Abstract
Cellular adaptation to diminished tissue oxygen tensions, hypoxia, is largely governed by the hypoxia inducible transcription factors, HIF-1 and HIF-2. Tumor hypoxia and high HIF protein levels are frequently associated with aggressive disease. In recent years, high tumor cell levels of HIF-2 and the oxygen sensitive subunit HIF-2α have been associated with unfavorable disease and shown to be highly expressed in tumor stem/initiating cells originating from neuroblastoma and glioma, respectively. In these cells, HIF-2 is active under nonhypoxic conditions as well, creating a pseudo-hypoxic phenotype with clear influence on tumor behavior. Neuroblastoma tumor initiating cells are immature with a neural crest-like phenotype and downregulation of HIF-2α in these cells results in neuronal sympathetic differentiation and the cells become phenotypically similar to the bulk of neuroblastoma cells found in clinical specimens. Knockdown of HIF-2α in neuroblastoma and glioma tumor stem/initiating cells leads to reduced levels of VEGF and poorly vascularized, highly necrotic tumors. As high HIF-2α expression further correlates with disseminated disease as demonstrated in neuroblastoma, glioma, and breast carcinoma, we propose that targeting HIF-2α and/or the pseudo-hypoxic phenotype induced by HIF-2 under normoxic conditions has great clinical potential.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sympathetic Nervous System
- VEGF Expression
- Tumor Stem Cell
- Clear Cell Renal Cell Carcinoma
- Glioma Stem Cell
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Mammalian cells, including tumor cells, require oxygen for maintenance of an efficient energy supply and lack of oxygen eventually leads to cell death due to impaired energy requiring processes. Cells can withstand fluctuations in oxygen levels by adapting to a decrease in oxygen involving reduction of energy consumption and increase in anaerobic metabolism. During the adaptation process, there is a dramatic shift in the expression of genes regulating a number of cellular functions including glucose transport and metabolism, angiogenesis, and cell survival. Central to this phenotypic shift are the hypoxia inducible factors (HIFs), HIF-1, and HIF-2. These factors are heterodimeric transcription factors composed of a unique alpha subunit and a beta subunit (ARNT/HIF-1β) shared by all three HIFs. Classically, HIFs are regulated by degradation of the alpha subunit at high oxygen levels and by stabilization at hypoxia (reviewed in Kaelin and Ratcliffe 2008). In the dimeric, active state, HIF-1 and HIF-2 bind to hypoxia responsive elements (HREs) located in genes regulated by hypoxia and HIFs. Although HIF-1 and HIF-2 seem to activate hypoxia-responsive genes by similar means (Tian et al. 1997; Wiesener et al. 1998), the HIF-α subunits work in a nonredundant manner and several differences in gene regulation have been proposed, many of which emphasize the predominant role of HIF-1 in regulating the transcriptional response to hypoxia (Iyer et al. 1998; Ryan et al. 1998; Hu et al. 2003; Park et al. 2003; Sowter et al. 2003). As detailed below, HIF-2 is also crucial for the hypoxic response, and as opposed to HIF-1 it is active at prolonged hypoxia as well (Holmquist-Mengelbier et al. 2006). The less studied HIF-3 is present in several splice variants lacking the C-terminal transactivation domain and is thought to negatively regulate HIF-1 and HIF-2 by sequestering the HIF-1 and HIF-2 alpha subunits, thereby blocking their binding to HREs (Makino et al. 2001; Maynard et al. 2007). Although the main mode of HIF activation is via stabilization of the alpha subunits, HIF-2, as well as HIF-1, can be stable and transcriptionally active at physiological or even higher oxygen tensions, as will be the central theme in this review. We propose that this phenomenon, at least regarding HIF-2, is largely linked to its role and regulation during normal development.
The phenotypes obtained by elimination of either Hif1a or Epas1/Hif2a clearly show that both genes are needed for proper development and that they are nonredundant. Importantly, and the reason for the focus on HIF-2α in this review, HIF-1α and HIF-2α are differently regulated in tumors such as neuroblastoma, breast cancer, nonsmall cell lung carcinoma (NSCLC), and glioma and seem to have different impact on tumor behavior and patient outcome in these tumors (Holmquist-Mengelbier et al. 2006; Helczynska et al. 2008; Heddleston et al. 2009; Kim et al. 2009b; Li et al. 2009; Noguera et al. 2009).
2 Phenotypic Effects of HIF-2α Elimination
While elimination of Hif1a has profound and repeatable effects on embryonal development, the effects of knocking out Hif2a have turned out to be much more complex and dependent on the genetic background, as summarized below. Despite displaying incompletely overlapping phenotypes, four different Hif2a knockout mice have been instrumental in identifying putative roles for HIF2A during normal development (Tian et al. 1998; Peng et al. 2000; Compernolle et al. 2002; Scortegagna et al. 2003). While Hif1a −/− animals are dead within embryonic day E11 with severe disorganization of vascular networks and gross neural tube defects, the effects of eliminating Hif2a – at least during early development – appears less general.
Hif2a expression during development is most abundant in vascular endothelial cells and disrupted vascular development of specific (although distinct) organs has been observed in various Hif2a −/− mice. Furthermore, whether or not attributable to vascular system defects, some Hif2a −/− mice have succumbed to embryonic death displaying hemorrhage. In particular, the Hif2a −/− mice created by Peng et al. showed varying degrees of vascular disorganization despite apparently normal blood vessel formation, suggesting that HIF-2α is required for normal remodeling/maturation postvasculogenesis (2000). Hif2a −/− mice in other studies, however, appeared normal in vascular development (despite hemorrhage) or displayed only subtle changes during late stages of pulmonary vascularization. In the Compernolle et al. study, Hif2a knockout mice died neonatally due to respiratory distress syndrome, apparently caused by impaired fetal lung maturation because of reduced VEGF levels and insufficient surfactant production (2002).
