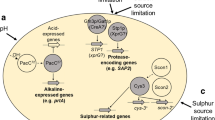
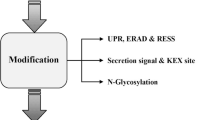
Abstract
The secretion of enzymes by many species of filamentous fungi is an essential feature of their lifestyle, whether it be to support saprophytic growth or pathogenicity. Fungi respond to their habitats by controlled gene expression and secretion of particular enzymes in response to environmental triggers. Although fungal enzymes have long been exploited by man, only recently have the detailed mechanisms of gene expression and enzyme secretion been investigated. Such investigations are still in their infancy and studies have necessarily concentrated on those species which are amenable to molecular approaches. Indeed, it is likely that only a small fraction of fungi have been studied at any level and, of those that have, only a minority are receptive to the techniques of molecular biology. In this chapter we have taken a broad view by discussing the impact of ecology and physiology on exoenzyme production but concentrating, where possible, on examples which have been studied at a detailed molecular level.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Aleshin, A., Golubev, A., Firsov, L.M. and Honzatko, R.B. (1992) Crystal structure of glucoamylase from Aspergillus awamori var. X100 to 2.2-Å resolution. Journal of Biological Chemistry, 267, 19291–8.
Alic, M. and Gold, M.H. (1991) Genetics and molecular biology of the lignin degrading basidiomycete Phanerochaete chrysosporium, in More Gene Manipulations in Fungi, (ed. J.W. Bennett and L.L. Lasure), Academic Press, London, pp. 319–41.
Allen, A.L. and Roche, CD. (1989) Effects of strain and fermentation conditions on production of cellulase by Trichoderma reesei. Biotechnology and Bioengineering, 33, 650–6.
Allison, D.S., Rey, M.W., Berka, R.M. et al. (1992) Transformation of the thermophilic fungus Humicola grisea var. thermoidea and overproduction of Humicola glucoamylase. Current Genetics, 21, 225–9.
Archer, D.B. (1994) Enzyme production by recombinant Aspergillus, in Recombinant Microbes for Industrial and Agricultural Applications, (ed. Y. Murooka), Marcel Dekker, New York, pp. 373–93.
Archer, D.B., Jeenes, D.J., MacKenzie, D.A. et al. (1990) Hen egg white lysozyme expressed in, and secreted from, Aspergillus niger is correctly processed and folded. BioTechnology, 8, 741–5.
Ashikari, T., Nakamura, N., Tanaka, Y. et al. (1986) Rhizopus raw-starch-degrading glucoamylase: its cloning and expression in yeast. Agricultural and Biological Chemistry, 50, 957–64.
Ball, C. (1984) Filamentous fungi, in Genetics and Breeding of Industrial Microorganisms, (ed. C. Ball), CRC Press, Boca Raton, FL, pp. 159–188.
Barrett, A.J. and Rawlings, N.D. (1991) Types and families of endopeptidases. Biochemical Society Transactions, 19, 707–15.
Bastande, K.B. (1992) Xylan structure, microbial xylanases, and their mode of action. World Journal of Microbiology and Biotechnology, 8, 353–68.
Berges, T., Boddy, L.M., Peberdy, J.F. and Barreau, C. (1993) Cloning of an invertase gene from Aspergillus niger by expression in Trichoderma reesei. Current Genetics, 24, 53–9.
Berka, R.M., Ward, M., Wilson, L.J. et al. (1990) Molecular cloning and deletion of the aspergillopepsin A gene from Aspergillus awamori. Gene, 86, 153–62.
Berka, R.M., Kodama, K.H., Rey, M.W. et al. (1991) The development of Aspergillus niger var. awamori as a host for the expression and secretion of heterologous gene products. Biochemical Society Transactions, 19, 681–5.
Berka, R.M., Carmona, C.L., Hayenga, K.J. et al. (1993) Isolation and characterization of the Aspergillus oryzae gene encoding aspergillopepsin O. Gene, 125, 195–8.
Berkholt, V. (1987) Amino acid sequence of endothiapepsin. European Journal of Biochemistry, 167, 327–38.
Boel, E. and Huge-Jensen, B. (1989) Recombinant Humicola lipase and process for the production of recombinant Humicola lipases. European Patent Application EP 0305216.
Boel, E., Hjort, I., Svensson, B. et al. (1984) Glucoamylase G1 and G2 from Aspergillus niger are synthesized from two different but closely related mRNAs. EMBO Journal, 3, 1097–102.
Boel, E., Huge-Jensen, B., Christensen, M. et al. (1988) Rhizomucor miehei triglyceride lipase is synthesized as a precursor. Lipids, 23, 701–6.
Brunt, S.A. and Silver, J.C. (1991) Molecular cloning and characterization of two distinct hsp85 sequences from the steroid responsive fungus Achyla ambisexualis. Current Genetics, 19, 383–8.
Bussink, H.J.D., Kester, H.C.M. and Visser, J. (1990) Molecular cloning, nucleotide sequence and expression of the gene encoding pre-pro-polygalacturonase II of Aspergillus niger. FEBS Letters, 273, 127–30.
Bussink, H.J.D., Buxton, F.P. and Visser, J. (1991a) Expression and sequence comparison of the Aspergillus niger and Aspergillus tubigensis genes encoding polygalacturonase II. Current Genetics, 19, 467–474.
Bussink, H.J.D., Brouwer, K.B., de Graaff, L.H. et al. (1991b) Identification and characterization of a second polygalacturonase gene of Aspergillus niger. Current Genetics, 20, 301–7.
Caddick, M.X. and Arst, H.N. (1987) Structural genes for phosphatases in Aspergillus nidulans. Genetics Research, 47, 83–91.
Calmels, T.P.G., Martin, F., Durand, H. and Tiraby, G. (1991) Proteolytic events in the processing of secreted proteins in fungi. Journal of Biotechnology, 17, 51–66.
Campbell-Platt, G. and Cook, P.E. (1989) Fungi in the production of food and food ingredients. Journal of Applied Bacteriology Symposium Supplement, pp. 117S–131S.
