Abstract
Background and aim: Alkaptonuria (AKU) clinical manifestations resemble severe arthritis. The Suitability of Nitisinone in Alkaptonuria 1 (SONIA 1) study is a dose-finding trial for nitisinone treatment of AKU patients. We tested a panel of serum and urinary biomarkers reflecting extracellular matrix remodelling (ECMR) of cartilage, bone and connective tissue in SONIA 1 patients to identify non-invasive and diagnostic biomarkers of tissue turnover in AKU.
Methods: Fasted serum and urine were retrieved from 40 SONIA 1 patients and 44 healthy controls. Established biomarkers of bone remodelling (CTX-I, P1NP, OC), cartilage remodelling (CTX-II, C2M, AGNx1) and inflammation (CRPM) as well as exploratory biomarkers of ECMR (C6M, VCANM, MIM, TIM) were measured at baseline in serum and urine by means of enzyme-linked immunosorbent assays (ELISAs) or automated systems (Elecsys 2010).
Results: The levels of bone resorption (CTX-I) and cartilage degradation (C2M) were elevated in AKU patients as compared to controls (p > 0.0001 and p = 0.03, respectively). Also tissue inflammation (CRPM) was elevated in AKU patients (p = 0.01). In addition all four exploratory biomarkers of ECMR (C6M, VCANM, MIM, TIM) were elevated in AKU patients compared to healthy controls. CTX-II was the only biomarker to be reduced in AKU patients. TIM was the only marker that showed a higher concentration than the normal assay range in AKU patients.
Conclusions: We have identified new potential biomarkers for assessment of cartilage, bone and cardiovascular remodelling in AKU and demonstrated the robustness of the assays used to measure the biomarker concentration in biological fluids.
Competing interests: None declared
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Urine Creatinine
- Cartilage Degradation
- Extracellular Matrix Remodelling
- Homogentisic Acid
- Cardiovascular Remodelling
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Alkaptonuria (AKU, OMIM disorder accession number 203500) is an ultra orphan autosomal recessive disorder caused by the deficiency of the enzyme homogentisate 1,2-dioxygenase (HGO). HGO is responsible for the breakdown of homogentisic acid (HGA) into 4-maleylacetoacetate during the tyrosine and phenylalanine catabolism. Accumulated HGA can be oxidised to a melanin-like polymeric pigment via benzoquinone acetic acid (BQA), which in turn is deposited in connective tissues in a process termed ochronosis. Cartilage appears to be a preferred site for ochronosis, and one of the earliest and most severe clinical manifestations is a premature osteoarthropathy resembling osteoarthritis, which begins to occur around the age of 30 years. Progressive arthritic pain affects the spine and weight-bearing joints, which develops into kyphoscoliosis, impaired spinal and thoracic mobility disc disease/prolapse and vertebral and long bone fractures. Hearing loss, cardiac arrhythmias and congestive cardiac failure also occur in 40, 40 and 10%, respectively of all AKU patients (Ranganath et al. 2013). AKU has an incidence of 1:250,000–1,000,000, which increases to 1:19,000 in restricted communities, e.g. in Slovakia (Zatkova 2011). AKU is diagnosed by urine homogentisic acid measurement following clinical suspicion in case of symptoms such as dark urine, staining of nappies in babies and pigmentation in the ears and eyes.
Because of the similarities and the differences of joint destruction in AKU and osteoarthritis and the rapid progression of joint disease in AKU, we decided to investigate biomarkers previously tested in arthritis in AKU. Several protease-dependent ECM remodelling biomarkers (neo-epitope) of cartilage degradation and bone resorption have been described in arthritis-related literature; the most characterised and validated are CTX-II (matrix metalloproteinase (MMP)-dependent type II collagen degradation) indicating subchondral remodelling (Dam et al. 2009a) and CTX-I (cathepsin K-dependent type I collagen degradation) and OC (osteocalcin) indicating bone resorption and formation, respectively (Garnero et al. 2003; Rosenquist et al. 1995). Other cartilage and bone biomarkers have shown promising results in arthritis, such as AGNxI (aggrecanase-mediated aggrecan degradation), C2M (MMP-mediated type II collagen degradation) (Siebuhr et al. 2014; Dorleijn et al. 2014; Bay-Jensen et al. 2014) and P1NP (type I collagen formation) (Siebuhr et al. 2012). Moreover, since the pathology of ochronosis may involve the cardiac cartilage, with clinical manifestations such as cardiac arrhythmias and congestive cardiac failure, our interest was to investigate also potential biomarkers of cardiac and vascular remodelling. TIM (MMP-mediated titin degradation) is a biomarker for cardiac remodelling (Vassiliadis et al. 2012), and VCANM (MMP-mediated versican degradation) and MIM (MMP-mediated mimecan degradation) were previously associated with atherosclerosis (Barascuk et al. 2013; Barascuk et al. 2011). The biomarker for MMP-mediated type VI collagen remodelling C6M and the inflammatory biomarker CRPM (MMP-mediated degradation of C-reactive protein) (Veidal et al. 2011; Skjot-Arkil et al. 2012) were also considered as possible AKU diagnostic biomarkers. Type VI collagen is a prominent component of articular cartilage (Soder et al. 2002), and CRPM was shown as a biomarker for local inflammation in another articular disease, ankylosing spondylitis (Skjot-Arkil et al. 2012).
