Abstract
The floral transition is a critical developmental change in a plant's life cycle that is marked by the switch from vegetative to reproductive growth. The transition is induced by leaf-derived signals that translocate through the phloem to the shoot apex where the shoot apical meristem is reprogrammed to adopt a floral fate. In maize, this occurs when the vegetative shoot meristem ceases leaf initiation and becomes consumed in the production of the tassel inflorescence primordium. Upper axillary shoot meristems are converted into ear inflorescence primordia soon after this period. This review highlights current understanding of the genes and molecular mechanisms regulating the floral transition in maize. We relate flowering control in maize to its progenitor teosinte, provide an overview of the quantitative nature of flowering in maize germplasm and describe what is currently known about the molecular components of the maize floral transition genetic network.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Overview of Maize Flowering
Floral transition, the switch from vegetative to reproductive growth, marks a critical event in the life cycle of higher plants. During vegetative growth, the shoot apical meristem (SAM), a population of totipotent cells at the growing point of the plant, gives rise to leaves and other above-ground organs. To switch to reproductive growth, the SAM becomes committed to the production of reproductive structures, such as branched inflorescences bearing flowers (Fig. 1). The period when the SAM is reprogrammed is called the floral transition and the timing of the transition largely determines when a plant flowers.
Maize vegetative and reproductive meristems. The floral transition occurs when the vegetative shoot apical meristem switches to reproductive growth to become the tassel primordium and the upper axillary meristems transition to become ear primordia. Both apical and axillary primordia develop into the male inflorescence (tassel) shedding pollen at the same time the female inflorescence (ear) exserts silks. Scale bar = 200 μm
Higher plants have developed sophisticated genetic mechanisms to ensure that flowering coincides with an optimal time for reproductive success. Early physiological studies revealed that environmental signals such as day length and temperature could alter the timing of the transition so that flowering occurs at the appropriate time. The underlying molecular components of floral inductive pathways are being elucidated at present, largely through the analysis of flowering time genes in the small model plant Arabidopsis thaliana. Although these studies show that diverse plant species share parts of the regulatory pathways defined in Arabidopsis, whether all components of these regulatory pathways exist in other plants is not apparent. Therefore further analysis of floral regulatory mechanisms in other species is required to define common pathways as well as uncover unique regulatory elements that may have evolved to accommodate particular environmental conditions and species-specific physiologies.
In maize, the floral transition is characterized by the cessation of leaf formation, the elongation of the SAM and the adoption of an inflorescence identity to create the tassel primordium (Fig. 1). A similar transition occurs later in time with the conversion of several axillary meristems into ear primordia. Schmidt and Vollbrecht describe the process of inflorescence formation in more detail in the Maize Development section, Chap. 2. Here, we focus on current understanding of the genetic and molecular events underlying floral induction in maize and some of the physiological changes associated with this key developmental process. Research on the maize floral transition has not received as much attention as other species; however, recent studies indicate that, while maize shares some common regulatory features with other model plants, it also employs unique components in a genetic network that controls flowering.
1.1 Teosinte: An Obligate Short-Day Plant
Domesticated maize is derived from a type of teosinte ( Zea mays ssp. parviglumis), a sub-tropical, wild grass species that originates in southwest Mexico (Doebley, 2004). Although modern maize and teosinte appear quite dissimilar from each other, most of these morphological distinctions can be traced to a handful of genetic differences (Beadle, 1939). Relevant to this chapter, one major difference between teosinte and maize grown in more northerly latitudes is that modern maize flowers after making a particular number of leaves, regardless of photoperiod, whereas teosinte requires short day (SD) photoperiods to induce flowering. That is, unlike its tropical ancestor, temperate maize is primarily photoperiod insensitive and some varieties are day-neutral (DN). The discovery that some plants require a defined photoperiod to induce flowering follows the work of Garner and Allard (1920) with tobacco. Inspired by these studies, Emerson (1924) performed the first analysis of photoperiod effects on teosinte and tropical maize. He showed that teosinte would flower several months early if given short day treatments (10 h day/14 h night), and would only flower in mid- to late October (in Ithaca, NY) if untreated, presumably because the shorter days of September trigger floral inductive signals.
Therefore, as early Native American farmers migrated to higher latitudes, they selected for maize that is less dependent on short-day photoperiods to flower. This is a necessity given that tropical maize grown at 40° N latitude or higher will flower in October, and therefore almost certainly will be killed by frost before seed set. The question arises as to what genetic changes in maize were selected in the shift from photoperiod dependency to day neutrality. Specifically, were mechanisms common in other species modified to permit day-neutral flowering, or was a novel inductive mechanism co-opted for this purpose?
