Abstract
The porphyrias are a group of inherited metabolic diseases resulting from enzymatic deficiencies of specific haem biosynthetic enzymes. They can be classified as primarily acute and non-acute types. Clinically, the acute hepatic porphyrias (AHPs) are characterised by acute neurovisceral attacks. Patients with AHP may be at increased risk for development of hepatocellular carcinoma (HCC). However, systematic studies on the occurrence of other malignancies in patients with the AHPs have not been performed to date. Here, we studied the development of HCC and distinct malignant tumours in patients with the AHPs registered in a single European porphyria specialist centre. A questionnaire was designed and sent to all individuals (n = 122) diagnosed between 1970 and 2012 of whom a valid address was available (n = 82), requesting information on their personal and family history of cancer. Statistical analysis was performed to calculate incidence, prevalence and relative risk of HCC. To calculate confidence intervals, a Poisson distribution was assumed. Forty-nine patients (59.8%) returned a completed questionnaire. Overall, HCC was diagnosed in one female (2.1%), and the remaining patients reported on six distinct malignancies. We were able to confirm that HCC is an important complication in AHP. The patients in our cohort had an approximately 35-fold increased risk of developing HCC, similar to observations in other European countries. In addition, we detected colon, breast, uterine and thyroid cancer as well as lymphoma and a liver metastasis in patients with AHP. However, considering the small number of tumours and patients studied here, the data should be interpreted with caution, and further studies on cancer occurrence in AHP patients will require a multicentre setting.
Competing interests: None declared
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute hepatic porphyrias
- Acute intermittent porphyria
- Hepatocellular carcinoma
- Hereditary coproporphyria
- Porphyrias
- Variegate porphyria
- δ-Aminolaevulinic acid dehydratase-deficient porphyria
Introduction
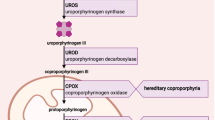
The porphyrias are a group of rare inherited metabolic diseases that result from a catalytic deficiency of one of the eight enzymes along the haem biosynthetic pathway. They can be classified as acute hepatic, hepatic cutaneous and erythroid cutaneous forms. The acute hepatic porphyrias (AHPs) are characterised by life-threatening acute neurovisceral attacks. The AHPs include autosomal dominant acute intermittent porphyria (AIP), variegate porphyria (VP), hereditary coproporphyria (HCP) and autosomal recessive δ-aminolaevulinic acid dehydratase-deficient porphyria (ADDP). Clinically, the AHPs are characterised by the sudden onset of unspecific neurovisceral symptoms, including colicky abdominal pain, nausea and vomiting, constipation, tachycardia, hypertension, paraesthesia, muscle and back pain, para- and tetraplegia, encephalopathy, paralysis, anxiety and acute psychosis. VP and HCP are sometimes referred to as neurocutaneous porphyrias because they can also manifest with blistering photosensitivity on sun-exposed areas of the body.
A serious complication in the AHPs is the development of hepatocellular carcinoma (HCC). HCC is the most common primary malignant liver tumour and one of the most common malignant tumours worldwide (Mendy and Walton 2009). More than 500,000 new cases are diagnosed each year, with the highest incidence being observed in Asia and Africa (Mendy and Walton 2009; Nordenstedt et al. 2010). However, the incidence has also increased in the United States and Europe during the past decade (Llovet et al. 2003). Well-known risk factors for the development of HCC include alcohol ingestion, hepatitis B and C virus infection and liver cirrhosis (Hardell et al. 1984; Fattovich et al. 2004). Several mortality and case–control studies have demonstrated an association between this type of liver cancer and the AHPs in Europe (Lithner and Wetterberg 1984; Bengtsson and Hardell 1986; Kauppinen and Mustajoki 1988; Andersson et al. 1996; Linet et al. 1999; Andant et al. 2000; Schneider-Yin et al. 2009; Innala and Andersson 2011; Elder et al. 2013; Sardh et al. 2013). Concerning other malignant tumour entities, however, systematic studies on their occurrence in AHP patients have not yet been conducted. To date, only few single cases have been reported (Scarlett et al. 1995; Schaffer et al. 2001; Forget et al. 2001; Davies and Whitaker 2002; Kristiansen and Langkjer 2006; Hanneken et al. 2009; Cunningham et al. 2010; Mañas Gómez et al. 2011).