Creating Hif2a −/− animals by hybrid mating allowed Scortegagna et al. (2003) to study effects of Hif2a loss in the postnatal mouse. These mice suffered from biochemical/metabolic abnormalities and multiple-organ pathology specifically in sites of high-energy demand including the heart, liver, testis, and bone marrow, indicating a syndrome related or similar to mitochondrial disease. Overall, adult Hif2a −/− mice showed greater oxidative stress as well as reduced response to oxidative stress, suggesting an important role for HIF-2α in ROS homeostasis. The fact that Hif2a itself is regulated by ROS may indicate a role as a primary sensor of oxidative stress. In support, ROS accumulation and improved response to radiation therapy by HIF2A inhibition was recently described in human tumor cells (Bertout et al. 2009). These findings may implicate a role for HIF2A in radiation and chemotherapy resistance in tumor and possibly normal stem cells.
In addition, Hif2a −/− mice display defects in hematopoietic development due to greatly reduced EPO levels in the kidney (Scortegagna et al. 2003, 2005; Rankin et al. 2007). Administration of exogenous EPO reverts this phenotype as well as some of the other defects associated with Hif2a elimination (Scortegagna et al. 2005). Further supporting a role for HIF2A in EPO production, a gain-of-function mutation in the HIF2A gene has been found associated with familial erythrocytosis (Percy et al. 2008a, b).
3 HIF-2 During Normal Sympathetic Nervous System (SNS) Development
In 1998, Steven McKnight and colleagues showed that Hif2a expression was transient but prominent in developing sympathetic ganglia and paraganglia (Organ of Zuckerkandl) (Tian et al. 1998). The latter organ is the main site of catecholamine synthesis during development and is thus tyrosine hydroxylase (TH) positive; it was later confirmed that HIF-2α is also expressed in developing human fetal SNS (Nilsson et al. 2005) (Fig. 1). Supporting a direct role for Hif2a in catecholamine production, the Hif2a deficient 129/SvJ mice contained substantially reduced levels of catecholamines, displayed bradycardia, and died at mid-gestation at a developmental stage corresponding to when Hif2a levels in the Organ of Zuckerkandl were the highest in heterozygous animals (Tian et al. 1998). Strikingly, the mid-gestational death was rescued by feeding the mothers DOPS, a substance that can directly convert into norepinephrine. Although a sympathetic phenotype was less pronounced in other Hif2a −/− mice – particularly as a cause of death – altered catecholamine content or DOPS-mediated rescue was recorded at least to some degree in all other Hif2a knockout animals (Peng et al. 2000; Compernolle et al. 2002; Scortegagna et al. 2003). These findings are consistent with the reported role of Hif2a in activating transcription of the DDC and DBH enzymes and thereby regulating catecholamine synthesis in fetal rat sympathoadrenal progenitor cells regardless of oxygen tension (Brown et al. 2009). In further support of a role for Hif2a in sympathetic development, mice lacking the HIF prolyl hydroxylase PHD3 displayed an increased number (but reduced functionality) of sympathetic cells in the adrenal medulla, carotid body, and the superior cervical ganglia due to reduced apoptosis (Bishop et al. 2008). A reasonable assumption based on the role of PHD3 in targeting HIFs for degradation is that HIF protein levels in general would be higher in PHD3 −/− animals, and in vitro studies have suggested that PHD3 is more important in regulation of HIF-2α than HIF-1α (Appelhoff et al. 2004; Henze et al. 2009). Indeed, the sympathetic phenotype of Phd3 −/− mice was intriguingly reverted by crossing animals with heterozygous Hif2a +/− (but not Hif1a +/−) mice, again indicating that proper control of Hif2a expression is crucial for normal SNS development (Bishop et al. 2008). This notion is perhaps embodied by the link between high HIF-2α expression and immature, aggressive phenotypes of the SNS malignancy neuroblastoma, as discussed below.
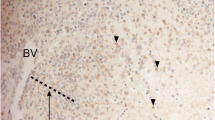
HIF-2α positive human paraganglia at fetal week 8.5. Paraganglia stained for tyrosine hydroxylase (TH) and HIF-2α in nonconsecutive but adjacent sections (ethical approval LU 389-98, Lund University, Sweden). Arrows indicate distinct nests of immature paraganglia cells positive for both TH and HIF-2α. These structures have been further characterized in (Hoehner et al. 1996)
4 Hypoxia in Solid Tumors and Relation to Tumor Aggressiveness
Direct measurements of oxygen tension in solid tumors and adjacent nonmalignant tissue reveal that tumors, generally, are less well oxygenated and that large parts of solid tumors are hypoxic (Höckel and Vaupel 2001). Although these hypoxic areas are often necrotic, the general histological pattern is such that tumor cells survive low oxygen tensions and can thus adapt to hypoxic conditions. In vitro studies support this conclusion as tumor cells established as cell lines can survive for several days at as low concentrations as 0.1% oxygen. Another interesting aspect of tumor hypoxia is the well-documented association between oxygen shortage and tumor aggressiveness (reviewed in Bertout et al. 2008). The mechanistic background is probably very complex, but involves cytotoxic resistance, insensitivity to radiation, decreased DNA repair capacity, increased vascularization, and increased metastatic potential (reviewed in Semenza 2003; Erler et al. 2006; Löfstedt et al. 2007) and as will be discussed in more detail below, dedifferentiation or loss of a differentiated tumor phenotype. As adaptation to hypoxia in tumor cells is largely mediated via stabilization and activation of HIF-1α and HIF-2α, high levels of HIF proteins have also been associated with disseminated disease and poor overall survival. In tumor cell lines – with few exceptions – both HIF-1α and HIF-2α are expressed and we recently postulated that HIF-1α is involved in adaptation to acute and HIF-2α to prolonged hypoxia (Holmquist-Mengelbier et al. 2006; Helczynska et al. 2008). For historical reasons, HIF-1α is the isoform that has been most extensively studied in clinical tumor materials and frequently been correlated to aggressive tumor disease, but in recent years high tumor levels of HIF-2α rather than HIF-1α have been shown to associate with negative overall survival and metastatic disease. In breast carcinoma for instance, earlier published data link HIF-1α, while later reports link HIF-2α to unfavorable disease (Schindl et al. 2002; Bos et al. 2003; Gruber et al. 2004; Dales et al. 2005; Generali et al. 2006; Giatromanolaki et al. 2006; Kronblad et al. 2006; Helczynska et al. 2008). Whether these contradicting observations reflect real differences in the tumor material analyzed or can be attributed to methodological shortcomings is presently unknown. Nevertheless, data from tumors of different derivations in which HIF-2 appears to be important for clinical behavior are exemplified in the next paragraph.
