Abstract
Acute myeloid leukemia (AML) is the most common form of leukemia in adults, and despite some recent progress in understanding the biology of the disease, AML remains the leading cause of leukemia-related deaths in adults and children. AML is a complex and heterogeneous disease, often involving multiple genetic defects that promote leukemic transformation and drug resistance. The cooperativity model suggests that an initial genetic event leads to maturational arrest in a myeloid progenitor cell, and subsequent genetic events induce proliferation and block apoptosis. Together, these genetic abnormalities lead to clonal expansion and frank leukemia. The purpose of this chapter is to review the biology of receptor tyrosine kinases (RTKs) in AML, exploring how RTKs are being used as novel prognostic factors and potential therapeutic targets.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute Myeloid Leukemia
- Small Molecule Inhibitor
- Acute Myeloid Leukemia Patient
- Acute Myeloid Leukemia Cell
- Internal Tandem Duplication
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Acute myeloid leukemia (AML) is the most common form of leukemia in adults, and despite some recent progress in understanding the biology of the disease, AML remains the leading cause of leukemia-related deaths in adults and children [132] AML is a complex and heterogeneous disease, often involving multiple genetic defects that promote leukemic transformation and drug resistance. The co-operativity model suggests that an initial genetic event leads to maturational arrest in a myeloid progenitor cell, and subsequent genetic events induce proliferation and block apoptosis. Together, these genetic abnormalities lead to clonal expansion and frank leukemia [64, 170, 127, 176].
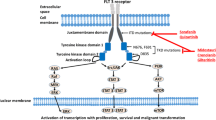
Mutations in receptor tyrosine kinases (RTKs) or their downstream effectors are extremely common in AML, with estimated 40–60% of AML patients harboring a mutation abnormality in RTKs [144, 89, 45, 49]. In addition, another 15–25% of AML patients will have a mutation in one of the downstream effectors in RTK pathways [144, 168, 113, 69, 27]. More than 50 different RTKs have been identified, which are classified into 20 subfamilies based on their structural and functional characteristics (reviewed in Ref. [89]) [14, 38, 120]. A meticulous examination of all the different RTKs has yet to be performed, but such studies are underway. These studies will most likely discover that an even greater percentage of AML patients harbor mutations or abnormalities in RTK pathways. Although several RTKs have been implicated in malignancies, the vast majority of RTK mutations in AML, thus far, have been found in the RTK subclass III family (a.k.a. the PDGFR family) [120, 3, 124]. The subclass III RTK family consists of FLT3, KIT, PDGFRA, PDGFRB, and CSF1R, and the majority of this review will examine the biology, prognosis, and potential therapeutic targets of these RTKs, focusing on FLT3 and KIT mutations, which are by far the most common RTKs known to be affected in AML. The role of CSFR1 (a.k.a. C-FMS) and PDGFR in myeloid malignancies will also be briefly discussed. In addition, we will assess the growing interest in small molecule inhibitors against RTKs as potential therapeutic targets for AML.
Receptor Tyrosine Kinase Activation and Downstream Effectors
RTKs play a critical role in myeloid proliferation, differentiation, and apoptosis [115, 167, 126, 44, 133, 97, 102, 99, 93]. Structurally, subclass III RTKs consist of an extracellular ligand-binding (E) domain, transmembrane (TM) dimerization domain, juxtamembrane (JM) domain, and an intracellular tyrosine kinase domain (Fig. 1A). In their inactive state, RTKs exist primarily as monomers, and multiple autoinhibitory or intrinsic repressive forces prevent dimerization (a.k.a. receptor activation) [52, 123]. While in the monomeric state, the RTK displays a “closed” conformation, which prevents easy phosphorylation of specific tyrosine residues within the intercellular domains and limits inappropriate activation (Fig. 1A). Activation begins when a ligand binds to the extracellular domain (or domains), causing a conformational change in the receptor. The new conformation reverses the intrinsic repulsive forces of the receptor, promoting dimerization with either itself or other membrane-bound RTKs (Fig. 1B). Together, these changes lead to an “open” confirmation of the receptor, which facilitates the transfer of a phosphate from ATP to the tyrosine substrate within the intracellular kinase domain (Fig. 1B). This activated conformational change and/or phosphorylated tyrosine also promote the docking of adaptor proteins (e.g., SHC), which also become activated. The activated adapter proteins then interact with downstream effectors, efficiently transmitting the extracellular signal to the appropriate intracellular pathways (Fig. 2). After activation, RTKs are rapidly internalized and degraded, such that within 20 min the signal will start to dissipate [178, 163]. This rapid degradation and downregulation of the receptor contributes to the tightly controlled signaling activity of RTKs.
Subclass III tyrosine kinase receptor structure and activation. (A) Inactive receptor. Subclass III RTKs consist of an extracellular ligand-binding domain (E), transmembrane dimerization domain (TM), juxtamembrane domain (JM), and an intracellular tyrosine kinase domain (K). In their inactive state, RTKs exist primarily as monomers, and multiple autoinhibitory or intrinsic repressive forces prevent dimerization (R). Note the “closed” conformation or the tyrosine kinase domain. (B) Activated receptor. RTKs become activated when a ligand (L) binds to their extracellular domain (E) causing a conformational change in the receptor. These changes lead to an “open” confirmation of the intracellular tyrosine kinase domain (K), which facilitates the transfer of phosphate (P) to the tyrosine kinase domain. This activated conformational change and/or phosphorylated tyrosine also promote the docking of adaptor proteins (A), which are then activated. The activated adapter proteins facilitate transmission of the extracellular signal to the appropriate intracellular pathways
Tyrosine kinase receptor pathway. Binding of a ligand (L) to tyrosine kinase receptor activates multiple downstream effectors, including the PI3K (phosphatidylinositol 3’ kinase) and RAS pathways. Solid arrows are more direct interactions, while broken arrows represent associations that probably involve multiple steps between the proteins. The activated RTK interacts with multiple adapter proteins: SH2-containing sequence proteins (SHC), SH2-containing inositol phosphatase (SHIP), GRB2, and others, which connect the RTK to the PI3K and RAS pathways. RAS activation stimulates the MAPK kinase pathway: RAF, MAPK/ERK kinases (MEK), extracellular-signal-regulated kinase kinases (ERK), and 90-kDa ribosomal protein S6 kinase (RSK). These downstream effectors activate cyclic adenosine monophosphate-response element-binding protein (CREB), ELK, and signal transducer and activators of transcription (STATs), which lead to transcription of specific genes that promote proliferation. Activated PI3K stimulates protein kinase B (PKB/AKT) and other members of the PI3K pathway (e.g., rapamycin or mTOR), which promotes translation. In addition, activated PI3K induces phosphorylation of the pro-apoptotic BCL2 family protein (BAD), which blocks apoptosis by binding BCL2. Both pathways probably also interact with each other and other pathways/effectors such as BRCA1, p21WAF1, and p27KIP1
Several different types of mutations (missense point mutations, deletions, insertions, and internal tandem duplications) in AML cells have been described in the different RTKs (mainly FLT3 and c-KIT, Fig. 3) [144, 89, 45, 49, 69, 101, 10]. Despite the different types of mutations, the RTK sequences tend to remain in frame, ensuring translation of the entire protein. These mutations have been found to be most frequent in JM domain or tyrosine kinase domain and will be described in more detail under the specific subclass RTKs; nevertheless, there are two general themes to these mutations. Those mutations involving the JM domain tend to be large insertions, which probably disrupt the intrinsic repulsive forces that naturally prevent dimerization. However, recently, small point mutations and deletions have been described in the JM domain of FLT3 [146]. Once these repulsive forces are disrupted, ligand-independent activation occurs. Whether the different types and sizes of mutations in the JM domain have a unique biological and clinical significance is currently being investigated [145, 116]. The other domain of RTKs that is frequently mutated is that of the tyrosine kinase domain (or activation loop domain) [175, 1, 160, 42, 140, 67]. Missense point mutations in the tyrosine kinase domain (a.k.a. TKD mutations or FLT3/ALM) also constitutively activate the receptor. Similar to JM mutations, TKD mutations also activate downstream effectors, inappropriately stimulating pathways that are critical in the normal regulation of differentiation, proliferation, and apoptosis [175, 1].
