Abstract
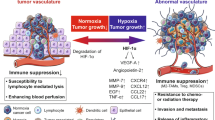
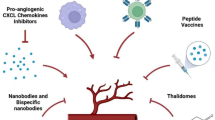
Cancer immunotherapies have yielded promising results in recent years, but new approaches must be utilized if more patients are to experience the benefits of these therapies. Angiogenesis and the tumor endothelium confer unique immune privilege to a growing tumor, with significant effects on diverse immunological processes such as hematopoietic cell maturation, antigen presentation, effector T cell differentiation, cytokine production, adhesion, and T cell homing and extravasation. Here, we review the role of angiogenesis and the tumor endothelium on regulation of the antitumor immune response. We place particular emphasis on the role of vascular endothelial growth factor (VEGF) in the suppression of numerous immunological processes that control tumor progression. Further, we describe the unique crosstalk between the VEGF and endothelin systems, and how their interactions may shape the antitumor immune response. These insights establish new targets for combinatorial approaches to modify existing cancer immunotherapies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vascular Endothelial Growth Factor
- Cancer Immunotherapy
- Antitumor Response
- Vascular Endothelial Growth Factor Signaling
- Serum Vascular Endothelial Growth Factor Level
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In recent years, cancer immunotherapy has had promising successes, resulting in objective clinical responses in patients with melanoma and other tumors (a historical perspective of cancer immunotherapy has been reviewed in detail by Rosenberg et al. 2008 and Gattinoni et al. 2006b). Conventionally, investigational approaches have centered on nonspecific immune modulation capitalizing on intrinsic tumor immunogenicity (e.g., therapeutic use of interleukin-2 [IL-2] or blocking antibody against cytotoxic T-lymphocyte antigen-4 [CTLA-4]) (Phan et al. 2003; Rosenberg et al. 1985, 1998), cancer vaccines (using tumor antigens or tumor antigen-pulsed antigen-presenting cells) (Chiang et al. 2010), or adoptive cell therapy (ACT) using expanded, autologous tumor-infiltrating lymphocytes (TILs) (Gattinoni et al. 2006a; Rosenberg et al. 2008). Several of these therapies have yielded substantial results (particularly ACT), while other strategies have been less successful (cancer vaccines), or have produced significant adverse effects (severe autoimmunity in anti-CTLA-4 treated patients) (Phan et al. 2003). Therefore, new combinatorial approaches toward cancer immunotherapy must be considered to improve the clinical outcome for all patients.
Although generating more effective antitumor immune response is extremely pertinent to the success of future immune therapies, a major obstacle impeding the success of cancer immunotherapy is the tumor microenvironment itself. The tumor microenvironment consists of the tumor cells, blood vessels, stromal cells, immune cells, extracellular matrix components, cytokines, and proteases (Hanahan and Weinberg 2000). The tumor microenvironment can impede the success of immune-based therapies through the suppression of homing, extravasation, and effector functions of effector lymphocytes (Witz 2008). In this review, we describe the underappreciated role of tumor angiogenesis, and vascular endothelial growth factor (VEGF) in particular, in modulating the antitumor response. Additionally, we review the crosstalk between VEGF and the endothelin signaling pathways, and its relationship to antitumor immunity.
2 Angiogenesis and Cancer
Proposed in 1971 by Judah Folkman (Folkman 1971) as an important mechanism for tumor growth, angiogenesis is now a well-established facet of tumor biology and is key to the progression of cancer. Angiogenesis is important for the supply of oxygen, nutrients, growth factors, and additional survival factors necessary for the cellular function and subsistence of tumors. Angiogenesis is considered a balance between pro- and antiangiogenic forces, and the “switch” to a proangiogenic phenotype is one of the hallmarks of malignant processes involved in cancer (Hanahan and Weinberg 2000). Importantly, increased vascularization and the expression of proangiogenic factors are commonly associated with an advanced tumor stage and a poor prognosis in cancer patients (Dvorak et al. 1995; Hicklin and Ellis 2005).
Angiogenesis is a multistep, complex process that begins with the recruitment of sprouting vessels from the existing vasculature and incorporation of endothelial progenitor cells into the newly developing vascular bed (Hicklin and Ellis 2005; Rafii et al. 2002). Endothelial cells proliferate, migrate, and invade the new area forming functional tubular structures that mature into fully formed vessels. Although the development and maturation of new vessel growth is multifaceted, requiring the precise and coordinated activation of a multitude of ligands and receptors (e.g., PDGF, Tie-1, Tie-2), the most pivotal regulator in both physiologic and pathologic angiogenesis is the VEGF and VEGF-receptor (VEGF-R) system (Hicklin and Ellis 2005; Rafii et al. 2002). VEGF signaling remains a critical rate-limiting agent in angiogenesis with pleiotropic effects controlling a multitude of angiogenic processes (Ferrara 2004).
VEGF overexpression is associated with tumorigenesis and a poor prognosis in a multitude of cancers, including gastric carcinoma (Maeda et al. 1996), colorectal carcinoma (Lee et al. 2000; Takahashi et al. 1995), lung cancer (Fontanini et al. 1997), melanoma (Gorski et al. 2003), prostate cancer (George et al. 2001), breast (Berns et al. 2003), and ovarian carcinoma (Paley et al. 1997). VEGF is upregulated in cancer cells in vivo by hypoxia and starvation (Zhang et al. 2002), and also by oncogene activation, which drives constitutive VEGF overexpression (Zhang et al. 2003b). VEGF directly promotes tumor angiogenesis through multiple mechanisms such as endothelial cell proliferation and survival, endothelial cell migration, vessel destabilization via Tie-2 (Zhang et al. 2003c), and enhancing chemotaxis of bone marrow-derived vascular precursor cells (e.g., endothelial cells, pericytes, vascular leukocytes) (Conejo-Garcia et al. 2004; Ellis and Hicklin 2008). In addition, VEGF promotes tumorigenesis through autocrine signaling, regulating tumor cell functions and driving tumor metastases (Ellis and Hicklin 2008). Important for cancer immunotherapy, VEGF has significant roles in modulation of the immune system and tuning the vascular endothelium, leading to the immune evasion by the tumor.