2 Breeding for Flowering Time
2.1 Quantitative Flowering Time Variation
Modern maize cultivars have been selected for a wide range in flowering time variation, allowing for adaptation to cultivation from short growing seasons at high latitudes to long growing seasons in tropical and subtropical climes. The earliest flowering maize variety, Gaspé Flint, reaches reproductive maturity, shedding pollen and exserting silks, in as few as 30–35 days after planting (Fig. 2). The floral transition in Gaspé Flint typically occurs within 7–10 days of germinating, with mature plants producing 7–9 leaves at maturity (Muszynski, unpublished observations). In contrast, late tropical varieties may require 4 months or more to flower, taking advantage of the longer growing season. Most U.S. Corn Belt lines flower between 75 and 120 days, with each line optimized to balance the extent of vegetative growth and duration of grain fill to its adapted geographic location.
Examples of maize flowering time variants. Gaspé Flint is an extremely early flowering variety, indeterminate1 (id1 ) is an extremely late flowering recessive mutant, delayed flowering1 (dlf1 ) is a moderately late flowering recessive mutant and Leafy (Lfy) is a moderately late flowering dominant mutant. All late flowering mutant plants produce more leaves and are taller than wild type siblings
Maize breeders have utilized the diversity in flowering time to study and manipulate this quantitatively inherited trait. Genetic mapping studies of quantitative trait loci (QTL) for flowering time indicate that this trait is controlled by the combined action of a limited number of loci with large effects and many loci with small effects (Veldboom et al., 1994; Austin et al., 2001; Chardon et al., 2004, 2005). Using near isogenic lines (NILs) developed from backcrossing Gaspé Flint into N28, a standard maturity inbred, and selection for early flowering, two QTL were identified on chromosome 8 termed vegetative to generative transition1 (Vgt1) and Vgt2 (Vladutu et al., 1999; Salvi et al., 2002). Both loci specifically affect the floral transition, with alleles from the Gaspé Flint parent mediating an earlier transition, leading to fewer leaves produced and days to shed pollen. Recent advances in positional cloning in maize allowed for the molecular isolation of the Vgt1 locus and suggest a possible hypothesis for its mode of action (Salvi et al., 2007) (see below). A number of QTL mapping studies have detected flowering time loci on most of the maize chromosomes, with repeated identification of single large effect loci on chromosomes 1 and 9 and two large effect loci on chromosomes 8 and 10 (Chardon et al., 2004). Such results suggest that numerous genes participate in the floral transition and subsequent developmental processes regulating inflorescence development that affect the timing of pollen shed and silk exsertion. Future studies are required to define the molecular determinants underlying each QTL and dissect the role each plays in regulating flowering time.
2.2 QTL Corresponding to Specific Genes
Association genetic analysis offers a complementary approach to bi-parental mapping for the identification of loci controlling quantitatively inherited phenotypes. Utilizing the vast range of phenotypic variation found in diverse maize germplasm, association genetic analysis correlates molecular polymorphisms within candidate gene sequences to quantitative phenotypic variation. In a survey of 92 diverse inbred lines, sequence variation at the dwarf8 (d8) locus, encoding a negative regulator of gibberellic acid (GA) signaling, was associated with quantitative variation for flowering time and plant height. Key to this result was the accurate estimation of population structure within this set of inbred lines (Thornsberry et al., 2001). Additionally, although linkage disequilibrium usually decays rapidly in maize, significant disequilibrium was detected for several polymorphisms within the d8 gene, suggesting that one or more of these polymorphisms are responsible for the flowering time variation. Finally, the distribution of nonsynonomous polymorphisms within d8 suggests that this locus has been under selection. Similar studies with a larger set of European inbreds and maize land races confirmed sequence polymorphisms in d8 are associated with variation for flowering time in that germ-plasm and may have led to the adaptation of tropical maize to a more temperate climate (Camus-Kulandaivelu et al., 2006).
The variation in activity level of regulatory genes can have profound effects on development that may be a target of selection. Associations with flowering time and other quantitative traits linked with maize domestication were correlated with differences in gene copy number of the maize FLORICAULA/LEAFY homologous genes zfl1 and zfl2 (Bomblies and Doebley, 2006). Increasing functional copies of zfl1 consistently showed earlier flowering as measured by total leaf number, a direct measure of the timing of the floral transition, as well as a modest but significant decrease in the number of days to pollen shed and silk exsertion. On the other hand, increasing the number of active copies of zfl2 is associated more with changes in plant architecture and inflorescence traits, such as the number of ears or lateral branches per plant and the number of kernel rows per ear. As more flowering time genes are identified (discussed in more detail in Sect. 4), investigations using various statistical methodologies will identify the chromosomal regions and loci that underlie the quantitative inheritance of flowering time in diverse and elite germplasm. Such information will enable direct and precise selection for the determinants of flowering time variation using molecular breeding to modulate this trait in germplasm enhancement programs.