The currently available treatment is mostly palliative, with lifelong analgesia and joint surgery. Suitability of Nitisinone in Alkaptonuria 1 (SONIA 1) is a randomised clinical study for nitisinone dose selection to treat AKU patients (Ranganath et al. 2014). Nitisinone is a competitive inhibitor of the enzyme 4-hydroxyphenylpyruvate dioxygenase, which metabolises 4-hydroxyphenylpyruvate to HGA, which therefore prevents HGA formation. A recent study has demonstrated that nitisinone blocks the formation of the ochronotic pigment in a mouse model of AKU (Preston et al. 2014). Here we analysed the mentioned biomarkers of extracellular matrix remodelling in the serum and urine of SONIA 1 patients, in order to identify possible biomarkers of disease status.
Methods
Clinical Cohort
SONIA 1 is a randomised, open-label, parallel-group study with a no-treatment control group. The study was conducted at two sites, Liverpool (United Kingdom) and Piešťany (Slovakia).
Patients were recruited if they were 18 years and older, with proven increased homogentisic acid excretion in the urine. Ochronosis was not a requirement for participation; however, 80% of the patients presented observable signs of ochronosis in the ears, eyes or skin at enrolment. Forty AKU patients were randomised into five groups (eight patients per group) to receive either 1, 2, 4 or 8 mg nitisinone once daily or no treatment (control) for 4 weeks. Serum and urine (first morning void) were collected at baseline, 2 weeks and 4 weeks. Detailed information on the study has previously been published (Ranganath et al. 2014). Forty-four healthy individuals were used as a control group. Serum and urine (first morning void, 40 samples) were collected in Denmark from healthy volunteers and elderly women enrolled in a variety of studies at CCBR, Ballerup (Dam et al. 2009b). Demographics of the patients enrolled in the study that were considered for this work and of healthy controls are detailed in Table 1.
Measurements
Selected biomarkers for extracellular matrix remodelling were measured in the serum and urine of AKU patients (SONIA 1 baseline) and healthy controls to detect which of the biomarkers had increased concentration in AKU in order to profile the turnover of cartilage and bone in AKU patients. Established biomarkers of bone formation and resorption and cartilage remodelling were measured together with more exploratory biomarkers for extracellular matrix remodelling. The biomarkers measured in the study and the bibliographic references for the corresponding assays are listed in Table 2. All commercial assays were run following manufacturer’s instructions. β-CrossLaps Elecsys 2010 was used for the measurement of CTX-I, U-CartiLaps ELISA for the measurement of CTX-II, N-MID Osteocalcin Elecsys 2010 for the measurement of OC and P1NP Elecsys 2010 for the measurement of P1NP. All other assays (C2M, AGNx1, C6M, CRPM, TIM, VCANM, MIM) were competitive ELISAs developed at Nordic Bioscience and performed according to the protocols detailed in the indicated references. CTX-II was measured in the urine, and CTX-I was measured in both the serum and urine. The concentration of the urinary biomarkers was normalised for urine creatinine. All other biomarkers were measured only in the serum.
Robustness Analysis
The robustness of the biomarkers in this cohort was evaluated by observing their dependence from demographic parameters, such as age, gender and BMI, from collection site and from collection time. To explore this last point, we used repeated samples from six SONIA 1 patients collected over 24 h: two baseline samples, two samples after 1 month of treatment with 8 mg/day nitisinone and two samples after 1 month in the non-treated arm. The variation is shown as % change from time 0. A % change <20% was considered in the limits of intrinsic variation of the assay. This evaluation was performed in serum assays only, since the 24 h collection of urine was done by acidifying the urine and this procedure was incompatible with the assays used. The concentration range of normal samples (normal range) specified in the ELISA protocols was used as standard to compare the values of the biomarkers measured in the study.
