, Huaiyong Z, Jiangwen L, Martins WN, Frost R, Daniel L, Yuenian S (2009) Mesoporous structure with size controllable anatase attached on silicate layers for efficient photocatalysis. J Phys Chem C 113:8243–8248
Zhang G, Ding X, Hu Y, Huang B, Zhang X, Qin X, Zhou J, Xie J (2008) Photocatalytic degradation of 4BS dye by N,S-codoped TiO2 pillared montmorillonite photocatalysts under visible-light irradiation. J Phys Chem C 112:17994–17997
Damardji B, Khalaf H, Duclaux L, David B (2009) Preparation of TiO2-pillared montmorillonite as photocatalyst. Part I. Microwave calcination, characterisation, and adsorption of a textile azo dye. Appl Clay Sci 44:201–205
Damardji B, Khalaf H, Duclaux L, David B (2009) Preparation of TiO2-pillared montmorillonite as photocatalyst. Part II. Photocatalytic degradation of a textile azo dye. Appl Clay Sci 45:98–104
Dvininov E, Popovici E, Pode R, Cocheci L, Barvinschi P, Nica V (2009) Synthesis and characterization of TiO2-pillared Romanian clay and their application for azoic dyes photodegradation. J Hazard Mater 167:1050–1056
Pode R, Popovici E, Vasile A, Cocheci L, Dvininov E (2009) Sorption and photocatalytic degradation of azoic dyes on TiO2-pillared montmorillonitic clay. Revue Roumaine de Chimie 54:313–321
Ooka C, Yoshida H, Takeuchi S, Maekawa M, Yamada Z, Hattori T (2004) Hydrogen peroxide improving crystallinity of TiO2 nanoparticle in layer compound. Catal Comm 5:49–54
Yuan XT, Yu J, Liu HZ, Li WJ (2004) Synthesis and characterization of novel organic/inorganic pillared clays. Acta Chimica Sinica 62:1049–1054
Yan SF, Yu J, Liu HZ, Sun TC (2007) Preparation of Ti-pillared montmorillonite tailored by surfactant. Chin J Process Eng (Guocheng Gongcheng Xuebao) 7:90–94
Ding X, An T, Li G, Zhang S, Chen J, Yuan J, Zhao H, Chen H, Sheng G, Fu J (2008) Preparation and characterization of hydrophobic TiO2 pillared clay: the effect of acid hydrolysis catalyst and doped Pt amount on photocatalytic activity. J Colloid Interface Sci 320:501–507
Li D, Qu J (2009) The progress of catalytic technologies in water purification: a review. J Environ Sci (Beijing, China) 21:713–719
von Sonntag C (2008) Advanced oxidation processes: mechanistic aspects. Water Sci Technol 58:1015–1021
Litter MI (2005) Introduction to photochemical advanced oxidation processes for water treatment. In: Hutzinger O (ed) Environmental photochemistry. Part II (series The Handbook of Environmental Chemistry), vol 2 M. Springer, Berlin, pp 325–366
Augugliaro V, Litter M, Palmisano L, Soria J (2006) The combination of heterogeneous photocatalysis with chemical and physical operations: a tool for improving the photoprocess performance. J Photochem Photobiol C: Photochem Rev 7:27–144
Jain DM (2005) Photo Fenton degradation for environmental application. J Ind Pollut Control 21:181–194
Waite TD (2002) Challenges and opportunities in the use of iron in water and wastewater treatment. Rev Environ Sci BioTechnol 1:9–15
Suty H, De Traversay C, Cost M (2004) Applications of advanced oxidation processes: present and future. Water Sci Technol 49:227–233
Sum OSN, Feng J, Hu X, Yue PL (2004) Pillared laponite clay-based Fe nanocomposites as heterogeneous catalysts for photo-Fenton degradation of acid black. Chem Eng Sci 59:5269–5275
Liu Y, Li YM, Wen LH, Li HY (2005)Fe pillared bentonite and photo-catalytic degradation of dye-Orange II. J Funct Mater (Gongneng Cailiao) 36:136–138
Bobu MM, Siminiceanu I, Lundanes E (2008) Mineralization of two phenyl urea herbicides in water by a heterogeneous photo-Fenton process. Environ Eng Manage J 7:37–40
Bobu M, Yediler A, Siminiceanu I, Schulte-Hostede S (2008) Degradation studies of ciprofloxacin on a pillared iron catalyst. Appl Catal B: Environ 83:15–23
De León MA, Castiglioni J, Bussi J, Sergio M (2008) Catalytic activity of an iron-pillared montmorillonitic clay mineral in heterogeneous photo-Fenton process. Catal Today 133–135:600–605
Chen Q, Wu P, Li Y, Zhu N, Dang Z (2009) Heterogeneous photo-Fenton photodegradation of reactive brilliant orange X-GN over iron-pillared montmorillonite under visible irradiation. J Hazard Mater 168:901–908
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Perathoner, S., Centi, G. (2010). Catalytic Wastewater Treatment Using Pillared Clays. In: Gil, A., Korili, S., Trujillano, R., Vicente, M. (eds) Pillared Clays and Related Catalysts. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-6670-4_7
Download citation
DOI: https://doi.org/10.1007/978-1-4419-6670-4_7
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-6669-8
Online ISBN: 978-1-4419-6670-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)