5 Differential Tumor HIF Expression in Relation to Patient Outcome
5.1 Neuroblastoma
Neuroblastoma is a childhood tumor that arises in precursor cells or immature neuroblasts of the SNS, which is derived from the neural crest. There is strong positive correlation between tumor aggressiveness (clinical stage and overall outcome) and immature phenotype (Fredlund et al. 2008). Although several cytogenetic aberrations linked to poor neuroblastoma prognosis have been identified, amplification of MYCN is the only aberration at the gene level that strongly associates with advanced disease, which in turn associates with activated MYC signaling and an immature phenotype (Fredlund et al. 2008). The fully disseminated disease, Stage 4, is highly aggressive and the overall survival of children with this disease stage is less than 40% (Matthay et al. 1999).
As stated above, HIF-2α is expressed during discrete periods of murine and human SNS development and the fact that neuroblastoma is an SNS-derived tumor appears to be important when the role(s) of HIFs in neuroblastoma is discussed. Both HIF-1α and HIF-2α proteins are expressed and become stabilized at hypoxia in neuroblastoma cell lines (Jögi et al. 2002). There is however a distinct difference in stabilization kinetics suggesting that HIF-1 is responsible for the acute, and HIF-2 for the prolonged response to hypoxia (Holmquist-Mengelbier et al. 2006). HIF-2α is also less sensitive than HIF-1α to oxygen-dependent degradation, and accumulates already at near-physiological oxygen tensions. Immunohistochemical analysis of HIF expression in neuroblastoma specimens reveals that both HIF-1α and HIF-2α proteins, as expected, can be detected in tumor cell layers adjacent to necrotic areas. While HIF-1α is mainly restricted to perinecrotic zones, HIF-2α protein is also expressed at other locations, most notably in cells adjacent to blood vessels. Presence of tumor cells staining intensely for HIF-2α, more so than high number of HIF-2α+ cells correlates positively to distant metastasis and negative overall survival (Holmquist-Mengelbier et al. 2006; Noguera et al. 2009). In contrast, HIF-1α protein expression did not correlate to aggressive disease or negative outcome (Noguera et al. 2009). As will be discussed below, we postulated that a fraction of the cells staining intensely for HIF-2α are the neuroblastoma tumor initiating or stem cells, which could explain why the presence of such cells so strongly associates with unfavorable disease (Pietras et al. 2008).
5.2 Breast Carcinoma
HIF-1α protein is not expressed in normal breast tissue or ductal hyperplastic lesions, but is detected in ductal carcinoma in situ (DCIS) and invasive breast cancers (Bos et al. 2001; Helczynska et al. 2003) In cell lines, both HIFs are expressed and hypoxia-regulated. Similar to the situation in neuroblastoma, HIF-1α is acutely and transiently upregulated, whereas HIF-2α protein is still present after prolonged hypoxia and appears to mediate a sustained hypoxic response, including expression of VEGF (Helczynska et al. 2008). Expression in tumors and association of HIFs to breast cancer aggressiveness appear to be a complex issue. Early studies on HIF-1α protein expression in various subgroups of breast cancers link high levels of the protein to poor outcome, although several of these reports contradict each other. In more recent studies, the overall relationship between HIF-1α protein and breast cancer specific death is meager and HIF-1α associates positively rather than negatively to favorable disease (Tan et al. 2007; Helczynska et al. 2008). However, there are early reports correlating high HIF-1α protein expression with shorter overall and disease-free survival time in patients with lymph node-positive breast cancer, whereas this association was not significant in lymph-node negative patients (Schindl et al. 2002; Kronblad et al. 2006). In contrast to these findings, two reports show association between high HIF-1α protein expression and poor outcome in node-negative but not in node-positive subgroups of patients (Bos et al. 2003; Generali et al. 2006). In addition, significant associations between HIF-1α protein expression and outcome without subgroup divisions (Dales et al. 2005) and unfavorable outcome in node-positive tumors, although restricted to T1/T2 tumors (Gruber et al. 2004) have been published. There are several putative explanations as to why these reports differ in predicting outcome and range from small or poorly defined clinical material to technical explanations. Our own experience is that commercial HIF antibodies vary in quality, also at the batch level, implicating that immunohistochemical stainings have to be interpreted with some caution. In summary, we conclude from published data that the prognostic impact of HIF-1α protein expression in breast cancer is at best restricted to subgroups of patients, which in such cases need to be verified in large prospective studies. Most studies, however, have common findings in that multi- and univariate analyses fail to reveal HIF-1α protein level as an independent prognostic factor.
