Abstract
Behavior patterns in a large, free-ranging social group of buffy-headed marmosets (Callithrix flaviceps) were monitored over a 7-month period during which four adults – one male and three females – emigrated in two separate events. Social interactions such as play and allogrooming were relatively frequent, but agonistic interactions were rare, being observed, on average, less than twice per observation day for a group with between seven and ten adult members at any given time. With the exception of events involving the breeding female, intra-sexual agonism was almost non-existent between adults, and male→female aggression was five times more frequent than female→male. Absent between males and rare in females, submissive behavior was almost invariably directed by non-breeding females towards males and the breeding female. Taken together, these interactions point to a three-tiered social hierarchy within the group, with the breeding female in the top tier, followed by males in the second, and finally, non-breeding females. However, no one male was more dominant socially than any other, nor was any non-breeding female more subordinate. Male and female group members dispersed under different circumstances, but there is little evidence in either case to suggest that emigrations were a consequence of intra-group agonism, related to social rank or to competition for resources.
Resumen
Patrones de comportamiento en un grupo de Callithrix flaviceps fueron monitoreados a lo largo de un período de siete meses durante el cual cuatro adultos –un macho y tres hembras- emigraron en dos eventos distintos. Las interacciones sociales como un comportamiento lúdico y acicalamiento fueron relativamente frecuentes, pero las interacciones agonísticas fueron raras, siendo observadas, en promedio, menos de dos veces por día de observación para un grupo de entre siete y diez miembros adultos en un momento dado. Con la excepción de los eventos que involucraron a la hembra en estado de reproducción, el agonismo intrasexual estuvo prácticamente ausente entre los adultos, y la agresión macho→hembra fue cinco veces más frecuente que entre hembra→macho. Ausente entre los machos, y raro entre las hembras, el comportamiento sumiso fue casi siempre dirigido hacia machos y a hembras reproductoras por hembras no reproductoras. Analizadas en conjunto, las interacciones indican que una jerarquia social de tres camaradas dentro de un grupo: hembra reproductora→macho→hembra no reproductora. Sin embargo, ningún macho fue más dominante socialmente que cualquier otro, y ninguna hembra fue más subordinada. Los miembros de los dos sexos se dispersaron bajo diferentes circunstancias, pero existe poca evidencia de que las emigraciones estuvieran relacionadas de alguna forma con agonismo dentro del grupo, sea relacionado con la posición social o a la competición por recursos.
Resumo
Padrões comportamentais em um grupo silvestre de sagüis-da-serra (Callithrix flaviceps) foram monitorados ao longo de um período de sete meses durante o qual quatro adultos – um macho e três fêmeas – emigraram em dois eventos distintos. Interações sociais como o comportamento lúdico, e a alocatação foram relativamente freqüentes, embora interações agonísticas foram raras. Agonismo foi observado menos do que duas vezes por dia, em média, neste grupo, que continha de sete a dez membros adultos, em um dado momento. Com a exceção de eventos que envolveram a fêmea reprodutora, o agonismo intrasexual foi praticamente ausente entre adultos, e a agressão macho→fêmea foi cinco vezes mais freqüente que fêmea→macho. Ausente entre machos, e raro em fêmeas, o comportamento submissivo foi quase sempre direcionado a machos e a fêmea reprodutora por fêmeas não reprodutivas. Analisadas em conjunto, as interações indicam uma hierarquia social de três camadas dentro do grupo, com a fêmea reprodutora na primeira camada, seguido pelos machos na segunda, e finalmente as fêmeas não reprodutivas. Entretanto, nenhum macho foi mais dominante socialmente do que qualquer outro, e nenhuma fêmea foi mais subordinada. Os emigrantes dos dois sexos dispersaram sob circunstâncias diferentes, mas existe pouca evidência de que as emigrações foram relacionadas de alguma forma com agonismo dentro do grupo, relacionado a posição social ou a competição por recursos.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Social behavior in groups of marmosets and tamarins (the Callitrichidae) is characterized by the dominance of a single reproductive female or breeding pair (Abbott et al. 1993; French 1997), even when polygynous breeding is the norm (e.g., Dietz and Baker 1993; Digby 1995a; Coutinho and Corrêa 1996). The cooperative breeding system mediates tolerance of mature, non-reproductive helpers by breeding animals, but it also creates the potential for increased competition for both resources and breeding positions. However, intra-group agonism is normally rare in free-ranging callitrichids (Goldizen and Terborgh, 1989; Digby 1995a), and in captivity, it generally occurs between members of the same sex (e.g., Callithrix jacchus: Epple 1975; Rothe 1978; Evans 1983; Abbott 1984; Stevenson and Rylands 1988).
