Abstract
Introduction: Sapropterin dihydrochloride (Kuvan®), a synthetic 6R-diastereoisomer of tetrahydrobiopterin (BH4), is approved in Europe for the treatment of patients aged ≥4 years with hyperphenylalaninaemia (HPA) due to BH4-responsive phenylalanine hydroxylase (PAH) deficiency, in conjunction with a phenylalanine-restricted diet, and also for the treatment of patients with BH4 deficiency.
Aims/methods: KAMPER is an ongoing, observational, multicentre registry with the primary objective of providing information over 15 years on long-term safety of sapropterin dihydrochloride treatment in patients with HPA. Here we report initial data on characteristics from patients recruited by the time of the third interim analysis and results at 1 year.
Results: Overall, 325 patients from 55 sites in seven European countries were included in the analysis: 296 (91.1%) patients with PAH deficiency (median [Q1, Q3] age, 10.3 [7.2, 15.0] years) and 29 (8.9%) with BH4 deficiency (12.8 [6.6, 18.9] years). Fifty-nine patients (18.2%) were aged ≥18 years; 4 patients were pregnant. No elderly patients (aged ≥65 years) or patients with renal or hepatic insufficiency were enroled in the study. Twelve-month data were available for 164 patients with PAH deficiency and 16 with BH4 deficiency. No new safety concerns were identified as of May 2013.
Conclusions: Initial data from KAMPER show that sapropterin dihydrochloride has a favourable safety profile. Registry data collected over time will provide insight into the management and outcomes of patients with PAH deficiency and BH4 deficiency, including long-term safety, impact on growth and neurocognitive outcomes and the effect of sapropterin dihydrochloride treatment on populations of special interest.
Competing interests: None declared
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- BH4-responsive phenylketonuria
- Hyperphenylalaninaemia
- Phenylketonuria
- Sapropterin
- Tetrahydrobiopterin deficiency
Introduction
Hyperphenylalaninaemia (HPA) is characteristic of phenylalanine hydroxylase (PAH) deficiency, a disorder caused by mutations in the PAH gene resulting in reduction or loss of PAH enzyme activity and associated with progressive neurocognitive impairment if untreated (Blau et al. 2010). PAH deficiency is predominantly managed with a phenylalanine-restricted diet, but treatment can be supplemented with synthetic tetrahydrobiopterin (BH4), an essential PAH cofactor, in BH4-responsive patients (Fiege and Blau 2007; Keil et al. 2013; Kure et al. 1999).
BH4 deficiencies affect 2% of individuals with HPA (Blau et al. 1996). BH4 is essential for the functioning of tyrosine hydroxylase, tryptophan hydroxylase (Friedman et al. 1972; Shiman et al. 1971) and nitric oxide synthase (Marletta 1993). Patients with most BH4 deficiencies require treatment with BH4 and neurotransmitter precursors (5-hydroxytryptophan and levodopa) to reduce neurological deterioration (Shintaku 2002).
Randomized, placebo-controlled studies have shown that sapropterin (sapropterin dihydrochloride, Kuvan®; Merck KGaA, Darmstadt, Germany; BioMarin, Novato, California, USA; and Asubio Pharma, Kobe, Japan), a synthetic 6R-diastereoisomer of BH4, can improve the control of blood phenylalanine concentration and allows greater phenylalanine consumption in BH4-responsive patients (Levy et al. 2007; Trefz et al. 2009). Sapropterin has been approved for the treatment of patients with HPA due to BH4-responsive PAH deficiency in the USA since December 2007, in Japan (as Biopten®) since July 2008, in Europe (for patients aged ≥4 years only) since December 2008 and in Canada since April 2010 (BioMarin Pharmaceutical Inc. 2010; Daiichi Sankyo 2013; Merck Serono 2013). Sapropterin is licensed for the treatment of patients of all ages with BH4 deficiencies in Europe and Japan (Daiichi Sankyo 2013; Merck Serono 2013).
The primary objective of the Kuvan® Adult Maternal Paediatric European Registry (KAMPER) is to provide information over 15 years on the long-term safety of sapropterin in patients with HPA, in accordance with a post-approval commitment with the European Medicines Agency. It is also designed to collect information on the use of sapropterin in maternal HPA and on the effects on childhood growth and neurocognitive outcomes. We report baseline data from patients recruited by the time of the third interim analysis and results at 1 year.