3 Long Distance Floral Inductive Signals
Early floral transition studies focused on the physiological changes associated with flowering and the signals that initiate the conversion of the shoot apex from vegetative to reproductive growth. Overall these studies established several key points regarding the transition (reviewed in Bernier and Perilleux, 2005). First, leaves are the source of the floral inductive signals; whether driven by environmental signals, such as photoperiod, or by endogenous signals, such as plant size or age. Second, the floral inductive signal, sometimes referred to as “florigen,” is transmitted through phloem tissue to the shoot apex to cause flowering. Finally, the shoot apex, where the SAM resides, must be competent to receive the florigenic signal in order for the transition to occur. That is, maize must pass through a juvenile phase that is incapable of flowering, and into an adult phase that is competent to perceive the floral inductive signal (Poethig, 1990). For most temperate inbreds the juvenile phase consists of the first five to seven leaves. Evidence supporting the unrespon-siveness of the juvenile apex to florigenic signals is based on in vitro culturing of shoot apices (Irish and Nelson, 1991). These experiments also gave the first clue that, like most physiological experiments with diverse plant species, maize leaves are the source of florigenic signals and the ability of maize to flower is not intrinsic to the shoot apex (Irish and Jegla, 1997).
A key tenet of the original florigen hypothesis is that floral inductive signals are universal and should act in the same way in diverse plants. However, until recently there was little evidence as to the biochemical nature of florigen. Now, recent discoveries, as described in Sect. 4, suggest that the movement of a small protein from leaf to apex through the phloem may act as a florigen. These studies of long distance flowering signals involved plants whose flowering is greatly accelerated by inductive photoperiods such as Arabidopsis and rice. Whether a similar system exists in maize has yet to be shown. Since maize is a day-neutral plant that relies on internal (or autonomous) inductive signals such as leaf number or plant size to induce flowering, does it use the same florigenic signals used by photoperiod sensitive plants? Or does the autonomous pathway co-opt metabolic products of plant growth to signal the floral transition? This is particularly relevant to temperate maize grown at higher latitudes, which relies almost exclusively on endogenous signals to flower. Evidence in other plants suggests that the redirection of assimilates to the shoot apex can affect flowering time (Corbesier et al., 1998; Ohto et al., 2001). However, it is difficult to distinguish cause and effect; for example, do greater levels of sucrose at the apex activate flowering genes, or is the increased accumulation of assimilates required to sustain the higher metabolic activities of the florally-induced apex? Although there is evidence for both possibilities (Bernier and Perilleux, 2005) , further evidence is required to define the mechanisms underlying the autonomous signaling pathway.
4 Molecular Mechanisms and Genetic Pathways
4.1 Photoperiod Effects on Flowering
Although most temperate maize varieties are considered to be day-neutral, they do retain some minor sensitivity to photoperiod. For most maize, sensitivity to SD photoperiod-induced acceleration of flowering is inversely correlated with distance from the equator. Under long-day (LD) photoperiods, different inbreds will flower after a genetically determined number of leaves are produced and growing degree units (GDUs) have accumulated. GDUs or heat units (HUs) are a measure of thermal time calculated from the average daily temperature and are a more accurate measure of flowering time than days alone (Zhang et al., 2005). Short-day (SD) photoperiods condition a minor reduction in leaf number and accumulated GDUs required to flower (Galinat and Naylor, 1951; Tollenaar and Hunter, 1983). The photoreceptor phytochrome is known to play a role in regulating flowering time in photoperiod sensitive species. In maize, a member of the phytochromeB (phyB) subfamily, phyB2, has been shown to play a predominant role in repressing flowering under LD and SD photoperiods (Sheehan et al., 2007). A naturally occurring deletion allele of phyB2 was found in the early flowering Northern flint inbred F2. A similar deletion allele was found in many of the early flint lines, suggesting this mutation contributes to early flowering in this germplasm. In extreme cases, such as teosinte and tropical maize, which have an absolute SD requirement to induce flowering, the photoreceptors and the circadian clock mechanisms are intact. However, the molecular components of this photoperiod induction pathway have yet to be elucidated.
4.2 Maize Flowering Time Mutants
Unlike Arabidopsis, where more than 20 mutants with specific effects on flowering time have been described, relatively few mutant loci in maize are known that have a discrete effect in altering flowering time. However, as described above in Sect. 2 , numerous QTL have been identified that are associated with altered flowering time (Chardon et al., 2004). The first mutation described to have a striking effect on maize flowering time, indeterminate, was discovered by Singleton (1946). Homozygous loss-of-function indeterminate mutants flower extremely late and often exhibit aberrant floral morphology, such as the absence of ears and reversion to vegetative growth (Galinat and Naylor, 1951) (Fig. 2). The indeterminate gene (later designated indeterminate1 [ id1]) was isolated by transposon tagging and found to encode a zinc finger protein (Colasanti et al., 1998). Subsequent analysis showed that ID1 protein is localized to nuclei and is able bind DNA in vitro, suggesting that ID1 has a role in regulating the transcription of other genes that control flowering time in maize (Kozaki et al., 2004; Wong and Colasanti, 2007) , although the identity of ID1 target genes have yet to be established. Comparative genomic analysis suggests that the id1 floral induction pathway may be unique to monocots, as no clear id1 homolog is present in the Arabidopsis genome (Colasanti et al., 2006).