Caprari, C., Richter, A., Bergmann, C. et al. (1993) Cloning and characterization of a gene encoding the endopolygalacturonase of Fusarium moniliforme. Mycological Research, 97, 497–505.
Cheetham, P.S.J. (1985) The applications of enzymes in industry, in Handbook of Enzyme Biotechnology, (ed. A. Wiseman), Ellis Horwood, Chichester, pp. 274–379.
Chiba, Y., Yamagata, Y., Nakajima, T. and Ichishima, E. (1992) A new high-mannose type N-linked oligosaccharide from Aspergillus carboxypeptidase. Bioscience, Biotechnology and Biochemistry, 56, 1371–2.
Christensen, T., Woeldike, H., Boel, E. et al. (1988) High level expression of recombinant genes in Aspergillus oryzae. BioTechnology, 6, 1419–22.
Civas, A., Eberhard, R., Le Dizet, P. and Petek, F. (1984) Glycosidases induced in Aspergillus tamarii. Secreted α-D-galactosidase and p-D-mannanase. Biochemical Journal, 219, 857–63.
Cleves, A.E. and Bankaitis, V.A. (1992) Secretory pathway function in Saccharomyces cerevisiae. Advances in Microbial Physiology, 33, 73–144.
Contreras, R., Carrez, D., Kinghorn, J.R. et al. (1991) Efficient KEX2-like processing of a glucoamylase-interleukin-6 fusion protein by Aspergillus nidulans and secretion of mature interleukin-6. BioTechnology, 9, 378–81.
Cooke, R.C. and Whipps, J.M. (1993). Ecophysiology of Fungi. Blackwell, Oxford.
Coughlan, M.P. and Amaral Collaco, M.T. (1990) Advances in Biological Treatment of Lignocellulosic Materials. Elsevier, London.
Cullen, D. and Kersten, P. (1992) Fungal enzymes for lignocellulose degradation, in Applied Molecular Genetics of Filamentous Fungi, (eds J.R. Kinghorn and G. Turner), Blackie, London, pp. 100–31.
Dal Degan, F., Ribadeau-Dumas, B. and Breddam, K. (1992) Purification and characterization of two serine carboxypeptidases from Aspergillus niger and their use in C-terminal sequencing of proteins and peptide synthesis. Applied and Environmental Microbiology, 58, 2144–52.
Datta, A. (1992) Purification and characterization of a novel protease from solid substrate cultures of Phanerochaete chrysosporium. Journal of Biological Chemistry, 267, 728–36.
Datta, A., Bettermann, A. and Kirk, T.K. (1991) Identification of a specific manganese peroxidase among ligninolytic enzymes secreted by Phanerochaete chrysosporium during wood decay. Applied and Environmental Microbiology, 57, 1453–60.
Davies, R.W. (1991) Molecular biology of a high-level recombinant protein production system in Aspergillus, in Molecular Industrial Mycology, (eds S.A. Leong and R.M. Berka), Marcel Dekker, New York, pp. 45–81.
Davis, M.A. and Hynes, M.J. (1991) Regulatory circuits in Aspergillus nidulans, in More Gene Manipulations in Fungi, (eds J.W. Bennett and L.L. Lasure), Academic Press, San Diego, pp. 151–89.
Dean, R.A. and Timberlake, W.E. (1989) Regulation of the Aspergillus nidulans pectate lyase gene (pelA). Plant Cell, 1, 275–84.
Deising, H., Nicholson, R.L., Haug, M. et al. (1992) Adhesion pad formation and the involvement of cutinase and esterases in the attachment of uredospores to the host cuticle. Plant Celt, 4, 1101–11.
Delaney, R., Wong, N.R.S., Meng, G. et al. (1987) Amino acid sequence of rhizopuspepsin isozyme pI5. Journal of Biological Chemistry, 262, 1461–7.
den Herder, I.F., Mateo Rosell, A.M., van Zuilen, CM. et al. (1992) Cloning and expression of a member of the Aspergillus niger gene family encoding a-galactosidase. Molecular and General Genetics, 233, 404–10.
De Viragh, P.A., Sanglard, D., Togni, G. et al. (1993) Cloning and sequencing of two Candida parapilosis genes encoding acid proteases. Journal of General Microbiology, 139, 335–42.
Dickinson, L., Harboe, M., van Meeswijck, R. et al. (1987) Expression of active Mucor miehei aspartic protease in Mucor circinelloides. Carlsberg Research Communications, 52, 243–52.
Disanto, M.E., Li, Q. and Logan, D.A. (1992) Purification and characterization of a developmentally regulated carboxypeptidase from Mucor racemosus. Journal of Bacteriology, 174, 447–55.
Dunn-Coleman, N.S., Bloebaum, P., Berka, R.M. et al. (1991) Commercial levels of chymosin production by Aspergillus. BioTechnology, 9, 976–81.
Durand, H., Clanet, M. and Tiraby, G. (1988) Genetic improvement of Trichoderma reesei for large scale cellulase production. Enzyme and Microbial Technology, 10, 341–6.
Durrands, P.K. and Cooper, R.M. (1988) Development and analysis of pectic screening media for use in the detection of pectinase mutants. Applied Microbiology and Biotechnology, 28, 463–7.
Elliott, T.J. (1985) The genetics and breeding of species of Agaricus, in The Biology and Technology of the Cultivated Mushroom, (eds P.B. Flegg, D.M. Spencer and D.A. Wood), Wiley, Chichester, pp. 111–29.
Ellis, R.J. and van der Vies, S.M. (1991) Molecular chaperones. Annual Review of Biochemistry, 60, 321–17.
Enari, T.M. and Niku-Paavola, M.L. (1987) Enzymatic hydrolysis of cellulose: is the current theory of the mechanisms of hydrolysis correct? Critical Reviews in Biotechnology, 5, 67–87.
Eriksson, K.E. and Kirk, T.K. (1985) Biopulping, biobleaching and treatment of kraft bleaching effluents with white rot fungi. Comprehensive Biotechnology, 3, 271–94.