3 Vascular Endothelial Growth Factor
The mammalian VEGF family is comprised of five proteins: VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placenta growth factor (PlGF). The most well-studied family member is VEGF-A (frequently referred to as simply VEGF) (Ellis and Hicklin 2008; Hicklin and Ellis 2005). Alternative splicing of VEGF leads to the expression of multiple functional isoforms of the VEGF protein containing 121, 165, 189, and 206 amino acids. VEGF165 is the predominant functional isoform (Ellis and Hicklin 2008; Hicklin and Ellis 2005). The VEGF ligands bind and activate three structurally similar receptors, tyrosine kinases, VEGF-R1 (also referred to as FLT1), VEGF-R2 (or KDR), and VEGF-R3 (or FTL4). The different VEGF ligands have unique binding specificities for each of these receptors, leading to a complex diversity of function following ligation (Ferrara 2004). In addition, neuropilins (NP-1 and -2) act as coreceptors, increasing the binding affinity of VEGF for VEGF-Rs (Soker et al. 1998). It has been proposed that NPs may signal independently of VEGF-Rs, but this has not been definitively demonstrated.
Ligation of the VEGF-Rs initiates multiple signal transduction cascades unique to each individual VEGF-R, and is responsible for activating the appropriate gene networks (Kowanetz and Ferrara 2006). VEGF-R2 is expressed primarily in the vasculature, and is the key mediator of VEGF-induced angiogenesis. VEGF-R1 is also expressed on the vasculature, and can also be found on other cell types. Although VEGF-R1 has a higher binding affinity relative to VEGF-R2, it induces less activation than VEGF-R2 (Waltenberger et al. 1994). Therefore, VEGF-R1 may act as a functional inhibitor of VEGF-R2 mediated angiogenesis through competitive binding (Hiratsuka et al. 1998). VEGF-R3 primarily binds VEGF-C and -D, and has important roles in cardiovascular development as well as lymphangiogenesis (Ellis and Hicklin 2008; Kukk et al. 1996).
4 Direct Effects of VEGF on Leukocytes
4.1 Dendritic Cell Defects in Cancer Patients and Mouse Models: A Role for VEGF
Dendritic cells (DCs) are central to the generation of an antitumor response. As professional antigen-presenting cells (APC), they present tumor antigens to both B cells and T cells, generating an antigen-specific antitumor response. Defective DC function, combined with a failure of DC maturation, is frequently observed in cancer patients and in tumor-bearing mice. These defects occur in DCs found in the blood, tumor tissue, or draining lymph nodes (Almand et al. 2000; Gabrilovich et al. 1996a,b, 1997; Nestle et al. 1997). The effects of defective DC function (i.e., defective antigen presentation) on the antitumor response are somewhat clear; lack of tumor antigen presentation means lack of effective antitumor response or even worse, active tolerance. Indeed, it has been speculated that immature or incompletely matured DCs may mediate tumor tolerance, inducing T cell anergy or the expansion of regulatory T cells (Tregs) (Lutz and Schuler 2002; Mahnke et al. 2002).
The clinical significance of DC dysfunction has been demonstrated in a study of patients with breast, neck/head, and lung cancer (Almand et al. 2000); DCs isolated from cancer patients were functionally impaired in a mixed leukocyte reaction, and this functional impairment corresponded to a more severe cancer diagnosis (higher stage) (Almand et al. 2000). Further, both the percentage and the total number of DCs were significantly reduced in the peripheral blood of cancer patients, and this observation correlated with an increase in the total number of immature hematopoietic cells. The increase of immature cells in the blood was closely correlated to serum VEGF levels, but not transforming growth factor-beta (TGF-β), IL-6, or granulocyte macrophage colony stimulating factor (GM-CSF) (Almand et al. 2000). Importantly, these aberrations in DCs were somewhat corrected following chemotherapy and anti-VEGF therapy (Almand et al. 2000).
DC defects can be induced by tumor-derived TGF-β (Geissmann et al. 1999) and IL-10 (Steinbrink et al. 1999). However, VEGF plays a significant role in the suppression of DC maturation and function. Although DC defects in cancer patients and tumor-bearing mice had been appreciated for several years prior, Gabrilovich and colleagues were the first to identify a soluble factor, released from tumor cells, that was capable of impairing both DC function and DC maturation from CD34+ hematopoietic precursors (Gabrilovich et al. 1996a). By using neutralizing blocking antibodies, the tumor-derived soluble factor was discovered to be VEGF, and antibodies against IL-10 or TGF-β were unable to reverse the suppression (Gabrilovich et al. 1996a). Similar observations of defective DCs in cancer patients, with a dependence or association with VEGF, have since been made (Della Porta et al. 2005; Takahashi et al. 2004; Yang and Carbone 2004). Experimentally, these finding have been recapitulated in the mouse, suggesting a common mechanism and inherent role for VEGF in the antitumor response. In particular, Ishida and colleagues demonstrated that tumor-bearing mice displayed defects in DC numbers as well as function, and that VEGF blocking antibody reversed these defects (Ishida et al. 1998).
Although several mechanisms may be involved in the generation of DC defects, VEGF can exert its immunosuppressive effects through the disruption of normal hematopoiesis. VEGF continually infused in mice, at levels commonly associated with cancer pathology, resulted not only in defects of DC maturation and function, but also in widespread changes in the differentiation of multiple hematopoietic lineages. For example, VEGF infusion induced a significant increase in B cells and Gr-1+ immature myeloid cells (Della Porta et al. 2005; Gabrilovich et al. 1996a, 1998; Ishida et al. 1998; Ohm and Carbone 2001; Ohm et al. 1999; Takahashi et al. 2004; Yang and Carbone 2004). It has been discovered that VEGF mediates the suppression of DC maturation through the impairment of normal nuclear factor-kappa B (NF-κB) signaling during hematopoiesis (Oyama et al. 1998), mediated through VEGF-R1 signaling on CD34+ hematopoietic progenitor cells (Dikov et al. 2005).
The effects of VEGF on DC maturation and function can be partially reversed through VEGF blockade. Treatment of patients with the VEGF blocking antibody, Bevacizumab, has been shown to partially reverse some of the DC defects. In an initial study by Almand et al., cancer patients receiving anti-VEGF antibody demonstrated a reversal of maturation defects of their DCs, and this observation has also been observed by others (Almand et al. 2000; Fricke et al. 2007; Osada et al. 2008). These observations have also been recapitulated experimentally in mouse tumor models (Gabrilovich et al. 1999; Nair et al. 2003; Roland et al. 2009). Therefore, VEGF blockade may be critical to the success of any cancer immunotherapeutic strategy.