Creatinine Assessment
The urinary biomarkers were normalised for levels of urine creatinine. Since AKU patients have high levels of homogentisic acid (HGA) in their urine, the possibility that HGA could interfere with the enzymatic creatinine assay was explored. In order to assess the possible interference of HGA with the creatinine assessment, the creatinine levels measured by a creatininase-based reaction assay and by a Jaffé-based reaction assay were compared. Creatinine was assessed in first void morning urine of AKU patients using two different commercial assays. Urine creatinine was measured on the Siemens Advia 1800 analyser, using an enzymatic creatinine assay, based upon the conversion of creatinine to glycine, formaldehyde and hydrogen peroxide, catalysed by creatininase and sarcosine oxidase. Urine creatinine was also measured using the creatinine Jaffé method on the COBAS 701 analyser where creatinine forms a yellow-orange complex with picrate at an alkaline pH.
Assessment of HGA Interference with Hydroxyperoxidase-Based ELISAs
To assess the possible interference of HGA with the peroxidase-based detection system in the ELISAs, three serum samples (one with no HGA, one with low HGA and one with high HGA) and three urine samples (one with no HGA, one with low HGA and one with high HGA) were spiked with twofold diluted HGA starting from 20 mM. The recovery of the analyte was investigated in the CRPM ELISA for serum and in the U-CartiLaps ELISA (CTX-II) for urine, taken as representative assays for serum and urine measurements, considering as 100% the concentration of the sample without HGA added.
Results
Robustness Analysis of the Biomarkers
P1NP, N-MID and VCANM showed a good stability over 24 h, presenting an average variation <10%. C6M, CRPM and TIM presented an average variation <20%, but they presented outliners that showed a higher variation. AGNx1, CTX-I, C2M and MIM showed an average variation >20% during the day. None of the biomarkers were age dependent. Only CTX-II/creatinine was significantly more elevated in the urine of female AKU patients than male. The same trend was observable in the control group although the difference was not significant (p = 0.06). Only CTX-I measured in the serum was significantly lower in obese patients compared to patients with normal weight. A similar tendency, although not significant, was observed in urine levels of CTX-I. Urine CTX-I and serum TIM levels were significantly different in patients enrolled in Liverpool and in Piešťany. The results of the robustness analysis are summarised in Fig. S1 and Table S1 of the supplementary data.
Interference of HGA with Enzyme-Based Assays
Figure S2 of the supplementary data shows the correlation between the creatinine concentration obtained using the two assays for creatinine measurement in urine samples with HGA/creatinine levels >600 μmol/mmol and in urine samples with HGA/creatinine <600 μmol/mmol. While the creatinine levels measured in the two assays were strongly correlated in samples containing HGA at very low levels (Fig. S2b), the creatininase-based assay underestimated the concentration of creatinine in samples with high HGA content (Fig. S2a). The Jaffé-based reaction assay was then used in the assessment of urine creatinine used to normalise the urinary marker CTX-II.
Table S2 and S3 show the recovery of analyte concentration in the assay for urinary CTX-I and serological CRPM. The average recovery was within the accepted values in the presence of decreasing concentrations of HGA spiked in the samples. This was particularly valid in alkaptonuric patient samples, in which HGA was already present. The initial concentration of 20 mM represents the maximum concentration present in the urine of AKU patients.
Diagnostic Markers of ECM Remodelling in AKU
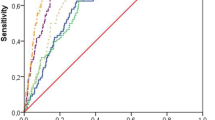
As summarised in Table 3, the biomarkers for cartilage degradation C2M and C6M, for bone resorption CTX-I, for chronic inflammation CRPM and for cardiovascular remodelling MIM, TIM and VCANM were significantly more elevated in SONIA 1 patients than in healthy controls. The biomarker for subchondral cartilage remodelling CTX-II was less concentrated in the urine of SONIA 1 patients than in healthy urine. TIM was the biomarker that showed the best separation between the two groups, with an area under the receiving operating curve close to 0.9 (Fig. 1). All biomarker concentration was within the concentration range indicated in the assay protocol, which is based on measurement on serum and urine from the normal population (Table 3), except TIM in SONIA 1 patients, which was markedly more elevated than the highest concentration range.