Mutations in tyrosine kinase receptor. Several different types of mutations have been described in tyrosine kinase receptors. The vast majority of these mutations are either in the juxtamembrane (JM) domain or in the tyrosine kinase domain (TKD). Internal tandem duplications (bright green) are the most common type of mutations in JM domain (star), while point mutations (bright blue) are the most common type of mutations in the TKD. Other labels include the extracellular (E) and transmembrane (TM) domains, plasma membrane (PM), extracellular space (ES), and intracellular cytosol (IC)
In addition to RTK mutations, mutations in downstream effectors within RTK pathways may also play a critical role in leukemogenesis. For example, RAS, a component of many RTK pathways, is mutated in approximately 20–25% of AML patients – usually either NRAS or KRAS [144, 89, 113, 35, 26, 104, 84, 17, 22, 119], and mutations in other members of the RTK pathways, such as PTNP11, have also been discovered in AML patients [27, 119, 138, 12]. Besides activating the RTK pathway, RTK mutations inappropriately activate other pathways such as JAK/STAT pathway [151, 54, 159, 13]. Recently, the JAK/STAT pathway has received attention due to novel small molecule inhibitors that make it a potential therapeutic target for several malignancies [95, 171, 174]. Whether it is the constitutive activation of the receptor by an RTK mutation [144, 89, 45, 49, 69, 101, 74, 2, 152, 130, 146, 175, 1, 19, 21, 9, 11, 24, 134, 129, 10, 160, 42, 140, 67, 143, 163, 117, 109], an autocrine/paracrine stimulation of the receptor by a ligand secreting tumor (e.g., VEGF) [161, 4, 48], or the activation of the downstream effectors [27, 119, 138, 12, 151, 54, 159, 13, 181, 96, 15, 76], inappropriate activation of RTK pathways directly contribute to pathogenesis of AML, progression of the disease, and its resistance to chemotherapy.
FLT3 Mutations
As FLT3 mutations have been implicated in the prognosis of AML, FLT3 mutations remain one of the most common genetic abnormalities in AML identified thus far [144, 89, 69, 101, 74, 2, 152, 130, 146, 175, 1, 145, 22, 157, 179, 59, 73, 37, 41]. As described above, FLT3 mutations occur within two specific regions within the FLT3 gene (juxtamembrane domain and tyrosine kinase domain). The most common type of a FLT3 mutation is that of internal tandem duplication (FLT3/ITD) in the JM domain, which occurs in 15–35% of AML patients [144, 89, 69, 74, 152, 130, 75, 90]. FLT3/ITDs are rare in infant AML, where only approximately 1% of children <1 year harbor a FLT3/ITD, but steadily increases with aging, such that 10–15% of pediatric and 20–35% of adult AML patients have FLT3/ITDs [144, 89, 69, 74, 152, 130, 75, 90]. FLT3/ITDs cause ligand-independent dimerization and autophosphorylation of the receptor, leading to constitutive activation of the FLT3 and many downstream effectors (SHC, RAS, ERK, AKT, and STAT5) [151, 54, 88, 71, 70, 94]. Besides FLT3/ITDs, smaller insertions, deletions, and missense point mutations have recently been described in the JM of AML patients [146]. These mutations are relatively rare, occurring in less than 5% of patients [146]. Although the clinical significance of these “non-ITD” mutations in the JM (a.k.a. FLT3-JM-PM) are currently unknown, Reindl et al. recently found that FLT3-JM-PMs promoted ligand-independent dimerization, autophosphorylation, and constitutive activation of the receptor; however, the activation seemed to be “weaker” than compared to classic FLT3/ITD transduced cells, as indicated by a lower level of phosphorylation of the receptor and less activation of downstream STAT5 in cells transduced with FLT3-JM-PM [116].
FLT3/ITDs are associated with rapid disease progression and resistance to conventional therapy [69, 74, 152, 130, 75]. Initial studies demonstrated a strong prognostic significance for the presence of FLT3/ITD, such that patients with this mutation had an extremely poor clinical outcome compared to the patients without FLT3/ITD [69, 75]. In more recent studies using contemporary chemotherapy, the prognostic significance of FLT3/ITD has been less dramatic, with an overall survival of approximately 30% for the FLT3/ITD population compared to 45% to that of patients without FLT3/ITD [74, 152]. However, these studies also identified a subclass of patients with FTL3/ITD, in which the mutant ITD to wild-type allele (ITD allelic ratio or ITD-AR) correlates with clinical outcome. ITD-ARs vary significantly from patient to patient, and this difference may have clinical implications [152, 18, 173]. For example, some AML patients have a predominantly mutant ITD product with little or no normal product (high ITD-AR), whereas others have an equal or higher distribution of normal product (low ITD-AR). The ITD-AR has been used to identify patients with FLT3/ITD at a higher risk of relapse and poor outcome in a number of clinical trials [152, 18, 173]. Although ITD-AR may become a critical tool in the risk identification of FLT3/ITD-positive patients, the exact ITD-AR threshold “cut-off” that identifies high-risk patients has not been established. Thiede et al. used ITD-AR threshold of 0.78 to define relapse risk in FLT3/ITD-positive patients [152]. Patients with low allelic ratio (AR ≤ 0.78) had an overall survival of nearly 60% compared to overall survival of 0% in patients with ITD-AR of >0.78 (p = 0.006) [152]. They also suggested that ITD allelic ratio may be a continuous variable, as changing the ITD-AR cut-off altered the clinical outcome. Similar findings were shown in a pediatric AML population from European cooperative studies. Using the ITD-AR median, Zwaan et al. demonstrated that patients with high ITD-AR (ITD-AR > median of 0.69) had a poor outcome, whereas the outcome for those with low ITD-AR (ITD-AR ≤ 0.69) was no different than patients without FLT3/ITD [173]. More recent analysis of ITD-AR in a cohort of 630 children treated in CCG-2941/2961 has revealed that ITD-AR of 0.4 can identify the highest proportion of FLT3/ITD-positive patients at high risk of relapse (personal communication by Meshinchi, submitted for publication). Underlying mechanisms for the allelic ratio variation is under study. We and others have presented data in support of loss of heterozygosity (LOH) in chromosome 13q12 as a possible mechanism for high ITD-AR in some of the AML patients [75, 18]. Other studies, however, have failed to demonstrate LOH in patients with high ITD-AR [152], suggesting that there may be additional factors responsible for variation in the ITD-AR.
Recently, we demonstrated that size of duplicated region in FLT3/ITD-positive AML may have prognostic significance. In a study of adult 151 AML patients treated, those AML patients with larger ITDs had a significantly higher relapse compared to those with smaller ITDs [145]. Ongoing studies in other populations are underway, but this data would reinforce the hypothesis that different sizes and types of mutations in the JM may have unique biological and clinical significance, adding yet another layer of complexity to the use of FLT3/ITDs as a prognostic marker [145, 116, 182].
FLT3 mutations in activation loop of the tyrosine kinase (FLT3/ALM) occur in approximately 5–10% of AML patients [89, 175, 1, 140] making FLT3/ALM the second most common type of FLT3 mutation. Like FLT3/ITDs, FLT3/ALM constitutively activates the FLT3 receptor and downstream effectors [175, 140, 67, 151, 54, 13, 73, 88, 71]. However, it remains to be determined whether FLT3/ITDs and FLT3/ALMs have similar biological consequences. Recent evidence would suggest that mutations in JM and TKD cause biologically different responses. When Grundler et al. transduced mice marrow with FLT3/ITDs, the mice developed the classic myeloproliferative syndrome, which had previously been described in other FLT3/ITD transduction murine models [147, 53]. However, mice transduced with FLT3/ALM developed an oligoclonal lymphoid disorder [147]. These data argue against the hypothesis that the biological consequences of FLT3/ITDs and FLT3/ALMs are the same. In addition, global RNA expression studies have found distinct expression patterns between FLT3/ITDs and FLT3/ALMs, which readily differentiate the two types of mutations, providing additional evidence that suggest biological differences between the two types of FLT3-activating mutations [65]. When one examines the clinical significance of FLT3/ALM, there appear to be clinical differences between patients with FLT3/ITDs and FLT3/AMLs. Unlike FLT3/ITDs, the frequency of FLT3/ALMs does not vary with age. In addition, available data suggest that patients with FLT3/ALM have a lower diagnostic white count, higher remission rates, lower relapse rate, and better overall survival than patients with FLT3/ITD [89, 152, 175, 78]. However, it must be noted that the frequency of FLT3/ALMs is considerably less than FLT3/ITDs, making it more difficult to obtain significant power to convincingly rule out a possible clinical significance for these mutations. Also, there have been fewer studies examining the clinical significance of FLT3/ALMs, since these mutations were first identified in 2001, which is approximately 5 years after FLT3/ITDs were discovered [101, 175, 1, 140].
