Abstract
Liver transplantation has been reported in patients with methylmalonic acidemia (MMA), but long-term outcome is controversial. Many patients with other approved indications for liver transplantation die before donor grafts are available. A 28-year-old man with MMA underwent cadaveric liver transplantation. His liver was used as a domino graft for a 61-year-old man with primary sclerosing cholangitis, who had low priority on the transplant waiting list. Surgical outcome was successful, and after transplantation both patients have excellent graft function. The patient with MMA showed substantial decrease in methylmalonate in urine (from 5,277 ± 1,968 preoperatively to 1,068 ± 384 mmol/mol creatinine) and plasma (from 445.9 ± 257.0 to 333.3 ± 117.7 μmol/l) over >1-year follow-up, while dietary protein intake increased from 0.6 to 1.36 ± 0.33 g/kg/day. The domino recipient maintained near-normal levels of plasma amino acids but did develop elevated methylmalonate in blood and urine while receiving an unrestricted diet (peak plasma methylmalonate 119 μmol/l and urine methylmalonate 84–209 mmol/mol creatinine, with 1.0–1.9 g/kg/day protein). Neither patient demonstrated any apparent symptoms of MMA or metabolic decompensation during the postoperative period or following discharge.
Conclusion: Liver transplantation substantially corrects methylmalonate metabolism in MMA and greatly attenuates the disease. In this single patient experience, a liver from a patient with MMA functioned well as domino graft although it did result in subclinical methylmalonic acidemia and aciduria in the recipient. Patients with MMA can be considered as domino liver donors for patients who might otherwise spend long times waiting for liver transplantation.
Competing interests: None declared
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Primary Sclerosing Cholangitis
- Familial Hypercholesterolemia
- Familial Hypercholesterolemia
- Maple Syrup Urine Disease
- Maple Syrup Urine Disease
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Methylmalonic acidemia (MMA) is a biomarker for a family of disorders in which the activity of methylmalonyl-CoA mutase is defective (Nyhan et al. 2012). Deficiency of the mutase apoenzyme (mut 0) is the most severe form, often leading to death in infancy or severe neurologic disability (Matsui et al. 1983). The mutase is involved in the catabolism of four essential amino acids, as well as odd chain fatty acids and the side chain of cholesterol (Fig. 1), so dietary restriction of the sources of methylmalonate is demanding. Excessive metabolite accumulation from diet or catabolic stresses can precipitate ketosis and major metabolic decompensation, which can be fatal without successful treatment. Failure to thrive and developmental delay are regular features. Kidney disease, including renal tubular acidosis and interstitial nephritis, may develop and lead to renal failure which may require renal transplantation (Walter et al. 1989).
Human propionate metabolism. The three carbon groups of the metabolic sources of propionyl-CoA are shown in red. Enzymes are labeled (PCC propionyl-CoA carboxylase, and Mut methylmalonyl-CoA mutase, MMCE methylmalonyl-CoA epimerase). The acylcarnitine species are labeled according to their common designations (C3-acylcarnitine = propionylcarnitine, C4DC-acylcarnitine = methylmalonylcarnitine)
Liver transplantation (LTx) in MMA has been found to eliminate life-threatening recurrent ketoacidosis in some, but not all, cases (Leonard et al. 2001; Kayler et al. 2002; Nagarajan et al. 2005; Morioka et al. 2007; Chen et al. 2010). The mutase remains deficient in extrahepatic tissues, so, not surprisingly, disease manifestations may persist. Late-onset kidney and neurologic disease progression is not prevented, and cerebrospinal fluid concentrations of methylmalonate remain high (Leonard et al. 2001; Nyhan et al. 2002; Kaplan et al. 2006). Patients with coexisting kidney disease have undergone simultaneous liver–kidney transplantation with good results (Nagarajan et al. 2005). It is clear that LTx can be lifesaving, but its long-term value in MMA is still debated (Morioka et al. 2007; Chapman et al. 2012).