None of the biomarkers changed following 1 month of treatment with nitisinone at any dose (Table S4).
Discussion
This is the first study exploring the protease-dependent cartilage and bone extracellular matrix remodelling in AKU patients. We have observed the basal levels of ECM turnover in patients with AKU enrolled in the SONIA 1 clinical study. We could not detect any change in the levels of the biomarkers following treatment, probably due to the short observation period; however, we were able to identify some promising diagnostic biomarkers, which can describe ochronosis features with respect to bone and cartilage remodelling.
To investigate the robustness of our biomarkers for their use in clinical studies, we evaluated the possible dependence of the biomarkers from age, BMI, gender and site of sample collection. The biomarkers were not different in various age and gender groups. CTX-I, a biomarker for bone resorption, was decreased in obese patients, suggesting that obese AKU patients present a reduced bone remodelling. Urinary levels of CTX-I were reduced, and serological levels of TIM were increased in patients enrolled in Liverpool compared to patients enrolled in Piešťany. The patients enrolled in Liverpool came from different European nations, and it is therefore not possible to identify a common trait in different lifestyle and eating habits that could explain the difference in the biomarkers. We evaluated the circadian variation of the biomarkers and showed that most of the serum biomarkers had little to none variation in the serum of SONIA 1 patients in 24 hours. The only biomarkers which showed a variation not imputable to the assay normal variation were AGNx1, CTX-I, C2M and MIM. This information is novel for the exploratory biomarkers MIM and for AGNx1 and C2M, but this tendency has already been demonstrated and discussed for CTX-I (Karsdal et al. 2008). It is possible to conclude that such variation did not affect the present study, since the sampling was performed at the same time in all patients and in all visits, but it must be taken into consideration in case of different study designs.
As part of the robustness analysis, we also investigated, following indications from previous literature (Curtis et al. 2014), whether the presence of high concentration of HGA in the urine of AKU patients could interfere with the enzymatic reaction of the creatininase used in the creatinine assay and of the peroxidase used in the ELISAs. The correlation analysis of the creatinine levels measured with a creatininase-based assay and a Jaffé-based assay, which is not influenced by HGA, demonstrated that high urinary HGA levels interfere with the creatininase, leading to a lower signal and an underestimation of creatinine concentration. Therefore, a Jaffé-based assay is to be used for a correct estimation of urine creatinine in AKU patients, especially pre-nitisinone when urine HGA is greatest. On the contrary the peroxidase-based serum and urine assays seemed not to be affected by high levels of HGA, as demonstrated by the spiking experiment.
In AKU it is commonly held that cartilage is the predominantly affected tissue, whereas unbalanced bone remodelling is limited. However, recent evidences suggest that changes within the bone compartment occur in AKU patients (Taylor et al. 2012a). Furthermore, these changes in bone were more frequent in AKU (100%) than OA (70%) (Taylor et al. 2012a). CTX-I and OC have been heavily studied as a measure of bone balance in arthritis. In ankylosing spondylitis CTX-I and OC separately were shown to be associated with disease duration, and an association between the biomarkers indicated the skewed turnover of bone in ankylosing spondylitis (Arends et al. 2012). CTX-I alone was studied in RA numerous times and was associated with disease progression and disease activity (Syversen et al. 2009). However, in OA the results on CTX-I were contrary (Karsdal et al. 2010). In arthritis CTX-II was shown to be related to joint erosions and revealed a predictive value for radiographic progression (Hashimoto et al. 2009). In addition, CTX-II was associated with disease activity of ankylosing spondylitis (Pedersen et al. 2011) and correlated with inflammation (Vosse et al. 2008). The cartilage biomarker, C2M, was shown to be able to discriminate between early responders and nonresponders in RA patients treated with tocilizumab (Bay-Jensen et al. 2014).