As only one or two helpers may make a significant contribution to a breeding female’s reproductive success (Goldizen 1987; Sánchez et al. 1999), it would seem reasonable to expect declining tolerance in groups with more than four adult members (Ferrari and Digby 1996). Many free-ranging marmoset (Callithrix) groups contain five or more adults, however, which suggests that such tolerance may depend on additional factors. The marmosets’ unique set of specializations for the dietary exploitation of plant exudates, which provides a stable resource base, and the relatedness of group members and the risks of dispersal is likely to help explain tolerance within large groups (Ferrari and Lopes 1989), as are the relatedness of group members and the risks of dispersal (Goldizen and Terborgh 1989; Ferrari and Digby 1996).
In the present study, agonistic behavior in a free-ranging group of buffy-headed marmosets, Callithrix flaviceps, is analyzed during a period when the group reached its maximum size (15 members) and four adult members emigrated. The results of the study point to a three-tiered, gender-based social hierarchy, but no evidence of any increase in agonism was found with decreasing resource availability, or in relation to the emigration of group members. Dispersal thus appeared to be triggered by external (availability of potential mates) rather than internal factors.
2 Methods
2.1 Study Site and Animals
The C. flaviceps study group was monitored at the Fazenda Montes Claros (19°50′S, 41°50′W) in the municipality of Caratinga, in Minas Gerais, Brazil, between 1985 and 1991 (see Ferrari 1988; Ferrari and Diego 1992). The observational records analyzed here cover the 7-month period between January and July, 1986, during which a pair of twins were born and four adult group members emigrated. During this period, group size varied between a maximum of 15 members (following the birth) and a minimum of 11, following emigrations (Table 8.1). The study subjects were fully habituated to the presence of human observers (observer-subject distances were often less than 1 m) and individually identifiable through differences in pelage markings.
2.2 Behavioral Data
Quantitative behavioral records were collected using instantaneous scan sampling (Altmann 1974), with a scan of 1-min duration carried out at 5-min intervals throughout the daily activity period of the study group (sleep-tree to sleep-tree) during 10 days each month. The behavior of each group member located during the scan was recorded, allowing the compilation of activity budgets (Ferrari 1988, 1992). Behavior sampling (Martin and Bateson 1993) was also used for the collection of records of social behavior. In all cases, the identity of the animals involved and the sequence of events were recorded whenever possible. For the purposes of the present study, only records of agonistic (dominant) and submissive (subordinate) behavior categories (Table 8.2) were analyzed. The dominant member of a dyad was defined according to the ratio of agonistic to submissive behavior. The characteristics of the data proscribed the use of a more systematic, quantitative measure, such as Nishida’s (1988) index of reciprocity. Resource availability was monitored throughout the main study period (for details, see Ferrari 1988).
As the composition of the group changed during the study period, comparisons between sexes and across months are based on the rate of a given behavior category per individual per standard observation month (10 days) rather than absolute values. Group composition only changed mid-month in June, at the end of the fifth day of observation. The data for this month are thus presented separately, with June 1 referring to the period preceding the emigration, and June 2 to that following the change in group composition. The distribution of events among individuals was tested using χ 2.