Two other mutations have been described which have discrete effects on maize flowering time; delayed flowering1 (dlf1) and Leafy (Lfy) both postpone the floral transition, leading to late flowering (Shaver, 1983; Neuffer et al., 1997) (Fig. 2). Leafy (unrelated to the Arabidopsis LEAFY gene or similar homologous genes) is a dominant, late flowering mutation which increases the number of leaves on mutant plants, specifically between the uppermost ear and tassel. Most normal maize plants produce 5–7 leaves between the uppermost ear and the tassel, while mutant Lfy plants can have 9–15 or more leaves above the uppermost ear, depending on genetic background. The Lfy mutation can delay flowering by 10–20 days and switches inflorescence maturation such that mutant plants exsert silks prior to shedding pollen. This effect can become so pronounced that silks on Lfy mutant plants senesce before pollen is shed, thereby preventing self-pollination (Muszynski, personal observation). Little is known about the morphological and developmental aspects of this dominant mutation and it has yet to be molecularly isolated. Although relatively uncharacterized from a genetic perspective, it has been used to a modest degree in maize breeding programs in Canada to increase leaf biomass as a means to improve yield (Dijak et al., 1999; Andrews et al., 2000; Costa et al., 2002; Subedi and Ma, 2005a, b; Subedi et al., 2006).
Positional cloning and association mapping recently pinpointed the molecular position of Vgt1, a major flowering time QTL on chromosome 8, to an intergenic region ̃70 kb upstream of an APETALA2 (AP2)-like transcription factor, designated ZmRap2.7 (Salvi et al., 2007). Variation in flowering time in various inbred lines was associated with sequence changes in this putative cis element that presumably controls the expression of ZmRap2.7. Transgenic analysis showed that overexpression of ZmRap2.7 cDNA caused late flowering, whereas down-regulation in antisense maize plants caused early flowering. Similarity to members of a family of Arabidopsis AP2-like genes that have a negative effect on flowering suggests a potential orthologous role for ZmRap2.7 (Aukerman and Sakai, 2003). Further, this finding provides further evidence that some aspects of maize flowering may be controlled by conserved floral regulatory mechanisms.
4.3 Conserved Elements of Maize Floral Induction
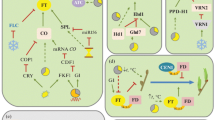
Mutations in dlf1 have a modest effect on flowering, with mutant plants flowering 1–2 weeks later than wild type sibs and also having minor inflorescence alterations. Molecular isolation and characterization of dlf1 showed that it encodes a protein with homology to basic leucine zipper (bZIP) transcription factors and likely functions through binding DNA (Muszynski et al., 2006). The dlf1 gene has high sequence similarity and a comparable expression pattern to the Arabidopsis FLOWER LOCUS D (FD) gene; fd mutants also exhibit a late-flowering pheno-type. Thus, FD and DLF1 proteins are predicted to share co-orthologous functions. In maize, double mutant analysis indicates dlf1 is downstream of id1 activity and, consistent with this result, dlf1 is misexpressed in id1 mutants (Muszynski et al., 2006). These data, in concert with expression analysis, provide the preliminary components of a maize flowering time genetic network (Fig. 3). In the proposed model, ID1 protein, perhaps in response to intrinsic attributes such as leaf number or assimilate levels, regulates the production or transmission of floral inductive signals in leaves. Transmission of the signal to the shoot apex activates dlf1 either transcriptionally or posttranscriptionally. The model network predicts several targets downstream of dlf1 , including an early target ( x) which feedback regulates dlf1 expression and one or more ZMM MADS-box gene (Muszynski et al., 2006). Because both single id1 and dlf1 mutants and the id1/dlf1 double mutant all flower eventually, an alternate floral induction pathway has been proposed that functions in parallel to the id1-dlf1 pathway (Fig. 3). The components of the alternate pathway are not known, but data supports the idea that it converges with the id1-dlf1 module downstream of dlf1. Likewise, the identity of the MADS-box gene(s) downstream of dlf1 has not been unambiguously determined, but recent studies point to ZMM4 and ZMM15 as likely candidates for maize floral meristem identity genes (Danilevskaya et al., 2008). In fact, ZMM4 and ZMM15 cluster as the closest maize homologs within the Arabidopsis FRUITFUL ( FUL) clade (Malcomber et al., 2006). In Arabidopsis, FUL, along with its paralogs CALIFLOWER (CAL) and APETALA1 (AP1) have redundant roles in specifying floral meristem identity downstream of the floral activators FD and FLOWER LOCUS T (FT) (Abe et al., 2005; Wigge et al., 2005). The extent of overlap versus distinctiveness between the Arabidopsis and maize floral networks is a fruitful area for future research.