Nearly 15,000 patients are on the waiting list for liver transplantation in the United States, and fewer than 6,000 are transplanted each year. Several strategies such as the use of partial and split grafts, and of non-heart-beating and other “marginal” donors, have been used to increase graft availability. Domino transplantation (transplantation of an organ removed from the prospective recipient of another organ) was first performed with heart transplantation in the late 1980s (Yacoub et al. 1990). Domino liver transplantation (DLTx) was first performed in the late 1990s (Ando et al. 1997; Furtado et al. 1997; Azoulay et al. 1999; Furtado 2000) for familial amyloid polyneuropathy (FAP) and has found wider indications (Kitchens 2011), including familial hypercholesterolemia (FH) (Popescu et al. 2009; Liu et al. 2010; Popescu and Dima 2012) and maple syrup urine disease (MSUD) (Barshop and Khanna 2005; Khanna et al. 2006). In each of these conditions, the “trait” is transplanted, but the donor disease either does not develop in the domino recipient (as in MSUD) or, if it does, is manageable for a period of time exceeding the patient’s expected survival from end-stage disease (as in FAP and FH).
We first reported DLTx using the liver from a patient with MSUD (Barshop and Khanna 2005; Khanna et al. 2006). This experience established that, in certain conditions, the recipient of the domino liver does not manifest the disease phenotype since the deficient enzyme is substantially extrahepatic. Since then, other centers have also performed DLTx using livers from donors with MSUD (Mazariegos et al. 2012; Badell et al. 2013).
This report describes the first experience with DLTx using the liver from a patient with MMA as a domino graft. Overall, favorable results were observed in both patients.
Methods
Patients
Patient 1 (domino donor) was a 28-year-old man with MMA, diagnosed in infancy during an episode of ketoacidosis and hyperammonemia. At birth, he required resuscitation due to umbilical cord prolapse and developed persistent metabolic acidosis and neutropenia, and elevated urine methylmalonate was found. He had a trial of cyanocobalamin (1,000 μg/day IV for >1 month), but showed no lowering of methylmalonate. His fibroblasts were shown to be in the mut 0 complementation group in the Rosenblatt laboratory (Raff et al. 1991), and he was subsequently documented to be homozygous for the c.322C>T (p.Arg108Cys) mutation in the MUT gene, previously shown to cause MMA (Worgan et al. 2006). For several years he was well controlled with management including dietary restriction of propiogenic amino acids and large intravenous doses of carnitine. He grew to adulthood and had relatively good quality of life with school, working, and driving. After the age of 21, he became inconsistent with diet and began to experience metabolic decompensation and episodes of altered mental status including paranoia and agitation and a decreasing functional status. Over the 3 years prior to LTx, he developed episodes of metabolic decompensation requiring multiple admissions to hospital with ketoacidosis. He also had increasing neurologic disability including seizures, altered gait, and slower speech. His biological MELD score was 10 (INR = 1, bilirubin = 0.7 mg/dl and creatinine 1.15 mg/dl) and was granted 30 MELD exception points after presentation to the United Network for Organ Sharing (UNOS) regional review board. The patient was lean, had no evidence of insulin resistance, and did not have elevated triglycerides, cholesterol, or liver enzymes, although liver biopsy at the time of explant for the domino procedure showed steatosis, as often seen in MMA (Fujisawa et al. 2013). The donor for patient 1 was an unrelated 30-year-old woman who suffered brain death, and the liver was procured locally.
Patient 2 (domino recipient) was a 61-year-old man with primary sclerosing cholangitis and biliary cirrhosis. He had a history of ulcerative colitis for which he underwent cholecystectomy and proctocolectomy 15 years previously. He had mild proctitis that was quiescent on medications, but had several episodes of bacterial cholangitis in the preceding year and poor quality of life. Bile duct brushings were negative for cancer. The patient had a MELD score of 16, which was far less than the average score at transplant for his blood type for our region (>25). He could not get extra points from the UNOS regional review board and had no living donor available and accordingly was offered the domino transplant. The Liver Transplant and Biochemical Genetics teams, including an independent donor advocate unassociated with the transplant program, spoke with him about domino liver transplantation, disease details in the donor, the metabolic condition, and the possibility of developing symptomatic MMA. Approval was obtained from the institutional ethics committee and the institutional review board.
Perioperative Courses
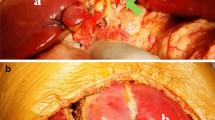
The liver of patient 1 (MMA patient/domino donor) was removed using the standard method, clamping the supra- and infrahepatic cava and conserving as much of the hepatic veins, portal vein, and hepatic artery as possible. Liver biopsy (Fig. 2) showed 25–30% macrosteatosis, but the transplant team felt that this was suitable for use as a domino graft. The liver transplant was completed in a piggyback fashion using well-described techniques.