HIF-2α and its association with the outcome in breast cancer patients has been far less studied, but published immunohistochemical data suggest that HIF-2α correlates to high metastatic potential and is an independent prognostic factor associated with breast cancer specific death (Helczynska et al. 2008). In two cohorts of breast cancer patients, both HIF-1α and HIF-2α correlated to increased VEGF expression, but only high HIF-2α protein exhibited significant correlation to reduced recurrence-free and breast cancer-specific survival, and was an independent prognostic factor. Importantly, high HIF-2α protein expression correlated to the presence of distal metastasis but to no other clinical feature analyzed (Helczynska et al. 2008). In another report, HIF-2α protein was analyzed in a small subset of infiltrating ductal breast carcinomas, which showed a significant relationship between high HIF-2α protein expression and increased vascular density as well as secondary deposits to multiple axillary lymph nodes. Multivariate analysis revealed HIF-2α as an independent factor relating to extensive nodal metastasis (Giatromanolaki et al. 2006).
5.3 Renal Cell Carcinoma
During normal kidney development, HIF-1α is expressed in most cell types whereas HIF-2α is mainly found in renal interstitial fibroblast-like cells and endothelial cells. In the fully developed normal kidney, HIF-1α expression is maintained, while HIF-2α expression disappears. The role of HIF-signaling during development is largely unclear, but the cell type- and stage-specific expression distribution of HIF-α subunits correlates with the expression of critical angiogenic factors such as VEGF and endoglin (Freeburg and Abrahamson 2003; Bernhardt et al. 2006). Conditional knockouts in renal proximal tubule cells of either HIF-1α or HIF-β alone do not generate an abnormal phenotype whereas conditional knockout of pVHL results in HIF-dependent development of tubular and glomerular cysts (Rankin et al. 2006).
Clear cell renal cell carcinoma (CCRCC) is characterized by extensive neovascularization. This is generally explained by impaired HIF-α subunit degradation due to mutation or hypermethylation of the VHL gene, found in approximately 60–70% of all CCRCCs (Gnarra et al. 1994; Herman et al. 1994). At normoxia, pVHL constitutes the recognition subunit of a larger E3 ubiquitin ligase complex that targets the HIF-α subunits for proteasomal degradation (Kaelin 2002). Thus, in CCRCCs where pVHL function has been lost the HIF-α subunits are constitutively expressed and a pseudo-hypoxic phenotype, including increased vascularization, is present. Intriguingly, there seems to be a bias towards HIF-2α expression as compared to HIF-1α expression in these VHL-deficient carcinoma cells (Maxwell et al. 1999; Krieg et al. 2000). The abundance of VHL-deficient RCC cell lines expressing HIF-2α but not HIF-1α (Maxwell et al. 1999) is also interesting as this contrasts with normal renal epithelial cells, where HIF-2α expression is absent during ischemia (Rosenberger et al. 2003). Furthermore, the HIF signaling pathways are activated early in the development of neoplastic lesions in VHL disease, with the HIF-1α isoform being expressed even in earliest foci while the HIF-2α protein is detected first in more advanced lesions (Mandriota et al. 2002). In pVHL-defective CCRCC, HIF-1 positively regulates BNIP3, an autophagy marker, but has no profound effect on cyclin D1, TGF-α, and VEGF expression, whereas HIF-2 negatively regulates BNIP3 but promotes cyclin D1, TGF-α, and VEGF expression (Raval et al. 2005). Thus, these differences in regulation of autophagy vs. cell growth and angiogenesis might be understood in the light of HIF-2α being expressed mainly during late CCRCC progression and in more advanced lesions. siRNA-mediated knockdown of HIF-2α represses tumor growth in pVHL-deficient CCRCC (Kondo et al. 2003; Zimmer et al. 2004), and overexpression of HIF-2α in the VHL wild type 786-O cells resulted in enhanced tumor formation (Raval et al. 2005). In contrast, overexpression of HIF-1α in 786-O cells diminished tumor xenograft growth (Raval et al. 2005). Finally, and in agreement with the HIF-1α overexpression xenograft data, HIF-1α has been reported in a clinical RCC material to be an independent prognostic factor predicting favorable outcome (Lidgren et al. 2005).
5.4 Nonsmall Cell Lung Carcinoma
HIF protein expression is virtually absent in normal lung tissue at normoxia, whereas both isoforms are accumulated during hypoxic conditions (Giatromanolaki et al. 2001). In the corresponding normal lung tissue examined from lung cancer patients, bronchial and alveolar epithelium adjacent to the tumor site show weak to intense cytoplasmic staining of the HIF proteins, whereas all other lung tissue components are negative for HIF expression (Giatromanolaki et al. 2001).
Intratumoral hypoxia in lung cancers correlates with decreased overall survival (Swinson et al. 2003; Le et al. 2006). Both HIF-1α and HIF-2α are frequently expressed in NSCLC, also during early progression of disease, but whereas HIF-2α causes, or is a surrogate marker for poor clinical prognosis (Giatromanolaki et al. 2001), the role of HIF-1α in predicting outcome is debated. Some reports demonstrate that HIF-1α expression has no impact on patient overall survival (Giatromanolaki et al. 2001; Kim et al. 2005), but potentially contradicting data exist (Volm and Koomagi 2000; Yohena et al. 2009).