Model comparing key floral induction and floral meristem identity genes in maize, rice and Arabidopsis. The circadian clock in Arabidopsis and rice drives signaling under inductive photoperiods to activate key leaf-expressed flowering time genes (GI and CO or OsGI and Hd1), which in turn activate expression of genes encoding the mobile floral stimulus (FT or Hd3a). Genes downstream of the circadian clock are unknown in Teosinte and designated by (?). The mobile floral stimulus protein transits from leaves through the phloem (red dotted line) to the shoot apex where it interacts with apex-expressed floral induction genes (FD or OsDlf1?) to activate expression of floral meristem identity MADS-box genes (AP1/CAL/FUL or OsMADS14). In day-neutral (DN) maize, a leaf-derived autonomous signal activates id1; the ID1 protein regulates the transmission or production of a mobile “florigenic factor” (F). The relationship between F and ZCN8, a possible maize FT ortholog, is not known. Signals downstream of F are transmitted to the shoot apex and regulate dlf1 expression or DLF1 activity. Interaction of DLF1 and ZCN8 presumably activates downstream targets x and the ZMM MADS-box floral identity genes. Redundant inductive signaling through an id1-independent alternate pathway converges downstream of dlf1 to activate x and the ZMM MADS genes too. The position of genes marked by (?) is speculative and the alternate pathway (blue dotted arrows) is hypothetical
4.4 Molecular Components of Maize Florigenic Signals
Recent studies suggest that the RAF kinase inhibitor-like FT gene of Arabidopsis and its co-orthologs in rice ( Heading date3a, Hd3a) and cucurbits comprise a key mobile component of the leaf-derived floral stimulus in these species (Corbesier et al., 2007; Lin et al., 2007; Tamaki et al., 2007). Thus the long sought molecular identity of the phloem-mobile florigen may be partially solved. The Arabidopsis FT gene product, activated by the circadian-controlled CONSTANS (CO) transcription factor in the leaf, has been shown to migrate from the leaf via the phloem to the shoot apex where it interacts with the FD transcription factor. The FT-FD complex then directly activates downstream floral meristem identity genes that mediate flowering (Fig. 3). Whether a similar CO-FT regulatory module exists in maize has not been shown. Similarly, a mobile maize FT-related protein has not been described. However, the maize genome does contain at least 25 FT-like and related paralogous TERMINAL FLOWER (TFL)-like genes, designated ZCN (for Zea mays CENTRORADIALIS), that could encode candidates for a conserved florigenic protein (Danilevskya et al., 2008, Plant Physiology, in press). Determining which family member(s) participate in florigenic signaling will require functional analysis of each gene but will be crucial for further elaboration of the maize genetic flowering time network.
One piece of the maize florigen puzzle that remains to be solved is the role of id1 in controlling maize flowering. Like CO, id1 is expressed and acts in leaves, specifically in immature, developing leaves (Colasanti et al., 1998). Therefore the ID1 transcription factor is believed to regulate the synthesis or facilitate the transmission of a leaf-derived mobile floral stimulus. In one scenario, ID1 may directly regulate expression of the maize equivalent of Arabidopsis FT; a possible candidate is the FT-related ZCN8 gene (Fig. 3) (Danilevskya et al., 2007, Plant Physiology, in press). Alternatively ID1 may control the synthesis or movement of a yet-to-be-identified leaf-based florigenic factor (“F” in Fig. 3). Evidence suggests that the ID1 protein itself is localized and acts in the leaf and does not migrate from leaf to apex (Wong and Colasanti, 2007). Further, although id1 defines a moderately sized zinc finger gene family found in all higher plants, the absence of a clear functional id1 equivalent in distantly related plants suggests that id1 may have a unique role in controlling flowering in maize and perhaps closely related species, such as sorghum and rice (Colasanti et al., 2006). Expression profiling of the molecular differences between normal maize and late-flowering id1 mutants revealed a small number of downstream target genes that could be associated with long distance signaling (Coneva et al., 2007). One intriguing finding of this study is that a large proportion of the differentially expressed genes in id1 mutants have roles associated with photosynthesis and C4 carbon assimilation. This preliminary finding suggests a possible link between assimilate partitioning and floral induction in maize. Further research into this connection, and the identification of direct targets of id1 should shed more light on a flowering time regulatory network that may be unique to maize.