In SONIA 1 patients’ serum CTX-I was elevated when compared to healthy control and the biomarker could separate efficiently the two groups (AUROC: 0.837). The levels of the biomarker in SONIA 1 patients were the same as previously observed in RA patients (data not shown). P1NP, a biomarker for bone formation, was elevated in AKU patients compared to controls despite the magnitude of the difference was not as elevated as for CTX-I. These results support the histological findings that pathological bone turnover is a feature of AKU. Nevertheless, OC was not elevated in this population, which is consistent with the focal uncoupling of resorption and formation in subchondral trabecular bone. Among the cartilage degradation biomarkers, C2M and CTX-II levels were significantly different between AKU patients and controls. C2M increased in AKU patients, with levels comparable to those observed in noninflammatory OA and early RA, but lower than those observed in inflammatory OA and severe RA (data not shown). The same observation can be done regarding the levels of CRPM, a biomarker for local chronic inflammation which, despite being more elevated in AKU patients than in the controls, presented lower levels than in other rheumatologic disease such as inflammatory OA and RA (data not shown). The urinary levels of CTX-II were on the contrary lower in SONIA 1 patients. This is likely to be related to the extensive pigmentation of cartilage making it resistant to proteolytic degradation. Histological studies on joints obtained from AKU patients at arthroplasty (Taylor et al. 2011), and from AKU mice (Preston et al. 2014; Taylor et al. 2012b), have in fact revealed that the earliest deposition of ochronotic pigment is in isolated chondrons in calcified cartilage. Subsequently the pigmentation proliferates into the hyaline articular cartilage and eventually to the articular surface. Pigmentation makes the cartilage stiff and resistant to proteolysis. Once the cartilage is extensively pigmented, there are severe focal changes in bone remodelling possibly as a result of stress shielding from the stiffened ochronotic cartilage. The subchondral plate can be completely resorbed by osteoclastic action (Taylor et al. 2011), and there is a focal uncoupling of resorption resulting in the formation of trabecular excrescences (Taylor et al. 2012a). However, a limitation of this study lies in the different ratio of female/male in the AKU group compared to the control group. Due to limitation in sample availability, the control cohort included mostly females, while SONIA 1 patients were prevalently males. This could have led to a misinterpretation of the CTX-II results, since this marker is the only one affected by gender, being higher in the urine of females. The higher mean values of CTX-II in the control group could then potentially be a consequence of such difference in the two groups. Moreover, despite it is not known whether females have different ochronosis levels, it is known that generally females develop arthropathy later than males. This has been taken into account by having an older control cohort for the serum marker measurements, but not for the urine measurements, and this could further have affected the CTX-II results. Another limitation lies in the use of controls and patients from different nations. Given the differences in some markers in patients enrolled in the two study sites, it is not possible to exclude that cultural differences, such as dietary habits, could influence the marker levels in serum and urine also in the control cohort.
A longitudinal study including measurements of disease severity will be required to investigate the burden of disease and prognostic potential of these biomarkers and observe similarity and differences with other rheumatic diseases.
Cardiovascular implications have been described in AKU for more than 20 years (Kenny et al. 1990). The working hypothesis is that the deposition of the pigment could lead to injury to the vascular endothelium causing formation of atheroma (Lok et al. 2013). The versican fragment VCANM was associated with the remodelling of the atherosclerotic plaque in patients with cardiovascular diseases (Barascuk et al. 2013). MIM, a fragment reflecting the MMP-mediated degradation of the proteoglycan mimecan, was also preliminarily associated with the cardiovascular remodelling in a mouse model of atherosclerosis (Barascuk et al. 2011). Therefore, the elevation of these biomarkers in the serum of SONIA 1 patients can reflect the atheroma remodelling occurring in AKU. TIM is a biomarker for heart remodelling, and it was found elevated in patients with coronary calcifications and other cardiovascular morbidities (Vassiliadis et al. 2012). We observed an elevation of this biomarker in the serum of AKU patients, and this finding is in accordance with previous reports of calcifications in the cardiac valves due to the accumulation of the ochronotic pigment and presence of inflammatory cells (Millucci et al. 2014a; Millucci et al. 2014b), which may produce the proteases responsible for the cleavage of the titin fragment identified by the TIM assay.
Conclusions
In this study we have presented robust and well-characterised tools to measure the levels of pathological extracellular matrix turnover related to bone, cartilage and cardiovascular remodelling in ochronotic patients. The biomarkers that showed an elevation in SONIA 1 patients may be used in a successive clinical study investigating the suitability of nitisinone treatment for a longer period. Such longitudinal study would allow identifying possible biomarkers of treatment efficacy and biomarkers for disease progression, to be used for personalised healthcare and patient management in the future.