Eriksson, K.E.L., Blanchette, R.A. and Ander, P. (1990) Microbial and Enzymatic Degradation of Wood and Wood Components. Springer Verlag, Berlin.
Evans, R., Ford, C., Sierks, M. et al. (1990) Activity and thermal stability of genetically truncated forms of Aspergillus glucoamylase. Gene, 91, 131–4.
Evans, C.S., Gallagher, I.M., Atkey, P.J. and Wood, D.A. (1991) Localisation of degradative enzymes in white-rot decay of lignocellulose. Biodegradation, 2, 93–106.
Eveleigh, D.E. (1987) Cellulase in perspective, in Technology in the 1990’s. Utilization of Lignocellulosic Wastes (eds B.S. Hartley, P.M.A. Broda and P.J. Senior), Philosophical Transactions of the Royal Society of London, pp. 321–447.
Farquhar, R., Honey, N., Murant, S.J. et al. (1991) Protein disulfide isomerase is essential for viability in Saccharomyces cerevisiae. Gene, 108, 81–9.
Farrell, R.L., Murtagh, K.E., Tien, M. et al. (1989) Physical and enzymatic properties of lignin peroxidase isoenzymes from Phanerochaete chrysosporium. Enzyme and Microbial Technology, 11, 322–8.
Fincham, J.R.S. (1989) Transformation in fungi. Microbiological Reviews, 53, 148–70.
Forrester, I.T., Graski, A.C., Mishra, C. et al. (1990) Characteristics and N-terminal amino acid sequence of a manganese peroxidase purified from Lentinula edodes cultures grown on a commercial wood substrate. Applied Microbiology and Biotechnology, 33, 359–65.
Frederick, G.D., Rombouts, P. and Buxton, F.P. (1993) Cloning and characterisation of pepC, a gene encoding a serine protease from Aspergillus niger. Gene, 125, 57–64.
Gems, D., Johnstone, I.L. and Clutterbuck, A.J. (1991) An autonomously replicating plasmid transforms Aspergillus nidulans at high frequency. Gene, 98, 61–7.
Gething, M-J. and Sambrook, J. (1992) Protein folding in the cell. Nature, 355, 33–45.
Gierasch, L.M. (1989) Signal sequences. Biochemistry, 28, 923–30.
Gines, M.J., Dove, M.J. and Seligy, V.L. (1989) Aspergillus oryzae has two nearly identical Taka-amylase genes, each containing eight introns. Gene, 79, 107–17.
Godfrey, A. and Reichelt, J. (1983) Industrial Enzymology. The Applications of Enzymes in Industry. McMillan, London, UK.
Gonzalez-Candelas, L. and Kolattukudy, P.E. (1992) Isolation and analysis of a novel inducible pectate lyase gene from the phytopathogenic fungus Fusarium solani f. sp. pisi (Nectria haematococca, mating population VI). Journal of Bacteriology, 174, 6343–9.
Goosen, T., Bos, C.J. and van den Broek, H. (1992) Transformation and gene manipulation in filamentous fungi: an overview, in Handbook of Applied Mycology, Vol. 4, (eds D.K. Arora, R.P. Elander and K.G. Mukerji), Marcel Dekker, New York, pp. 151–95.
Grainger, J.M., Jones, K.L., Hotten, P.M. and Rees, J.F. (1984) Estimation and control of microbial activity in landfill, in Microbiological Methods for Environmental Biotechnology, (eds J.M. Grainger and J.M. Lynch), Society for Applied Bacteriology Technical Series 19, Academic Press, London, pp. 259–73.
Gray, G.L., Hayenga, K., Cullen, D. et al. (1986) Primary structure of Mucor miehei aspartyl protease: evidence for a zymogen intermediate. Gene, 48, 41–53.
Grove, S.N. (1978) The cytology of hyphal tip growth, in The Filamentous Fungi, vol. 3, (eds J.E. Smith and D.R. Berry), Arnold, London, pp. 28–50.
Gysler, C., Harmsen, J.A.M., Kester, H.C.M. et al. (1990) Isolation and structure of the pectin lyase D-encoding gene from Aspergillus niger. Gene, 89, 101–8.
Hammel, K.E. (1989) Organopollutant degradation by lignolytic fungi. Enzyme and Microbial Technology, 11, 776–7.
Harkki, A., Uusitalo, J., Bailey, M. et al. (1989) A novel fungal expression system: secretion of active calf chymosin from the filamentous fungus Trichoderma reesei. BioTechnology, 7, 596–603.
Harkki, A., Mäntylä, A., Penttilä, M. et al. (1991) Genetic engineering of Trichoderma to produce strains with novel cellulase profiles. Enzyme and Microbial Technology, 13, 227–33.
Harmsen, J.A.M., Kusters van Someren, M.A. and Visser, J. (1990) Cloning and expression of a second Aspergillus niger pectin lyase gene (pelA): indications of a pectin lyase gene family in Aspergillus niger. Current Genetics, 18, 161–6.
Hata, Y., Kitamoto, K., Gomi, K. et al. (1991). The glucoamylase cDNA from Aspergillus oryzae: its cloning, nucleotide sequence, and expression in Saccharomyces cerevisiae. Agricultural and Biological Chemistry, 55, 941–9.
Hayashida, S. and Flor, P.Q. (1981) Raw starch-digestive glucoamylase productivity of protease-less mutant from Aspergillus awamori var. kawachi. Agricultural and Biological Chemistry, 45, 2675–81.
Hayashida, S. and Teramoto, Y. (1986) Production and characteristics of raw starch-digesting a-amylase from a protease-negative Aspergillus ficum mutant. Applied and Environmental Microbiology, 52, 1068–73.
Hayashida, S., Kuroda, K., Ohta, K. et al. (1989) Molecular cloning of the glucoamylase I gene of Aspergillus awamori var. kawachi for localization of the raw-starch affinity site. Agricultural and Biological Chemistry, 53, 923–9.