Extensive expression profiling, along with the release of a completed maize genome sequence will allow the identification of all putative flowering time genes from diverse plant species. This information, in combination with reverse genetics and refined QTL analysis, will eventually lead to a comprehensive understanding of the molecular components controlling maize flowering.
5 Future and Perspectives
Understanding the mechanisms that control the transition to flowering in maize may provide a glimpse into the evolution of a new regulatory function. The extremely rapid evolution of modern temperate maize from tropical teosinte necessitated selection for flowering under non-inductive photoperiods that maize progenitors depend on to induce flowering. Current molecular evidence suggests that maize utilizes components of the floral transition regulatory pathway common to other plant species, but also may have developed a regulatory pathway that is unique to maize and perhaps other agronomically important grasses. The transition to flowering is a central event in the life of all higher plants; hence there are probably many inputs from both environmental and endogenous signals that can be co-opted to optimize flowering time. Most likely both endogenous (autonomous) and environmental mechanisms exist in all plants, and the balance can shift, depending more on one pathway or the other, conditioned by the geographic location and climate where a plant grows. Therefore, studies of maize flowering should reveal key pieces to the puzzle of what cause plants to flower that may not be apparent from studies of other model plants. Thus, elucidating the maize flowering gene network will be fundamental to translating knowledge from model systems to plants of economic importance.
In a more practical sense, understanding the molecular mechanisms underlying the control of flowering can be directly applied to improve crop productivity. Maize breeders select for maturities exquisitely adapted to different geographical locations to flower as late as possible, but early enough to assure that yield is maximized. Inbreds developed for one area of adaptation are rarely used in another due to limitations of flowering, grain fill or kernel maturation. Thus, breeding between inbreds with different maturities is uncommon, leading to a reduction in germplasm diversity and impeding the transfer of superior alleles to new lines. A comprehensive understanding of the genetic determinants regulating flowering would enable breeders to manipulate maturity through molecular breeding or transgenic methods and in this way increase the diversity of germplasm utilized in a selection program.
The intimate connection between flowering time and assimilate partitioning has a direct impact on maize yield. However, a plant that flowers too early produces fewer leaves and thus has less ability to capture sunlight and produce assimilates. It may be possible to characterize genes that control the rate of leaf production in order to develop maize that initiates leaves more quickly before flowering. A comprehensive understanding of the genetic and molecular mechanisms underlying flowering could lead to accentuated breeding where flowering time can be further fine-tuned to maximize yield. Further, a more sophisticated biotechnological approach could involve the creation of maize harboring floral transition genes under the control of promoters that allow the farmer to apply an external stimulus to promote or retard flowering in response to unforeseen but imminent abiotic stresses such as drought or prolonged cold periods that can have a severe impact on yield. Another future challenge will be to uncouple the floral transition from assimilate redirection to prolong the grain fill period. In any case, being able to adjust flowering to maximize yield would make maize an even better food, feed and fuel source.
References
Abe, M., Kobayashi, Y., Yamamoto, S., Daimon, Y., Yamaguchi, A., Ikeda, Y., Ichinoki, H., Notaguchi, M., Goto, K., and Araki, T. (2005). FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex . Science 309, 1052–1056.
Andrews, C.J., Dwyer, L.M., Stewart, D.W., Dugas, J.A., and Bonn, P. (2000) . Distribution of carbohydrate during grain-fill in leafy and normal maize hybrids . Can J Plant Sci 80, 87–95.
Aukerman, M.J., and Sakai, H. (2003). Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes . Plant Cell 15, 2730–2741.
Austin, D.F., Lee, M., and Veldboom, L.R. (2001). Genetic mapping in maize with hybrid progeny across testers and generations: Plant height and flowering . TAG Theor Appl Genet 102, 163–176.
Beadle, G.W. (1939). Teosinte and the origin of maize. J Hered 30, 245–247.
Bernier, G., and Perilleux, C. (2005). A physiological overview of the genetics of flowering time control. Plant Biotechnol J 3, 3–16.
Bomblies, K., and Doebley, J.F. (2006). Pleiotropic effects of the duplicate maize FLORICAULA/ LEAFY genes zfl1 and zfl2 on traits under selection during maize domestication . Genetics 172, 519–531.
Camus-Kulandaivelu, L., Veyrieras, J.-B., Madur, D., Combes, V., Fourmann, M., Barraud, S., Dubreuil, P., Gouesnard, B., Manicacci, D., and Charcosset, A. (2006). Maize adaptation to temperate climate: Relationship between population structure and polymorphism in the Dwarf8 gene. Genetics 172, 2449–2463.
Chardon, F., Virlon, B., Moreau, L., Falque, M., Joets, J., Decousset, L., Murigneux, A., and Charcosset, A. (2004). Genetic architecture of flowering time in maize as inferred from quantitative trait loci meta-analysis and synteny conservation with the rice genome . Genetics 168, 2169–2185.