Liver biopsy of explant from domino donor. Images (H&E stain, left: 40×, right: 400× magnification) show approximately 30% macrovesicular steatosis and an additional 10–20% microvesicular steatosis which is azonal, with focal ballooning degeneration and rare foci of lobular inflammation. There was no fibrosis identified on trichrome stain (not shown)
Suprahepatic vena caval anastomosis was performed on patient 2 between the donor vena cava and recipient confluence of the middle, left, and right hepatic veins. Hepatic arterial anastomosis was performed between donor iliac artery jump graft and recipient common hepatic artery at the level of the gastroduodenal takeoff. A Roux-en-Y choledochojejunostomy was performed to reconstruct the biliary system because of history of primary sclerosing cholangitis.
Patient 1 (MMA patient, domino donor, and recipient of the cadaveric liver transplant) had excellent graft function but developed ascites and pleural effusion post-liver transplant. Imaging showed narrowing of the suprahepatic cava, probably related to clamp injury. An angiogram confirmed the presence of hemodynamically significant gradient, and segmental balloon angioplasty was performed with excellent recovery. The postoperative period was also complicated by acute kidney injury, deep venous thrombosis of the subclavian vein, and pulmonary embolism which responded to anticoagulation. He was initially discharged from the hospital on postoperative day (POD) 84. He was readmitted to hospital on POD 104–106 for the management of renal insufficiency (including hemodialysis) and subsequently recovered. He was treated in hospital on POD 132–136 because of elevated liver enzymes, and biopsy indicated mild rejection that was treated to resolution. He had a seizure episode and purulent meningitis of unknown etiology was documented by lumbar puncture, which responded to an empiric course of antibiotics and antifungal and antituberculosis therapy, including hospitalization (POD 183–191). A pseudomonas pneumonia was diagnosed, and treatment was completed during another hospitalization (POD 206–219). He was initially given tacrolimus for immunosuppression, subsequently changed to cyclosporine following the seizure episode, and then to sirolimus, because of compromised renal function. Throughout his postoperative period, despite these complications, he showed no sign of metabolic decompensation or ketoacidosis. He has remained stable at home performing activities of daily living and has maintained normal liver chemistry and metabolic stability.
Patient 2 (domino recipient) had excellent graft function and was discharged on postoperative day 16 with tacrolimus for immunosuppression. He was admitted again (POD 153–156) because of febrile illness when a pulmonary opacity was identified and then readmitted (POD 158–165) when bronchoscopy confirmed Nocardia brasiliensis pneumonia. He responded well to a prolonged course of trimethoprim–sulfamethoxazole and moxifloxacin. He was noted to have nephrolithiasis and was hospitalized (POD 183–184) for management of a ureteral stone. At no point was there evidence of metabolic acidosis or ketonuria.
Biochemical Testing
Quantitative evaluation of urine and plasma organic acids and plasma carnitine and acylcarnitine profile was performed prior to and following transplantation at regular intervals during clinic visits and hospitalizations. Plasma samples were analyzed on an automated amino acid analyzer. Urine organic acids were analyzed by gas chromatography–mass spectrometry (Hoffmann et al. 1989).
Results
Domino Donor
Following LTx, dietary protein intake for the domino donor (MMA patient, liver transplant patient 1, recipient of the cadaveric liver transplant) was liberalized in small increments, and the time course is shown in Fig. 3, along with the course of laboratory test results. Plasma propionylcarnitine (C3-acylcarnitine) rose initially during the period of early postoperative complications and then settled at a plateau slightly lower than pretransplantation levels (63.2 ± 25.4 μmol/l preoperatively, 234.6 ± 71.8 over the first 50 postoperative days, and 58.3 ± 30.1 μmol/l beyond day 90). There was a drop in plasma methylmalonate from the pretransplantation level of 445.9 ± 257.0 μmol/l (N = 3) to 76.0 μmol/l in the postoperative period when protein was severely limited, and as protein was reintroduced, the level of plasma MMA was moderately elevated (801.4 ± 344.4 μmol/l from POD 7–40, while the patient had postoperative complications) and then reached a plateau (333.3 ± 117.7 μmol/l from 3 to 11 months posttransplant). Pretransplantation urinary methylmalonate was 5,277 ± 1,968 mmol/mol creatinine (reference range 0–5) and 1,068 ± 384 mmol/mol creatinine from month 3 to 11 (Fig. 3) despite liberalization of diet to 1.0–1.9 g/kg/day (daily average 1.4 ± 0.3, equivalent to >45 g/day), an amount which could not be considered previously.