Hoch, H.C. (1986) Freeze-substitution of fungi, in Ultrastructure Techniques for Microorganisms, (eds H.C. Aldrich and W.J. Todd), Plenum Press, New York, pp. 183–212.
Holzbaur, E.L.F., Andrawis, A. and Tien, M. (1991) Molecular biology of lignin peroxidases from Phanerochaete chrysosporium, in Molecular Industrial Mycology (eds S.A. Leong and R.M. Berka), Marcel Dekker, New York, pp. 197–223.
Horiuchi, H., Yanai, K., Okazaki, T. et al. (1988a) Isolation and sequencing of a genomic clone encoding aspartic proteinase of Rhizopus niveus. Journal of Bacteriology, 170, 272–8.
Horiuchi, H., Yanai, K., Takagi, M. et al. (1988b) Primary structure of a base non-specific ribonuclease from Rhizopus niveus. Journal of Biochemistry 103, 408–18.
Horiuchi, H., Ashikari, T., Amachi, T. et al. (1990) High-level secretion of a Rhizopus niveus aspartic proteinase in Saccharomyces cerevisae. Agricultural and Biological Chemistry, 54, 1771–9.
Hsu, I-N., Delbaere, L.T.J., Jarvis, M.N.G. and Hofmann, T. (1977) Penicillopepsin from Penicillium janthinellum crystal structure at 2.8Å and sequence homology with porcine pepsin. Nature, 266, 140–5.
Huge-Jensen, B., Andreasen, F., Christensen, T. et al. (1989) Rhizomucor miehei triglyceride lipase is processed and secreted from transformed Aspergillus oryzae. Lipids, 24, 781–5.
Impoolsup, A., Bhumiratana, A. and Flegel, T.W. (1981) Isolation of alkaline and neutral proteases from Aspergillus flavus var. columnaris, a soy sauce koji mold. Applied and Environmental Microbiology, 42, 619–28.
Inaka, K., Taniyama, K., Kikuchi, M. et al. (1991) The crystal structure of a mutant human Iysozyme C77/95A with increased secretion efficiency in yeast. Journal of Biological Chemistry, 266, 12599–603.
Jaton-Ogay, K., Suter, M., Crameri, R. et al. (1992) Nucleotide sequence of a genomic and a cDNA clone encoding an extracellular alkaline protease of Aspergillus fumigatus. FEMS Microbiology Letters, 92, 163–8.
Jeenes, D.J., MacKenzie, D.A., Roberts, I.N. and Archer, D.B. (1991), Heterologous protein production by filamentous fungi. Biotechnology and Genetic Engineering Reviews 9, 327–67.
Jeenes, D.J., Marczinke, B., MacKenzie, D.A. and Archer, D.B. (1993) A truncated glucoamylase gene fusion for heterologous protein secretion from Aspergillus niger. FEMS Microbiology Letters, 107, 267–72.
Judelson, H.S. and Michelmore, R.W. (1989) Structure and expression of a gene encoding heat shock protein hsp70 from the oomycete fungus Bremia lactucae. Gene, 79, 207–17.
Kester, H.C.M. and Visser, J. (1990) Purification and characterization of polygalacturonases produced by the hyphal fungus Aspergillus niger. Biotechnology and Applied Biochemistry, 12, 150–60.
Khanh, N.Q., Albrecht, H., Ruttowski, E. et al. (1990) Nucleotide sequence and derived amino acid sequence of a pectinesterase cDNA isolated from Aspergillus niger strain RH5344. Nucleic Acids Research, 18, 4262.
Kikuchi, M. and Ikehara, M. (1991) Conformational features of signal sequences and folding of secretory proteins in yeasts. Trends in Biotechnology, 9, 208–11.
Kimura, A., Takata, M., Sakai, O. et al. (1992) Complete amino acid sequence of crystalline a-glucosidase from Aspergillus niger. Bioscience, Biotechnology and Biochemistry 56, 1368–70.
Kinderlerer, J.L. (1989) Volatile metabolites of filamentous fungi and their role in food flavour. Journal of Applied Bacteriology Symposium Supplement, pp. 133S–144S.
Kirk, T.K. and Farrell, R.L. (1987) Enzymatic ‘combustion’: The microbial degradation of lignin. Annual Review of Microbiology, 41, 465–505.
Kirk, T.K., Croan, S., Tien, M. et al. (1986) Production of multiple ligninases by Phanerochaete chrysosporium. Effect of selected growth conditions and use of a mutant strain. Enzyme and Microbial Technology, 8, 7–32.
Knowles, J., Teeri, T.T., Lehtovaara, P. et al. (1988) The use of gene technology to investigate fungal cellulolytic enzymes, in Biochemistry and Genetics of Cellulose Degradation, (eds J.-P. Aubert, P. Beguin and J. Millet), Academic Press, London, pp. 153–169.
Kobayashi, H., Sekibata, S., Shibuya, H. et al. (1989) Cloning and sequence analysis of cDNA for Irpex lacteus aspartic proteinase. Agricultural and Biological Chemistry, 53, 1927–33.
Koh-Luar, S.I., Parish, J.H., Bleasby, A.J. et al. (1989) Exported proteins of Neurospora crassa: 1-glucoamylase. Enzyme and Microbial Technology, 11, 692–5.
Kolattukudy, P.G. (1985) Enzymatic penetration of the plant cuticle by fungal pathogens. Annual Review of Phytopathology, 23, 223–50.
Korman, D.R., Bayliss, F.T., Barnett, C.C. et al. (1990) Cloning, characterization, and expression of two α-amylase genes from Aspergillus niger var awamori. Current Genetics, 17, 203–12.
Kornfeld, R. and Kornfeld, S. (1985) Assembly of asparagine-linked oligosaccharides. Annual Review of Biochemistry, 54, 631–44.
Kramer, G.F.H., Gunning, A.P., Morris, V.J. et al. (1993) Scanning tunnelling microscopy of Aspergillus niger glucoamylases. Journal of the Chemical Society. Faraday Transactions, 89, 2595–602.