Chardon, F., Hourcade, D., Combes, V., and Charcosset, A. (2005). Mapping of a spontaneous mutation for early flowering time in maize highlights contrasting allelic series at two-linked QTL on chromosome 8 . Theor Appl Genet 112, 1–11.
Colasanti, J., Yuan, Z., and Sundaresan, V. (1998). The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize . Cell 93, 593–603.
Colasanti, J., Tremblay, R., Wong, A.Y.M., Coneva, V., Kozaki, A., and Mable, B.K. (2006). The maize INDETERMINATE1 flowering time regulator defines a highly conserved zinc finger protein family in higher plants . BMC Genomics 7, 1–15.
Coneva, V. , Zhu, T., and Colasanti, J. (2007). Expression differences between normal and indeter-minate1 maize suggest downstream targets of ID1, a floral transition regulator in maize. Journal of Experimental Botany, 58, 3679–3693.
Corbesier, L., Lejeune, P., and Bernier, G. (1998). The role of carbohydrates in the induction of flowering in Arabidopsis thaliana: Comparison between the wild type and a starchless mutant . Planta (Berlin) 206, 131–137.
Corbesier, L., Vincent, C., Jang, S., Fornara, F., Fan, Q., Searle, I., Giakountis, A., Farrona, S., Gissot, L., Turnbull, C., and Coupland, G. (2007) . FT protein movement contributes to longdistance signaling in floral induction of Arabidopsis. Science 316, 1030–1033.
Costa, C., Dwyer, L.M., Stewart, D.W., and Smith, D.L. (2002). Nitrogen effects on grain yield and yield components of leafy and nonleafy maize genotypes . Crop Sci 42, 1556–1563.
Danilevskaya, O.N., Meng, X., Hou, Z., Ananiev, E.V., Simmons, C.R. (2008). A Genomic and Expression Compendium of the Expanded PEBP Gene Family from Maize. Plant Physiol. 146, 250–264.
Danilevskaya, O.N., Meng, X., Selinger, D.A., Deschamps, S., Hermon, P. et al. (2008). Involvement of the MADS-Box Gene ZMM4 in Floral Induction and Inflorescence Development in Maize. Plant Physiol. 147, 2054–2069.
Dijak, M., Modarres, A.M., Hamilton, R.I., Dwyer, L.M., Stewart, D.W., Mather, D.E., and Smith, D.L. (1999). Leafy reduced-stature maize hybrids for short-season environments. Crop Sci 39 1106–1110.
Doebley, J. (2004). The genetics of maize evolution. Annu Rev Genet 38, 37–59.
Emerson, R.A. (1924). Control of flowering in teosinte. J Hered 15, 41–48.
Galinat, W.C., and Naylor, A.W. (1951). Relation of photoperiod to inflorescence proliferation in Zea mays L . Am J Bot 38, 38–47.
Garner, W.W., and Allard, H.A. (1920). Effect of the relative effect of day and night and other factors of the environment on growth and reproduction in plants . J Agric Res 18, 553–606.
Irish, E., and Jegla, D. (1997). Regulation of extent of vegetative development of the maize shoot meristem. Plant J 11, 63–71.
Irish, E.E., and Nelson, T.M. (1991). Identification of multiple stages in the conversion of maize meristems from vegetative to floral development . Dev 112, 891–898.
Kozaki, A., Hake, S., and Colasanti, J. (2004). The maize ID1 flowering time regulator is a zinc finger protein with novel DNA binding properties . Nucleic Acids Res 32, 1710–1720.
Lin, M.-K., Belanger, H., Lee, Y.-J., Varkonyi-Gasic, E., Taoka, K.-I., Miura, E., Xoconostle-Cazares, B., Gendler, K., Jorgensen, R.A., Phinney, B., Lough, T.J., and Lucas, W.J. (2007). FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the cucurbits. Plant Cell 19, 1488–1506.
Malcomber, S.T., Preston, J.C., Reinheimer, R., Kossuth, J., and Kellogg, E.A. (2006). Developmental gene evolution and the origin of grass inflorescence diversity . Adv Bot Res 44 426–481.
Muszynski, M.G., Dam, T., Li, B., Shirbroun, D.M., Hou, Z., Bruggemann, E., Archibald, R., Ananiev, E.V., and Danilevskaya, O.N. (2006). Delayed flowering1 encodes a basic Leucine Zipper protein that mediates floral inductive signals at the shoot apex in maize . Plant Physiol 142, 1523–1536.
Neuffer, M.G., Coe, E.H., and Wessler, S.R. (1997). Mutants of Maize. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press .