VEGF likely exerts effects on the immune system beyond its role in the suppression of hematopoiesis. B7-H1 is expressed on tumor cells, but it is also highly expressed on tumor-associated myeloid DCs in ovarian cancer patients (Curiel et al. 2003). Interestingly, incubation of blood myeloid DCs with VEGF induced robust expression of B7-H1 on the cell surface (Curiel et al. 2003). B7-H1 is a cell surface protein belonging to the B7 family of costimulatory molecules. B7-H1 may inhibit T cell growth by ligation of the programmed death-1 (PD-1) receptor, as well as promote programmed cell death of effector T cells through an unknown mechanism (Curiel et al. 2003). Therefore, expression of B7-H1 is associated with suppression of T cell effector functions. Thus, VEGF has potential roles in multiple aspects of immunosuppression mediated through DCs.
4.2 Effects of VEGF on T Cells
In the context of cancer immunotherapy, T cells have a well-appreciated role in the antitumor response, and cancer immunotherapies rely on the use of autologous, tumor-reactive T cells to mediate tumor regression (Rosenberg et al. 2008). In ovarian cancer, our lab has demonstrated that the presence of intratumoral T cells (also called intraepithelial T cells) was significantly associated with an increase in the five-year overall survival rate (Zhang et al. 2003a). Specifically, the five-year overall survival rate was 38% for patients with intratumoral T cells, and only 4.5% in patients whose tumor islets contained no T cells (Zhang et al. 2003a). This observation is not unique to ovarian cancer as the infiltration of T cells into tumors has been associated with positive clinical outcomes in breast (Marrogi et al. 1997), prostate (Vesalainen et al. 1994), esophageal (Schumacher et al. 2001), and colorectal cancers (Naito et al. 1998). The effects of VEGF extend to many cell types in the hematopoietic system, and are not exclusive to DCs (Gabrilovich et al. 1998; Huang et al. 2007). VEGF-Rs are expressed on many additional cell types, notably T cells. Interestingly, we observed that ovarian tumors expressing high levels of VEGF were rarely associated with intratumoral T cells (Zhang et al. 2003a). Whether this observation is mediated by VEGF through direct or indirect action on T cells remains to be determined.
Thymic atrophy is a common characteristic of cancer patients (Ohm et al. 2003). Although most cancer patients tend to be older, premature thymic atrophy occurs in many childhood cancers, which is partially reversible upon treatment (Ohm et al. 2003). Further, thymic involution occurs in tumor-bearing mice, suggesting a common mechanism (Ohm et al. 2003). In addition to negative effects on DC maturation, VEGF is also believed to suppress proper T cell development (Huang et al. 2007; Ohm et al. 2003). Treatment of mice with pathologic levels of VEGF, comparable to that seen in cancer patients, induced a robust thymic atrophy, and a significant reduction in CD4+ and CD8+ T cells (Ohm et al. 2003). Further, VEGF blockade in tumor-bearing mice partially reversed the thymic atrophy (Ohm et al. 2003). The immunosuppressive effects of VEGF on T cells occurred on bone marrow precursors, as VEGF did not appreciably disrupt maturation of T cells already in the thymus (Ohm et al. 2003). These effects likely occur through VEGF-R2 signaling on bone marrow precursor cells (Huang et al. 2007). Although pathologic levels of VEGF clearly influence the proper development of T cells, the relevance of these findings and their impact on the antitumor response remain undefined.
Tregs control peripheral tolerance through the suppression of autoreactivity, but are believed to also suppress antitumor immunity. CD4+CD25+Foxp3+ Tregs isolated from tumors were recently demonstrated to suppress tumor-specific T cell immunity both in vitro and in vivo, and importantly, an accumulation of tumor Tregs was associated with reduced survival and a high death hazard (Curiel et al. 2004). However, the precise mechanisms controlling the activation and accumulation of Tregs into tumors remain poorly defined. NP-1, a coreceptor that interacts with VEGF-R1 and -R2, has been detected on CD4+CD25+ Tregs (Sarris et al. 2008). Enhanced activation of NP-1 increased CD4+CD25+ Treg interactions with DCs in preference to T helper (Th) cells (Sarris et al. 2008). Although not specifically demonstrated, enhanced VEGF signaling, in conjunction with NP-1, may enhance Treg activation, creating a tolerogenic environment and tumor evasion. Additionally, VEGF treatment of mouse splenocytes during T cell stimulation has been demonstrated to induce IL-10 production from T cells while suppressing IFN-γ production (Shin et al. 2009). This immunosuppressive effect was attributed to VEGF-R1 expressed on T cells (Shin et al. 2009). Therefore, although it remains to be specifically demonstrated, direct VEGF signaling on T cells may enhance T cell regulatory functions, contributing to an immunosuppressive environment.
In contrast to the observations above, it has been suggested that direct VEGF signaling on T cells may enhance T cell functions (Mor et al. 2004). Coincubation with VEGF of concanavalin A or antigen-stimulated T cells supported Th1 differentiation, enhanced IFN-γ production, and suppressed IL-10 production (Mor et al. 2004). Further, VEGF treatment of T cells during peptide stimulation enhanced the severity of an adoptive transfer model of experimental allergic encephalomyelitis (Mor et al. 2004). In addition, both VEGF-R1 and -R2 were expressed in memory phenotype CD4+CD45RO+ cells in human T cells, but not naïve cells (Basu et al. 2010). VEGF treatment of these cells activated the MAPK and the PI3K-Akt pathways and enhanced IFN-γ production. Further, VEGF was chemotactic for the CD4+CD45RO+ T cells (Basu et al. 2010).
Clearly, the direct effects of VEGF on T cell functions remain inconclusive. However, insights into the roles of VEGF on the T cell antitumor response, either direct or indirect, can be gleaned from studies using VEGF blocking antibodies. In one single-arm clinical trial of a tumor vaccine combined with anti-VEGF therapy (Bevacizumab), it has been shown that the combination is associated with a high rate of T cell specific immune response, characterized by increased IFN-γ levels and T cell proliferation following stimulation with antigen (Rini et al. 2006). Supporting this observation, VEGF-R2 blockade in mice using an anti-VEGF-R2 antibody has been demonstrated to induce a de novo T cell-mediated antitumor response in mice (Manning et al. 2007). VEGF-R2 blockade resulted in spontaneous infiltration of CD4+ and CD8+ T cells that produced IFN-γ, and VEGF-R2 blockade protected against subsequent tumor challenge in a tumor vaccine model (Manning et al. 2007). However, VEGF-R2 blockade resulted in a substantial increase in serum VEGF levels. Therefore, it is unknown whether the antitumor T cell response was generated through blockade of tumorigenic angiogenesis, or increased serum VEGF enhanced activation of T cells through VEGF-R1 signaling. On the other hand, consistent with a role for VEGF signaling in CD4+CD25+ Tregs, VEGF-R2 blockade in this study enhanced T cell effector functions in a tolerized mouse tumor model system (Manning et al. 2007). This observation is supported by the demonstration that anti-VEGF treatment in mice reduced the number of Tregs, decreased Foxp3 expression, enhanced cytotoxic lymphocyte (CTL) induction, and increased tumor vaccine efficacy (Li et al. 2006). In conclusion, VEGF or VEGF-R blockade predominantly enhances T cell antitumor immunity, an effect most consistent with the concept that VEGF has direct immunosuppressive functions on T cells.