Kriechbaum, M., Heilmann, H.J., Wientjes, F.J. et al. (1989) Cloning and DNA sequence analysis of the glucose oxidase gene from Aspergillus niger NRRL-3. FEBS Letters, 255, 63–6.
Kubicek, C.P., Messner, R., Gruber, F. et al. (1993) The Trichoderma cellulase regulatory puzzle: from the interior life of a secretory fungus. Enzyme and Microbial Technology, 15, 90–9.
Kukuruzinska, M.A., Bergh, M.L.E. and Jackson, B.J. (1987) Protein glycosylation in yeast. Annual Review of Biochemistry, 56, 915–44.
Kusters van Someren, M., Flipphi, M., de Graaff, L. et al. (1992) Characterization of the Aspergillus niger pelB gene: structure and regulation of expression. Molecular and General Genetics, 234, 113–20.
Lejohn, H.B. and Braithwaite, C.E. (1984) Heat and nutritional shock-induced proteins of the fungus Achyla are different and under independent transcriptional control. Canadian Journal of Biochemistry and Cell Biology, 62, 837–46.
Lowe, D.A. (1992) Fungal enzymes, in Handbook of Applied Mycology, Vol. 4, (eds D.K. Arora, R.P. Elander and K.G. Mukerji), Marcel Dekker, New York, pp. 681–706.
MacKenzie, D.A., Jeenes, D.J., Belshaw, N.J. and Archer, D.B. (1993) Regulation of secreted protein production by filamentous fungi: recent developments and perspectives. Journal of General Microbiology, 139, 2295–307.
MacRae, W.D., Buxton, F.P., Sibley, S. et al. (1988) A phosphate-repressible phosphatase gene from Aspergillus niger: its cloning, sequencing and transcriptional analysis. Gene, 71, 339–48.
Maldonado, M.C., Strasser de Saad, A.M. and Callieri, D. (1989) Catabolite repression of the synthesis of inducible polygalacturonase and pectinesterase by Aspergillus niger sp. Current Microbiology, 18, 303–6.
Marciano, P., Di Lenna, P. and Magro, P. (1982) Polygalacturonase isoenzymes produced by Sclerotinia sclerotiorum in vivo and in vitro. Physiological Plant Pathology, 20, 201–12.
Marth, E.H. and Yousef, A.E. (1991) Fungi and dairy products, in Handbook of Applied Mycology, Vol. 3 (eds D.K. Arora, K.G. Mukerji and E.H. Marth) Marcel Dekker, New York, pp. 375–414.
Matcham, S.E., Jordan, B.R. and Wood, D.A. (1984) Methods for assessment of fungal growth on solid substrates, in Microbiological Methods for Environmental Biotechnology (eds J.M. Grainger and J.M. Lynch), Academic Press, London, pp. 5–18.
Marsuura, Y., Kusunoki, M., Harada, W. and Kakudo, M. (1984) Structure and possible catalytic residues of Taka amylase A EC 3.2.1.1. Journal of Biochemistry, 95, 697–702.
Mattern, I.E., van Noort, J.M., van den Berg, P. et al. (1992) Isolation and characterization of mutants of Aspergillus niger deficient in extracellular proteases. Molecular and General Genetics, 234, 332–6.
Müller, M. (1992) Proteolysis in protein import and export: signal peptide processing in eu-and prokaryotes. Experientia, 48, 118–29.
Murakami, K., Ishida, Y., Masaki, A. et al. (1991) Isolation and characterization of the alkaline protease gene of Aspergillus oryzae. Agricultural and Biological Chemistry, 55, 2807–11.
Nakadai, T. (1985) The roles of enzymes produced by shoyu-koji-molds. Journal of the Japan Soy Sauce Research Institute, 11, 67–79.
Nandan, R. and Raisuddin, S. (1992) Fungal degradation of industrial wastes and wastewater, in Handbook of Applied Mycology, (eds D.K. Arora, R.P. Elander and K.G. Mukerji), Marcel Dekker, New York, pp. 931–61.
Nevalainen, K.M., Penttilä, M.E., Harkki, A. et al., (1991). The molecular biology of Trichoderma and its application to the expression of both homologous and heterologous genes, in Molecular Industrial Mycology, (eds S.A. Leong and R.M. Berka), Marcel Dekker, New York, pp. 129–18.
Nevalainen, K.M.H. and Palva, E.T. (1979) Improvement of amyloglucosidase production of Aspergillus awamori by mutagenic treatments. Journal of Chemical Technology and Biotechnology, 29, 390–5.
Nout, M.J.R. and Rombouts, F.M. (1990) Recent developments in tempe research. Journal of Applied Bacteriology, 69, 609–33.
Nunberg, J.H., Meade, J.H., Cole, G. et al. (1984) Molecular cloning and characterization of the glucoamylase gene of Aspergillus awamori. Molecular and Cellular Biology, 4, 2306–15.
Nyyssönen, E., Penttilä, M., Harkki, A. et al, (1993) Efficient production of antibody fragments by the filamentous fungus Trichoderma reesei. BioTechnology, 11, 59–5.
Ogawa, K., Ohara, H. and Toyama, N. (1988) Interspecific hybridization of Aspergillus awamori var. kawachi and Aspergillus oryzae by protoplast fusion. Agricultural and Biological Chemistry, 52, 1985–91.
Omura, F., Taniyama, Y. and Kikuchi, M. (1991) Behavior of cysteine mutants of human lysozyme in de novo synthesis and in vivo secretion. European Journal of Biochemistry, 198, 477–84.
Ono, K., Shintani, K., Shigata, S. and Oka, S. (1988) Various molecular species in glucoamylase from Aspergillus niger. Agricultural and Biological Chemistry, 52, 1689–98.
Orpin, C.G. (1975) Studies on the rumen flagellate Neocallimastix frontalis. Journal of General Microbiology, 91, 249–62.
Pfanner, N. and Neupert, W. (1990) The mitochondrial protein import apparatus. Annual Review of Biochemistry, 59, 331–53.