Ohto, M., Onai, K., Furukawa, Y., Aoki, E., Araki, T., and Nakamura, K. (2001). Effects of sugar on vegetative development and floral transition in Arabidopsis . Plant Physiol 127, 252–261.
Poethig, R.S. (1990). Phase-change and the regulation of shoot morphogenesis in plants. Science 250, 923–930.
Salvi, S., Tuberosa, R., Chiapparino, E., Maccaferri, M., Veillet, S., van Beuningen, L., Isaac, P., Edwards, K., and Phillips, R.L. (2002). Toward positional cloning of Vgt1, a QTL controlling the transition from the vegetative to the reproductive phase in maize . Plant Mol Biol 48, 601–613.
Salvi, S., Sponza, G., Morgante, M., Tomes, D., Niu, X., Fengler, K.A., Meeley, R., Ananiev, E.V., Svitashev, S., Bruggemann, E., Li, B., Hainey, C.F., Radovic, S., Zaina, G., Rafalski, J.A., Tingey, S.V., Miao, G.-H., Phillips, R.L., and Tuberosa, R. (2007). Conserved noncoding genomic sequences associated with a flowering-time quantitative trait locus in maize . PNAS 104, 11376–11381.
Shaver, D.L. (1983). Genetics and breeding of maize with extra leaves above the ear. In Proceedings of the 38th Annual Corn and Sorghum Industry Research Conference (Chicago, IL: American Seed Trade Association, Washington, DC), pp. 161–180.
Sheehan, M.J., Kennedy, L.M., Costich, D.E., and Brutnell, T.P. (2007). Subfunctionalization of PhyB1 and PhyB2 in the control of seedling and mature plant traits in maize . Plant J 49 338–353.
Singleton, W.R. (1946). Inheritance of indeterminate growth in maize. J Hered 37, 61–64.
Subedi, K.D., and Ma, B.L. (2005a). Ear position, leaf area, and contribution of individual leaves to grain yield in conventional and leafy maize hybrids . Crop Sci 45, 2246–2257.
Subedi, K.D., and Ma, B.L. (2005b). Nitrogen uptake and partitioning in stay-green and leafy maize hybrids. Crop Sci 45, 740–747.
Subedi, K.D., Ma, B.L., and Smith, D.L. (2006). Response of a leafy and non-leafy maize hybrid to population densities and fertilizer nitrogen levels . Crop Sci 46, 1860–1869.
Tamaki, S., Matsuo, S., Wong, H.L., Yokoi, S., and Shimamoto, K. (2007). Hd3a protein is a mobile flowering signal in rice. Science 316, 1033–1036.
Thornsberry, J.M., Goodman, M.M., Doebley, J., Kresovich, S., Nielsen, D., and Buckler, E.S. (2001). Dwarf8 polymorphisms associate with variation in flowering time . Nat Genet 28, 286–289.
Tollenaar, M., and Hunter, R.B. (1983). A photoperiod and temperature sensitive period for leaf number of maize . Crop Sci 23, 457–460.
Veldboom, L.R., Lee, M., and Woodman, W.L. (1994). Molecular marker-facilitated studies in an elite maize population: I . Linkage analysis and determination of QTL for morphological traits. TAG Theor Appl Genet 88, 7–16.
Vladutu, C., McLaughlin, J., and Phillips, R.L. (1999). Fine mapping and characterization of linked quantitative trait loci involved in the transition of the maize apical meristem from vegetative to generative structures . Genetics 153, 993–1007.
Wigge, P.A., Kim, M.C., Jaeger, K.E., Busch, W., Schmid, M., Lohmann, J.U., and Weigel, D. (2005) . Integration of spatial and temporal information during floral induction in Arabidopsis . Science 309, 1056–1059.
Wong, A.Y.M., and Colasanti, J. (2007). Maize floral regulator protein INDETERMINATE1 is localized to developing leaves and is not altered by light or the sink/source transition . J Exp Bot 58, 403–414.
Zhang, Y.-M., Mao, Y., Xie, C., Smith, H., Luo, L., and Xu, S. (2005). Mapping quantitative trait loci using naturally occurring genetic variance among commercial inbred lines of maize (Zea mays L.). Genetics 169, 2267–2275.
Acknowledgments
We thank Mei Guo, Olga Danilevskaya and Evgueni Ananiev for meristem images and Olga Danilevskaya and Carl Simmons for sharing data prior to publication.
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer Science + Business Media, LLC
About this chapter
Cite this chapter
Colasanti, J., Muszynski, M. (2009). The Maize Floral Transition. In: Bennetzen, J.L., Hake, S.C. (eds) Handbook of Maize: Its Biology. Springer, New York, NY. https://doi.org/10.1007/978-0-387-79418-1_3
Download citation
DOI: https://doi.org/10.1007/978-0-387-79418-1_3
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-79417-4
Online ISBN: 978-0-387-79418-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)