References
Arends S, Spoorenberg A, Houtman PM, Leijsma MK, Bos R, Kallenberg CG, Groen H, Brouwer E, van der V (2012) The effect of three years of TNFalpha blocking therapy on markers of bone turnover and their predictive value for treatment discontinuation in patients with ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res Ther 14:R98
Barascuk N, Vassiliadis E, Zheng Q, Wang Y, Wang W, Larsen L, Rasmussen LM, Karsdal MA (2011) Levels of circulating MMCN-151, a degradation product of mimecan, reflect pathological extracellular matrix remodeling in apolipoprotein E knockout mice. Biomark Insights 6:97–106
Barascuk N, Genovese F, Larsen L, Byrjalsen I, Zheng Q, Sun S, Hosbond S, Poulsen TS, Diederichsen A, Jensen JM, Mickley H, Register TC, Rasmussen LM, Leeming DJ, Christiansen C, Karsdal MA (2013) A MMP derived versican neo-epitope is elevated in plasma from patients with atherosclerotic heart disease. Int J Clin Exp Med 6:174–184
Bay-Jensen AC, Liu Q, Byrjalsen I, Li Y, Wang J, Pedersen C, Leeming DJ, Dam EB, Zheng Q, Qvist P, Karsdal MA (2011) Enzyme-linked immunosorbent assay (ELISAs) for metalloproteinase derived type II collagen neoepitope, CIIM–increased serum CIIM in subjects with severe radiographic osteoarthritis. Clin Biochem 44:423–429
Bay-Jensen AC, Platt A, Byrjalsen I, Vergnoud P, Christiansen C, Karsdal MA (2014) Effect of tocilizumab combined with methotrexate on circulating biomarkers of synovium, cartilage, and bone in the LITHE study. Semin Arthritis Rheum 43:470–478
Curtis SL, Roberts NB, Ranganath LR (2014) Interferences of homogentisic acid (HGA) on routine clinical chemistry assays in serum and urine and the implications for biochemical monitoring of patients with alkaptonuria. Clin Biochem 47:640–647
Dam EB, Byrjalsen I, Karsdal MA, Qvist P, Christiansen C (2009a) Increased urinary excretion of C-telopeptides of type II collagen (CTX-II) predicts cartilage loss over 21 months by MRI. Osteoarthritis Cartilage 17:384–389
Dam EB, Loog M, Christiansen C, Byrjalsen I, Folkesson J, Nielsen M, Qazi AA, Pettersen PC, Garnero P, Karsdal MA (2009b) Identification of progressors in osteoarthritis by combining biochemical and MRI-based markers. Arthritis Res Ther 11:R115
Dorleijn DM, Luijsterburg PA, Bay-Jensen AC, Siebuhr AS, Karsdal MA, Rozendaal RM, Bos PK, Bierma-Zeinstra SM (2014) Association between biochemical cartilage markers and clinical symptoms in patients with hip osteoarthritis: cohort study with 2-year follow-up. Osteoarthritis Cartilage 23:57–62
Garnero P, Ferreras M, Karsdal MA, Nicamhlaoibh R, Risteli J, Borel O, Qvist P, Delmas PD, Foged NT, Delaisse JM (2003) The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone Miner Res 18:859–867
Hashimoto J, Garnero P, van der HD, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Yoshikawa H, Nishimoto N (2009) A combination of biochemical markers of cartilage and bone turnover, radiographic damage and body mass index to predict the progression of joint destruction in patients with rheumatoid arthritis treated with disease-modifying anti-rheumatic drugs. Mod Rheumatol 19:273–282
Karsdal MA, Byrjalsen I, Riis BJ, Christiansen C (2008) Investigation of the diurnal variation in bone resorption for optimal drug delivery and efficacy in osteoporosis with oral calcitonin. BMC Clin Pharmacol 8:12
Karsdal MA, Byrjalsen I, Bay-Jensen AC, Henriksen K, Riis BJ, Christiansen C (2010) Biochemical markers identify influences on bone and cartilage degradation in osteoarthritis–the effect of sex, Kellgren-Lawrence (KL) score, body mass index (BMI), oral salmon calcitonin (sCT) treatment and diurnal variation. BMC Musculoskelet Disord 11:125
Kenny D, Ptacin MJ, Bamrah VS, Almagro U (1990) Cardiovascular ochronosis: a case report and review of the medical literature. Cardiology 77:477–483