In lung adenocarcinomas, mutations in KRAS are common and the presence of KRAS mutations predicts poor outcome (Huncharek et al. 1999). Mice conditionally expressing a nondegradable HIF-2α and mutated Kras (Kras G12D) in the lungs display severed tumor burden and decreased survival, compared to mice expressing Kras G12D only, suggesting that HIF-2α play a pivotal role in lung cancer pathogenesis (Kim et al. 2009b). In agreement with a role for HIF-2α in NSCLC tumorigenesis, in a clinical material HIF-2α was an independent prognostic marker with high protein expression correlating to poor outcome (Giatromanolaki et al. 2001).
5.5 Glioblastoma
Glioblastoma multiforme (GBM) is characterized by a rich vasculature network (Hossman and Bloink 1981; Blasberg et al. 1983; Groothuis et al. 1983) and intratumoral necrosis (Raza et al. 2002). Both HIF-1α and HIF-2α proteins are expressed in human glioblastomas (Jensen 2006; Li et al. 2009) with HIF-1α expression being mostly concentrated in areas of necrosis and at the tumor margin (Zagzag et al. 2000). Studies on a small set of brain tumors have suggested that HIF-1α protein correlates positively to brain tumor grade and vascularity (Zagzag et al. 2000).
Based on published data, the role of HIF-2α in glioblastoma formation and aggressiveness is not fully clear, as HIF-2α has been attributed a tumor-suppressor role (Acker et al. 2005), as well as a marker for poor prognosis (Li et al. 2009). Overexpression of HIF-2α protein in rat glioblastomas suppressed tumor growth despite overall enhanced vascularization. This was in part explained by increased tumor cell apoptosis, and knockdown of HIF-2α in hypoxic human glioblastoma cells reduced the apoptotic rate of these cells (Acker et al. 2005). Recent work on GBM has focused highly on the small fraction of tumor cells with stem cell characteristics that are thought to initiate and maintain tumor growth (Hemmati et al. 2003; Singh et al. 2003; Galli et al. 2004; Singh et al. 2004). Several markers identifying a glioma stem cell population have been proposed, including CD133, Nestin (Singh et al. 2003), and A2B5 (Ogden et al. 2008). HIF-2α was recently shown to be expressed at high levels in CD133+ glioma stem cells grown in vitro (McCord et al. 2009), and to co-localize with stem cell markers in tumor specimens (Li et al. 2009), suggesting that HIF-2α is an independent marker for glioma stem cells. Interestingly, HIF-2α is specifically expressed in brain tumor stem cells but not in neural progenitor cells, in contrast to HIF-1α, which is expressed in both cell types. As in neuroblastoma, a proportion of the HIF-2α positive cells are located adjacent to blood vessels in the tumor specimens, indicating that HIF-2α is expressed by a small but significant number of tumor cells, also in nonhypoxic regions (Pietras et al. 2008, 2009; Li et al. 2009). Finally, analyzing gliomas at the mRNA level, HIF2A, but not HIF1A expression, correlates with poor patient survival (Li et al. 2009).
6 Hypoxia and Tumor Cell Differentiation
As mentioned above, hypoxia has profound effects on cellular phenotypes. One aspect of adaptation to hypoxia, which is of particular importance in tumor cells, is the effect on the tumor cell differentiation status and newly discovered links between HIF-2α expression and tumor initiating/stem cells. Initially described in cultured neuroblastoma and breast cancer cells and in breast tumor specimens, hypoxia can push tumor cells towards an immature stem cell-like phenotype (Jögi et al. 2002; Helczynska et al. 2003). The phenomenon has also been observed in glioma (Heddleston et al. 2009) recently suggesting that the dedifferentiating effect of hypoxia could be general and not restricted to specific tumor forms. These observations have potential direct clinical impact, since at least in neuroblastoma and breast carcinoma, immature stages of differentiation correlate to aggressive tumor behavior and unfavorable outcome. Thus, we have proposed that the hypoxia-induced immature stem cell features work in concert with other hypoxia-driven changes in establishing an aggressive tumor phenotype (Jögi et al. 2002; Helczynska et al. 2003; Axelson et al. 2005).
7 HIF-2α and Tumor Initiating/Stem Cells
HIF-2α is expressed during discrete periods of murine SNS development as determined by in situ hybridization (Tian et al. 1998) and the expression is both strong and selective as most other tissues either lack or only show week HIF-2α expression (Jögi et al. 2002), suggesting that HIF-2α in the developing SNS is regulated at the transcriptional level. By immunohistochemistry we could further demonstrate HIF-2α protein in human SNS paraganglia at fetal week 8.5 (Nilsson et al. 2005), which developmentally corresponds to mouse embryonal day E16, a time point when mouse SNS paraganglia express HIF-2α as determined by in situ hybridizations (Tian et al. 1998; Jögi et al. 2002). Using the same immunohistochemical protocol that detects HIF-2α in developing human paraganglia, staining of human neuroblastoma specimens highlights small subsets of cells intensely expressing HIF-2α protein, and the presence of such cells strongly correlates to disseminated disease (high clinical stage) and tumor related death (Holmquist-Mengelbier et al. 2006). Further immunohistochemical characterization of these cells reveals that they are frequently perivascularly located, lack the expression of SNS markers like TH and NSE found in the bulk of neuroblastoma tumor cells, but express neural crest and early SNS progenitor markers such as NOTCH-1, HES-1, Vimentin, and HAND2 (Pietras et al. 2008). Histologically, these cells were classified as tumor cells, although ambiguous cases exist. However, in most cases it could be excluded that the HIF-2α+ cells were tumor-associated macrophages, reported to express HIF-2α, and contributed to adverse outcome when present in breast cancer specimens (Leek et al. 2002). To verify that the HIF-2α+ cells indeed were tumor cells proper and not stromal cells, MYCN amplification was demonstrated by in situ FISH in perivascularly located, strongly HIF-2α immunofluorescing cells in tumors harboring an amplified MYCN gene. We hypothesized that these immature stem cell-like HIF-2α+ cells could be neuroblastoma stem or tumor initiating cells (TICs) (Pietras et al. 2008).