Methods
Study Design
KAMPER is an ongoing observational, multicentre drug registry (ClinicalTrials.gov identifier NCT01016392) conducted in accordance with the protocol and protocol amendments, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guideline for Good Clinical Practice (ICH Topic E6), applicable local regulations and the Declaration of Helsinki. Patients receive the standard of care for the management of HPA with no additional study-related dietary or other protocol restrictions. Patients undergo clinical assessments and receive medications and treatments as recommended by their study physician, including sapropterin treatment as per the summary of product characteristics (Merck Serono 2013).
The registry is being established in European countries where sapropterin is marketed, which reflects different geographical areas, lifestyles, genetic backgrounds and varied incidence rates of PAH deficiency and BH4 deficiencies. At the time of the third interim analysis (May 2013), the registry was established in Austria, France, Germany, Italy, the Netherlands, Slovakia and Spain.
Study Population
Patients with HPA due to PAH deficiency (including patients with mild phenylketonuria and mild HPA with phenylalanine levels >360 μmol/L) aged ≥4 years, or BH4 deficiency (no age limit) and who meet the study inclusion/exclusion criteria, are being recruited from December 2009 to December 2019, with follow-up until 2025. Patients are eligible for the study if they are currently receiving sapropterin treatment at a participating centre, are responsive to BH4 or sapropterin (≥30% reduction in blood phenylalanine level or attainment of physician-defined therapeutic blood phenylalanine targets) and are willing and able to provide written, signed, informed consent (or by parent/guardian where appropriate). Exclusion criteria include known hypersensitivity to sapropterin and breastfeeding.
Eligible patients are identified at a baseline visit and followed in accordance with routine care practices of the participating sites. Eligible patients who are pregnant and unable to lower blood phenylalanine levels with a phenylalanine-restricted diet, or who become pregnant while participating in the study and decide to continue sapropterin therapy, are invited to enrol in the maternal sub-registry. Patients may have received diet therapy before sapropterin therapy and may have received BH4 formulations other than sapropterin before the study.
Study Variables
The primary endpoint is the incidence and description of adverse events (AEs) and serious AEs (SAEs), including the incidence in specific populations (elderly patients aged ≥65 years, paediatric patients aged <18 years and patients with renal or hepatic insufficiency). Secondary outcomes include study population characteristics (PAH deficiency and BH4 deficiencies, PAH genotype, electrocardiogram [ECG] results), adherence to treatment (sapropterin and diet therapy), phenylalanine tolerance and metabolic control during follow-up (blood phenylalanine and tyrosine levels and long-term sensitivity to sapropterin treatment). Secondary outcomes also include auxological/nutritional outcomes (growth, clinical and biological micronutrients [including vitamin D, serum iron, serum folate and serum B12] and bone density), neurological and neuropsychiatric outcomes and maternal sub-registry outcomes. Information was also collected regarding type of treatment (PAH deficiency, sapropterin with/without diet therapy; BH4 deficiencies, sapropterin with/without neurotransmitter precursors). Information regarding recommended data collection is available in Supplementary Table 1.
Assessments
Study visits may vary widely between patients and between study years. Patients attend a baseline visit followed by quarterly to annual visits according to the routine practice at each participating centre and the individual needs of patients. For the maternal sub-registry, additional data are gathered, e.g. ultrasound results on the mother during pregnancy and foetal outcomes.
Study Size
In total, 625 patients from 100 study sites in Europe are planned for enrolment to have an evaluable population of ≥500 at the end of the study. Five hundred evaluable patients are estimated to represent 20% among an initial population of 2,500 patients with HPA, which is the estimated number of patients with PKU who are sapropterin responsive (20% of total PKU population) in Europe (Burton et al. 2007).
Minimizing Bias
To minimize selection bias, broad eligibility criteria are used, sites are expected to enrol all eligible patients treated according to the approved label for sapropterin in each country (Merck Serono 2013), and patients are recruited from a diverse pool of countries and clinical sites. To minimize measurement bias, sites receive systematic, standardized protocol training and use standardized data collection forms at enrolment and follow-up assessments.