Pitson, S.M., Seviour, R.J. and McDougal, B.M. (1993) Noncellulolyric fungal β-glucanases: their physiology and regulation. Enzyme and Microbial Technology, 15, 178–92.
Pourrat, H., Barthomeuf, C., Texier, O. and Pourrat, A. (1988) Production of semi-alkaline protease by Aspergillus niger. Journal of Fermentation Technology, 66, 383–8.
Priest, F.G. (1984) Extracellular enzymes. Aspects of Microbiology, vol. 9. Van Nostrand Reinhold, Wokingham.
Punt, P.J., Zegers, N.D., Busscher, M. et al. (1991) Intracellular and extracellular production of proteins in Aspergillus under the control of expression signals of the highly expressed Aspergillus nidulans gpdA gene. Journal of Biotechnology, 17, 19–34.
Raj, H.G., Saxena, M., Allameh, A. and Mukerji, K.G. (1992) Metabolism of foreign compounds by fungi, in Handbook of Applied Mycology, (eds D.K. Arora, R.P. Elander and K.G. Mukerji), Marcel Dekker, New York, pp. 881–904.
Ramasamy, K., Kelley, R.L. and Reddy, C.A. (1985) Lack of lignin degradation by glucose oxidase-negative mutants of Phanerochaete chrysosporium. Biochemical Biophysical Research Communications, 131, 436–41.
Reid, I.D. (1989) Solid-state fermentations for biological delignification. Enzyme and Microbial Technology, 11, 786–803.
Reid, G.A. (1991) Protein targeting in yeast. Journal of General Microbiology, 137, 1765–73.
Rexach, M., d’Enfert, C., Wuestehube, L. and Schekman, R. (1992) Genes and proteins required for vesicular transport from the endoplasmic reticulum. Antonie van Leeuwenhoek, 61, 87–92.
Roberts, I.N., Jeenes, D.J., MacKenzie, D.A. et al. (1992) Heterologous gene expression in Aspergillus niger: a glucoamylase-porcine pancreatic prophospholipase A2 fusion protein is secreted and correctly processed to yield mature enzyme. Gene, 122, 155–61.
Rombouts, F.M. and Pilnik, W. (1980) Pectic enzymes, in Microbial Enzymes and Bioconversions (ed. A.H. Rose), Academic Press, London, pp. 227–82.
Rombouts, F.M., Voragen, A.G.J., Searle-van Leeuwen, M.F. et al. (1988) The arabinanases of Aspergillus niger — purification and characterization of two α-L-arabino-furanosidases and an enrfo-l,5-α-L-arabinase. Carbohydrate Polymers, 9, 25–47.
Rossignol, J.-L. and Picard, M. (1991) Ascobolus immersus and Podospora anserina: sex, recombination, silencing and death, in More Gene Manipulations in Fungi, (eds J.W. Bennett and L.L. Lasure), Academic Press, San Diego, pp. 266–90.
Rothman, J.E. and Orci, L. (1992) Molecular dissection of the secretory pathway. Nature, 355, 409–15.
Rouvinen, J., Bergfors, T., Teeri, T. et al. (1990) Three-dimensional structure of cellobiohydrolase II from Trichoderma reesei. Science, 249, 380–6.
Saha, B.C. and Zeikus, J.G. (1989) Microbial glucoamylases: biochemical and biotechnological features. Starch, 2, 57–64.
Sakaguchi, K., Gomi, K., Takagi, M. and Horiuchi, H. (1992) Fungal enzymes used in oriental food and beverage industries, in Applied Molecular Genetics of Filamentous Fungi, (eds J.R. Kinghorn and G. Turner), Blackie, Glasgow, pp. 54–99.
Salovuori, I., Makarow, M., Rauvala, H. et al. (1987) Low molecular weight high-mannose type glycans in a secreted protein of the filamentous fungus Trichoderma reesei. BioTechnology, 5, 152–6.
Satyanarayana, T., Johri, B.N. and Klein, J. (1992) Biotechnological potential of thermophilic fungi, in Handbook of Applied Mycology, Vol. 4, (eds D.K. Arora, R.P. Elander and K.G. Mukerji), Marcel Dekker, New York, pp. 729–61.
Scott-Craig, J.S., Panaccione, D.G., Cervone, F. and Walton, J.D. (1990) Endopolygalacturonase is not required for pathogenicity of Cochliobolus carbonum on maize. Plant Cell, 2, 1191–200.
Selker, E.U. (1990) Premeiotic instability of repeated sequences in Neurospora crassa. Annual Review of Genetics, 24, 579–613.
Shibuya, I., Gomi, K., Iimura, Y. et al. (1990). Molecular cloning of the glucoamylase gene of Aspergillus shirousami and its expression in Aspergillus oryzae. Agricultural and Biological Chemistry, 54, 1905–14.
Shoemaker, S., Schweickart, V., Ladner, M. et al. (1983) Molecular cloning of exo-cellobiohydrolase I derived from Trichoderma reesei strain L27. BioTechnology, 1, 691–6.
Sirianuntapiboon, S., Somchai, P., Ohmomo, S. and Atthasampunna, P. (1988) Screening of filamentous fungi having the ability to decolorize molasses pigments. Agricultural and Biological Chemistry, 52, 387–92.
Smith, D.C., Bhat, K.M. and Wood, T.M. (1991) Xylan-hydrolysing enzymes from thermophilic and mesophilic fungi. World Journal of Microbiology and Biotechnology, 7, 475–84.
Sodhi, H.S., Elliott, T.J., Wood, D.A. and Stephens, S. (1991) Studies on enzyme mutants in Coprinus bilanatus. Mushroom Science, 13, 131–8.
Soliday, C.L., Flurkey, W.H., Okita, T.W. and Kolattukudy, P.E. (1984) Cloning and structure determination of cDNA for cutinase, an enzyme involved in fungal penetration of plants. Proceedings of the National Academy of Sciences of the United States of America, 81, 3939–43.