Recently, David Kaplan’s laboratory isolated neuroblastoma cells from patient bone marrows and showed that these cells grow and form neurospheres in neural stem cell promoting medium (Hansford et al. 2007). These cells are highly tumorigenic in an orthotopic xenograft mouse model (Hansford et al. 2007) and are by this functional definition TICs. The neuroblastoma TICs virtually lack expression of SNS markers but express neural crest markers including NOTCH1, HES1, ID2, and VIM (Pietras et al. 2009). As the TICs also have high levels of HIF-2α at normoxic conditions, they strongly share phenotypic characteristics with the earlier identified HIF-2α+, SNS marker−, and neural crest marker+ cells in neuroblastoma specimens (Pietras et al. 2008). The relation between isolated immature neuroblastoma bone marrow TICs and the phenotypically similar cells in neuroblastoma specimens, and that between immature stem cell-like cells in tumor specimens and the bulk of neuroblastoma cells expressing SNS markers have not been established. However, down-regulation of HIF-2α in the cultured neuroblastoma TICs by an shHIF2A approach releases the tumor cells from a differentiation block resulting in expression of the early SNS markers ASCL1/HASH1, ISL1, and SCG10. When removed from the stem cell-promoting medium and grown in vivo as subcutaneous tumors, the shHIF-2α-transduced TICs develop into a more mature neuroblastoma phenotype with expression of classical SNS markers such as tyrosine hydroxylase and chromogranin A, thus acquiring a phenotype similar to that of the bulk cells of clinical neuroblastomas (Pietras et al. 2009). We conclude that HIF-2α keeps the neuroblastoma TICs in a stem cell-like state and that these cells have properties in keeping with what could be expected of a neuroblastoma stem cell. Our current view of the relation between neuroblastoma TICs, circulating neuroblastoma cells, tumor bulk, and HIF protein expression is summarized in Fig. 2. The phenotypic similarities between bone marrow-derived TICs and the HIF-2α+ tumor cells located adjacent to blood vessels in neuroblastoma specimens suggest that these cells are related and we postulate that circulating neuroblastoma cells are the connecting link as has been demonstrated in melanoma, breast, and colon tumor model systems by Massague and co-workers (Kim et al. 2009a). In tumors, we further postulate that the HIF-2α+ neuroblastoma stem cells will by unknown mechanisms spontaneously differentiate bulk cells expressing SNS markers such as CHGA, TH, and GAP-43. The bulk cells have reduced normoxic VEGF expression and thus reduced angiogenic capacity due to lowered HIF-2α protein levels and when such cells experience hypoxia, they dedifferentiate and acquire stem cell neural crest-like features (Jögi et al. 2002).
A putative interplay between neuroblastoma (NB) tumor-initiating cells (TICs), tumor bulk, HIF-2α, sympathetic nervous system (SNS) differentiation, and oxygen status. We postulate that bone marrow-derived neuroblastoma TICs communicate with primary neuroblastomas as circulating tumor cells. In neuroblastoma tumors, TICs will by unknown mechanisms, spontaneously lose their HIF-2α protein expression, differentiate, and acquire expression of SNS markers. In hypoxic regions of neuroblastomas, tumor cells lose their differentiated phenotype and become stem cell-like (Jögi et al. 2002). In this model, HIF-1α protein expression is strictly linked to a hypoxic cellular environment
As briefly touched upon above, there is also experimental support from other cell systems for the role of HIF-2α during early development and maintenance of a (tumor) stem cell phenotype (reviewed in Keith and Simon 2007). In embryoid bodies, overexpression of HIF-2α results in maintained pluripotency and potentiation of tumorigenic growth (Covello et al. 2006). These effects were a result of direct transcriptional activation of the POU transcription factor OCT4 by HIF-2α, as silencing of OCT4 in HIF-2α knock-in cells reverted the stem cell phenotype and reduced tumor growth (Covello et al. 2006). In glioma, cell populations enriched for tumor stem cell properties have high HIF-2α protein levels and as in neuroblastoma, HIF-2α has been suggested to be a marker of glioma stem cells. In addition, downregulation of HIF-2α in such cells results in decreased tumor initiating capacity and glial differentiation (Li et al. 2009; Heddleston et al. 2009). In line with these findings are the observations that HIF-2α protein expression correlates to breast cancer specific death and distant metastasis – the latter process most likely dependent on the presence of cells with tumor stem cell properties. Based on the published observations that HIF-2α is intimately and functionally linked to immature neural tumor stem cell phenotypes and appears to counteract early steps in SNS and glial differentiation, we hypothesize that HIF-2α might be a general marker of tumor stem cells with a dedifferentiating function similar to that in glioma and neuroblastoma stem/TICs.