Together, the data suggest that FLT3/ITDs, FLT3/ALM, and FLT3/JM/PM all promote constitutive activation of the receptor, but probably have biological differences that impact their prognostic significance. Therefore, in evaluating the prognostic relevance of FLT3-activating mutations, one must keep in mind not only the presence or absence of a mutation but also location, type, and allelic ratio of the mutation. The identification of a high-risk population in FLT3/ITD-positive patients is of great importance, as it may identify a significant number of patients who are destined for poor outcome and may benefit from alternative treatments such as early hematopoietic stem cell transplant. The ITD-AR or ITD size may provide additional tools to better risk-stratify AML patients. Besides understanding how these different FLT3 mutations respond to chemotherapy, it will be critical to determine how these different mutations impact the responsiveness to small molecule inhibitors. However, at this time, investigators are left with trying to better risk-stratify AML patients based on a variety of poorly understood surrogate FLT3 prognostic markers.
KIT Mutations
Activating mutations of KIT receptor gene have been reported in a variety of myeloid malignancies. Early studies implicated KIT mutations in the pathogenesis of mastocytosis [42, 87]; however, recent studies have found that these mutations may also be involved in the pathogenesis of AML, especially those with t(8;21) or inv(16) [170, 176, 89, 49, 27, 19, 24, 134, 129, 10, 85, 23]. Activating mutations in KIT receptor gene usually occur in either the JM domain, which regulates receptor dimerization, or the intracellular tyrosine kinase domain of the receptor gene. Mutations in both regions lead to constitutive activation of the KIT receptor [160, 42, 103, 68, 162].
KIT mutations have been reported in 3–15% of adult and pediatric AML [49, 24, 85] and in significantly higher proportion of those with t(8;21) or inv(16), where KIT mutations were observed in nearly 40–50% of AML involving the core-binding factor (CBF) [49]. Data suggest that KIT-activating mutations may co-operate with t(8;21) translocation to contribute to myeloid leukemogenesis, where KIT mutation leads to proliferative advantage in cells which have undergone maturation arrest due to t(8;21) translocations [170]. Although presence of KIT mutations may not display prognostic significance in AML patients at large, their presence may have prognostic and possible therapeutic implications in AML patients involving CBF [49, 19, 21, 134]. Cairoli et al. evaluated 67 patients with t(8;21) or inv(16) and demonstrated that 46% of the AML patients harbored a missense, insertion, deletion, or internal tandem duplication mutations in either exon 8, 11, or 17 of the KIT receptor gene [19, 164]. Missense mutations in the tyrosine kinase domain (TKD) of the receptor (D816) was the most common mutation observed, where 20/67 patients (29%) had D816 missense mutations. Correlation of the D816 mutation in KIT with clinical outcome demonstrated that those patients with D816 mutations had a significantly higher relapse rate and worse survival. A similar study reported an adverse outcome for pediatric AML patients, in which these investigators identified mutations in the KIT TKD in 17% of pediatric AML patients with t(8;21) [134]. In contrast, Care et al. found more prognostic significance for KIT mutations in exon 8 in CBF leukemias harboring Inv16, in which 24% of patients with Inv16 had a mutation in exon 8 [24]. Besides the potential prognostic implication of these mutations, KIT mutations may have therapeutic implications, as there are data to suggest that primary leukemic cells that harbor some forms of KIT mutations may be susceptible to pro-apoptotic effects of Imatinib Mesylate [77], thus providing a therapeutic modality for some AML patients with CBF abnormalities at high risk of relapse.
Other RTKs, c-FMS, and PDGFR
Activating mutations in CSF1R (a.k.a. c-FMS) were initially described in myeloid cell lines, MDS, and AML, having an estimated frequency of 2–10% in myeloid malignancies [50, 125, 165, 118, 153]; however, a later study in AML patients did not identify any CSF1R mutations in their AML population [32]. The true prevalence of CSF1R mutations remains to be defined, but the importance of this receptor for the development of leukemia should not be underestimated. CSF1R resides on chromosome 5q33-35 and aberrant regulation of the receptor through methylation or loss of heterozygosity may play a critical role in leukemogenesis [141, 16]. If disruption of the CSF1R occurs at an “inappropriate” time during myeloid differentiation, studies have found that it may predispose cells to malignant transformation [36].
With respect to mutations in the PDGFR1α (chromosome 4q11-13) and PDGFR1β (5q31-32) genes, large-scale mutation analyses of the two genes have not been conducted; however, translocations involving PDGFR1β and TEL (a.k.a. ETV6, 12p13) have been identified in chronic myelomonocytic leukemia, activating PDGR1β and promoting malignant transformation [122, 50, 25, 155]. Recently, translocations involving PDGFR1α and FIP1L1 (4q12) have also been found in hypereosinophilic syndrome and chronic eosinophilic leukemia [154, 28, 30, 166, 51], suggesting a possible role for PDGFR1α in malignant transformation.
In addition to the activating mutations at the receptor level, activating mutations of the secondary mediators of the RTKs (e.g., RAS, BRAF genes) have also been reported in AML [144, 113, 27, 138, 96, 29, 60, 100, 121, 135, 86, 40]. Some of these activating mutations, such as those involving RAS, are common, occurring in 10–20% of AML patients. While the clinical significance of these mutations remains uncertain, ongoing studies are investigating how these mutations may co-operate with other genetic abnormalities to promote leukemogenesis and affect prognosis [89, 113, 69].
Small Molecule Inhibitors as Therapeutic Options
Recently, there has been a surge in the development of small molecule inhibitors for myeloid leukemia [111]. Imatinib Mesylate (Gleevec) has proven to be a major advancement for the treatment of chronic myeloid leukemia in chronic phase (CML-CP). Imatinib induces a high percentage of complete cytogenetic remission for patients with CML-CP, and many of these patients have maintained this remission for 3–5 years [33, 58]. The results for Imatinib have been less impressive in more advanced forms of CML (e.g., accelerated or blast phase), in which patients will sometimes obtain responses, but almost universally relapse with resistant disease [5]. Multiple factors, including mutations in the bcr-abl gene, genomic amplification of the bcr-abl, and/or other genetic abnormalities, may account for some of the disparity in the responses between CML-CP and its more advanced counterparts [34]. To counteract the resistance secondary to bcr-abl mutations, newer small molecule inhibitors such as BMS-354825 (a.k.a. Dasatinib) and AMN 107 have been developed that display activity against cells harboring the mutated bcr-abl gene [57, 106]. Although early clinical trials seem promising for these drugs, long-term studies will be necessary to ensure that resistance against these newer small molecule inhibitors does not develop. Unlike CML-CP, AML is a much more heterogeneous disease, as indicated by the variety of different cytogenetic abnormalities and mutations within AML cells. Therefore, the development of a “universal” small molecule inhibitor for AML will be more challenging, if not impossible. Yet, the RTK pathways offer the “potential” for such drug development, and we will briefly describe some of the novel tyrosine kinase inhibitors (TKIs) that have recently been developed that target RTK pathways.
FLT3 Inhibitors
The FLT3 pathway is an obvious target for TKIs. FLT3 mutations are one of the most common mutations in AML. Because FLT3 mutations constitutively activate the receptor’s pathway and contribute to leukemogenesis, small molecule inhibitors that block their activation may have therapeutic benefits for many AML patients. In addition, an increased expression of the wild-type (WT) receptor may also play a role in leukemogenesis for some AML patients [128], suggesting that FLT3 inhibitors may be effective in more than just AML patients with the FLT3 mutations. Initial in vitro studies using non-specific TKI (herbimycin A, AG1296, and AG1295) found that these drugs blocked constitutive activation of FLT3/ITDs and preferentially killed cells harboring FLT3/ITDs [7, 180, 83, 158]. However, all these compounds are highly toxic in humans, initiating searches for more selective and less toxic drugs for clinical use. Through molecular screening, numerous compounds have now been identified (MLN518, PKC412, SU5416, SU5614, SU11248, CEP-701, CEP-5214), and we will briefly describe the progress and limitations of these compounds.
MLN518 (a.k.a. CT53518 from Millennium) has been found to inhibit the activation of FLT3/ITDs and growth potential of cells harboring these mutations [92]. Like many TKIs, MLN518 is not specific for the FLT3/ITD receptor, also inhibiting WT forms of FLT3, PDGF, and KIT. Heinrich et al. published their results of a phase I study for high-risk AML patients in 2002, which found that two of six patients had > 50% reduction in bone blasts [66]. A phase II study evaluated the efficacy of MLN518 in 18 FLT3/ITD-positive AML patients with relapsed or refractory disease that were unfit for conventional chemotherapy. In this study, 6 of 18 patients demonstrated an objective response, as measured by a decrease in the peripheral blood blast by a mean of 92% (range 85–100%) [55]. However, no complete responses (CRs) were achieved.