5 VEGF, the Tumor Vascular Endothelium, and Immune Evasion
5.1 The Vascular Endothelium
The tumor vascular endothelium presents a significant challenge to the success of immune therapy, as it provides a physical barrier through which tumor-reactive T cells must extravasate, recognize tumors, and exert their cytotoxic effects. The vascular endothelial barrier, frequently prohibitive to tumor-reactive T cells, is maintained by locally expressed cytokines, growth factors, and the nature and quantity of adhesion molecules expressed by the endothelium (Zitvogel et al. 2006). In many of the T cell immune therapies that have been conducted, it has been noted that while activated T cells could be found in the periphery, they often failed to infiltrate the tumor itself (Boon et al. 2006; Dudley et al. 2002; Lurquin et al. 2005). Thus, successful transmigration through the tumor endothelial barrier is required for activated or administered lymphocytes to execute their effector functions, resulting in tumor regression. Precisely how the tumor vasculature establishes immune privilege is not well known, but the ongoing processes of angiogenesis may participate in immune escape. Specifically, tumor-derived VEGF may play a pivotal role in reducing leukocyte homing to and extravasation through the vascular endothelium.
5.2 VEGF and Adhesion Molecule Expression
T cells extravasate through the endothelium to the tumor in a multistep process that includes binding to adhesion molecules expressed on endothelial cells, and is followed by diapedesis. VEGF has been demonstrated to increase the expression of many endothelial cell adhesion molecules (CAMs), particularly in the context of angiogenesis (reviewed in detail by Francavilla et al. 2009). In agreement with this observation, VEGF-induced enhancement of CAM expression has been associated with increased leukocyte adhesion both in vitro and in vivo (Detmar et al. 1998; Min et al. 2005). However, understanding the role of VEGF in leukocyte adhesion is complicated by reports that demonstrate VEGF may actually inhibit adhesion molecule expression on endothelial cells (Bouzin et al. 2007; Detmar et al. 1998; Dirkx et al. 2003; Griffioen et al. 1996a,b; Min et al. 2005).
Although the role of VEGF signaling and leukocyte adhesion may be difficult to discern, in the context of a proinflammatory environment the emerging concept is that angiogenic growth factors impair immune cell adhesion (Bouzin et al. 2007; Griffioen et al. 1996a, b). For example, Griffioen and colleagues demonstrated reduced expression of adhesion molecules intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule (VCAM-1) after treatment of tumor necrosis factor-alpha (TNF-α) stimulated HUVEC with basic fibroblast growth factor (bFGF) or VEGF (Griffioen et al. 1996a). In a similar manner, Bouzin and colleagues observed reductions in ICAM-1 and VCAM-1 expression in TNF-α stimulated HUVECs as early as 2 h after VEGF addition (Bouzin et al. 2007). Although these effects on adhesion molecule expression were transient, longer treatment times demonstrated a disruption of adhesion molecule organization and clustering on the cell surface (Bouzin et al. 2007). This response was associated with a perturbation of the spatial organization and clustering of ICAM-1, and was dependent on caveolin-1 and nitric oxide (Bouzin et al. 2007).
6 The Tumor Endothelium and VEGF Crosstalk: A Role for the Endothelin System
6.1 The Endothelin System
Members of the endothelin system have been identified in a broad array of tissue types, including neuronal, renal, and vascular tissues, and regulate a number of critical physiological processes including reproduction, embryonic development, and cardiovascular homeostasis (Grant et al. 2003; Kedzierski and Yanagisawa 2001; Meidan and Levy 2007; Yanagisawa et al. 1998). The endothelin system has well-known roles in regulating vasoconstriction and mediates both cardiovascular and renal disorders (Bagnato and Rosano 2008; Nelson et al. 2003). Particularly, the endothelin system is an important regulator of physiologic and pathogenic angiogenesis, and VEGF signaling is intimately involved in dynamic crosstalk with the endothelin system (Bagnato and Rosano 2008; Nelson et al. 2003).
The endothelin system is comprised of four endothelin (ET) peptide ligands, ET-1, ET-2, ET-3, and ET-4 (Saida et al. 1989; Yanagisawa and Masaki 1989) that signal through their two G protein-coupled receptors (GPCR), ETAR and ETBR (Frommer and Muller-Ladner 2008; Meidan and Levy 2007). Biologically active ETs are derived from precursor proteins following cleavage by membrane-bound metalloproteinases termed endothelin-converting enzymes (ECE) (Valdenaire et al. 1995). Amongst the four endothelin ligands, ET-1 is the most potent ligand and is widely expressed in multiple cells types, notably endothelial cells (Luscher and Barton 2000). Binding of the ETAR and ETBR by ET peptides triggers downstream signal transduction pathways, including, but not limited to, the RAF/MEK/MAPK pathway and PI3K/AKT pathway (Nelson et al. 2003).
The endothelin axis has been speculated to play significant roles in tumorigenesis. Endothelin or the endothelin receptors or both are upregulated in a number of cancers including ovarian, breast, renal, colon, and prostate cancer (Bagnato and Rosano 2008; Nelson et al. 2003). Importantly, the use of specific endothelin receptor antagonists has been demonstrated to slow tumor growth in patients, or prevent tumor growth in mouse models (Bagnato and Rosano 2008; Nelson et al. 2003). In addition to its role in angiogenesis described in more detail below, the endothelin axis is believed to activate autocrine/paracrine loops that promote proliferation, protection from apoptosis, immune evasion, vasculogenesis, and invasion and metastatic dissemination of tumors (Bagnato and Rosano 2008; Nelson et al. 2003).
6.2 Endothelin and Tumor Angiogenesis
The interactions between endothelin and VEGF regulate multiple aspects of angiogenesis including endothelial cell proliferation, migration, invasion, vessel formation, and neovascularization (Nelson et al. 2003). Further, endothelin and VEGF signaling influence the regulation of vascular permeability (Nelson et al. 2003). In the context of angiogenesis, ET-1 upregulates the expression of the extra domain-B containing fibronectin (EDB+ FN) in human vascular endothelial cells (Bagnato and Spinella 2003; Khan et al. 2005). EDB+ FN has been suggested as a marker of angiogenesis in human cancers and is believed to control ocular neovascularization in patients with proliferative diabetic retinopathy (Bagnato and Spinella 2003; Khan et al. 2005). Additionally, the expression of endothelins, or their receptors, correlates with high expression of VEGF in a multitude of tumor types (Boldrini et al. 2006; Salani et al. 2000a; Wulfing et al. 2004), and elevated expression of ET-1 and VEGF was associated with lymphatic vessel invasion and poor outcomes in invasive ductal breast carcinoma (Gasparini et al. 1994).