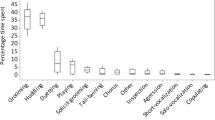
Standardized rates were calculated for each sex (Tables 8.3 and 8.4), and for the group as a whole (Fig. 8.1). For the χ 2 analyses, the individual values (number of events) were used, and compared to a homogeneous distribution.
3 Results
Infant care-giving behavior and changes in the composition of the C. flaviceps group during the present study period have been described elsewhere (Ferrari 1992; Ferrari and Diego 1992). Overall, there was little difference in the contribution of males and non-breeding females to the rearing of infants (carrying and food sharing). The adult male Simão left the study group at the end of May to join a neighboring group containing a single adult female and two adult males. In mid-June, the non-breeding females Cuba, Dida, and Spock left the study group to join the two males from the group into which Simão had immigrated for the formation of a new social unit of five adults; they were subsequently observed within the home range of the main study group on a regular basis.
During the 7 months considered here, social behavior, predominantly play, allogrooming, and scent marking, accounted for 10.4% of scan sample records (n = 40713). Agonistic behavior accounted for no more than 0.2% of records in any one month. Even when behavioral sampling is included, agonism was observed at a maximum rate of 3.4 records per adult in any 1 month (March), and physical contact was extremely rare. The maximum monthly rate for submissive categories was similar (3.8), as might be expected given the complementary nature of these categories.
Agonistic interactions between males were virtually nonexistent, with a single record collected in January (Table 8.3). Interactions between females were more frequent, but account for a little over one tenth of the records, and almost invariably involved the breeding female (Maggie). Maggie was aggressor in all but one record of intra-female agonism, and received two-thirds of intra-female submissions (Table 8.4).
Intra-female agonism was concentrated in the first 3 months of the study period, when dependent infants were present in the group, and basically resulted from the breeding female’s monitoring of caregivers. When infant distress vocalizations were insistent, the breeding female normally reacted by approaching, threatening, and even attacking adult caregivers (both male and female). This occurred in the context of both infant carrying and the solicitation of food items (see Ferrari 1987). By contrast, no agonism between females was recorded in June, the month during which Cuba, Dida, and Spock emigrated.
Most of the inter-sexual agonism involving the breeding female was also related to the monitoring of caregivers. While males were aggressive towards Maggie on two occasions, she was never observed exhibiting submissive behavior towards individuals of either sex, which would appear to confirm her dominant position within the group. Similarly, a male was submissive towards a non-breeding female on only one occasion, in contrast with 170 records of non-breeding female→male submission. Males were also five times more aggressive towards non-breeding females than vice versa. Overall, then, the data indicate that males were socially dominant over non-breeding females.
While males as a whole were clearly dominant over non-breeding females, the almost total lack of intra-gender agonism obscures any possible linear hierarchy. There was also no clear tendency for a given male to be more aggressive than others (January to May: χ 2 = 4.96, df = 3, p > 0.20), nor for a given non-breeding female to be more submissive (χ 2 = 6.22, df = 3, p > 0.05).
Despite being able to exploit plant exudates systematically as a substitute source of carbohydrates during periods of fruit scarcity, marmosets are vulnerable to seasonal fluctuations in the abundance of arthropod prey, which declined progressively during the present study period, accompanying the transition from the wet to the dry season (Ferrari 1988). Over the year as a whole, feeding on animal material declined significantly with decreasing arthropod abundance, as did foraging efficiency and prey selectivity. However, the relative contribution of animal material to the C. flaviceps diet varied little between January (17.4% of feeding records) and June (19.8%), whereas it fell to 9.3% in July, that is, after the emigrations.
The exact consequences of such seasonal changes are unclear, but the fact that Maggie gave birth to a set of healthy twins towards the end of September appears to support the idea that nutritional stress was not a factor. While the observed changes do suggest that intra-specific competition increased during the course of the study period, there is no evidence to suggest that this resulted in any change in the relationships between group members (see Fig. 8.1), in particular with regard to the possibility that emigrations were stimulated by an increase in intra-group agonism. Inter-gender aggression was in fact recorded less frequently than expected (homogeneous distribution) in May (χ 2 = 3.83, df = 1, p > 0.05) prior to the emigration of Simão, and barely more than average in June 1 (χ 2 = 0.18, df = 1, p > 0.70).