Leeming DJ, Larsen DV, Zhang C, Hi Y, Veidal SS, Nielsen RH, Henriksen K, Zheng Q, Barkholt V, Riis BJ, Byrjalsen I, Qvist P, Karsdal MA (2010) Enzyme-linked immunosorbent serum assays (ELISAs) for rat and human N-terminal pro-peptide of collagen type I (PINP)–assessment of corresponding epitopes. Clin Biochem 43:1249–1256
Lok ZS, Goldstein J, Smith JA (2013) Alkaptonuria-associated aortic stenosis. J Card Surg 28:417–420
Millucci L, Ghezzi L, Paccagnini E, Giorgetti G, Viti C, Braconi D, Laschi M, Geminiani M, Soldani P, Lupetti P, Orlandini M, Benvenuti C, Perfetto F, Spreafico A, Bernardini G, Santucci A (2014a) Amyloidosis, inflammation, and oxidative stress in the heart of an alkaptonuric patient. Mediators Inflamm 2014:258471
Millucci L, Ghezzi L, Braconi D, Laschi M, Geminiani M, Amato L, Orlandini M, Benvenuti C, Bernardini G, Santucci A (2014b) Secondary amyloidosis in an alkaptonuric aortic valve. Int J Cardiol 172:e121–e123
Pedersen SJ, Sorensen IJ, Garnero P, Johansen JS, Madsen OR, Tvede N, Hansen MS, Thamsborg G, Andersen LS, Majgaard O, Loft AG, Erlendsson J, Asmussen K, Jurik AG, Moller J, Hasselquist M, Mikkelsen D, Skjodt T, Lambert R, Hansen A, Ostergaard M (2011) ASDAS, BASDAI and different treatment responses and their relation to biomarkers of inflammation, cartilage and bone turnover in patients with axial spondyloarthritis treated with TNFalpha inhibitors. Ann Rheum Dis 70:1375–1381
Preston AJ, Keenan CM, Sutherland H, Wilson PJ, Wlodarski B, Taylor AM, Williams DP, Ranganath LR, Gallagher JA, Jarvis JC (2014) Ochronotic osteoarthropathy in a mouse model of alkaptonuria, and its inhibition by nitisinone. Ann Rheum Dis 73:284–289
Ranganath LR, Jarvis JC, Gallagher JA (2013) Recent advances in management of alkaptonuria (invited review; best practice article). J Clin Pathol 66:367–373
Ranganath LR, Milan AM, Huges AT (2014) Suitability of nitisinone in alkaptonuria 1 (SONIA 1): an international, multicenter, randomized, open-label, no-treatment controlled, parallel-group, dose–response study to investigate the effect of once daily nitisinone on 24-hour urinary homogentisic acid excretion in patients with alkaptonuria after 4 weeks of treatment. Ann Rheum Dis 0:1–6
Rosenquist C, Qvist P, Bjarnason N, Christiansen C (1995) Measurement of a more stable region of osteocalcin in serum by ELISA with two monoclonal antibodies. Clin Chem 41:1439–1445
Siebuhr AS, Wang J, Karsdal M, Bay-Jensen AC, Jin Y, Zheng Q (2012) Matrix metalloproteinase-dependent turnover of cartilage, synovial membrane, and connective tissue is elevated in rats with collagen induced arthritis. J Transl Med 10:195
Siebuhr AS, Petersen KK, Rendt-Nielsen Z, Egsgaard LL, Eskehave T, Christiansen C, Simonsen O, Hoeck HC, Karsdal MA, Bay-Jensen AC (2014) Identification and characterisation of osteoarthritis patients with inflammation derived tissue turnover. Osteoarthritis Cartilage 22:44–50
Skjot-Arkil H, Schett G, Zhang C, Larsen DV, Wang Y, Zheng Q, Larsen MR, Nawrocki A, Bay-Jensen AC, Henriksen K, Christiansen C, Alexandersen P, Leeming DJ, Karsdal MA (2012) Investigation of two novel biochemical markers of inflammation, matrix metalloproteinase and cathepsin generated fragments of C-reactive protein, in patients with ankylosing spondylitis. Clin Exp Rheumatol 30:371–379
Soder S, Hambach L, Lissner R, Kirchner T, Aigner T (2002) Ultrastructural localization of type VI collagen in normal adult and osteoarthritic human articular cartilage. Osteoarthritis Cartilage 10:464–470
Syversen SW, Goll GL, van der HD, Landewe R, Gaarder PI, Odegard S, Haavardsholm EA, Kvien TK (2009) Cartilage and bone biomarkers in rheumatoid arthritis: prediction of 10-year radiographic progression. J Rheumatol 36:266–272
Taylor AM, Boyde A, Wilson PJ, Jarvis JC, Davidson JS, Hunt JA, Ranganath LR, Gallagher JA (2011) The role of calcified cartilage and subchondral bone in the initiation and progression of ochronotic arthropathy in alkaptonuria. Arthritis Rheum 63:3887–3896