The domino donor had normal liver enzymes and liver function but impaired renal function. Preoperative eGFR was >60, and serum creatinine was 1.10 ± 0.32 mg/dl, changing to 2.91 ± 1.61 over the first 100 postoperative days with estimated GFR as low as 29 ml/min/1.73 m2 on POD 24, but the renal function improved progressively (creatinine 2.86 ± 1.08 mg/dl and eGFR 29.9 ± 10.8 over POD 100–200, creatinine 2.40 ± 0.50 mg/dl and eGFR 33.2 ± 8.2 over POD 200–300, and creatinine 1.67 ± 0.50 mg/dl with eGFR 51.0 ± 12.1 ml/min/m2 beyond POD 300).
Domino Recipient
Following LTx, the domino recipient (liver transplant patient number 2) developed appreciable plasma methylmalonate levels, undetectable preoperatively, 32 μmol/l (normal 0–0.01 μmol/l) in the immediate postoperative period, and 63.5 ± 32.8 μmol/l during the months following LTx (Fig. 4). Pretransplantation urine methylmalonate was ≤2 mmol/mol creatinine. Following LTx urine methylmalonate increased to 161 mmol/mol creatinine in the immediate postoperative period and averaged 128 ± 53.5 during the next 9 months. Plasma propionylcarnitine (C3-acylcarnitine) was 0.21 ± 0.02 μmol/l prior to surgery and 8.0 ± 4.9 μmol/l over 9 months of follow-up. The domino recipient maintained normal renal and liver functions, has resumed his normal activities of daily living, and is following an unrestricted diet.
Discussion
Methylmalonic acidemia is a disease in which death and/or mental retardation is common, and liver transplantation has been used in an attempt to prevent the characteristic potentially lethal metabolic decompensation. In that respect, transplantation has been largely successful, and most patients have not had ketoacidosis following transplantation. Positive outcomes and improved quality of life have been reported (Kayler et al. 2002; Nagarajan et al. 2005), but limited impact on clinical outcome or progressive neurologic disorder has been reported in some cases, so the role of liver transplantation in MMA is debated (Kaplan et al. 1996, 2006; Chakrapani et al. 2002; Nyhan et al. 2002; Kasahara et al. 2006; Chen et al. 2010).
Our previous experience with domino liver transplantation for MSUD led us to speculate that transplanting a methylmalonic acid domino graft would not cause symptomatic methylmalonic acidemia in the recipient, but this transplant sequence had never been performed previously or documented in the peer review literature. The recipient of the domino organ from the patient with MSUD maintained near-normal levels of plasma amino acids and normal levels of urine branched-chain keto and hydroxy acids, and he did not develop symptomatic MSUD. As in the case of the branched-chain keto acid dehydrogenase in MSUD, methylmalonyl-CoA mutase is expressed widely in body tissues. We found that domino LTx from our patient with MMA led to appreciable levels of methylmalonate in the recipient, but the levels were much lower than to those of the domino donor.
The observation that methylmalonate levels continue to be quite elevated in patients with methylmalonic acidemia following LTx has been made previously (Kasahara et al. 2006; Chen et al. 2010). It is expected that in the domino recipient, peripherally produced methylmalonate is broken down normally since all extrahepatic cells contain active mutase. However, elevated methylmalonate levels suggest that the amount produced by the mutase-deficient liver exceeds the metabolic capacity of peripheral cells.
Our domino donor developed seizures following LTx that may have been caused or exacerbated by the dosing of tacrolimus or an infection. It is true that LTx has been reported to be less than curative for MMA and posttransplant CSF methylmalonate levels continue to be elevated (Kaplan et al. 2006); metabolic stroke has been reported as much as 5 years following LTx (Chakrapani et al. 2002).
Tubulointerstitial nephritis and progressive renal function impairment have been associated with MMA (Rutledge et al. 1993). There are several reports of combined liver–kidney transplantation in patients with MMA (Nagarajan et al. 2005; Kasahara et al. 2006). In a recent review, reduction in methylmalonate levels to 13.8 ± 9.2% of preoperative levels was observed following LTx, but 4/18 cases had renal insufficiency and 3/18 had postoperative neurologic disability (Kasahara et al. 2006). Patients with MMA who have had LTx can subsequently develop cyclosporine or tacrolimus nephrotoxicity superimposed on the preexisting tubulointerstitial nephritis. Our MMA patient developed transient renal failure during the postoperative period and required dialysis transiently. However, eGFR returned to >50 ml/min. He will require ongoing monitoring to determine if kidney function worsens. Total follow-up duration at the time this manuscript was finalized is more than 2.5 years.