Here, we sought to assess the frequency of HCC and other malignancies in patients with AHP and their first-degree relatives in a single European porphyria specialist centre and, in particular, whether the frequency of HCC differs from that encountered in other European countries.
Material and Methods
Patients and Questionnaire
In order to gain insight into individual medical histories, we developed a questionnaire (Fig. 1) that we sent to all patients with AHP (n = 122) from our porphyria registry, comprising 97 patients with AIP, 20 with VP, 4 with HCP and one patient with ADDP. Most of these patients resided in North Rhine-Westphalia (NRW), which currently has 17.84 million inhabitants and is the most populated state of Germany. We requested personal and family histories of malignant tumours and symptoms of the disease as well as health behaviour. Additionally, patients with the neurocutaneous porphyrias (VP and HCP) received a second questionnaire regarding sun exposure, ingestion of phototoxic and photoprotective drugs and cutaneous symptoms (data not shown). All participants in this study provided informed consent, in accordance with guidelines set forth by the Ethics Committee of the Heinrich Heine University Düsseldorf and the Declaration of Helsinki principles.
Incidence and Prevalence of AHP and Relative Risk of HCC
The incidence of AHP in our cohort was calculated as the ratio of the number of symptomatic patients who were first diagnosed between January 2007 and December 2009 to the number of patient-years corresponding to the reference population in that period, according to Elder et al. (2013). The prevalence was calculated as the product of the incidence rate and the mean duration of disease. As mean duration of disease, 45 years was used for AIP and 40 years for VP, in accordance with Elder et al. (2013)
To calculate confidence intervals, a Poisson distribution was assumed for the observed cases (Ulm 1990). The relative risk for developing HCC was measured by the ratio of the observed number of HCC cases among porphyric patients to the number of expected HCC cases to be among them (Kauppinen and Mustajoki 1988; Andant et al. 2000). The number of expected cases was calculated by multiplying, within each 5-year age group, the corresponding mean incidence of HCC in NRW for the years 2010–2011, according to data derived from the cancer registry NRW database (http://www.krebsregister.nrw.de), by the number of patient-years in our AHP cohort. To standardise for gender, the incidence rates in the age groups were calculated as a weighted mean of the respective gender-specific incidence rates, where the weights correspond to the age group-specific gender distribution in the AHP cohort.
The population of NRW was obtained from the State Office for Data Processing and Statistics NRW website (http://www.it.nrw.de) and averaged across year values. The populations of the European countries were obtained from the Eurostat website (http://epp.eurostat.ec.europa.eu) and averaged across year values between January 2007 and January 2010 (reference date January 1st). HCC cases in 5-year age groups for HCC in NRW were obtained from the cancer registry NRW website that was first established in 2005. Raw incidence rates were calculated based on the HCC cases using the NRW population in 5-year age groups as available from the State Office for Data Processing and Statistics NRW website. Data were analysed using the software R, version 3.0.2 (R Core Team, 2013).
Results
Questionnaire
Only 82 of the 122 AHP patients registered in our centre could be reached by mail. 49 of these 82 patients (59.8%) returned a completed questionnaire, including 38 with AIP, 10 with VP and 1 patient with ADDP. None of the patients with HCP contributed to our study. Patients reported on a total of seven malignant tumours (Table 1).
Malignant Tumours
The group of malignant tumours contained one HCC, one colon carcinoma, one liver metastasis of a colon carcinoma, one breast carcinoma, one malignant uterus tumour, one thyroid carcinoma and one non-Hodgkin lymphoma (Table 1).
Incidence of AHP
The incidence rate of AHP in NRW in the years 2007–2009 was 0.26 per million per year (95% CI: 0.14–0.44). Considering the two most frequent types of AHP separately, the incidence rate of AIP was 0.20 per million per year (95% CI: 0.10–0.37), and the incidence rate of VP was 0.06 per million per year (95% CI: 0.01–0.16).
Prevalence of AHP
The prevalence of AIP for NRW in the years 2007–2009 was 9.19 per million (95% CI: 4.59–16.44) and the prevalence of VP was 2.23 per million (95% CI: 0.46–6.51).
Relative Risk of Developing HCC
AHP patients in NRW had an approximately 35-fold increased risk of developing HCC (relative risk: 34.64, 95% CI: 0.88–193.01).