Statistical Analyses
This analysis included patients enrolled between 8 December 2009 and 26 November 2012 who had available baseline data. All analyses were descriptive; 95% confidence intervals (CIs) were calculated for the primary endpoint. Categorical variables were summarized as n (%) of patients; continuous variables were summarized using descriptive statistics (median, Quartile 1 [Q1], Quartile 3 [Q3]). No imputation for missing data was performed; for categorical variables, percentages were calculated with ‘missing’ as a category. Height, weight and body mass index (BMI) Z-scores were calculated for patients with PAH deficiency and BH4 deficiencies using normative data from the World Health Organization 2007 reference population (World Health Organization 2011). Height and BMI Z-scores used 19-year-olds as a reference group for patients aged >19 years; weight Z-scores were calculated for patients aged ≤10 years only. Z-scores between −2 and 2 were considered to be within the normal range.
Results
Patients
Patients were recruited from 55 sites in seven European countries (Supplementary Fig. 1). In total, 329 patients were enrolled and 325 were included in the present analysis; 296 (91.1%) patients with PAH deficiency and 29 (8.9%) with a BH4 deficiency (Supplementary Fig. 2). Fifty-nine patients (18.2%) were aged ≥18 years at enrolment, and four patients (1.2%) entered the maternal sub-registry. Two patients (0.6%) with PAH deficiency discontinued from the study: one was considered a nonresponder after 14 months of treatment, and one pregnant patient discontinued early due to her concerns about drug use during pregnancy. No elderly patients (≥65 years of age) or patients with renal or hepatic insufficiency were enrolled. Data were available for the 1-year follow-up analysis for 180 patients (87.8%; 164 with PAH deficiency; 16 patients with a BH4 deficiency).
At baseline, median (Q1, Q3) age was 10.3 (7.2, 15.0) years in patients with PAH deficiency and 12.8 (6.6, 18.9) years in patients with a BH4 deficiency (Table 1). Newborn screening for HPA was performed in most patients with PAH deficiency (259/296; 87.5%) and BH4 deficiencies (26/29; 89.7%), with the majority (234/296 [79.1%] and 27/29 [93.1%], respectively) undergoing confirmatory testing and approximately a third (101/296 [34.1%] and 10/29 [34.5%], respectively) undergoing a second confirmatory testing.
PAH gene analysis was performed in 212/296 (71.6%) patients with PAH deficiency; 91 individual mutations and 149 different genotypes were identified (n=210). The most frequent mutations are shown in Table 2.
Overall, 85 patients with PAH deficiency (28.7%) and six with a BH4 deficiency (20.7%) had ≥1 medical condition at baseline. Intellectual disability was diagnosed in 10 patients (7 [2.4%] with PAH deficiency and 3 [10.3%] with a BH4 deficiency). The majority of patients with a BH4 deficiency (22/23; 95.7%) were receiving either concomitant levodopa or carbidopa/levodopa.
BH4 Responsiveness
Sapropterin/BH4 response test data were available for 307 patients: 291/296 (98.3%) with PAH deficiency and 16/29 (55.2%) with a BH4 deficiency. Further information regarding BH4 responsiveness testing is available in Supplementary Table 2. Of 291 patients with PAH deficiency, 282 (96.9%) demonstrated ≥30% decrease in blood phenylalanine levels during the response test. Of 16 patients with BH4 deficiency, 15 (93.8%) demonstrated ≥30% decrease in blood phenylalanine levels during the response test; data were missing for one patient.
Safety
A total of 101 AEs, including 7 SAEs, were reported in 61 patients (Table 3). Headache was the most frequently reported AE, occurring in 8 (2.7%) patients with PAH deficiency and 1 (3.4%) patient with a BH4 deficiency. No deaths were reported. AEs were mild or moderate in intensity, with the exception of one SAE of severe headache that led to hospitalization (PAH deficiency group) and was considered possibly related to sapropterin treatment.
In patients with PAH deficiency, 88 AEs occurred in 55 patients, including five SAEs in three patients; nine AEs were considered possibly related to sapropterin treatment (Table 3). Most AEs were mild in severity (n = 65), 22 were moderate, and one was severe. The incidence of total AEs per patient-year was 19.0% (95% CI 14.1%, 25.6%) in year 1 (n = 43) and 9.0% (95% CI 5.7%, 14.3%) in year 2 (n = 18); the number of patient-years was 226.3 and 199.5, respectively.