St Leger, R.J., Charnley, A.K. and Cooper, R.M. (1986) Cuticle-degrading enzymes of entomopathogenic fungi. Synthesis in culture on cuticle. Journal of Invertebrate Pathology, 48, 85–95.
St Leger, R.J., Charnley, A.K. and Cooper, R.M. (1987a) Characterisation of cuticle degrading proteases produced by the entomopathogen Metarhizium anisopliae. Archives of Biochemistry and Biophysics, 253, 221–32.
St Leger, R.J., Cooper, R.M. and Charnley, A.K. (1987b) Production of cuticle degrading enzymes by the entomopathogen Metarhizium anisopliae during infection of cuticles from Calliphora vomitoria and Manduca sexta. Journal of General Microbiology, 133, 1371–82.
St Leger, R.J., Frank, D.C., Roberts, D.W. and Staples, R.C. (1992) Molecular cloning and regulatory analysis of the cuticle-degrading protease structural gene from the entomopathogenic fungus Metarhizium anisopliae. European Journal of Biochemistry, 204, 991–1001.
Stahl, D.J. and Schafer, W. (1992) Cutinase is not required for fungal pathogenicity on pea. Plant Cell, 4, 621–9.
Stepanov, V.M. (1985) Fungal aspartyl proteinases, in Aspartyl Proteinases and Their Inhibitors, (ed. V. Kostka), Walter de Gruyter, New York, pp. 27–40.
Stephens, S.K., Elliott, T.J. and Wood, D.A. (1991) Extracellular enzyme mutants of Coprinus bilanatus. Enzyme and Microbial Technology, 13, 976–81.
Sugihara, A., Shimada, Y. and Tominaga, Y. (1988) Purification and characterization of Aspergillus niger lipase. Agricultural and Biological Chemistry, 52, 1591–2.
Svensson, B., Pedersen, T.G., Svendsen, I.B. et al. (1982) Characterization of two forms of glucoamylase from Aspergillus niger. Carlsberg Research Communications, 47, 55–69.
Svensson, B., Larsen, K., Svendsen, I. and Boel, E. (1983) The complete amino acid sequence of the glycoprotein glucoamylase Gl, from Aspergillus niger. Carlsberg Research Communications, 48, 529–44.
Svensson, B., Larsen, K. and Gunnarsson, A. (1986) Characterization of a glucoamylase G2 from Aspergillus niger. European Journal of Biochemistry, 154, 497–502.
Tachikawa, H., Miura, T., Katakura, Y. and Mizunaga, T. (1991) Molecular structure of a yeast gene PDI1 encoding protein disulfide isomerase that is essential for cell growth. Journal of Biochemistry, 110, 306–13.
Tada, S., Iimura, Y., Gomi, K. et al. (1989) Cloning and nucleotide sequence of the genomic Taka-amylase A gene of Aspergillus oryzae. Agricultural and Biological Chemistry, 53, 593–9.
Tada, S., Gomi, K., Kitamoto, K. et al. (1991) Construction of a fusion gene comprising the Taka-amylase A promoter and the Escherichia coli β-glucuronidase gene and analysis of its expression in Aspergillus oryzae. Molecular and General Genetics, 229, 301–6.
Takahashi, K., Inoue, H., Sakai, K. et al. (1991) The primary structure of Aspergillus niger acid proteinase A. Journal of Biological Chemistry, 266, 19480–3.
Taniyama, Y., Kuroki, R., Omura, F. et al. (1991) Evidence for intramolecular disulfide bond shuffling in the folding of mutant human lysozyme. Journal of Biological Chemistry, 266, 6456–61.
Taniyama, Y., Ogasahara, K., Yutani, K. and Kikuchi, M. (1992) Folding mechanism of mutant human lysozyme C77/95A with increased secretion efficiency in yeast. Journal of Biological Chemistry, 267, 4619–24.
Tao, J., Ginzberg, I., Koltin, Y. and Bruenn, J.A. (1993) Mutants of Ustilago maydis KP6 toxin defective in production of one of two polypepudes from one preprotoxin. Molecular and General Genetics, 238, 234–40.
Tatsumi, H., Murakami, S., Tsuji, R.F. et al. (1991) Cloning and expression in yeast of a cDNA done encoding Aspergillus oryzae neutral protease II, a unique metalloprotease. Molecular and General Genetics, 228, 97–103.
Tenkamen, M., Puls, J. and Poutanen, K. (1992) Two major xylanases of Trichoderma reesei. Enzyme and Microbial Technology, 14, 566–74.
Theodorou, M.K., King-Spooner, C. and Beever, D.E. (1989) Presence or Absence of Anaerobic Fungi in Landfill Refuse. UK Department of Energy (ETSU B1246).
Thompson, S.A. (1991), Fungal aspartic proteases, in Molecular Industrial Mycology, (eds S.A. Leong and R.M. Berka), Marcel Dekker, New York, pp. 107–28.
Thompson, J.A. (1993) Molecular biology of xylan degradation. FEMS Microbiology Reviews, 104, 65–82.
Törrönen, A., Mach, R.L., Messner, R. et al. (1992) The two major xylanases from Trichoderma reesei: characterisation of both enzymes and genes. BioTechnology, 10, 1461–4.
Ushijima, S., Nakadai, T. and Uchida, K. (1990) Further evidence on the specific protoplast fusion between Aspergillus oryzae and Aspergillus sojae and subsequent haploidization, with special reference to their production of some hydrolysing enzymes. Agricultural and Biological Chemistry, 54, 2393–9.