4 Discussion
The social dominance of a single breeding female or male/female pair is a well-documented characteristic of callitrichid social organization, one which has a well-defined role in the reproductive biology of these primates (Abbott et al. 1993; French 1997). In general, relationships between other group members have received less attention, although tolerance is the norm (at least in the wild), even between unrelated group members or potentially reproductive adult males. Captivity often accentuates intra-group agonism (Epple 1975; Rothe 1978; Box and Morris 1980; Abbott 1984; Snowdon and Soini 1988; Moura 2003), which may result in the formation of a distinct, intra-sexual hierarchy (Rothe 1978; Fuchs et al. 1991). But up to now, little evidence has been found of social dominance between the sexes (Evans and Poole 1984; Sutcliffe and Poole 1984; Digby 1995a), as might be expected for such a sexually monomorphic mammal (Kleiman 1977).
The gender-based social hierarchy described here thus represents a somewhat novel interpretation of the social organization of a callitrichid species. Unfortunately, with the exception of data collected at the present study site (Ferrari and Diego 1992; Guimarães 1998), virtually nothing is known of the behavior of C. flaviceps, so it remains unclear whether the patterns reported here are typical of the species, and even less so, to what extent similar patterns might be expected in other members of the genus. In fact, with one exception (Digby 1995a), very little is known of intra-group relations in Callithrix species. Digby found some evidence of an age-based hierarchy in the common marmoset, Callithrix jacchus, although the social context was different from that in C. flaviceps, in particular the presence of two breeding females in each of the three study groups.
What does seem most likely, from these scant data, is that social structure and intra-group relations in marmoset species are as variable as their mating patterns (Ferrari and Digby 1996; Ferrari et al. 1996). As for mating patterns, group composition may play at least as important a role as ecological variables. Except for recruitment through births, the composition of the C. flaviceps study group remained unchanged for at least 18 months prior to the emigrations reported here, and it seems likely that most if not all group members were closely related (Ferrari and Diego 1992). All three C. jacchus groups appeared to be less stable, by contrast, suffering losses of both adult and immature members (Digby and Barreto 1993), in addition to at least one infanticide (Digby 1995b). The latter, in particular, appears to reflect more intense competition for reproductive success, as might be expected from the presence of two breeding females in each group.
The relatedness of group members may be a key factor in intra-group relations, and may account for much of the difference between captive and field data, given that the stable resource base provided by the marmosets’ gum-feeding specializations is common to all species (Soini 1982; Ferrari and Digby 1996; Ferrari et al. 1996; Passamani 1996; Veracini 1997, this volume Chapt. 12). Ideally, it would be valuable to compare systematically the social relations in groups of different composition, although up to now, there has been only one field study in which the genetic relatedness of all group members is known (see Faulkes et al. Chapt. 5 this volume). Systematic differences between marmosets and tamarins (Leontopithecus and Saguinus) might also be expected, given the ecological and demographic differences between these two groups (Ferrari and Lopes 1989).
The present study also reinforces the highly systematic nature of dispersal and group formation in this species, at least. The only pattern observed in the present study was an apparent increase in agonistic interactions between the breeding female and non-breeding females at the time of the birth. Certainly, there is no evidence to suggest that intra-group agonism was influenced in any way by resource abundance or intra-group competition, nor that emigrations were related to either factor. It thus seems more likely that the primary determinant of both emigrations was the availability of potential mates in neighboring groups. Timing may also have been important, given that Simão’s emigration coincided with the period during which Maggie would have conceived the litter born in late September.