PKC412 (from Novartis) was initially developed as a VEGF receptor inhibitor, but studies found that this benzoylstaurosporine blocked FLT3, including FLT3 mutated receptors [128, 31]. Armstrong et al. also found that MLL cells over-expressing WT FLT3 were preferentially killed by PKC412 [128,172]. A phase II trial examined the efficacy of PKC412 as a single agent for relapsed or refractory AML patients with poor performance status [6]. The PKC412 was given orally at 75 mg three times a day. Initial results found the drug to be well tolerated, with the most common side effect being nausea. However, no CRs were obtained in this heavily pretreated population. Additional follow-up of this study was recently presented at the American Society of Hematology (ASH) conference. A total of 20 FLT/ITD-positive AML patients with mutant FLT3 with either relapsed/refractory AML or high-grade myelodysplasia were treated with single agent PKC412. The peripheral blood blasts decreased by 50% in 6 of the 20 patients, with 2 responders obtaining a blast percentage of <5%. Again, no CRs were observed in this high-risk population [150] but autophosphorylation of the mutant receptor was blocked in most of the responding patients, indicating an in vivo target response using the dose in the study. Given these results, PKC412 has been combined with daunorubicin and cytarabine for induction of AML patients [148, 46]. In a phase I trial examining this combination approach, investigators recently reported at ASH that those AML patients harboring FLT3/ITDs had a CR rate of 91% (10/11) compared to 53% (17/32) for those without FLT3/ITDs (p = 0.033) [46]. Although not a randomized study, these data suggest that combination therapies adding TKI with chemotherapy may improve remission induction for those AML patients harboring FLT3/ITDs, although its efficacy for improving survival remains to be defined.
Sugen has several drugs under development as potential TKIs (SU5416, SU5614, and SU11248) [38, 149, 105]. Similar to most of the other TKIs, these agents also block the activities of other tyrosine kinase receptors (KIT, PDGFR, and VEGF) [38, 149, 105]. Giles et al. treated 55 patients with refractory or relapsed AML with SU5416 at a dose of 145 mg/m2 [177]. AML patients without FLT3 mutations were also included in the trial. Grade 3/4 toxicities were few, but included headaches (14%), dyspnea (14%), infusion-related reactions (11%), and thrombotic episodes (7%). As a single agent, only three patients (5%) obtain a partial response. Another phase I trial examined SU11248 in 32 patients with advanced AML. Again, the drug was relatively well tolerated, with major dose-limiting toxicity being fatigue [38, 47]. Although approximately 50% of the AML patients had a >50% reduction in their blast count percentage, complete remission was not achieved [38, 47].
CEP-701 and CEP-5214 (from Cephalon) are two indolocarbazole compounds that inhibit autophosphorylation of the FLT3-WT and FLT3-mutant receptors [39, 81]. Unlike some of the other TKIs, these drugs have less activity against other RTKs such as KIT, FMS, and PDGF, and most clinical studies have focused on CEP-701 (Lestaurtinib). Initial studies with CEP-701 demonstrated the ability of this agent to kill FLT3-mutated primary AML cells from patients, and subsequent murine studies have found that CEP-701 extends the survival of mice injected with BaF3 cells transformed using FLT3/ITD [39]. A phase I/II trial evaluated CEP-701 as a single agent for patients with refractory, relapsed, or poor risk AML expressing FLT3-activating mutations. Fourteen heavily pretreated AML patients with FLT3 mutations were treated with CEP-701. CEP-701 toxicities were minimal, with nausea, fatigue, and neutropenia being most commonly described. Five patients obtained objective clinical responses, which correlate closely with a block in the constitutive phosphorylation of the mutated receptor. Clinical responses included significant reductions in bone marrow and peripheral blood blasts; however, no CRs were observed [80, 137]. These investigators recently opened a study to examine if the addition of CEP-701 to conventional chemotherapy may improve clinical outcomes. A total of 48 AML patients with FLT3 mutations in first relapse were randomized to either receiving standard chemotherapy or standard chemotherapy with CEP-701. Of the 24 patients who received CEP-701, 5 achieved complete CR and another 5 obtained a CR with incomplete count recovery. For those patients receiving only standard chemotherapy, three achieved CR and an additional three obtained CR with incomplete count recovery. Accrual is continuing on this trial, and it is too early to know if the addition of CEP-701 will benefit these high-risk patients, but Levis et al. found that CEP-701 and chemotherapy killed a cell line harboring a FLT3/ITD in a synergistic fashion [136], suggesting that using CEP-701 in combination with chemotherapy may be beneficial.
Together, these results suggest that current TKIs probably are not extremely effective as single agents in heavily pretreated AML patients with FLT3 mutations. How are TKIs in previously untreated AML patients with FLT3 mutations remain to be determined? However, there is a push to use these agents in combination with more “standard” chemotherapy, believing that this offers the most potential for providing a therapeutic advantage with this class of drugs. Therefore, the efficacy of TKIs as a single agent in de novo AML patients may not ever be fully investigated. It will be critical to determine which AML patients with FLT3 mutations may have the highest likelihood of response with these novel drugs. As previously described, AML is a very heterogeneous disease event within the subgroup of patients with FLT3 mutations.
KIT and Other Small Molecule Inhibitors
There are few selective KIT inhibitors, and small molecules against KIT have not been extensively used for the treatment of AML patients. Many TKIs (e.g., SU5416, SU6668, Gleevec) have some activity against KIT [82, 56]. A recent study also found that both SU5416 and SU6668 inhibited KIT autophosphorylation and downstream effectors [56], and there has been at least one case report of an individual with refractory AML obtaining CR with SU5416 as monotherapy [139]. In addition, Gleevec has significant activity against WT-KIT and KIT harboring mutations in JM domain [82]. However, Gleevec has no activity against KIT with activating point mutations in the tyrosine kinase domain [82]; therefore, Gleevec’s role in AML therapy may be limited to a highly selected group of AML patients, if at all. However, a newer TKI (BMS-354825 or Dasatinib) has been found to inhibit constitutive activation of KIT receptors with mutations in both the JMD and the TKD [106], suggesting that it may have a broader therapeutic application for AML with KIT mutations. To date, few large studies have actually targeted these drugs for AML patients with KIT mutations. This limited experience is probably due to lower prevalence of KIT mutations in AML as compared to FLT3 mutations, but given the recent discovery of the high rate of KIT mutations in AML involving CBF, additional studies using these drugs in AML patients with KIT mutations will certainly be developed [124].
In addition, small molecule inhibitors directed toward PDGF [91] or downstream effectors in the RTK pathway have been developed [43, 98, 156, 142, 108, 110, 169]. These drugs are currently in a variety of different stages of investigation. Because RAS mutations occur frequently in many different types of tumors [144, 113, 96, 29, 60, 100, 121, 135, 86, 40], RAS has been a natural target of the RTK pathway for many years, and farnesyltransferase inhibitors have led the way in trying to block the effect of constitutive activation of RAS in AML [114, 112, 107]. In early clinical trials examining farnesyltransferase inhibitors, the response rates as a single agent have been encouraging, ranging from 20 to 35%, but the efficacy of these compounds have not correlated with RAS mutations [107]. Details about the biology and clinical implications of farnesyltransferase inhibitors are beyond the scope of this review and have recently been extensively discussed by the leaders in this field [61, 131, 62, 63]. Other inhibitors have targeted downstream effectors such as MEK or RAF [Morgan, 2001 #1171;Rahmani, 2005 #2428;Sridhar, 2005 #2423;Ouyang, 2006 #2425;Wallace, 2006 #2427; Baines, 2000 #1356]. Although some studies suggest efficacy of these agents, major responses have been limited in AML patients.
Future Directions
There are many challenges ahead in the treatment of AML. One of the major challenges will be to begin to interrogate our understanding of the RTK pathways into the development of novel therapeutic approaches for the treatment of AML. As a start, it would be critical to be able to better risk-stratify AML patients harboring abnormalities in RTK pathways. In order to do this successfully, additional investigations will need to determine the exact frequency of mutations in RTK pathway and the clinical significance of these mutations. In addition, it will also be important to understand how the different types of mutations affect the RTK pathways. These investigations will be difficult, given the large number of RTKs, downstream effectors, and heterogeneity within AML.