ET-1 induces the expression of VEGF in cancer cell lines in vitro (Rosano et al. 2003; Salani et al. 2000b; Spinella et al. 2002, 2007). ET-1 increases VEGF production through HIF-1α (Salani et al. 2000b) by ovarian cancer cells via ETAR activation (Spinella et al. 2004). Additionally, ovarian tumor growth in nude mice was inhibited after treatment with the ETAR-selective antagonist ABT-627, an effect associated with reduced VEGF expression (Spinella et al. 2004). ETBR activation counters ET-1/ETAR activity by increasing production of nitric oxide, promoting ET-1 clearance, triggering apoptotic pathways, and blocking cell growth. However, it is unclear whether this antagonism occurs in tumor cells (Lalich et al. 2007). As such, there may also be role for ETBR in tumor angiogenesis and cancer development (Bagnato and Rosano 2008). ET-1 has been shown to directly promote tumor angiogenesis by inducing endothelial cell survival, proliferation, and invasion in an ETBR-dependent manner (Salani et al. 2000b). ETBR may promote angiogenesis indirectly by upregulating VEGF production in the vasculature (Jesmin et al. 2006). Furthermore, there is a strong correlation between ETBR and VEGF expression in a number of different tumor specimens (Kato et al. 2001). In summary, the interaction of the endothelin system and angiogenesis, and VEGF in particular, may be a significant regulator of tumorigenesis.
6.3 ETBR and the Tumor Endothelial Barrier to T Cell Homing
ETBR is overexpressed in melanoma and is associated with aggressive tumor phenotype (Bachmann-Brandt et al. 2000). Highlighting the role of ETBR in melanoma, the receptor antagonist BQ-788 inhibited the growth of human melanoma cell lines and reduced human melanoma tumor growth in a nude mouse model (Lahav 2005; Lahav et al. 1999). ETBR is also overexpressed in ovarian cancer, Kaposi’s sarcoma, glioblastoma, and breast cancer (Bagnato et al. 2004; Egidy et al. 2000; Kefford et al. 2007; Rosano 2003). Interestingly, ETBR upregulation predicts poor outcome in both breast and ovarian cancers (Grimshaw et al. 2004; Wulfing et al. 2003), and ETBR overexpression has even been proposed as tumor progression marker (Demunter et al. 2001).
Our laboratory has recently demonstrated a novel role for ETBR in tumor immunotherapy (Buckanovich et al. 2008). Microarray analysis was conducted using the endothelial cells isolated using laser capture microdissection. ETBR was discovered as one of the few genes overexpressed in the endothelial cells of tumors lacking TILs (Buckanovich et al. 2008). Immunohistochemical staining of ovarian cancer tumors confirmed this result, and ETBR was localized to the endothelium and the stroma. Importantly, ETBR overexpression was associated with poor survival, likely due to lack of TILs, which was previously demonstrated as an indicator of a good prognosis (Zhang 2003). Further, recombinant human ET-1 blocked the adhesion of activated T cells to human umbilical vein endothelial cells (HUVECs) in vitro (Buckanovich et al. 2008). This effect was reversed if HUVECs were treated with the specific ETBR antagonist, BQ-788. ET-1 signaling through ETBR was discovered to block T cell adhesion to the endothelium through suppression of ICAM-1 expression. ETBR blockade upregulated ICAM-1, promoted ICAM-1 clustering, and restored T cell adhesion (Buckanovich et al. 2008). Thus, these data provide a mechanistic link between the observations made in ovarian cancer patients.
TNF-α is a major inflammatory cytokine implicated in carcinogenesis, tumor angiogenesis, and progression; and it is upregulated in ovarian cancer (Merogi et al. 1997). It has been previously reported that the overall TNF-α mRNA levels are similar in ovarian tumors with or without intraepithelial T cells (Zhang et al. 2003a). This was counterintuitive, as TNF-α is a major factor activating endothelium and promoting adhesion of T cells. It has now been found that ET-1 efficiently blocks adhesion of T cells to endothelial cells even when endothelial cells are activated with TNF-α (Buckanovich et al. 2008). This observation explains the paradox of how tumors may exhibit inflammation yet be prohibitive to T cell infiltration, thus establishing immune privilege even in the face of inflammation.
Based on the above data, the effectiveness of ETBR blockade, using the receptor antagonist BQ-788, was determined using a tumor vaccine therapy that controls tumor growth very poorly. In this context, the tumor vaccine had little effect on tumor growth, but ETBR blockade significantly enhanced the antitumor effect by permitting the infiltration of tumor antigen-specific T cells into the tumor site (Buckanovich et al. 2008). The benefits of ETBR blockade were attenuated with the use of an ICAM-1 neutralizing antibody, indicating that adhesion molecule interactions between the endothelium and T cells were responsible for the antitumor effects of ETBR blockade (Buckanovich et al. 2008). Thus, in tumors there is likely a hyperactivation of ET-1/ETBR signaling that is responsible for the suppression of T cell homing. Furthermore, these results establish a vascular mechanism of tumor immune evasion mediated by the endothelium, and also present a new opportunity to target the ETBR to prevent tumor growth and enhance cancer immunotherapy.
7 Concluding Statements
The mechanisms regulating the overexpression of ETBR on the tumor endothelium are unknown. However, the overexpression of ETBR may participate in a feed-forward loop of autocrine/paracrine ET-1 production and ET receptor signaling between the tumor and the vascular endothelium. Thus, enhanced ET-1 signaling in tumor and endothelial cells through ETBR would lead to enhanced NO and HIF-1α production, followed by increased VEGF production by tumor cells. In the context of inflammation, ETBR and VEGF signaling on endothelial cells would shut down the capacity of T cells to extravasate through the endothelium to attack the tumor through a reduction in adhesion molecule expression, particularly ICAM-1. Further, enhanced VEGF production would support ongoing maturation defects in DCs and possibly T cells, while enhancing Treg activation, leading to reduced antigen presentation and immune evasion.