8 HIFs and Vascularization
HIFs were implicated early in the tumor angiogenic process when it became clear that hypoxia promotes VEGF expression (Shweiki et al. 1992; Forsythe et al. 1996). With neuroblastoma as a model system we showed that there is a temporal shift in the usage of the HIFs during hypoxia-driven VEGF expression; whereas the VEGF expression is HIF-1 dependent at an acute phase, the expression during prolonged hypoxia is primarily HIF-2 driven (Holmquist-Mengelbier et al. 2006). In a follow-up study using large clinical neuroblastoma tissue microarray material immunohistochemically stained for HIF-1α, HIF-2α, VEGF, and blood vessel endothelial cells (CD31), tumor cells staining intensely for HIFs correlated to VEGF positivity, while the HIF-1α and HIF-2α staining did not fully correlate to each other (Noguera et al. 2009). Furthermore, HIF-1α and VEGF, but not HIF-2α, showed negative correlation to the number of blood vessels, in agreement with the observation that strongly HIF-2α positive tumor cells express VEGF and frequently locate adjacent to blood vessels. As opposed to HIF-2α, presence of HIF-1α positive cells did not correlate to tumor aggressiveness or disseminated disease (Noguera et al. 2009). We conclude from in vitro and in vivo neuroblastoma data that both HIF-1α and HIF-2α become stabilized at hypoxia and that the HIF-2α protein level can be positively regulated by additional, not fully explored mechanisms.
The HIF-2α+ neuroblastoma and glioma stem/TICs strongly express VEGF (Holmquist-Mengelbier et al. 2006; Bao et al. 2006; Calabrese et al. 2007; Pietras et al. 2008, 2009; Li et al. 2009) and the perivascular location of phenotypically similar cells in tumor specimens might implicate that tumor stem cells actively take part in the process of tumor vascularization. The hypothesis is supported by the observation that knockdown of HIF-2α in neuroblastoma and glioma stem/TICs results in poorly vascularized tumors. Moreover, vascularization is affected in mice with eliminated Hif2a (Peng et al. 2000; Rankin et al. 2008) and overexpression of Hif2a in embryoid bodies results in early and extensive formation of a vascular network (Covello et al. 2006). The underlying molecular mechanisms behind the perivascular localization of neural tumor stem cells are presently not understood and are topics for future investigations.
9 HIF-2α and the Pseudo-Hypoxic Phenotype: Targets for Tumor Treatment
One striking feature of both neuroblastoma and glioma stem/TICs is their pseudo-hypoxic phenotype due to high expression of active HIF-2α at physiological oxygen tensions (Holmquist-Mengelbier et al. 2006; Pietras et al. 2008, 2009; Li et al. 2009). As a consequence, several genes considered to be hypoxia-regulated are highly expressed at nonhypoxic conditions in these HIF-2α+ tumor cells, thus creating a pseudo-hypoxic phenotype, presumably similar to that in a majority of CCRCCs. Whether the pseudo-hypoxic phenotype is drastically different from a corresponding bona fide hypoxic phenotype is not known, but as discussed above, VEGF expression and presumably vascularization would be one important determinant of the high HIF-2α expression at nonhypoxic conditions. This conclusion, in addition to data indicating that HIF-2 appears to play a pivotal role in aggressive and disseminated growth of neuroblastoma, glioma, breast carcinoma, nonsmall cell lung carcinoma, and CCRCC, strongly suggests HIF-2α and the pseudo-hypoxic phenotype are attractive therapeutic targets in at least these tumor types. By pushing HIF-2α+ tumor stem cells toward a more mature tumor bulk-like phenotype by interfering with the expression or activity of HIF-2, the tumor stem cell pool as well as the bulk of tumor cells, targeted by existing, efficient treatment protocols, would be reduced in numbers. The demonstration that both neuroblastoma and glioma stem/initiating cells differentiate in vivo when HIF-2α is knocked down (Pietras et al. 2009; Heddleston et al. 2009; Li et al. 2009) can be seen as proof of principle. Diminished HIF-2α activity also leads to reduced VEGF expression in these two tumor stem cell models, and as would be anticipated, neuroblastoma and glioma tumors with reduced HIF-2α expression are highly necrotic (Pietras et al. 2009; Li et al. 2009; Heddleston et al. 2009) suggesting that targeting HIF-2α will result in both an antiangiogenic effect and a reduction of the tumor stem cell pool. As high HIF-2α protein levels associate with disseminated disease, targeting of HIF-2α or the HIF-2α-driven pseudo-hypoxic phenotype, might also affect tumor spread and transition into advanced clinical stages.