Uusitalo, J.M., Nevalainen, K.M.H., Harkki, A.M. et al. (1991) Enzyme production by recombinant Trichoderma reesei strains. Journal of Biotechnology, 17, 35–50.
van den Hondel, C.A.M.J.J., Punt, P.J. and van Gorcom, R.F.M. (1991) Heterologous gene expression in filamentous fungi, in More Gene Manipulations in Fungi, (eds J.W. Bennett and L.L. Lasure), Academic Press, San Diego, pp. 396–428.
van den Hondel, C.A.M.J.J., Punt, P.J. and van Gorcom, R.F.M. (1992) Production of extracellular proteins by the filamentous fungus Aspergillus. Antonie van Leeuwenhoek, 61, 153–60.
van Gorcom, R.F.M., van Hartingsveldt, W., van Parldon, P.A.B. et al. (1991) Cloning and expression of microbial phytase. European Patent Application 0420358A1.
van Hartingsveldt, W., van Zeijl, C.M.J., Veenstra, A.E. et al. (1990) Heterologous gene expression in Aspergillus: analysis of chymosin production in single-copy transformants of A. niger, in Proceedings of the 6th International Symposium on the Genetics of Industrial Microorganisms, Strasbourg, pp. 107–16.
van Noort, J.M., van den Berg, P. and Mattern, I.E. (1991) Visualization of proteases within a complex sample following their selective retention on immobilized bacitracin, a peptide antibiotic. Analytical Biochemistry, 198, 385–90.
von Heijne, G. (1990) The signal peptide. Journal of Membrane Biology, 115, 195–201.
Wang, H.L., Swain, E.W. and Hesseltine, C.W. (1980) Phytase of molds used in oriental food fermentation. Journal of Food Science, 45, 1262–6.
Ward, M. (1989) Production of calf chymosin by Aspergillus awamori, in Genetics and Molecular Biology of Industrial Microorganisms (eds C.L. Hershberger, S.W. Queener and G. Hegeman), American Society for Microbiology, Washington, pp. 288–94.
Ward, M. (1991) Chymosin production in Aspergillus, in Molecular Industrial Mycology, (eds S.A. Leong and R.M. Berka), Marcel Dekker, New York, pp. 83–105.
Ward, M. and Kodama, K.H. (1991) Introduction to fungal proteinases and expression in fungal systems. Advances in Experimental and Medical Biology, 306, 149–60.
Ward, M., Wilson, L.J., Kodama, K.H. et al. (1990) Improved production of chymosin in Aspergillus by expression as a glucoamylase-chymosin fusion. BioTechnology, 8, 435–40.
Williamson, G., Belshaw, N.J. and Williamson, M.P. (1992) O-Glycosylation in Aspergillus glucoamylase: conformation and role in binding. Biochemical Journal, 282, 423–8.
Wirsel, S., Lachmund, A., Wildhardt, G. and Ruttkowski, E. (1989) Three a-amylase genes of Aspergillus oryzae exhibit identical intron-exon organization. Molecular Microbiology, 3, 3–14.
Wong, K.K.Y., Tan, L.U.L. and Saddler, J.N. (1988) Multiplicity of β-1,4 xylanases in microorganisms: functions and applications. Microbiological Reviews, 52, 305–17.
Wood, D.A. (1985) Production and roles of extracellular enzymes during morphogenesis of basidiomycete fungi, in Developmental Biology of Higher Fungi, (eds D. Moore, L.A. Casselton, D.A. Wood and J.C. Frankland), Cambridge University Press, Cambridge, pp. 375–87.
Wood, D.A. (1989) Mushroom biotechnology. International Industrial Biotechnology, 9, 5–9.
Wood, D.A. and Smith, J.F. (1987) The cultivation of mushrooms, in Essays in Food Microbiology, (eds J.R. Norris and G.L. Pettipher), Wiley, Chichester, pp. 309–43.
Wood, T.M. and Garcia-Campoyo, V. (1990) Enzymology of cellulose degradation. Biodegradation, 1, 147–61.
Wood, T.M. and Mcrae, S.I. (1979) Synergism between enzymes ssinvolved in the solubilisation of native cellulose. Advances in Chemistry Series, 181, 181–209.
Wösten, H.A.B., Moukha, S.M., Sietsma, J.H. and Wessels, J.G.H. (1991) Localization of growth and secretion of proteins in Aspergillus niger. Journal of General Microbiology, 137, 2017–23.
Xue, G-P., Gobius, K.S. and Orpin, C.G. (1992) A novel polysaccharide hydrolase cDNA (ca/D) from Neocallimastix patriciarum encoding three multi-functional catalytic domains with high endoglucanase, cellobiohydrolase and xylanase activities. Journal of General Microbiology, 138, 2397–403.
Yamashita, I. (1989) The threonine and serine-rich tract of the secretory glucoamylase can direct β-galactosidase to the cell envelope. Agricultural and Biological Chemistry, 53, 483–9.
Yang, R. and Kenealy, W.R. (1992) Regulation of restrictocin production in Aspergillus restrictus. Journal of General Microbiology, 138, 1421–7.
Yokotsuka, T. (1991a) Proteinaceous fermented foods and condiments prepared with koji molds, in Handbook of Applied Mycology, Vol.3, (eds D.K. Arora, K.G. Mukerji and E.H. Marth), Marcel Dekker, New York, pp. 329–73.
Yokotsuka, T. (1991b) Non-proteinaceous fermented foods and beverages produced with koji molds, in Handbook of Applied Mycology, Vol. 3, (eds D.K. Arora, K.G. Mukerji and E.H. Marth), Marcel Dekker, New York, pp. 293–328.
Zhu, W-S., Wojdyla, K., Donlon, K. et al. (1990) Extracellular proteases of Aspergillus flavus. Diagnostic Microbiology and Infectious Disease, 13, 491–7.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1995 Neil A.R. Gow and Geoffrey M. Gadd
About this chapter
Cite this chapter
Archer, D.B., Wood, D.A. (1995). Fungal Exoenzymes. In: Gow, N.A.R., Gadd, G.M. (eds) The Growing Fungus. Springer, Dordrecht. https://doi.org/10.1007/978-0-585-27576-5_7
Download citation
DOI: https://doi.org/10.1007/978-0-585-27576-5_7
Publisher Name: Springer, Dordrecht
Print ISBN: 978-0-412-46600-7
Online ISBN: 978-0-585-27576-5
eBook Packages: Springer Book Archive