If this hypothesis is correct, targeted therapies to break the cyclical enhancement of VEGF, ET-1, and NO production should be the key components of any cancer immunotherapy. The use of ETBR receptor antagonists combined with anti-VEGF antibody administration may function synergistically to sanction the tumor environment to attack by the immune system. Thus, new complimentary approaches to existing cancer immunotherapies may enhance the existing therapies and extend their benefits to more patients.
Abbreviations
- ACT:
-
Adoptive cell transfer
- APC:
-
Antigen-presenting cell
- CD:
-
Cluster of differentiation
- CTLA-4:
-
Cytotoxic T-lymphocyte antigen-4
- DC:
-
Dendritic cell
- ECE:
-
Endothelin-converting enzymes
- EDB+ FN:
-
Extra domain-B containing fibronectin
- ET:
-
Endothelin
- ETAR:
-
Endothelin receptor A
- ETBR:
-
Endothelin receptor B
- GM-CSF:
-
Granulocyte macrophage colony stimulating factor
- GPCR:
-
G protein-coupled receptors
- HUVEC:
-
Human umbilical vascular endothelial cell
- ICAM-1:
-
Intercellular adhesion molecule-1
- IFN-γ:
-
Interferon-gamma
- IL:
-
Interleukin
- NF-κB:
-
Nuclear factor-kappa B
- NO:
-
Nitric oxide
- PD-1:
-
Programmed death-1
- PlGF:
-
Placenta growth factor
- TGF-β:
-
Transforming growth factor-beta
- Th:
-
T helper
- TIL:
-
Tumor-infiltrating lymphocyte
- TNF-α:
-
Tumor necrosis factor-alpha
- Treg:
-
Regulatory T cell
- VCAM-1:
-
Vascular cell adhesion molecule-1
- VEGF:
-
Vascular endothelial growth factor
- VEGF-R:
-
Vascular endothelial growth factor receptor
References
Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI (2000) Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res 6:1755–1766
Bachmann-Brandt S, Bittner I, Neuhaus P, Frei U, Schindler R (2000) Plasma levels of endothelin-1 in patients with the hepatorenal syndrome after successful liver transplantation. Transpl Int 13:357–362
Bagnato A, Rosano L (2008) The endothelin axis in cancer. Int J Biochem Cell Biol 40:1443–1451
Bagnato A, Spinella F (2003) Emerging role of endothelin-1 in tumor angiogenesis. Trends Endocrinol Metab 14:44–50
Bagnato A, Rosano L, Spinella F, Di Castro V, Tecce R, Natali PG (2004) Endothelin B receptor blockade inhibits dynamics of cell interactions and communications in melanoma cell progression. Cancer Res 64:1436–1443
Basu A, Hoerning A, Datta D, Edelbauer M, Stack MP, Calzadilla K, Pal S, Briscoe DM (2010) Cutting edge: Vascular endothelial growth factor-mediated signaling in human CD45RO+ CD4+ T cells promotes Akt and ERK activation and costimulates IFN-gamma production. J Immunol 184:545–549
Berns EM, Klijn JG, Look MP, Grebenchtchikov N, Vossen R, Peters H, Geurts-Moespot A, Portengen H, van Staveren IL, Meijer-van Gelder ME, Bakker B, Sweep FC, Foekens JA (2003) Combined vascular endothelial growth factor and TP53 status predicts poor response to tamoxifen therapy in estrogen receptor-positive advanced breast cancer. Clin Cancer Res 9:1253–1258
Boldrini L, Pistolesi S, Gisfredi S, Ursino S, Ali G, Pieracci N, Basolo F, Parenti G, Fontanini G (2006) Expression of endothelin 1 and its angiogenic role in meningiomas. Virchows Arch 449:546–553
Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P (2006) Human T cell responses against melanoma. Annu Rev Immunol 24:175–208
Bouzin C, Brouet A, De Vriese J, Dewever J, Feron O (2007) Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J Immunol 178:1505–1511
Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O'Brien-Jenkins A, Gimotty PA, Coukos G (2008) Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med 14:28–36
Chiang CL-L, Benencia F, Coukos G (2010) Whole tumor antigen vaccines. Semin Immunol 22:132–143
Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, Wagner DS, Katsaros D, Caroll R, Coukos G (2004) Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med 10:950–958
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W (2003) Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 9:562–567
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949
Della Porta M, Danova M, Rigolin GM, Brugnatelli S, Rovati B, Tronconi C, Fraulini C, Russo Rossi A, Riccardi A, Castoldi G (2005) Dendritic cells and vascular endothelial growth factor in colorectal cancer: correlations with clinicobiological findings. Oncology 68:276–284
Demunter A, De Wolf-Peeters C, Degreef H, Stas M, van den Oord JJ (2001) Expression of the endothelin-B receptor in pigment cell lesions of the skin. Evidence for its role as tumor progression marker in malignant melanoma. Virchows Arch 438:485–491
Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK (1998) Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol 111:1–6
Dikov MM, Ohm JE, Ray N, Tchekneva EE, Burlison J, Moghanaki D, Nadaf S, Carbone DP (2005) Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol 174:215–222
Dirkx AE, Oude Egbrink MG, Kuijpers MJ, van der Niet ST, Heijnen VV, Bouma-ter Steege JC, Wagstaff J, Griffioen AW (2003) Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res 63:2322–2329
Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA (2002) Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298:850–854
Dvorak HF, Brown LF, Detmar M, Dvorak AM (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146:1029–1039
Egidy G, Eberl LP, Valdenaire O, Irmler M, Majdi R, Diserens AC, Fontana A, Janzer RC, Pinet F, Juillerat-Jeanneret L (2000) The endothelin system in human glioblastoma. Lab Invest 80:1681–1689
Ellis LM, Hicklin DJ (2008) VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8:579–591
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25:581–611
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
Fontanini G, Lucchi M, Vignati S, Mussi A, Ciardiello F, De Laurentiis M, De Placido S, Basolo F, Angeletti CA, Bevilacqua G (1997) Angiogenesis as a prognostic indicator of survival in non-small-cell lung carcinoma: a prospective study. J Natl Cancer Inst 89:881–886
Francavilla C, Maddaluno L, Cavallaro U (2009) The functional role of cell adhesion molecules in tumor angiogenesis. Semin Cancer Biol 19:298–309
Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Lee JH, Sosman JA, Gabrilovich DI (2007) Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res 13:4840–4848
Frommer KW, Muller-Ladner U (2008) Expression and function of ETA and ETB receptors in SSc. Rheumatology (Oxford) 47(Suppl 5):v27–v28
Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP (1996a) Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2:1096–1103
Gabrilovich DI, Ciernik IF, Carbone DP (1996b) Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol 170:101–110
Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP (1997) Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res 3:483–490
Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP (1998) Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92:4150–4166
Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP (1999) Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res 5:2963–2970
Gasparini G, Weidner N, Bevilacqua P, Maluta S, Dalla Palma P, Caffo O, Barbareschi M, Boracchi P, Marubini E, Pozza F (1994) Tumor microvessel density, p53 expression, tumor size, and peritumoral lymphatic vessel invasion are relevant prognostic markers in node-negative breast carcinoma. J Clin Oncol 12:454–466
Gattinoni L, Powell DJ Jr, Rosenberg SA, Restifo NP (2006) Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 6:383–393
Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, Amigorena S, Hermine O, Durandy A (1999) TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol 162:4567–4575
George DJ, Halabi S, Shepard TF, Vogelzang NJ, Hayes DF, Small EJ, Kantoff PW (2001) Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone-refractory prostate cancer treated on Cancer and Leukemia Group B 9480. Clin Cancer Res 7:1932–1936
Gorski DH, Leal AD, Goydos JS (2003) Differential expression of vascular endothelial growth factor-A isoforms at different stages of melanoma progression. J Am Coll Surg 197:408–418
Grant K, Loizidou M, Taylor I (2003) Endothelin-1: a multifunctional molecule in cancer. Br J Cancer 88:163–166
Griffioen AW, Damen CA, Blijham GH, Groenewegen G (1996a) Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood 88:667–673
Griffioen AW, Damen CA, Martinotti S, Blijham GH, Groenewegen G (1996b) Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res 56:1111–1117
Grimshaw MJ, Hagemann T, Ayhan A, Gillett CE, Binder C, Balkwill FR (2004) A role for endothelin-2 and its receptors in breast tumor cell invasion. Cancer Res 64:2461–2468
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23:1011–1027
Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M (1998) Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA 95:9349–9354
Huang Y, Chen X, Dikov MM, Novitskiy SV, Mosse CA, Yang L, Carbone DP (2007) Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood 110:624–631
Ishida T, Oyama T, Carbone DP, Gabrilovich DI (1998) Defective function of Langerhans cells in tumor-bearing animals is the result of defective maturation from hemopoietic progenitors. J Immunol 161:4842–4851
Jesmin S, Miyauchi T, Goto K, Yamaguchi I (2006) Down-regulated VEGF expression in the diabetic heart is normalized by an endothelin ETA receptor antagonist. Eur J Pharmacol 542:184–185
Kato T, Kameoka S, Kimura T, Soga N, Abe Y, Nishikawa T, Kobayashi M (2001) Angiogenesis as a predictor of long-term survival for 377 Japanese patients with breast cancer. Breast Cancer Res Treat 70:65–74
Kedzierski RM, Yanagisawa M (2001) Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol 41:851–876
Kefford R, Beith JM, Van Hazel GA, Millward M, Trotter JM, Wyld DK, Kusic R, Shreeniwas R, Morganti A, Ballmer A, Segal E, Nayler O, Clozel M (2007) A phase II study of bosentan, a dual endothelin receptor antagonist, as monotherapy in patients with stage IV metastatic melanoma. Invest New Drugs 25:247–252
Khan ZA, Chan BM, Uniyal S, Barbin YP, Farhangkhoee H, Chen S, Chakrabarti S (2005) EDB fibronectin and angiogenesis – a novel mechanistic pathway. Angiogenesis 8:183–196
Kowanetz M, Ferrara N (2006) Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res 12:5018–5022
Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K (1996) VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development 122:3829–3837
Lahav R (2005) Endothelin receptor B is required for the expansion of melanocyte precursors and malignant melanoma. Int J Dev Biol 49:173–180
Lahav R, Heffner G, Patterson PH (1999) An endothelin receptor B antagonist inhibits growth and induces cell death in human melanoma cells in vitro and in vivo. Proc Natl Acad Sci USA 96:11496–11500
Lalich M, McNeel DG, Wilding G, Liu G (2007) Endothelin receptor antagonists in cancer therapy. Cancer Invest 25:785–794
Lee JC, Chow NH, Wang ST, Huang SM (2000) Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer 36:748–753
Li B, Lalani AS, Harding TC, Luan B, Koprivnikar K, Huan Tu G, Prell R, VanRoey MJ, Simmons AD, Jooss K (2006) Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res 12:6808–6816
Lurquin C, Lethe B, De Plaen E, Corbiere V, Theate I, van Baren N, Coulie PG, Boon T (2005) Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J Exp Med 201:249–257
Luscher TF, Barton M (2000) Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation 102:2434–2440
Lutz MB, Schuler G (2002) Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol 23:445–449
Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Sowa M (1996) Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 77:858–863
Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H (2002) Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol 80:477–483
Manning EA, Ullman JG, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, Hicklin DJ, Jaffee EM, Emens LA (2007) A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res 13:3951–3959
Marrogi AJ, Munshi A, Merogi AJ, Ohadike Y, El-Habashi A, Marrogi OL, Freeman SM (1997) Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer 74:492–501
Meidan R, Levy N (2007) The ovarian endothelin network: an evolving story. Trends Endocrinol Metab 18:379–385
Merogi AJ, Marrogi AJ, Ramesh R, Robinson WR, Fermin CD, Freeman SM (1997) Tumor-host interaction: analysis of cytokines, growth factors, and tumor-infiltrating lymphocytes in ovarian carcinomas. Hum Pathol 28:321–331
Min JK, Lee YM, Kim JH, Kim YM, Kim SW, Lee SY, Gho YS, Oh GT, Kwon YG (2005) Hepatocyte growth factor suppresses vascular endothelial growth factor-induced expression of endothelial ICAM-1 and VCAM-1 by inhibiting the nuclear factor-kappaB pathway. Circ Res 96:300–307
Mor F, Quintana FJ, Cohen IR (2004) Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol 172:4618–4623
Nair S, Boczkowski D, Moeller B, Dewhirst M, Vieweg J, Gilboa E (2003) Synergy between tumor immunotherapy and antiangiogenic therapy. Blood 102:964–971
Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H (1998) CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58:3491–3494
Nelson J, Bagnato A, Battistini B, Nisen P (2003) The endothelin axis: emerging role in cancer. Nat Rev Cancer 3:110–116
Nestle FO, Burg G, Fah J, Wrone-Smith T, Nickoloff BJ (1997) Human sunlight-induced basal-cell-carcinoma-associated dendritic cells are deficient in T cell co-stimulatory molecules and are impaired as antigen-presenting cells. Am J Pathol 150:641–651
Ohm JE, Carbone DP (2001) VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res 23:263–272
Ohm JE, Shurin MR, Esche C, Lotze MT, Carbone DP, Gabrilovich DI (1999) Effect of vascular endothelial growth factor and FLT3 ligand on dendritic cell generation in vivo. J Immunol 163:3260–3268
Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP (2003) VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 101:4878–4886
Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK, Clay T, Morse MA (2008) The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother 57:1115–1124
Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI (1998) Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol 160:1224–1232
Paley PJ, Staskus KA, Gebhard K, Mohanraj D, Twiggs LB, Carson LF, Ramakrishnan S (1997) Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer 80:98–106
Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA (2003) Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA 100:8372–8377
Rafii S, Lyden D, Benezra R, Hattori K, Heissig B (2002) Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer 2:826–835
Rini BI, Weinberg V, Fong L, Conry S, Hershberg RM, Small EJ (2006) Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer 107:67–74
Roland CL, Lynn KD, Toombs JE, Dineen SP, Udugamasooriya DG, Brekken RA (2009) Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS One 4:e7669
Rosano L, Spinella F, Salani D, Di Castro V, Venuti A, Nicotra MR, Natali PG, Bagnato A (2003) Therapeutic targeting of the endothelin a receptor in human ovarian carcinoma. Cancer Res 63:2447–2453
Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT et al (1985) Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 313:1485–1492
Rosenberg SA, Yang JC, White DE, Steinberg SM (1998) Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg 228:307–319
Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME (2008) Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 8:299–308
Saida K, Mitsui Y, Ishida N (1989) A novel peptide, vasoactive intestinal contractor, of a new (endothelin) peptide family. Molecular cloning, expression, and biological activity. J Biol Chem 264:14613–14616
Salani D, Di Castro V, Nicotra MR, Rosano L, Tecce R, Venuti A, Natali PG, Bagnato A (2000a) Role of endothelin-1 in neovascularization of ovarian carcinoma. Am J Pathol 157:1537–1547
Salani D, Taraboletti G, Rosano L, Di Castro V, Borsotti P, Giavazzi R, Bagnato A (2000b) Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol 157:1703–1711
Sarris M, Andersen KG, Randow F, Mayr L, Betz AG (2008) Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 28:402–413
Schumacher K, Haensch W, Roefzaad C, Schlag PM (2001) Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res 61:3932–3936
Shin JY, Yoon IH, Kim JS, Kim B, Park CG (2009) Vascular endothelial growth factor-induced chemotaxis and IL-10 from T cells. Cell Immunol 256:72–78
Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92:735–745
Spinella F, Rosano L, Di Castro V, Natali PG, Bagnato A (2002) Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1alpha in ovarian carcinoma cells. J Biol Chem 277:27850–27855
Spinella F, Rosano L, Di Castro V, Nicotra MR, Natali PG, Bagnato A (2004) Inhibition of cyclooxygenase-1 and -2 expression by targeting the endothelin a receptor in human ovarian carcinoma cells. Clin Cancer Res 10:4670–4679
Spinella F, Rosano L, Di Castro V, Decandia S, Nicotra MR, Natali PG, Bagnato A (2007) Endothelin-1 and endothelin-3 promote invasive behavior via hypoxia-inducible factor-1alpha in human melanoma cells. Cancer Res 67:1725–1734
Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH (1999) Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood 93:1634–1642
Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM (1995) Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 55:3964–3968
Takahashi A, Kono K, Ichihara F, Sugai H, Fujii H, Matsumoto Y (2004) Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines. Cancer Immunol Immunother 53:543–550
Valdenaire O, Rohrbacher E, Mattei MG (1995) Organization of the gene encoding the human endothelin-converting enzyme (ECE-1). J Biol Chem 270:29794–29798
Vesalainen S, Lipponen P, Talja M, Syrjanen K (1994) Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer 30A:1797–1803
Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH (1994) Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 269:26988–26995
Witz IP (2008) Tumor-microenvironment interactions: dangerous liaisons. Adv Cancer Res 100:203–229
Wulfing P, Diallo R, Kersting C, Wulfing C, Poremba C, Rody A, Greb RR, Bocker W, Kiesel L (2003) Expression of endothelin-1, endothelin-A, and endothelin-B receptor in human breast cancer and correlation with long-term follow-up. Clin Cancer Res 9:4125–4131
Wulfing P, Kersting C, Tio J, Fischer RJ, Wulfing C, Poremba C, Diallo R, Bocker W, Kiesel L (2004) Endothelin-1-, endothelin-A-, and endothelin-B-receptor expression is correlated with vascular endothelial growth factor expression and angiogenesis in breast cancer. Clin Cancer Res 10:2393–2400
Yanagisawa M, Masaki T (1989) Molecular biology and biochemistry of the endothelins. Trends Pharmacol Sci 10:374–378
Yanagisawa H, Hammer RE, Richardson JA, Williams SC, Clouthier DE, Yanagisawa M (1998) Role of Endothelin-1/Endothelin-A receptor-mediated signaling pathway in the aortic arch patterning in mice. J Clin Invest 102:22–33
Yang L, Carbone DP (2004) Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res 92:13–27
Zhang L, Conejo-Garcia J-R, Yang N, Huang W, Mohamed-Hadley A, Yao W, Benencia F, Coukos G (2002) Different effects of glucose starvation on expression and stability of VEGF mRNA isoforms in murine ovarian cancer cells. Biochem Biophys Res Commun 292:860–868
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G (2003a) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213
Zhang L, Yang N, Katsaros D, Huang W, Park J-W, Fracchioli S, Vezzani C, Rigault de la Longrais IA, Yao W, Rubin SC, Coukos G (2003b) The Oncogene Phosphatidylinositol 3'-Kinase Catalytic Subunit alpha Promotes Angiogenesis via Vascular Endothelial Growth Factor in Ovarian Carcinoma. Cancer Res 63:4225–4231
Zhang L, Yang N, Park J-W, Katsaros D, Fracchioli S, Cao G, O'Brien-Jenkins A, Randall TC, Rubin SC, Coukos G (2003c) Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res 63:3403–3412
Zitvogel L, Tesniere A, Kroemer G (2006) Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 6:715–727
Acknowledgments
This work was supported by NIH R01-CA098951; NIH P50-CA083638 Ovarian Cancer SPORE; and NIH R01-CA112162.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kandalaft, L.E., Motz, G.T., Busch, J., Coukos, G. (2010). Angiogenesis and the Tumor Vasculature as Antitumor Immune Modulators: The Role of Vascular Endothelial Growth Factor and Endothelin. In: Dranoff, G. (eds) Cancer Immunology and Immunotherapy. Current Topics in Microbiology and Immunology, vol 344. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2010_95
Download citation
DOI: https://doi.org/10.1007/82_2010_95
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-14135-5
Online ISBN: 978-3-642-14136-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)