In patients with a BH4 deficiency, 13 AEs occurred in six patients, including two SAEs in two patients but no treatment-related AEs (Table 3). In terms of AE severity, five were mild, eight were moderate, and none was severe. All patients with a BH4 deficiency who experienced an AE were treated concomitantly with carbidopa/levodopa; notable AEs were chorea (facial movements of a choreic nature) and tic (persistent facial tic) in one patient and hypertonia in one patient. No other relevant neurological AEs were reported in any other patients. The incidence of total AEs per patient-year was 26.7% (95% CI 12.0%, 53.9%) in year 1 (n = 6); the number of patient-years was 22.5.
Baseline ECG results were available for 17 patients with PAH deficiency and five with a BH4 deficiency; results were normal except for one patient with PAH deficiency (sinus bradycardia) and one with a BH4 deficiency (non-specific ventricular repolarization and first-degree atrioventricular block). At 1-year follow-up, data were available for eight patients with PAH deficiency and three with a BH4 deficiency; all were reported to be normal and were performed in patients who had not undergone a baseline ECG assessment.
Of four pregnant patients, a term live birth was reported for three, and one pregnancy was ongoing; no development problems or AEs related to the pregnancies have been reported.
Sapropterin Treatment
The median (Q1, Q3) sapropterin dose was 12.7 (10.0, 18.9) mg/kg/day in patients with PAH deficiency (n = 245) and 5.0 (3.0, 7.5) mg/kg/day in those with a BH4 deficiency (n = 25). In the pregnant patients, one was treated with sapropterin at a dose of 3 mg/kg/day and another received 10 mg/kg/day, and there was no alteration in dose during the course of either pregnancy. Another pregnant patient received sapropterin 8 mg/kg/day initially, reduced to 4 mg/kg/day, and another received sapropterin at doses between 9 and 17 mg/kg/day.
Blood Phenylalanine Concentrations at Baseline, 6 Months and 12 Months
In patients with PAH deficiency, median (Q1, Q3) blood phenylalanine concentration was 414 (289, 561) μmol/L before treatment with sapropterin (n=215), 349 (258, 503) μmol/L at 6 months (n = 133) and 340 (248, 486) μmol/L at 12 months (n = 121). Median blood phenylalanine concentration in patients with PAH deficiency varied between age groups at baseline, but observations suggest stability within age groups over 12 months (Supplementary Table 3).
In patients with a BH4 deficiency, median (Q1, Q3) blood phenylalanine concentration was 91 (67, 313) μmol/L before treatment with sapropterin (n = 20), 103 (81, 254) μmol/L at 6 months (n = 11) and 89 (76, 117) μmol/L at 12 months (n = 6).
Natural Protein and Actual Phenylalanine Intake at Baseline, 6 Months and 12 Months
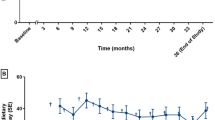
In patients with PAH deficiency who completed their dietary records, the observed median (Q1, Q3) natural protein intake was higher at 12 months than baseline (before sapropterin treatment) (Fig. 1; Supplementary Table 3). The observed median (Q1, Q3) dietary phenylalanine intake in patients with PAH deficiency varied over time, from 718 (385, 1514) mg/day at baseline (n = 136) to 1,525 mg/day (750, 2,298) at 6 months (n = 79) and 1,205 (600, 2,549) mg/day at 12 months (n = 70); however, median dietary phenylalanine intake was higher at 12 months than baseline for all age groups, including adults (Supplementary Table 3).
Growth
Median growth measurements were considered to be within the normal range for patients with PAH deficiency and those with a BH4 deficiency. In patients with PAH deficiency, median height, weight and BMI Z-scores were similar at baseline and 12 months. Median (Q1, Q3) Z-scores were: for height, −0.1 (−0.8, 0.6; n = 244) at baseline and −0.2 (−0.9, 0.6; n = 115) at 12 months; for weight, 0.3 (−0.2, 1.2; n = 118) at baseline and 0.4 (−0.4, 1.2; n = 58) at 12 months; and for BMI, 0.4 (−0.4, 1.2; n = 244) at baseline and 0.4 (−0.3, 1.3; n = 115) at 12 months. In patients with a BH4 deficiency, median baseline (Q1, Q3) Z-scores were: for height, −0.1 (−1.4, 0.2; n = 23); for weight, −0.1 (−1.2, 1.0; n = 12); and for BMI, 0.3 (−1.3, 1.2; n = 23); data are not reported for 12-month follow-up owing to small patient numbers (<10).