References
Abbott DH (1984) Behavioral and physiological suppression of fertility in subordinate marmoset monkeys. Am J Primatol 6:169–186
Abbott DH, Barrett J, George LM (1993) Comparative aspects of the social suppression of reproduction in female marmosets and tamarins. In: Rylands AB (ed) Marmosets and tamarins: systematics, behaviour, and ecology. Oxford University Press, Oxford, pp 152–163
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 48:1–41
Box HO, Morris JM (1980) Behavioural observations on captive pairs of wild caught tamarins (Saguinus mystax). Primates 21:53–65
Coutinho PEG, Corrêa HKM (1996) Polygyny in a free-ranging group of buffy-tufted-ear marmosets, Callithrix aurita. Folia Primatol 65:25–29
Dietz JM, Baker AJ (1993) Polygyny and female reproductive success in golden lion tamarins, Leontopithecus rosalia. Anim Behav 46:1067–1078
Digby LJ (1995a) Social organization in a wild population of Callithrix jacchus. II. Intragroup social behavior. Primates 36:361–375
Digby LJ (1995b) Infanticide, infant care, and female reproductive strategies in a wild population of common marmosets. Behav Ecol Sociobiol 37:51–61
Digby LJ, Barreto CE (1993) Social organization in a wild population of Callithrix jacchus. Part I: group composition and dynamics. Folia Primatol 6:123–134
Epple G (1975) The behavior of marmoset monkeys (Callitrichidae). In: Rosenblum LA (ed) Primate Behavior, vol 4. Academic, New York, pp 195–239
Evans S (1983) The pair-bond of the common marmoset, Callithrix jacchus jacchus: an experimental investigation. Anim Behav 31:651–658
Evans S, Poole TB (1984) Long-term changes and maintenance of the pair-bond in common marmosets, Callithrix jacchus jacchus. Folia Primatol 42:33–41
Faulkes C, Arruda MF, Monteiro da Cruz MAO (this volume) Genetic structure within and among populations of the common marmoset, Callithrix jacchus: implications for cooperative breeding. In: Ford SM, Porter LM, Davis LC (eds) The smallest anthropoids: the marmoset/callimico radiation. Springer, New York, pp 103–117
Ferrari SF (1987) Food transfer in a wild marmoset group. Folia Primatol 48:203–206
Ferrari SF (1988) The behaviour and ecology of the buffy-headed marmoset, Callithrix flaviceps (O. Thomas, 1903). Ph.D thesis, University of London.
Ferrari SF (1992) The care of infants in a wild marmoset, Callithrix flaviceps, group. Am J Primatol 26:109–118
Ferrari SF, Diego VH (1992) Long-term changes in a wild marmoset group. Folia Primatol 58:215–218
Ferrari SF, Digby LJ (1996) Wild Callithrix groups: stable extended families? Am J Primatol 38:19–27
Ferrari SF, Lopes MA (1989) A re-evaluation of the social organisation of the Callitrichidae, with reference to the ecological differences between genera. Folia Primatol 52:132–147
Ferrari SF, Corrêa HKM, Coutinho PEG (1996) Ecology of the “southern” marmosets (Callithrix aurita and Callithrix flaviceps): how different, how similar? In: Norconk MA, Rosenberger AL, Garber PA (eds) Adaptive Radiations of Neotropical Primates. Plenum, New York, pp 157–171
French JA (1997) Proximate regulation of singular breeding in callitrichid primates. In: Solomon RG, French JA (eds) Cooperative Breeding in Mammals. Cambridge University Press, Cambridge, pp 34–75
Fuchs E, Rosenbusch J, Anzenberger G (1991) Urinary protein pattern reflects social rank in male common marmosets (Callithrix jacchus). Folia Primatol 57:177–180
Goldizen AW (1987) Facultative polyandry and the role of infant carrying in wild saddle-back tamarins (Saguinus fuscicollis). Behav Ecol Sociobiol 20:99–109
Goldizen AW (1989) Social relationships in a cooperatively polyandrous group of tamarins (Saguinus fuscicollis). Behav Ecol Sociobiol 24:79–89