Researchers are examining how inhibitors of RTK pathways may be useful in the treatment of AML. As single agents, current compounds unfortunately have not induced complete responses in AML patients, but the potential of combining these agents with standard chemotherapy regimens may be more beneficial. In addition, combinations of small molecule inhibitors blocking inappropriate RTK activation at several points along the pathway may also be more effective than using them as a single agent. The major limitation to such approaches may be toxicity, given that the RTK pathway is critical in the regulation of the normal function of the hematopoietic system.
References
Abu-Duhier FM, Goodeve AC, Wilson GA, Care RS, Peake IR, Reilly JT. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113:983–988.
Abu-Duhier FM, Goodeve AC, Wilson GA, et al. FLT3 internal tandem duplication mutations in adult acute myeloid leukaemia define a high-risk group. Br J Haematol. 2000;111:190–195.
Agnes F, Shamoon B, Dina C, Rosnet O, Birnbaum D, Galibert F. Genomic structure of the downstream part of the human FLT3 gene: exon/intron structure conservation among genes encoding receptor tyrosine kinases (RTK) of subclass III. Gene. 1994;145:283–288.
Aguayo A, Estey E, Kantarjian H, et al. Cellular vascular endothelial growth factor is a predictor of outcome in patients with acute myeloid leukemia. Blood. 1999;94:3717–3721.
Anstrom KJ, Reed SD, Allen AS, Glendenning GA, Schulman KA. Long-term survival estimates for imatinib versus interferon-alpha plus low-dose cytarabine for patients with newly diagnosed chronic-phase chronic myeloid leukemia. Cancer. 2004;101:2584–2592.
Armstrong SA, Kung AL, Mabon ME, et al. Inhibition of FLT3 in MLL. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell. 2003;3:173–183.
Armstrong SA, Staunton JE, Silverman LB, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30:41–47.
Beghini A, Larizza L, Cairoli R, Morra E. c-kit activating mutations and mast cell proliferation in human leukemia. Blood. 1998;92:701–702.
Beghini A, Peterlongo P, Ripamonti CB, et al. C-kit mutations in core binding factor leukemias. Blood. 2000;95:726–727.
Beghini A, Ripamonti CB, Cairoli R, et al. KIT activating mutations: incidence in adult and pediatric acute myeloid leukemia, and identification of an internal tandem duplication. Haematologica. 2004;89:920–925.
Beghini A, Ripamonti CB, Castorina P, et al. Trisomy 4 leading to duplication of a mutated KIT allele in acute myeloid leukemia with mast cell involvement. Cancer Genet Cytogenet. 2000;119:26–31.
Bentires-Alj M, Paez JG, David FS, et al. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 2004;64:8816–8820.
Birkenkamp KU, Geugien M, Lemmink HH, Kruijer W, Vellenga E. Regulation of constitutive STAT5 phosphorylation in acute myeloid leukemia blasts. Leukemia. 2001;15:1923–1931.
Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365.
Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689.
Boultwood J, Rack K, Kelly S, et al. Loss of both CSF1R (FMS) alleles in patients with myelodysplasia and a chromosome 5 deletion. Proc Natl Acad Sci USA. 1991;88:6176–6180.
Bowen DT, Frew ME, Hills R, et al. RAS mutation in acute myeloid leukemia is associated with distinct cytogenetic subgroups but does not influence outcome in patients younger than 60 years. Blood. 2005;106:2113–2119.
Brandts CH, Sargin B, Rode M, et al. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res. 2005;65:9643–9650.
Cairoli R, Beghini A, Grillo G, et al. Prognostic impact of c-KIT mutations in core binding factor leukemias. An Italian retrospective study. Blood. 2006. In press.
Cairoli R, Beghini A, Morello E, et al. Imatinib mesylate in the treatment of Core Binding Factor leukemias with KIT mutations. A report of three cases. Leuk Res. 2005;29:397–400.
Cairoli R, Grillo G, Beghini A, et al. C-Kit point mutations in core binding factor leukemias: correlation with white blood cell count and the white blood cell index. Leukemia. 2003;17:471–472.
Callens C, Chevret S, Cayuela JM, et al. Prognostic implication of FLT3 and Ras gene mutations in patients with acute promyelocytic leukemia (APL): a retrospective study from the European APL Group. Leukemia. 2005;19:1153–1160.
Cammenga J, Horn S, Bergholz U, et al. Extracellular KIT receptor mutants, commonly found in core binding factor AML, are constitutively active and respond to imatinib mesylate. Blood. 2005;106:3958–3961.
Care RS, Valk PJ, Goodeve AC, et al. Incidence and prognosis of c-KIT and FLT3 mutations in core binding factor (CBF) acute myeloid leukaemias. Br J Haematol. 2003;121:775–777.
Carroll M, Tomasson MH, Barker GF, Golub TR, Gilliland DG. The TEL/platelet-derived growth factor beta receptor (PDGF beta R) fusion in chronic myelomonocytic leukemia is a transforming protein that self-associates and activates PDGF beta R kinase-dependent signaling pathways. Proc Natl Acad Sci USA. 1996;93:14845–14850.
Casey G, Rudzki Z, Roberts M, Hutchins C, Juttner C. N-ras mutation in acute myeloid leukemia: incidence, prognostic significance and value as a marker of minimal residual disease. Pathology. 1993;25:57–62.
Christiansen DH, Andersen MK, Desta F, Pedersen-Bjergaard J. Mutations of genes in the receptor tyrosine kinase (RTK)/RAS-BRAF signal transduction pathway in therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2005;19:2232–2240.
Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–1214.
Cools J, Stover EH, Gilliland DG. Detection of the FIP1L1-PDGFRA fusion in idiopathic hypereosinophilic syndrome and chronic eosinophilic leukemia. Methods Mol Med. 2006;125:177–187.
Cools J, Stover EH, Wlodarska I, Marynen P, Gilliland DG. The FIP1L1-PDGFRalpha kinase in hypereosinophilic syndrome and chronic eosinophilic leukemia. Curr Opin Hematol. 2004;11:51–57.
De Angelo DJ, Stone RM, Heaney ML, et al. Phase II evaluation of the tyrosine kinase inhibitor MLN518 in patients with acute myeloid leukemia (AML) bearing a FLT3 internal tandem duplication (ITD) mutation. Blood. 2004;104:1792a.
Dello Sbarba P, Pollard JW, Stanley ER. Alterations in CSF-1 receptor expression and protein tyrosine phosphorylation in autonomous mutants of a CSF-1 dependent macrophage cell line. Growth Factors. 1991;5:75–85.
Druker BJ, Lydon NB. Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J Clin Invest. 2000;105:3–7.
Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042.
Farr CJ, Saiki RK, Erlich HA, McCormick F, Marshall CJ. Analysis of RAS gene mutations in acute myeloid leukemia by polymerase chain reaction and oligonucleotide probes. Proc Natl Acad Sci USA. 1988;85:1629–1633.
Felgner J, Kreipe H, Heidorn K, et al. Increased methylation of the c-fms protooncogene in acute myelomonocytic leukemias. Pathobiology. 1991;59:293–298.
Fenski R, Flesch K, Serve S, et al. Constitutive activation of FLT3 in acute myeloid leukaemia and its consequences for growth of 32D cells. Br J Haematol. 2000;108:322–330.
Foran J, O'Farrell AM, Fiedler W, et al. An innovative single dose clinical study shows potent inhibition of FLT3 phosphorylation by SU11248 in vivo: a clinical and pharmacodynamic study in AML patients. Blood. 2002;100:2196a.
Foran J, Paquette R, Cooper M, et al. A phase I study of repeated oral dosing with SU11248 for the treatment of patients with acute myeloid leukemia who have failed, or are not eligible for conventional chemotherapy. Blood. 2002;100:2195a.
Forrester K, Almoguera C, Han K, Grizzle WE, Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. Nature. 1987;327:298–303.
Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–4380.
Furitsu T, Tsujimura T, Tono T, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest. 1993;92:1736–1744.
Furuta T, Sakai T, Senga T, et al. Identification of potent and selective inhibitors of PDGF receptor autophosphorylation. J Med Chem. 2006;49:2186–2192.
Gabbianelli M, Pelosi E, Montesoro E, et al. Multi-level effects of flt3 ligand on human hematopoiesis: expansion of putative stem cells and proliferation of granulomonocytic progenitors/monocytic precursors. Blood. 1995;86:1661–1670.
Gari M, Goodeve A, Wilson G, et al. c-kit proto-oncogene exon 8 in-frame deletion plus insertion mutations in acute myeloid leukaemia. Br J Haematol. 1999;105:894–900.