In the 59 patients with PAH deficiency and available baseline bone density data, osteopenia was detected in five patients (ages 11.9, 17.8, 18.4, 23.9 and 27.3 years) and osteoporosis in two patients (ages 16.7 and 23.0 years). At 12 months, two cases of osteopenia and one case of osteoporosis were recorded (n = 34 patients with available data). Osteopenia and osteoporosis had not been recorded in these patients at baseline. Baseline bone density data were only available for one patient with a BH4 deficiency; these data were normal with no osteopenia or osteoporosis detected. No follow-up data were available.
Discussion
At the third interim analysis, KAMPER had accumulated data from 296 patients with PAH deficiency and 29 patients with a BH4 deficiency, with 1-year follow-up data available for 180 patients. Most patients were <18 years of age at enrolment. No elderly patients (≥65 years) or patients with renal or hepatic insufficiency had been enrolled. As this was an analysis of baseline and 1-year data, there are currently insufficient data to make meaningful evaluations of observed long-term changes over time; however, such reporting will improve over the course of this 15-year study.
Information regarding sapropterin/BH4 response testing was available for most patients. The most frequent test dose was 20 mg/kg, and most tests were conducted over a 24- to 48-h period. Test doses and test periods were consistent with a previous report of clinical practice in Europe (Keil et al. 2013) and with recommendations for assessing BH4 response (Blau et al. 2009). Sapropterin test data were only available for 55% of patients with a BH4 deficiency. Although more patients may have been tested during the neonatal period, data are not readily available. In addition, physicians may use other diagnostic tests, such as pterin analysis, for assessing BH4 deficiency.
No new safety concerns were identified, with AEs consistent with those reported in previous studies (Levy et al. 2007; Trefz et al. 2009). While no AEs in patients with BH4 deficiency were considered related to sapropterin treatment, two patients experienced neurological AEs (chorea, tic and hypertonia) that may have been a result of concomitant carbidopa/levodopa or the primary condition. The summary of product characteristics for sapropterin provides warnings and precautions regarding neurological AEs if co-administered with other medicinal products, including levodopa (BioMarin Pharmaceutical Inc. 2010; Merck Serono 2013). AEs will continue to be evaluated as follow-up data are accrued.
In the current study, four patients were pregnant. Three patients have since delivered live infants with no reported developmental issues, and the fourth pregnancy was ongoing at the time of this interim analysis. There is increasing support for the use of sapropterin during pregnancy in women with BH4-responsive PAH deficiency who cannot achieve recommended blood phenylalanine concentrations, with or without diet therapy (Feillet et al. 2014; Grange et al. 2014).
Among patients with PAH deficiency and available dietary intake information, an increase from baseline in median actual phenylalanine intake was observed at 12 months in all age groups. These findings are consistent with previous studies showing that sapropterin may permit higher phenylalanine consumption (Hennermann et al. 2012; Keil et al. 2013; Lambruschini et al. 2005; Shintaku et al. 2004; Trefz et al. 2009). However, limitations related to sample size when stratified by age group and variability of dietary intake data must be considered. There are insufficient data to evaluate dietary intake in patients with BH4 deficiency.
Although an observation period of 1 year is considered too short to see clinically significant changes in growth, growth was similar to the normal population in both patients with PAH deficiency and BH4 deficiency; however, there were a limited number of patients with a BH4 deficiency at the 1-year follow-up. The proportion of patients with osteoporosis and osteopenia was generally in line with a recent report on the prevalence of mineral bone disease in patients with PAH deficiency, in which none of the patients treated with BH4 (n = 12), for an average of 7.1 years, developed mineral bone disease (Miras et al. 2013).