Discussion
Previously, systematic studies on the spectrum of malignant tumours in patients with AHP have not yet been conducted in any porphyria specialist centre. In this respect, it is important to state that our data showed an incidence of AHP in our cohort that is similar to that encountered in other European countries (Elder et al. 2013). In contrast to most epidemiological studies on the incidence of tumours, which are usually carried out on large cohorts from the general population, this work focuses on a small group of rare hereditary metabolic diseases, which affect only a few individuals. However, the increased incidence of certain tumour entities within a group of rare diseases could provide clues as to whether any of these tumours might have an immediate association with a specific acute porphyria variant. Thus, the work presented here aims at contributing to a better understanding of the occurrence of cancer in patients with AHP.
One of the most severe complications in the AHPs is the development of HCC, a tumour with poor prognosis. Since the first report on the concomitant occurrence of HCC and AIP in 1984, several studies have shown an association between this type of primary liver cancer and the AHPs in Europe (Lithner and Wetterberg 1984; Hardell et al. 1984; Bengtsson and Hardell 1986; Tidman et al. 1989; Grabczynska et al. 1996; Andersson et al. 1996; Linet et al. 1999; Andant et al. 2000; Schneider-Yin et al. 2009; Innala and Andersson 2011; Elder et al. 2013; Sardh et al. 2013). However, some of the aforementioned studies linking HCC to AHP certainly include cases in which there were further risk factors. Whenever case numbers are small, even one or two of these factors can introduce bias. Although we tried to address such a putative bias by sending a questionnaire to our patients, we are aware that this questionnaire may not be exhaustive regarding individual risk profiles since we did not ask for, e.g. alcohol ingestion and cigarette smoking. Further, questionnaire-based assessments may not be entirely reliable.
In our study, we detected a female with AIP who developed a HCC. Based on these data, patients with AHPs in NRW have a 35-fold increased risk to develop a HCC (relative risk: 34.64, 95% CI: 0.88–193.01). This result is consistent with the data of other European porphyria centres, in particular France (Andant et al. 2000). In these studies, AHP patients in France had a 36-fold relative risk and those from Finland a 61-fold relative risk to develop HCC (Kauppinen and Mustajoki 1988; Andant et al. 2000). The considerable amount of uncertainty in our estimate is due to the small number of AHP patients developing a HCC in our cohort.
Studies on the association between AHPs and HCC were also conducted in Switzerland, Sweden and Great Britain. However, the results did not include relative risks for the development of this tumour. In Switzerland, two AIP patients and two VP patients with HCC were observed over a period of 15 years (Schneider-Yin et al. 2009). Sweden is the only European country from which data derived from different geographic regions and porphyria centres are available. In 1986, Bengtsson et al. reported on five patients with AIP who developed HCC (Bengtsson and Hardell 1986). In a retrospective study from 1996, Andersson and colleagues identified nine patients with AIP from Northern Sweden who had HCC (Andersson et al. 1996). Later, the same group reported on 22 patients with AIP and HCC (Innala and Andersson 2011). Recently, Sardh et al. detected 23 primary liver tumours in patients with AHP (Sardh et al. 2013). An overview of the major studies on the concomitant occurrence of HCC and AHP is given in Table 2. In 1989 and 1996, two patients with VP and HCC were reported from Great Britain (Tidman et al. 1989; Grabczynska et al. 1996).
To date, the pathological mechanisms underlying the development of HCC in patients with AHP are largely unknown (Andant et al. 2000). Although in the general population cirrhosis is the most important aetiological factor, it seems to be only of minor importance in patients with AHP (Fattovich et al. 2004; Mullhaupt et al. 2008; Deybach and Puy 2011; Zhou et al. 2014). In support of this notion, we did not find any patient with liver cirrhosis in our study. Furthermore, only one patient with AIP and concomitant hepatitis C virus infection was detected.
Regarding the occurrence of non-hepatic tumours in patients with AHP, there are reports on breast cancer, thyroid cancer and non-Hodgkin lymphoma (Scarlett et al. 1995; Schaffer et al. 2001; Forget et al. 2001; Davies and Whitaker 2002; Kristiansen and Langkjer 2006; Mañas Gómez et al. 2011). However, these were only few and mostly incidental cases and systematic studies from a porphyria specialist centre have not been performed yet. Interestingly, malignant tumours of the gastrointestinal tract have so far not been reported in association with AHP.