Giles F, Schiffer C, Kantarjian H, et al. Phase 1 study of PKC412, an oral FLT3 kinase inhibitor, in sequential and concomitant combinations with daunorubicin and cytarabine (DA) induction and high-dose cytarabine consolidation in newly diagnosed patients with AML. Blood. 2004;104:262a.
Giles FJ, Stopeck AT, Silverman LR, et al. SU5416, a small molecule tyrosine kinase receptor inhibitor, has biologic activity in patients with refractory acute myeloid leukemia or myelodysplastic syndromes. Blood. 2003;102:795–801.
Gille H, Kowalski J, Yu L, et al. A repressor sequence in the juxtamembrane domain of Flt-1 (VEGFR-1) constitutively inhibits vascular endothelial growth factor-dependent phosphatidylinositol 3′-kinase activation and endothelial cell migration. Embo J. 2000;19:4064–4073.
Goemans BF, Zwaan CM, Miller M, et al. Mutations in KIT and RAS are frequent events in pediatric core-binding factor acute myeloid leukemia. Leukemia. 2005;19:1536–1542.
Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994;77:307–316.
Gotlib J, Cools J, Malone JM, 3rd, Schrier SL, Gilliland DG, Coutre SE. The FIP1L1-PDGFRalpha fusion tyrosine kinase in hypereosinophilic syndrome and chronic eosinophilic leukemia: implications for diagnosis, classification, and management. Blood. 2004;103:2879–2891.
Griffith J, Black J, Faerman C, et al. The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol Cell. 2004;13:169–178.
Grundler R, Miething C, Thiede C, Peschel C, Duyster J. FLT3-ITD and tyrosine kinase domain mutants induce 2 distinct phenotypes in a murine bone marrow transplantation model. Blood. 2005;105:4792–4799.
Hayakawa F, Towatari M, Kiyoi H, et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000;19:624–631.
Heinrich MC, Druker BJ, Curtin PT, et al. A “first in man” study of the safety and PK/PD of an oral FLT3 inhibitor (MLN518) in patients with AML or high risk myelodyspsia. Blood. 2002;100:1305a.
Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925–932.
Hochhaus A, Kreil S, Corbin AS, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002;16:2190–2196.
Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432.
Iwai T, Yokota S, Nakao M, et al. Internal tandem duplication of the FLT3 gene and clinical evaluation in childhood acute myeloid leukemia. The Children's Cancer and Leukemia Study Group, Japan. Leukemia. 1999;13:38–43.
Janssen JW, Steenvoorden AC, Lyons J, et al. RAS gene mutations in acute and chronic myelocytic leukemias, chronic myeloproliferative disorders, and myelodysplastic syndromes. Proc Natl Acad Sci USA. 1987;84:9228–9232.
Karp JE, Lancet JE, Kaufmann SH, et al. Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemias: a phase 1 clinical-laboratory correlative trial. Blood. 2001;97:3361–3369.
Karp JE, Lancet JE. Development of the farnesyltransferase inhibitor tipifarnib for therapy of hematologic malignancies. Fut Oncol. 2005;1:719–731.
Karp JE, Lancet JE. Targeting the process of farnesylation for therapy of hematologic malignancies. Curr Mol Med. 2005;5:643–652.
Kelly LM, Kutok JL, Williams IR, et al. PML/RARalpha and FLT3-ITD induce an APL-like disease in a mouse model. Proc Natl Acad Sci USA. 2002;99:8283–8288.
Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, Gilliland DG. FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood. 2002;99:310–318.
Kelly LM, Yu J, Boulton CL, et al. CT53518, a novel selective FLT3 antagonist for the treatment of acute myelogenous leukemia (AML). Caner Cell. 2002.
Kindler T, Breitenbuecher F, Kasper S, et al. Identification of a novel activating mutation (Y842C) within the activation loop of FLT3 in patients with acute myeloid leukemia (AML). Blood. 2005;105:335–340.
Kitayama H, Kanakura Y, Furitsu T, et al. Constitutively activating mutations of c-kit receptor tyrosine kinase confer factor-independent growth and tumorigenicity of factor-dependent hematopoietic cell lines. Blood. 1995;85:790–798.
Kiyoi H, Naoe T, Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–3080.
Kiyoi H, Ohno R, Ueda R, Saito H, Naoe T. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene. 2002;21:2555–2563.
Kiyoi H, Towatari M, Yokota S, et al. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12:1333–1337.
Kohl TM, Schnittger S, Ellwart JW, Hiddemann W, Spiekermann K. KIT exon 8 mutations associated with core-binding factor (CBF)-acute myeloid leukemia (AML) cause hyperactivation of the receptor in response to stem cell factor. Blood. 2005;105:3319–3321.
Kondo M, Horibe K, Takahashi Y, et al. Prognostic value of internal tandem duplication of the FLT3 gene in childhood acute myelogenous leukemia. Med Pediatr Oncol. 1999;33:525–529.
Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759.
Kottaridis PD, Gale RE, Langabeer SE, Frew ME, Bowen DT, Linch DC. Studies of FLT3 mutations in paired presentation and relapse samples from patients with acute myeloid leukemia: implications for the role of FLT3 mutations in leukemogenesis, minimal residual disease detection, and possible therapy with FLT3 inhibitors. Blood. 2002;100:2393–2398.
Kubo K, Naoe T, Kiyoi H, et al. Clonal analysis of multiple point mutations in the N-ras gene in patients with acute myeloid leukemia. Jpn J Cancer Res. 1993;84:379–387.
Kuchenbauer F, Feuring-Buske M, Buske C. AML1-ETO needs a partner: new insights into the pathogenesis of t(8;21) leukemia. Cell Cycle. 2005;4:1716–1718.
Lacayo NJ, Meshinchi S, Kinnunen P, et al. Gene Expression Profiles at Diagnosis in de novo Childhood AML Patients Identify FLT3 Mutations with Good Clinical Outcomes. Blood. 2004.
Lancet JE, Karp JE. Farnesyltransferase inhibitors in hematologic malignancies: new horizons in therapy. Blood. 2003;102:3880–3889.
Levis M, Allebach J, Fai-Tse K, et al. FLT3-targeted inhibitors kill FLT3-dependent modeled cells, leukemia-derived cell lines, and primary AML blasts in vitro and in vivo. Blood. 2001;89:721a.
Levis M, Allebach J, Tse KF, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99:3885–3891.
Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important in order to achieve synergistic cytotoxic effects. Blood. 2004;104:1145–1150.
Levis M, Tse KF, Smith BD, Garrett E, Small D. A FLT3 tyrosine kinase inhibitor is selectively cytotoxic to acute myeloid leukemia blasts harboring FLT3 internal tandem duplication mutations. Blood. 2001;98:885–887.
Liang DC, Shih LY, Fu JF, et al. K-Ras mutations and N-Ras mutations in childhood acute leukemias with or without mixed-lineage leukemia gene rearrangements. Cancer. 2006;106:950–956.
Longley BJ, Jr., Metcalfe DD, Tharp M, et al. Activating and dominant inactivating c-KIT catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc Natl Acad Sci USA. 1999;96:1609–1614.
McCoy MS, Toole JJ, Cunningham JM, Chang EH, Lowy DR, Weinberg RA. Characterization of a human colon/lung carcinoma oncogene. Nature. 1983;302:79–81.
Mead A, Linch D, Hills R, Wheatley K, Burnett A, Gale R. Favourable prognosis associated with FLT3 tyrosine kinase domain mutations in AML in contrast to the adverse outcome associated with internal tandem duplications. Blood. 2005;106:334a.
Meshinchi S, Alonzo TA, Gerbing R, Lang B, Radich J. FLT3 internal tandem duplication (FLT3/ITD) is a prognostic factor for poor outcome in pediatric AML: a CCG2961 study. Blood. 2003;102:335a.
Meshinchi S, Stirewalt DL, Alonzo TA, et al. Activating mutations of RTK/ras signal transduction pathway in pediatric acute myeloid leukemia. Blood. 2003;102:1474–1479.
Meshinchi S, Woods WG, Stirewalt DL, et al. Prevalence and prognostic significance of FLT3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97:89–94.
Mesters RM, Padro T, Bieker R, et al. Stable remission after administration of the receptor tyrosine kinase inhibitor SU5416 in a patient with refractory acute myeloid leukemia. Blood. 2001;98:241–243.
Minami Y, Kiyoi H, Yamamoto Y, et al. Selective apoptosis of tandemly duplicated FLT3-transformed leukemia cells by Hsp90 inhibitors. Leukemia. 2002;16:1535–1540.