The present analysis has several limitations. As KAMPER is an observational, registry study, some follow-up assessment data, such as blood assessments, were limited; however, missing data are common in observational studies. Furthermore, collection of data not routinely obtained during the assessment of patients with PAH and BH4 deficiencies (e.g. ECG data) may impede data availability, and data regarding PAH classification were unavailable at the time of the analysis. As recruitment is ongoing, any observed changes over time should be interpreted with caution. Observational cohort studies may be subject to potential bias; however, the potential for selection, measurement or information bias was minimized. Country selection included different geographic areas, lifestyles and incidence rates of PAH deficiency and BH4 deficiencies to minimize selection bias, but the limited number of patients in specific subgroups may limit generalization of the results. As yet there are no patients in the special populations of interest; however, the patient population reflects real-world practice, and the short duration of the registry to date and recruitment of special populations should improve over time. Enrolment of patients aged >65 years, however, is not expected in the time span of this study.
In conclusion, the initial data obtained from KAMPER show that sapropterin has a good safety profile. Registry data collected over time will provide insight into the management and outcomes of patients with PAH deficiency and BH4 deficiencies, including long-term safety, impact on phenylalanine tolerance, growth and neurocognitive outcomes and the effect of sapropterin treatment on populations of special interest.
Abbreviations
- AE:
-
Adverse event
- BH4 :
-
Tetrahydrobiopterin
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- ECG:
-
Electrocardiogram
- HPA:
-
Hyperphenylalaninaemia
- ICH:
-
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
- KAMPER:
-
Kuvan® Adult Maternal Paediatric European Registry
- PAH:
-
Phenylalanine hydroxylase
- Q:
-
Quarter
- SAE:
-
Serious adverse event
References
BioMarin Pharmaceutical Inc. (2010) Kuvan (sapropterin dihydrochloride) tablets. Full Prescribing Information. http://www.kuvan.com/hcp/kuvan-full-prescribing-information.html. Accessed 21 Aug 2014
Blau N, Barnes I, Dhondt JL (1996) International database of tetrahydrobiopterin deficiencies. J Inherit Metab Dis 19:8–14
Blau N, Belanger-Quintana A, Demirkol M et al (2009) Optimizing the use of sapropterin (BH(4)) in the management of phenylketonuria. Mol Genet Metab 96:158–163
Blau N, van Spronsen FJ, Levy HL (2010) Phenylketonuria. Lancet 376:1417–1427
Burton BK, Grange DK, Milanowski A et al (2007) The response of patients with phenylketonuria and elevated serum phenylalanine to treatment with oral sapropterin dihydrochloride (6R-tetrahydrobiopterin): a phase II, multicentre, open-label, screening study. J Inherit Metab Dis 30:700–707
Daiichi Sankyo (2013) Daiichi Sankyo launches natural tetrahydrobiopterin agent Biopten® Granules 10%. http://www.daiichisankyo.com/media_investors/media_relations/press_releases/detail/006042/20131129_479_E.pdf. Accessed 21 Aug 2014
Feillet F, Muntau AC, Debray FG et al (2014) Use of sapropterin dihydrochloride in maternal phenylketonuria. A European experience of eight cases. J Inherit Metab Dis. doi:10.1007/s10545-014-9716-5
Fiege B, Blau N (2007) Assessment of tetrahydrobiopterin (BH4) responsiveness in phenylketonuria. J Pediatr 150:627–630
Friedman PA, Kappelman AH, Kaufman S (1972) Partial purification and characterization of tryptophan hydroxylase from rabbit hindbrain. J Biol Chem 247:4165–4173
Grange DK, Hillman RE, Burton BK et al (2014) Sapropterin dihydrochloride use in pregnant women with phenylketonuria: an interim report of the PKU MOMS sub-registry. Mol Genet Metab 112:9–16
Hennermann JB, Roloff S, Gebauer C, Vetter B, von Arnim-Baas A, Mönch E (2012) Long-term treatment with tetrahydrobiopterin in phenylketonuria: treatment strategies and prediction of long-term responders. Mol Genet Metab 107:294–301
Keil S, Anjema K, van Spronsen FJ et al (2013) Long-term follow-up and outcome of phenylketonuria patients on sapropterin: a retrospective study. Pediatrics 131:e1881–e1888