In conclusion, domino liver transplantation from patients with MMA is feasible. In this case, the course of the domino donor was stabilized, and the domino recipient clearly benefited. In carefully selected patients it is a valuable strategy and a ready resource for a donor liver for a select group of patients who would otherwise die waiting for an organ.
References
Ando Y, Ericzon BG, Suhr OB, Tashima K, Ando M (1997) Reuse of a Japanese familial amyloidotic polyneuropathy patient’s liver for a cancer patient: the domino liver transplantation procedure. Intern Med 36:847
Azoulay D, Samuel D, Castaing D et al (1999) Domino liver transplants for metabolic disorders: experience with familial amyloidotic polyneuropathy. J Am Coll Surg 189:584–593
Badell IR, Hanish SI, Hughes CB et al (2013) Domino liver transplantation in maple syrup urine disease: a case report and review of the literature. Transplant Proc 45:806–809
Barshop BA, Khanna A (2005) Domino hepatic transplantation in maple syrup urine disease. N Engl J Med 353:2410–2411
Chakrapani A, Sivakumar P, McKiernan PJ, Leonard JV (2002) Metabolic stroke in methylmalonic acidemia five years after liver transplantation. J Pediatr 140:261–263
Chapman KA, Summar ML, Enns GM (2012) Propionic acidemia: to liver transplant or not to liver transplant? Pediatr Transplant 16:209–210
Chen PW, Hwu WL, Ho MC et al (2010) Stabilization of blood methylmalonic acid level in methylmalonic acidemia after liver transplantation. Pediatr Transplant 14:337–341
Fujisawa D, Nakamura K, Mitsubuchi H et al (2013) Clinical features and management of organic acidemias in Japan. J Hum Genet 58:769–774
Furtado A (2000) Domino liver transplantation using livers from patients with familial amyloidotic polyneuropathy. Curr Opin Organ Transplant 13:59–66
Furtado A, Tome L, Oliveira FJ, Furtado E, Viana J, Perdigoto R (1997) Sequential liver transplantation. Transplant Proc 29:467–468
Hoffmann G, Aramaki S, Blum-Hoffmann E, Nyhan WL, Sweetman L (1989) Quantitative analysis for organic acids in biological samples: batch isolation followed by gas chromatographic-mass spectrometric analysis. Clin Chem 35:587–595
Kaplan PE, Clinchot DM, Arnett JA (1996) Cognitive deficits after hepatic transplantation: relevance to the rehabilitation potential. Brain Inj 10:599–607
Kaplan P, Ficicioglu C, Mazur AT, Palmieri MJ, Berry GT (2006) Liver transplantation is not curative for methylmalonic acidopathy caused by methylmalonyl-CoA mutase deficiency. Mol Genet Metab 88:322–326
Kasahara M, Horikawa R, Tagawa M et al (2006) Current role of liver transplantation for methylmalonic acidemia: a review of the literature. Pediatr Transplant 10:943–947
Kayler LK, Merion RM, Lee S et al (2002) Long-term survival after liver transplantation in children with metabolic disorders. Pediatr Transplant 6:295–300
Khanna A, Hart M, Nyhan WL, Hassanein T, Panyard-Davis J, Barshop BA (2006) Domino liver transplantation in maple syrup urine disease. Liver Transpl 12:876–882
Kitchens WH (2011) Domino liver transplantation: indications, techniques, and outcomes. Transplant Rev 25:167–177
Leonard JV, Walter JH, McKiernan PJ (2001) The management of organic acidaemias: the role of transplantation. J Inherit Metab Dis 24:309–311
Liu C, Niu DM, Loong CC et al (2010) Domino liver graft from a patient with homozygous familial hypercholesterolemia. Pediatr Transplant 14:E30–E33
Matsui SM, Mahoney MJ, Rosenberg LE (1983) The natural history of the inherited methylmalonic acidemias. N Engl J Med 308:857–861
Mazariegos GV, Morton DH, Sindhi R et al (2012) Liver transplantation for classical maple syrup urine disease: long-term follow-up in 37 patients and comparative United Network for Organ Sharing experience. J Pediatr 160:116–121.e1
Morioka D, Kasahara M, Horikawa R, Yokoyama S, Fukuda A, Nakagawa A (2007) Efficacy of living donor liver transplantation for patients with methylmalonic acidemia. Am J Transplant 7:2782–2787
Nagarajan S, Enns GM, Millan MT, Winter S, Sarwal MM (2005) Management of methylmalonic acidaemia by combined liver-kidney transplantation. J Inherit Metab Dis 28:517–524