Minami Y, Yamamoto K, Kiyoi H, Ueda R, Saito H, Naoe T. Different antiapoptotic pathways between wild-type and mutated FLT3: insights into therapeutic targets in leukemia. Blood. 2003;102:2969–2975.
Mizuki M, Fenski R, Halfter H, et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96:3907–3914.
Mizuki M, Schwable J, Steur C, et al. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood. 2003;101:3164–3173.
Mizuki M, Schwaeble J, Steur C, et al. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood. 2003;101(8):3164–3173.
Moore TA, Zlotnik A. Differential effects of Flk-2/Flt-3 ligand and stem cell factor on murine thymic progenitor cells. J Immunol. 1997;158:4187–4192.
Morgan MA, Dolp O, Reuter CW. Cell-cycle-dependent activation of mitogen-activated protein kinase kinase (MEK-1/2) in myeloid leukemia cell lines and induction of growth inhibition and apoptosis by inhibitors of RAS signaling. Blood. 2001;97:1823–1834.
Murray LJ, Young JC, Osborne LJ, Luens KM, Scollay R, Hill BL. Thrombopoietin, flt3, and kit ligands together suppress apoptosis of human mobilized CD34+ cells and recruit primitive CD34+ Thy-1+ cells into rapid division. Exp Hematol. 1999;27:1019–1028.
Nakagawa T, Saitoh S, Imoto S, et al. Multiple point mutation of N-ras and K-ras oncogenes in myelodysplastic syndrome and acute myelogenous leukemia. Oncology. 1992;49:114–122.
Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918.
Namikawa R, Muench MO, de Vries JE, Roncarolo MG. The FLK2/FLT3 ligand synergizes with interleukin-7 in promoting stromal-cell-independent expansion and differentiation of human fetal pro-B cells in vitro. Blood. 1996;87:1881–1890.
Nanri T, Matsuno N, Kawakita T, et al. Mutations in the receptor tyrosine kinase pathway are associated with clinical outcome in patients with acute myeloblastic leukemia harboring t(8;21)(q22;q22). Leukemia. 2005;19:1361–1366.
Neubauer A, Dodge RK, George SL, et al. Prognostic importance of mutations in the ras proto-oncogenes in de novo acute myeloid leukemia. Blood. 1994;83:1603–1611.
O'Farrell AM, Abrams TJ, Yuen HA, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–3605.
O'Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505.
Oliff A. Farnesyltransferase inhibitors: targeting the molecular basis of cancer. Biochimica et Biophysica Acta. 1999;1423:C19–30.
Ouyang B, Knauf JA, Smith EP, et al. Inhibitors of Raf kinase activity block growth of thyroid cancer cells with RET/PTC or BRAF mutations in vitro and in vivo. Clin Cancer Res. 2006;12:1785–1793.
Padua RA, Guinn BA, Al-Sabah AI, et al. RAS, FMS and p53 mutations and poor clinical outcome in myelodysplasias: a 10-year follow-up. Leukemia. 1998;12:887–892.
Panka DJ, Wang W, Atkins MB, Mier JW. The Raf inhibitor BAY 43-9006 (Sorafenib) induces caspase-independent apoptosis in melanoma cells. Cancer Res. 2006;66:1611–1619.
Pellegata NS, Sessa F, Renault B, et al. K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res. 1994;54:1556–1560.
Prendergast GC, Davide JP, deSolms SJ, et al. Farnesyltransferase inhibition causes morphological reversion of ras-transformed cells by a complex mechanism that involves regulation of the actin cytoskeleton. Mol Cell Biol. 1994;14:4193–4202.
Radich JP, Kopecky KJ, Willman CL, et al. N-ras mutations in adult de novo acute myelogenous leukemia: prevalence and clinical significance. Blood. 1990;76:801–807.
Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem. 2005;280:35217–35227.
Ray RJ, Paige CJ, Furlonger C, Lyman SD, Rottapel R. Flt3 ligand supports the differentiation of early B cell progenitors in the presence of interleukin-11 and interleukin-7. Eur J Immunol. 1996;26:1504–1510.
Reindl C, Bagrintseva K, Vempati S, et al. Point mutations found in the juxtamembrane domain of FLT3 define a new class of activating mutations in AML. Blood. 2006.
Ridge SA, Worwood M, Oscier D, Jacobs A, Padua RA. FMS mutations in myelodysplastic, leukemic, and normal subjects. Proc Natl Acad Sci USA. 1990;87:1377–1380.
Ridge SA, Worwood M, Oscier D, Jacobs A, Padua RA. FMS mutations in myelodysplastic, leukemic, and normal subjects. Proc Natl Acad Sci USA. 1990;87:1377–1380.
Ritter M, Kim TD, Lisske P, Thiede C, Schaich M, Neubauer A. Prognostic significance of N-RAS and K-RAS mutations in 232 patients with acute myeloid leukemia. Haematologica. 2004;89:1397–1399.
Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557.
Rodenhuis S, van de Wetering ML, Mooi WJ, Evers SG, van Zandwijk N, Bos JL. Mutational activation of the K-ras oncogene. A possible pathogenetic factor in adenocarcinoma of the lung. N Engl J Med. 1987;317:929–935.
Rosenbauer F, Wagner K, Kutok JL, et al. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nat Genet. 2004;36:624–630.
Roskoski R, Jr. Structure and regulation of Kit protein-tyrosine kinase–the stem cell factor receptor. Biochem Biophys Res Commun. 2005;338:1307–1315.
Rosnet O, Birnbaum D. Hematopoietic receptors of class III receptor-type tyrosine kinases. Crit Rev Oncog. 1993;4:595–613.
Roussel MF, Downing JR, Rettenmier CW, Sherr CJ. A point mutation in the extracellular domain of the human CSF-1 receptor (c-fms proto-oncogene product) activates its transforming potential. Cell. 1988;55:979–988.
Rusten LS, Lyman SD, Veiby OP, Jacobsen SE. The FLT3 ligand is a direct and potent stimulator of the growth of primitive and committed human CD34+ bone marrow progenitor cells in vitro. Blood. 1996;87:1317–1325.
Schessl C, Rawat VP, Cusan M, et al. The AML1-ETO fusion gene and the FLT3 length mutation collaborate in inducing acute leukemia in mice. J Clin Invest. 2005;115:2159–2168.
Schittenhelm MM, Shiraga S, Schroeder A, et al. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66:473–481.
Schnittger S, Kohl TM, Haferlach T, et al. KIT-D816 mutations in AML1-ETO-positive AML are associated with impaired event-free and overall survival. Blood. 2006;107:1791–1799.
Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66.
Sebti SM, Der CJ. Opinion: searching for the elusive targets of farnesyltransferase inhibitors. Nat Rev Cancer. 2003;3:945–951.
SEER Cancer Statistics Review 1975-2001, Vol. 2004. National Cancer Institute; 2004.http://seer.cancer.gov/csr/1975_2004/. Accessed on 15 July, 2009.
Shah AJ, Smogorzewska EM, Hannum C, Crooks GM. Flt3 ligand induces proliferation of quiescent human bone marrow CD34+CD38− cells and maintains progenitor cells in vitro. Blood. 1996;87:3563–3570.
Shimada A, Taki T, Tabuchi K, et al. KIT mutations, and not FLT3 internal tandem duplication, are strongly associated with a poor prognosis in pediatric acute myeloid leukemia with t(8;21): a study of the Japanese Childhood AML Cooperative Study Group. Blood. 2006;107:1806–1809.
Slebos RJ, Kibbelaar RE, Dalesio O, et al. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med. 1990;323:561–565.
Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676.
Smith BD, Levis M, Brown P, et al. Single agent CEP-701, a novel FLT-3 inhibitor, shows initial response in patients with refractory acute myeloid leukemia. Blood. 2002;100:314a.
Smith ML, Snadden J, Neat M, et al. Mutation of BRAF is uncommon in AML FAB type M1 and M2. Leukemia. 2003;17:274–275.
Smolich BD, Yuen HA, West KA, Giles FJ, Albitar M, Cherrington JM. The antiangiogenic protein kinase inhibitors SU5416 and SU6668 inhibit the SCF receptor (c-kit) in a human myeloid leukemia cell line and in acute myeloid leukemia blasts. Blood. 2001;97:1413–1421.
Spiekermann K, Bagrintseva K, Schoch C, Haferlach T, Hiddemann W, Schnittger S. A new and recurrent activating length mutation in exon 20 of the FLT3 gene in acute myeloid leukemia. Blood. 2002;100:3423–3425.