Kure S, Hou DC, Ohura T et al (1999) Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. J Pediatr 135:375–378
Lambruschini N, Perez-Duenas B, Vilaseca MA et al (2005) Clinical and nutritional evaluation of phenylketonuric patients on tetrahydrobiopterin monotherapy. Mol Genet Metab 86(Suppl 1):S54–S60
Levy HL, Milanowski A, Chakrapani A et al (2007) Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study. Lancet 370:504–510
Marletta MA (1993) Nitric oxide synthase structure and mechanism. J Biol Chem 268:12231–12234
Merck Serono (2013) Kuvan 100 mg soluble tablets: summary of product characteristics. http://www.medicines.org.uk/emc/medicine/21362/SPC. Accessed 21 Aug 2014
Miras A, Boveda MD, Leis MR et al (2013) Risk factors for developing mineral bone disease in phenylketonuric patients. Mol Genet Metab 108:149–154
Shiman R, Akino M, Kaufman S (1971) Solubilization and partial purification of tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem 246:1330–1340
Shintaku H (2002) Disorders of tetrahydrobiopterin metabolism and their treatment. Curr Drug Metab 3:123–131
Shintaku H, Kure S, Ohura T et al (2004) Long-term treatment and diagnosis of tetrahydrobiopterin-responsive hyperphenylalaninemia with a mutant phenylalanine hydroxylase gene. Pediatr Res 55:425–430
Trefz FK, Burton BK, Longo N et al (2009) Efficacy of sapropterin dihydrochloride in increasing phenylalanine tolerance in children with phenylketonuria: a phase III, randomized, double-blind, placebo-controlled study. J Pediatr 154:700–707
World Health Organization (2011) The WHO Child Growth Standards. http://www.who.int/childgrowth/en/. Accessed 21 Aug 2014
Acknowledgments
This study (EMR700773_001) was supported by Merck Serono SA Geneva, Switzerland, a subsidiary of Merck KGaA, Darmstadt, Germany. Writing assistance was provided by Alyson Bexfield and Jane Davies of Caudex Medical, Oxford, UK (supported by Merck Serono SA Geneva, Switzerland). The authors thank Charles Edward Jefford of EMD Serono, Inc., Billerica, MA, USA, a subsidiary of Merck KGaA, Darmstadt, Germany, for support in the development of this manuscript.
The authors would also like to thank the KAMPER study investigators: Austria: Michaela Brunner-Krainz, Daniela Karall and Dorothea Möslinger; France: Magalie Barth, Nathalie Bednarek, Antoine Bedu, Thierry Billette de Villemeur, Pierre Broué, Brigitte Chabrol, Dries Dobbelaere, Cécile Dumesnil, Didier Eyer, Alain Fouilhoux, François Labarthe, Delphine Lamireau, Gilles Morin, Jean-Claude Netter, Vassili Valayannopoulos and Kathy Wagner-Mahler; Germany: Philipp Guder, Julia Hennermann, Jürgen Herwig, Ralf Husain, Martin Lindner, Amelie S. Lotz-Havla, Klaus Mohnike, Alexandra Puchwein-Schwepcke, Dagmar Scheible and Michael Staudigl; Italy: Generoso Andria, Milva Orquidea Bal, Roberto Cerone, Daniela Concolino, Antonio Correra, Vincenzo Leuzzi, Concetta Meli, Enrica Riva and Iris Scala; Netherlands: Annet M Bosch, Maria Estela Rubio-Gozalbo and Ans van der Ploeg; Slovakia: Katarina Hálová and Ludmila Potocnakova; Spain: Javier Blasco Alonso, Jaume Campistol Plana, Maria Luz Couce Pico, Maria García Jimenez, David Gil Ortega, Domingo González-Lamuño, Mónica Ruiz Pons, Angeles Rúiz Gomez, Félix Sánchez-Valverde and Pablo Sanjurjo Crespo.
Author information
Authors and Affiliations
Consortia
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: Nenad Blau, PhD
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Appendices
Synopsis
Initial data from the ongoing, 15-year, Kuvan® Adult Maternal Paediatric European Registry show that sapropterin dihydrochloride has a good safety profile in patients with phenylalanine hydroxylase deficiency or tetrahydrobiopterin deficiency.
Compliance with Ethics Guidelines
Conflicts of Interest
F. K. Trefz has served as a member on Merck Serono SA Geneva, Switzerland, advisory boards or similar committees; has current or recent participation in a clinical trial sponsored by Merck Serono SA Geneva, Switzerland; has assisted in the design of and/or participated in clinical studies using products manufactured by Merck Serono SA Geneva, Switzerland; and has received consulting fees or other remuneration including speaker fees from Merck Serono SA Geneva, Switzerland.