In our cohort, one patient with VP developed colorectal cancer with hepatic metastasis (Hanneken et al. 2009). Of note, three other patients with VP had a positive family history with regard to colon cancer. Even more interestingly, two patients suffered from Crohn’s disease, a chronic inflammatory bowel disorder that is a known risk factor for the development of malignant intestinal tumours such as colon cancer (Cunningham et al. 2010). Considering these data, the question arises, if VP could confer some degree of susceptibility for the development of gastrointestinal disease and colorectal cancer. However, there are certain statistical limitations of observational studies suggesting a putative association or a causative link between two conditions of which one is very rare. Thus, we are well aware that the number of patients studied here is too small to draw such a conclusion. Furthermore, we cannot compare our data with that from other porphyria centres at this time.
With regard to other types of cancer, one individual with AIP was diagnosed with breast cancer, and three other patients with AIP had a positive family history regarding this malignancy. Additionally, one AIP patient in our cohort had cervical cancer, one patient with AIP had thyroid cancer, and another individual with VP suffered from non-Hodgkin lymphoma.
Certainly, our data should be interpreted with caution because the sample studied was relatively small and contained only one patient with HCC. Therefore, the likelihood of error cannot be completely ruled out, in particular since the sample may not have captured all the characteristics and variables of the target population, making it difficult to distinguish real differences from random variation. Notwithstanding, our data indicate that the patients with AHP in our cohort have an approximately 35-fold increased risk of developing HCC. This is in support of previous reports from other European countries, which may suggest that the AHP reflect an important aetiological risk factor for this tumour entity. For the first time, we systematically studied the occurrence of malignant tumours other than HCC within a cohort of AHP patients. In order to obtain results based on a larger number of cases than in our study focusing on the NRW region of Germany, our data on the frequency of non-hepatic malignancies in the AHPs require confirmation from other porphyria specialist centres, preferably in a multicentre setting. Without such information, our findings regarding the occurrence of malignancies other than hepatic cancer are difficult to interpret.
Abbreviations
- ADDP:
-
δ-Aminolaevulinic acid dehydratase-deficient porphyria
- AHP:
-
Acute hepatic porphyria
- AHPs:
-
Acute hepatic porphyrias
- AIP:
-
Acute intermittent porphyria
- HCC:
-
Hepatocellular carcinoma
- HCP:
-
Hereditary coproporphyria
- VP:
-
Variegate porphyria
References
Andant C, Puy H, Bogard C et al (2000) Hepatocellular carcinoma in patients with acute hepatic porphyria: frequency of occurrence and related factors. J Hepatol 32:933–939
Andersson C, Bjersing L, Lithner F (1996) The epidemiology of hepatocellular carcinoma in patients with acute intermittent porphyria. J Intern Med 240:195–201
Bengtsson NO, Hardell L (1986) Porphyrias, porphyrins and hepatocellular cancer. Br J Cancer 54:115–117
Cunningham D, Atkin W, Lenz H-J et al (2010) Colorectal cancer. Lancet 375:1030–1047. doi:10.1016/S0140-6736(10)60353-4
Davies JH, Whitaker SJ (2002) Chlorambucil and acute intermittent porphyria. Clin Oncol (R Coll Radiol) 14:491–493
Deybach J-C, Puy H (2011) Hepatocellular carcinoma without cirrhosis: think acute hepatic porphyrias and vice versa. J Intern Med 269:521–524. doi:10.1111/j.1365-2796.2011.02358.x
Elder G, Harper P, Badminton M et al (2013) The incidence of inherited porphyrias in Europe. J Inherit Metab Dis 36:849–857. doi:10.1007/s10545-012-9544-4
Fattovich G, Stroffolini T, Zagni I, Donato F (2004) Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 127:S35–S50
Forget F, Awada A, Klastersky J (2001) Anticancer chemotherapy in a patient with prior history of acute intermittent porphyria. A case report and review of the literature. Support Care Cancer 9:465–466
Grabczynska SA, McGregor JM, Hawk JL (1996) Late onset variegate porphyria. Clin Exp Dermatol 21:353–356
Hanneken S, Kuerten V, Hoernke M, Neumann NJ (2009) Metastatic colon cancer triggering an acute attack of variegate porphyria. Int J Colorectal Dis 24:127–128. doi:10.1007/s00384-008-0537-6