Taylor AM, Boyde A, Davidson JS, Jarvis JC, Ranganath LR, Gallagher JA (2012a) Identification of trabecular excrescences, novel microanatomical structures, present in bone in osteoarthropathies. Eur Cell Mater 23:300–308
Taylor AM, Preston AJ, Paulk NK, Sutherland H, Keenan CM, Wilson PJ, Wlodarski B, Grompe M, Ranganath LR, Gallagher JA, Jarvis JC (2012b) Ochronosis in a murine model of alkaptonuria is synonymous to that in the human condition. Osteoarthritis Cartilage 20:880–886
Vassiliadis E, Rasmussen LM, Byrjalsen I, Larsen DV, Chaturvedi R, Hosbond S, Saabye L, Diederichsen AC, Genovese F, Duffin KL, Zheng Q, Chen X, Leeming DJ, Christiansen C, Karsdal MA (2012) Clinical evaluation of a matrix metalloproteinase-12 cleaved fragment of titin as a cardiovascular serological biomarker. J Transl Med 10:140
Veidal SS, Karsdal MA, Vassiliadis E, Nawrocki A, Larsen MR, Nguyen QH, Hagglund P, Luo Y, Zheng Q, Vainer B, Leeming DJ (2011) MMP mediated degradation of type VI collagen is highly associated with liver fibrosis–identification and validation of a novel biochemical marker assay. PLoS One 6:e24753
Vosse D, Landewe R, Garnero P, van der HD, van der LS, Geusens P (2008) Association of markers of bone- and cartilage-degradation with radiological changes at baseline and after 2 years follow-up in patients with ankylosing spondylitis. Rheumatology (Oxford) 47:1219–1222
Wang B, Chen P, Jensen AC, Karsdal MA, Madsen SH, Sondergaard BC, Zheng Q, Qvist P (2009) Suppression of MMP activity in bovine cartilage explants cultures has little if any effect on the release of aggrecanase-derived aggrecan fragments. BMC Res Notes 2:259
Zatkova A (2011) An update on molecular genetics of Alkaptonuria (AKU). J Inherit Metab Dis 34:1127–1136
Acknowledgements
This study was funded by the European Union Seventh Framework Programme (project 304985) and by the Danish Research Fund (“Den Danske Forskningsfond”).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: Verena Peters
Synopsis
Robust assays for non-invasive assessment of extracellular matrix turnover biomarkers can be used to describe the cartilage, bone and cardiovascular remodelling in alkaptonuria patients.
Compliance with Ethics Guidelines
Conflict of Interests
Federica Genovese is a full-time employee at Nordic Bioscience.
Anne Sofie Siebuhr is a full-time employee at Nordic Bioscience.
Kishwar Musa is a full-time employee at Nordic Bioscience.
Morten Karsdal is a full-time employee and holds shares at Nordic Bioscience.
Anne-Christine Bay-Jensen is a full-time employee and holds shares at Nordic Bioscience.
James Gallagher, Anna Milan, Jozef Rovensky and Lakshminarayan Ranganath declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Details of the Contributions of Individual Authors
Federica Genovese, Anne-Christine Bay-Jensen, Morten Karsdal, Jozef Rovensky and Lakshminarayan Ranganath conceived and designed the study; Kishwar Musa and Anna Milan performed the analysis; Federica Genovese, Anna Milan, Anne Sofie Siebuhr and Anne-Christine Bay-Jensen interpreted the data; Federica Genovese, Anne Sofie Siebuhr, James Gallagher, Anna Milan and Anne-Christine Bay-Jensen drafted the manuscript; and Anne-Christine Bay-Jensen, James Gallagher and Morten Karsdal revised the manuscript critically for important intellectual content.
Electronic Supplementary Material
Rights and permissions
Copyright information
© 2015 SSIEM and Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Genovese, F. et al. (2015). Investigating the Robustness and Diagnostic Potential of Extracellular Matrix Remodelling Biomarkers in Alkaptonuria. In: Zschocke, J., Baumgartner, M., Morava, E., Patterson, M., Rahman, S., Peters, V. (eds) JIMD Reports, Volume 24. JIMD Reports, vol 24. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2015_430
Download citation
DOI: https://doi.org/10.1007/8904_2015_430
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-48226-1
Online ISBN: 978-3-662-48227-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)