Goldizen AW, Terborgh J (1989) Demography and dispersal patterns of a tamarin population: possible causes of delayed breeding. Am Nat 134:208–224
Guimarães A (1998) Ecology and social behaviour of buffy-headed marmosets, Callithrix flaviceps. Neotrop Primates 6:51–52
Kleiman DG (1977) Monogamy in mammals. Q Rev Biol 52:39–69
Martin P, Bateson P (1993) Measuring Behaviour: an introductory Guide. Cambridge University Press, Cambridge
Moura ACA (2003) Sibling age and intragroup aggression in captive Saguinus midas midas. Int J Primatol 24:639–652
Nishida T (1988) Development of social grooming between mother and offspring in wild chimpanzees. Folia Primatol 50:109–123
Passamani M (1996) Ecologia e Comportamento de um Grupo de Sagüi-da-Cara-branca (Callithrix geoffroyi) em um Fragmento de Mata Atlântica no Espírito Santo. Universidade Federal de Minas Gerais, Belo Horizonte, M.Sc dissertation
Rothe H (1978) Sub-grouping behaviour in captive Callithrix jacchus families: a preliminary investigation. In: Rothe H, Wolters HJ, Hearn JP (eds) Biology and Behaviour of Marmosets. Eigenverlag H. Rothe, Göttingen, pp 233–257
Sánchez S, Peláez F, Gil-Burmann C, Kaumanns W (1999) Costs of infant-carrying in the cotton-top tamarin (Saguinus oedipus). Am J Primatol 48:99–111
Snowdon CT, Soini P (1988) The tamarins, genus Saguinus. In: Mittermeier RA, Rylands AB, Coimbra-Filho AF, da Fonseca GAB (eds) Ecology and behavior of neotropical primates, vol. 2. World Wildlife Fund, Washington, pp 223–298
Soini P (1982) Ecology and population dynamics of the pygmy marmoset, Cebuella pygmaea. Folia Primatol 39:1–21
Stevenson MF, Poole TB (1976) Ethogram of the common marmoset (Callithrix jacchus jacchus): general behavioural respertoire. Anim Behav 24:428–451
Stevenson MF, Rylands AB (1988) The marmosets, genus Callithrix. In: Mittermeier RA, Rylands AB, Coimbra-Filho AF, da Fonseca GAB (eds) Ecology and Behavior of Neotropical Primates, vol 2. World Wildlife Fund, Washington, pp 131–222
Sutcliffe MG, Poole TB (1984) Intragroup agonistic behavior in captive groups of the common marmoset (Callithrix jacchus jacchus). Int J Primatol 5:473–489
Veracini C (1997) O comportamento alimentar de Callithrix argentata (Linnaeus 1771) (Primata, Callitrichinae). In: Lisboa PLB (ed) Caxiuanã. MCT/CNPq, Belém, pp 437–446
Veracini C (this volume) Habitat use and ranging behavior of the silvery marmoset (Mico argentatus) at Caxiuanã National Forest (eastern Brazilian Amazonia). In: Ford SM, Porter LM, Davis LC (eds) The smallest anthropoids: the marmoset/callimico radiation. Springer, New York, pp 221–240
Acknowledgements
This study was supported by the Brazilian National Research Council (CNPq, process no. 307506/2003-7), the Medical Research Council of Great Britain, the A.H. Schultz-Stiftung, London University Central Research Fund, the Boise Fund and the Leakey Trust. I am especially grateful to Bob Martin, Cida Lopes, and Vânia Diego. An early version of this paper was presented at the 15th IPS Congress, in the symposium on “New World primates with parental care” organized by Hilary Box and Gisela Epple.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Ferrari, S.F. (2009). Social Hierarchy and Dispersal in Free-Ranging Buffy-Headed Marmosets (Callithrix flaviceps). In: Ford, S., Porter, L., Davis, L. (eds) The Smallest Anthropoids. Developments in Primatology: Progress and Prospects. Springer, Boston, MA. https://doi.org/10.1007/978-1-4419-0293-1_8
Download citation
DOI: https://doi.org/10.1007/978-1-4419-0293-1_8
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4419-0292-4
Online ISBN: 978-1-4419-0293-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)