A. C. Muntau has participated in strategic advisory boards for Merck Serono SA Geneva, Switzerland; has assisted in the design of and/or participated in clinical studies using products manufactured by Merck Serono SA Geneva, Switzerland; and has received honoraria as a consultant and as a speaker from Merck Serono SA Geneva, Switzerland.
F. B. Lagler has served as a member on Merck Serono SA Geneva, Switzerland, advisory boards and has received research grants from Merck GesmbH, Austria.
F. Moreau is an employee of EMD Serono, Inc., Billerica, MA, USA.
J. Alm has served as a member on Merck Serono SA Geneva, Switzerland, advisory boards or similar committees; has assisted in the design of and/or participated in clinical studies using products manufactured by Merck Serono SA Geneva, Switzerland; and has received honoraria as a consultant from Merck AB Sweden.
A. Burlina has served as a member on Merck Serono SA Geneva, Switzerland, advisory boards or similar committees; has current or recent participation in a clinical trial sponsored by Merck Serono SA Geneva, Switzerland; has assisted in the design of and/or participated in clinical studies using products manufactured by Merck Serono SA Geneva, Switzerland; and has received consulting fees or other remuneration including speaker fees from Merck Serono SA Geneva, Switzerland.
F. Rutsch has served as a member on Merck Serono SA Geneva, Switzerland, advisory boards or similar committees; has assisted in the design of and/or participated in clinical studies using products manufactured by Merck Serono SA Geneva, Switzerland; and has received consulting fees or other remuneration including speaker fees from Merck Serono SA Geneva, Switzerland.
A. Bélanger-Quintana has participated in strategic advisory boards and received grants and fees for presentations from Merck Serono SA Geneva, Switzerland, and Nutricia.
F. Feillet has served as a member on Merck Serono SA Geneva, Switzerland, advisory boards or similar committees; has current or recent participation in a clinical trial sponsored by Merck Serono SA Geneva, Switzerland; has assisted in the design of and/or participated in clinical studies using products manufactured by Merck Serono SA Geneva, Switzerland; and has received consulting fees or other remuneration including speaker fees from Merck Serono SA Geneva, Switzerland.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national), with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guideline for Good Clinical Practice (ICH topic E6, 1996) and with the Helsinki Declaration. Informed consent was obtained from all patients included in the registry.
Contributions of Individual Authors
F.K. Trefz was substantially involved in the conception and design of the study protocol; the interpretation of data; and the preparation, review and approval of the manuscript.
A.C. Muntau was substantially involved in the conception and design of the study protocol; the interpretation of data; and the preparation, review and approval of the manuscript.
F.B. Lagler was substantially involved in the conception and design of the study protocol; the interpretation of data; and the preparation, review and approval of the manuscript.
F. Moreau was substantially involved in the conception and design of the study protocol and the development of the statistical analysis plan; the analysis and interpretation of data; and the preparation, review and approval of the manuscript.
J. Alm was substantially involved in the conception and design of the study protocol; the interpretation of data; and the preparation, review and approval of the manuscript.
A. Burlina was substantially involved in the conception and design of the study protocol; the interpretation of data; and the preparation, review and approval of the manuscript.
F. Rutsch was substantially involved in collection and interpretation of data and the preparation, review and approval of the manuscript.
A. Bélanger-Quintana was substantially involved in the conception and design of the study protocol; the interpretation of data; and the preparation, review and approval of the manuscript.
F. Feillet is the principal investigator of the KAMPER study and was substantially involved in the conception and design of the study protocol; the interpretation of data; and the preparation, review and approval of the manuscript.
Rights and permissions
Copyright information
© 2015 SSIEM and Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Trefz, F.K. et al. (2015). The Kuvan® Adult Maternal Paediatric European Registry (KAMPER) Multinational Observational Study: Baseline and 1-Year Data in Phenylketonuria Patients Responsive to Sapropterin. In: Zschocke, J., Baumgartner, M., Morava, E., Patterson, M., Rahman, S., Peters, V. (eds) JIMD Reports, Volume 23. JIMD Reports, vol 23. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2015_425
Download citation
DOI: https://doi.org/10.1007/8904_2015_425
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-47466-2
Online ISBN: 978-3-662-47467-9
eBook Packages: MedicineMedicine (R0)