Hardell L, Bengtsson NO, Jonsson U et al (1984) Aetiological aspects on primary liver cancer with special regard to alcohol, organic solvents and acute intermittent porphyria–an epidemiological investigation. Br J Cancer 50:389–397
Innala E, Andersson C (2011) Screening for hepatocellular carcinoma in acute intermittent porphyria: a 15-year follow-up in northern Sweden. J Intern Med 269:538–545. doi:10.1111/j.1365-2796.2010.02335.x
Kauppinen R, Mustajoki P (1988) Acute hepatic porphyria and hepatocellular carcinoma. Br J Cancer 57:117–120
Kristiansen C, Langkjer ST (2006) Treatment and treatment considerations in a patient with advanced breast cancer and acute intermittent porphyria. Acta Oncol 45:337–339. doi:10.1080/02841860500434697
Linet MS, Gridley G, Nyrén O et al (1999) Primary liver cancer, other malignancies, and mortality risks following porphyria: a cohort study in Denmark and Sweden. Am J Epidemiol 149:1010–1015
Lithner F, Wetterberg L (1984) Hepatocellular carcinoma in patients with acute intermittent porphyria. Acta Med Scand 215:271–274
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362:1907–1917. doi:10.1016/S0140-6736(03)14964-1
Mañas Gómez MJ, González O, Vilallonga R, Armengol Carrasco M (2011) Severe hyponatremia in postoperatory of papillary thyroid carcinoma as a manifestation of acute intermittent porphyria. Med Clin (Barc) 136:556–557. doi:10.1016/j.medcli.2010.09.037
Mendy M, Walton R (2009) Molecular pathogenesis and early detection of hepatocellular carcinoma–perspectives from West Africa. Cancer Lett 286:44–51. doi:10.1016/j.canlet.2009.04.039
Mullhaupt B, Junker C, Wuest E, Renner EL (2008) Mortality from primary liver cancer in Switzerland from 1975 to 1994. Swiss Med Week 138:313–316
Nordenstedt H, White DL, El-Serag HB (2010) The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis 42(Suppl 3):S206–S214. doi:10.1016/S1590-8658(10)60507-5
Sardh E, Wahlin S, Björnstedt M et al (2013) High risk of primary liver cancer in a cohort of 179 patients with Acute Hepatic Porphyria. J Inherit Metab Dis 36:1063–1071. doi:10.1007/s10545-012-9576-9
Scarlett JD, Corry J, Jeal PN (1995) Cytotoxic chemotherapy and radiotherapy in a patient with breast cancer and variegate porphyria (VP). Aust N Z J Med 25:742–743
Schaffer M, Schaffer PM, Panzer M et al (2001) Porphyrias associated with malignant tumors: results of treatment with ionizing irradiation. Onkologie 24:170–172
Schneider-Yin X, Harms J, Minder EI (2009) Porphyria in Switzerland, 15 years experience. Swiss Med Week 139:198–206
Tidman MJ, Higgins EM, Elder GH, MacDonald DM (1989) Variegate porphyria associated with hepatocellular carcinoma. Br J Dermatol 121:503–505
Ulm K (1990) A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol 131:373–375
Zhou Y-M, Zhang X-F, Li B et al (2014) Prognosis after resection of hepatitis B virus-related hepatocellular carcinoma originating from non-cirrhotic liver. Ann Surg Oncol. doi:10.1245/s10434-014-3505-0
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: John Christodoulou, MB BS PhD FRACP FRCPA
Appendices
Synopsis
We performed a systematic analysis of the occurrence of malignant tumours in patients with acute hepatic porphyrias, which indicates that German patients with this type of porphyria have an approximately 35-fold increased risk of developing hepatocellular carcinoma.
Author Contribution
E.L. and J.F. were involved in conception and design of the study, analysis and interpretation of data and drafting the article. M.S. and H.S. were involved in analysis and interpretation of data and critical revision of the manuscript. N.J.N. was involved in conception and design of the study and critical revision of the manuscript.
Compliance with Ethics Guidelines
Conflict of Interest
Estefanía Lang, Martin Schäfer, Holger Schwender, Norbert J. Neumann, and Jorge Frank declare that they have no conflict of interest.
Rights and permissions
Copyright information
© 2015 SSIEM and Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Lang, E., Schäfer, M., Schwender, H., Neumann, N.J., Frank, J. (2015). Occurrence of Malignant Tumours in the Acute Hepatic Porphyrias. In: Zschocke, J., Baumgartner, M., Morava, E., Patterson, M., Rahman, S., Peters, V. (eds) JIMD Reports, Volume 22. JIMD Reports, vol 22. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2015_406
Download citation
DOI: https://doi.org/10.1007/8904_2015_406
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-47452-5
Online ISBN: 978-3-662-47453-2
eBook Packages: MedicineMedicine (R0)