Nyhan WL, Gargus JJ, Boyle K, Selby R, Koch R (2002) Progressive neurologic disability in methylmalonic acidemia despite transplantation of the liver. Eur J Pediatr 161:377–379
Nyhan WL, Barshop BA, Al-Aqeel A (2012) Methylmalonic acidemia. In: Atlas of inherited metabolic disease. Hodder Arnold, London, pp 19–32
Popescu I, Dima SO (2012) Domino liver transplantation: how far can we push the paradigm? Liver Transpl 18:22–28
Popescu I, Habib N, Dima S et al (2009) Domino liver transplantation using a graft from a donor with familial hypercholesterolemia: seven-yr follow-up. Clin Transplant 23:565–570
Raff ML, Crane AM, Jansen R, Ledley FD, Rosenblatt DS (1991) Genetic characterization of a MUT locus mutation discriminating heterogeneity in mut0 and mut- methylmalonic aciduria by interallelic complementation. J Clin Invest 87:203–207
Rutledge SL, Geraghty M, Mroczek E, Rosenblatt D, Kohout E (1993) Tubulointerstitial nephritis in methylmalonic acidemia. Pediatr Nephrol 7:81–82
Walter JH, Michalski A, Wilson WM, Leonard JV, Barratt TM, Dillon MJ (1989) Chronic renal failure in methylmalonic acidaemia. Eur J Pediatr 148:344–348
Worgan LC, Niles K, Tirone JC, Hofmann A, Verner ASA, Kucic T, Lepage P, Rosenblatt DS (2006) Spectrum of mutations in mut methylmalonic acidemia and identification of a common Hispanic mutation and haplotype. Hum Mutat 27:31–43
Yacoub MH, Banner NR, Khaghani A et al (1990) Heart-lung transplantation for cystic fibrosis and subsequent domino heart transplantation. J Heart Transplant 9:459–466, discussion 466–457
Acknowledgments
Supported in part by the National Institutes of Health Grants UL1TR000100 (BAB, WLN). We thank Dr. Grace Lin for the preparation and analysis of the histologic samples and Jon Gangoiti for the organic acid and acylcarnitine analyses.
Preliminary results from this study were presented orally at the Society for the Study of Inborn Errors of Metabolism Annual Symposium in Birmingham, England, 5 September, 2012.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: John H Walter, MD FRCPCH
Appendices
Compliance with Ethics Guidelines
-
This manuscript has been circulated among the coauthors and approved by them.
-
There is no previous similar or simultaneous publication of this information.
-
All coauthors contributed substantially to the work (in conception and design, analysis and interpretation of data, drafting the article, and/or critically revising the manuscript for important intellectual content).
-
All coauthors have agreed to this submission.
-
Ajai Khanna, Robert Gish, Susan Winter, William Nyhan, and Bruce Barshop declare that they have no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.
Details of the Contributions of Individual Authors
Dr. Bruce Barshop was responsible for the conception and planning of this project, did the majority of writing, and prepared the graphical figures.
Dr. Ajai Khanna was responsible for the conception and planning of this project, performed the surgery, and substantially contributed to the writing.
Dr. Robert Gish assisted in planning this project, performed medical management, and contributed to the writing.
Dr. Susan Winter provided clinical information and contributed to the writing.
Dr. William Nyhan assisted in planning this project and contributed to the writing.
Rights and permissions
Copyright information
© 2015 SSIEM and Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Khanna, A., Gish, R., Winter, S.C., Nyhan, W.L., Barshop, B.A. (2015). Successful Domino Liver Transplantation from a Patient with Methylmalonic Acidemia. In: Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Peters, V. (eds) JIMD Reports, Volume 25. JIMD Reports, vol 25. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2015_480
Download citation
DOI: https://doi.org/10.1007/8904_2015_480
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-49667-1
Online ISBN: 978-3-662-49668-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)