Springall F, O'Mara S, Shounan Y, Todd A, Ford D, Iland H. c-fms point mutations in acute myeloid leukemia: fact or fiction? Leukemia. 1993;7:978–985.
Sridhar SS, Hedley D, Siu LL. Raf kinase as a target for anticancer therapeutics. Mol Cancer Ther. 2005;4:677–685.
Stephenson SA, Slomka S, Douglas EL, Hewett PJ, Hardingham JE. Receptor protein tyrosine kinase EphB4 is up-regulated in colon cancer. BMC Mol Biol. 2001;2:15.
Stirewalt DL, Kopecky KJ, Meshinchi S, et al. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97:3589–3595.
Stirewalt DL, Kopecky KJ, Meshinchi S, et al. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood. 2006;107:3724–3726.
Stirewalt DL, Meshinchi S, Kussick SJ, et al. Novel FLT3 point mutations within exon 14 found in patients with acute myeloid leukaemia. Br J Haematol. 2004;124:481–484.
Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–665.
Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60.
Stone RM, Fischer T, Paquette R, et al. Phase 1B study of PKC412, an oral FLT3 kinase inhibitor, in sequential and simultaneous combinations with daunorubicin and cytarabine (DA) induction and high-dose consolidation in newly diagnosed patients with AML. Blood. 2005;106:404a.
Stone RM, Klimek V, J. DD, et al. PKC412, an oral FLT3 inhibitor, has activity in mutant FLT3 acute myeloid leukemia (AML): a phase II clinical trial. Blood. 2002;100:316a.
Tartaglia M, Martinelli S, Iavarone I, et al. Somatic PTPN11 mutations in childhood acute myeloid leukaemia. Br J Haematol. 2005;129:333–339.
Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335.
Tobal K, Pagliuca A, Bhatt B, Bailey N, Layton DM, Mufti GJ. Mutation of the human FMS gene (M-CSF receptor) in myelodysplastic syndromes and acute myeloid leukemia. Leukemia. 1990;4:486–489.
Tomasson MH, Sternberg DW, Williams IR, et al. Fatal myeloproliferation, induced in mice by TEL/PDGFbetaR expression, depends on PDGFbetaR tyrosines 579/581. J Clin Invest. 2000;105:423–432.
Tomasson MH, Williams IR, Hasserjian R, et al. TEL/PDGFbetaR induces hematologic malignancies in mice that respond to a specific tyrosine kinase inhibitor. Blood. 1999;93:1707–1714.
Tong FK, Chow S, Hedley D. Pharmacodynamic monitoring of BAY 43-9006 (Sorafenib) in phase I clinical trials involving solid tumor and AML/MDS patients, using flow cytometry to monitor activation of the ERK pathway in peripheral blood cells. Cytometry B Clin Cytom. 2006;70(3):107–114.
Towatari M, Iida H, Tanimoto M, Iwata H, Hamaguchi M, Saito H. Constitutive activation of mitogen-activated protein kinase pathway in acute leukemia cells. Leukemia. 1997;11:479–484.
Tse KF, Allebach J, Levis M, Smith BD, Bohmer FD, Small D. Inhibition of the transforming activity of FLT3 internal tandem duplication mutants from AML patients by a tyrosine kinase inhibitor. Leukemia. 2002;16:2027–2036.
Tse KF, Mukherjee G, Small D. Constitutive activation of FLT3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia. 2000;14:1766–1776.
Tsujimura T, Furitsu T, Morimoto M, et al. Ligand-independent activation of c-kit receptor tyrosine kinase in a murine mastocytoma cell line P-815 generated by a point mutation. Blood. 1994;83:2619–2626.
Tsujimura T, Kanakura Y, Kitamura Y. Mechanisms of constitutive activation of c-kit receptor tyrosine kinase. Leukemia. 1997;11 Suppl 3:396–398.
Tsujimura T, Morimoto M, Hashimoto K, et al. Constitutive activation of c-kit in FMA3 murine mastocytoma cells caused by deletion of seven amino acids at the juxtamembrane domain. Blood. 1996;87:273–283.
Turner AM, Lin NL, Issarachai S, Lyman SD, Broudy VC. FLT3 receptor expression on the surface of normal and malignant human hematopoietic cells. Blood. 1996;88:3383–3390.
Ueda S, Ikeda H, Mizuki M, et al. Constitutive activation of c-kit by the juxtamembrane but not the catalytic domain mutations is inhibited selectively by tyrosine kinase inhibitors STI571 and AG1296. Int J Hematol. 2002;76:427–435.
van der Geer P, Hunter T. Identification of tyrosine 706 in the kinase insert as the major colony-stimulating factor 1 (CSF-1)-stimulated autophosphorylation site in the CSF-1 receptor in a murine macrophage cell line. Mol Cell Biol. 1990;10:2991–3002.
Vandenberghe P, Wlodarska I, Michaux L, et al. Clinical and molecular features of FIP1L1-PDFGRA (+) chronic eosinophilic leukemias. Leukemia. 2004;18:734–742.
Veiby OP, Lyman SD, Jacobsen SE. Combined signaling through interleukin-7 receptors and flt3 but not c-kit potently and selectively promotes B-cell commitment and differentiation from uncommitted murine bone marrow progenitor cells. Blood. 1996;88:1256–1265.
Vogelstein B, Civin CI, Preisinger AC, et al. RAS gene mutations in childhood acute myeloid leukemia: a Pediatric Oncology Group study. Genes Chromosomes Cancer. 1990;2:159–162.
Wallace EM, Lyssikatos J, Blake JF, et al. Potent and selective mitogen-activated protein kinase kinase (MEK) 1,2 inhibitors. 1. 4-(4-bromo-2-fluorophenylamino)-1-methylpyridin-2(1H)-ones. J Med Chem. 2006;49:441–444.
Wang YY, Zhou GB, Yin T, et al. AML1-ETO and C-KIT mutation/overexpression in t(8;21) leukemia: implication in stepwise leukemogenesis and response to Gleevec. Proc Natl Acad Sci USA. 2005;102:1104–1109.
Weiner HL, Zagzag D. Growth factor receptor tyrosine kinases: cell adhesion kinase family suggests a novel signaling mechanism in cancer. Cancer Invest. 2000;18:544–554.
Weisberg E, Boulton C, Kelly LM, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1:433–443.
Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61:7233–7239.
Wiener JR, Kassim SK, Yu Y, Mills GB, Bast RC, Jr. Transfection of human ovarian cancer cells with the HER-2/neu receptor tyrosine kinase induces a selective increase in PTP-H1, PTP-1B, PTP-alpha expression. Gynecol Oncol. 1996;61:233–240.
Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439.
Yamashita N, Osato M, Huang L, et al. Haploinsufficiency of Runx1/AML1 promotes myeloid features and leukaemogenesis in BXH2 mice. Br J Haematol. 2005;131:495–507.
Yee KW, O'Farrell AM, Smolich BD, et al. SU5416 and SU5614 inhibit kinase activity of wild-type and mutant FLT3 receptor tyrosine kinase. Blood. 2002;100:2941–2949.
Yee NS, Langen H, Besmer P. Mechanism of kit ligand, phorbol ester, and calcium-induced down-regulation of c-kit receptors in mast cells. J Biol Chem. 1993;268:14189–14201.
Yokota S, Kiyoi H, Nakao M, et al. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia. 1997;11:1605–1609.
Zhao M, Kiyoi H, Yamamoto Y, et al. In vivo treatment of mutant FLT3-transformed murine leukemia with a tyrosine kinase inhibitor. Leukemia. 2000;14:374–378.
Zheng R, Levis M, Piloto O, et al. FLT3 ligand causes autocrine signaling in acute myeloid leukemia cells. Blood. 2004;103:267–274.
Zwaan CM, Meshinchi S, Radich JP, et al. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood. 2003;102:2387–2394.
Acknowledgments
Support for the authors was provided by National Institutes of Health grants (K23 CA92405 and CA 114563). This work was supported by National Institute of Health grants no. K23 CA92405-01 and CA18029.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Stirewalt, D.L., Meshinchi, S. (2009). Receptor Tyrosine Kinase Alterations in AML – Biology and Therapy. In: Nagarajan, L. (eds) Acute Myelogenous Leukemia. Cancer Treatment and Research, vol 145. Springer, New York, NY. https://doi.org/10.1007/978-0-387-69259-3_6
Download citation
DOI: https://doi.org/10.1007/978-0-387-69259-3_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-69257-9
Online ISBN: 978-0-387-69259-3
eBook Packages: MedicineMedicine (R0)