References
Acker T, Diez-Juan A, Aragones J, Tjwa M, Brusselmans K, Moons L, Fukumura D, Moreno-Murciano MP, Herbert JM, Burger A, Riedel J, Elvert G, Flamme I, Maxwell PH, Collen D, Dewerchin M, Jain RK, Plate KH, Carmeliet P (2005) Genetic evidence for a tumor suppressor role of HIF-2alpha. Cancer Cell 8:131–141
Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM (2004) Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 279:38458–38465
Axelson H, Fredlund E, Ovenberger M, Landberg G, Påhlman S (2005) Hypoxia-induced dedifferentiation of tumor cells–a mechanism behind heterogeneity and aggressiveness of solid tumors. Semin Cell Dev Biol 16:554–563
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN (2006) Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 66:7843–7848
Bernhardt WM, Schmitt R, Rosenberger C, Munchenhagen PM, Grone HJ, Frei U, Warnecke C, Bachmann S, Wiesener MS, Willam C, Eckardt KU (2006) Expression of hypoxia-inducible transcription factors in developing human and rat kidneys. Kidney Int 69:114–122
Bertout JA, Patel SA, Simon MC (2008) The impact of O2 availability on human cancer. Nat Rev Cancer 8:967–975
Bertout JA, Majmundar AJ, Gordan JD, Lam JC, Ditsworth D, Keith B, Brown EJ, Nathanson KL, Simon MC (2009) HIF2alpha inhibition promotes p53 pathway activity, tumor cell death, and radiation responses. Proc Natl Acad Sci USA 106:14391–14396
Bishop T, Gallagher D, Pascual A, Lygate CA, de Bono JP, Nicholls LG, Ortega-Saenz P, Oster H, Wijeyekoon B, Sutherland AI, Grosfeld A, Aragones J, Schneider M, van Geyte K, Teixeira D, Diez-Juan A, Lopez-Barneo J, Channon KM, Maxwell PH, Pugh CW, Davies AM, Carmeliet P, Ratcliffe PJ (2008) Abnormal sympathoadrenal development and systemic hypotension in PHD3-/- mice. Mol Cell Biol 28:3386–3400
Blasberg RG, Kobayashi T, Horowitz M, Rice JM, Groothuis D, Molnar P, Fenstermacher JD (1983) Regional blood flow in ethylnitrosourea-induced brain tumors. Ann Neurol 14:189–201
Bos R, Zhong H, Hanrahan CF, Mommers EC, Semenza GL, Pinedo HM, Abeloff MD, Simons JW, van Diest PJ, van der Wall E (2001) Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J Natl Cancer Inst 93:309–314
Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ, van der Wall E (2003) Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer 97:1573–1581
Brown ST, Kelly KF, Daniel JM, Nurse CA (2009) Hypoxia inducible factor (HIF)-2 alpha is required for the development of the catecholaminergic phenotype of sympathoadrenal cells. J Neurochem 110:622–630
Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11:69–82
Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P (2002) Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 8:702–710
Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B (2006) HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev 20:557–570
Dales JP, Garcia S, Meunier-Carpentier S, Andrac-Meyer L, Haddad O, Lavaut MN, Allasia C, Bonnier P, Charpin C (2005) Overexpression of hypoxia-inducible factor HIF-1alpha predicts early relapse in breast cancer: retrospective study in a series of 745 patients. Int J Cancer 116:734–739
Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ (2006) Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440:1222–1226
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613
Fredlund E, Ringner M, Maris JM, Påhlman S (2008) High Myc pathway activity and low stage of neuronal differentiation associate with poor outcome in neuroblastoma. Proc Natl Acad Sci USA 105:14094–14099
Freeburg PB, Abrahamson DR (2003) Hypoxia-inducible factors and kidney vascular development. J Am Soc Nephrol 14:2723–2730
Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A (2004) Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 64:7011–7021
Generali D, Berruti A, Brizzi MP, Campo L, Bonardi S, Wigfield S, Bersiga A, Allevi G, Milani M, Aguggini S, Gandolfi V, Dogliotti L, Bottini A, Harris AL, Fox SB (2006) Hypoxia-inducible factor-1alpha expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clin Cancer Res 12:4562–4568
Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL (2001) Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer 85:881–890
Giatromanolaki A, Sivridis E, Fiska A, Koukourakis MI (2006) Hypoxia-inducible factor-2 alpha (HIF-2 alpha) induces angiogenesis in breast carcinomas. Appl Immunohistochem Mol Morphol 14:78–82
Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM et al (1994) Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet 7:85–90
Groothuis DR, Pasternak JF, Fischer JM, Blasberg RG, Bigner DD, Vick NA (1983) Regional measurements of blood flow in experimental RG-2 rat gliomas. Cancer Res 43:3362–3367
Gruber G, Greiner RH, Hlushchuk R, Aebersold DM, Altermatt HJ, Berclaz G, Djonov V (2004) Hypoxia-inducible factor 1 alpha in high-risk breast cancer: an independent prognostic parameter? Breast Cancer Res 6:R191–R198
Hansford LM, McKee AE, Zhang L, George RE, Gerstle JT, Thorner PS, Smith KM, Look AT, Yeger H, Miller FD, Irwin MS, Thiele CJ, Kaplan DR (2007) Neuroblastoma cells isolated from bone marrow metastases contain a naturally enriched tumor-initiating cell. Cancer Res 67:11234–11243
Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN (2009) The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle 8:3274–3284
Helczynska K, Kronblad A, Jögi A, Nilsson E, Beckman S, Landberg G, Påhlman S (2003) Hypoxia promotes a dedifferentiated phenotype in ductal breast carcinoma in situ. Cancer Res 63:1441–1444
Helczynska K, Larsson AM, Holmquist Mengelbier L, Bridges E, Fredlund E, Borgquist S, Landberg G, Påhlman S, Jirstrom K (2008) Hypoxia-inducible factor-2alpha correlates to distant recurrence and poor outcome in invasive breast cancer. Cancer Res 68:9212–9220
Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI (2003) Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA 100:15178–15183
Henze AT, Riedel J, Diem T, Wenner J, Flamme I, Pouyseggur J, Plate KH, Acker T (2009) Prolyl hydroxylases 2 and 3 act in gliomas as protective negative feedback regulators of hypoxia-inducible factors. Cancer Res 70(1):357–366
Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S, Samid D, Duan DS, Gnarra JR, Linehan WM et al (1994) Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci USA 91:9700–9704
Höckel M, Vaupel P (2001) Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 93:266–276
Hoehner JC, Gestblom C, Hedborg F, Sandstedt B, Olsen L, Påhlman S (1996) A developmental model of neuroblastoma: differentiating stroma-poor tumors’ progress along an extra-adrenal chromaffin lineage. Lab Invest 75:659–675
Holmquist-Mengelbier L, Fredlund E, Löfstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, Poellinger L, Påhlman S (2006) Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell 10:413–423
Hossman KA, Bloink M (1981) Blood flow and regulation of blood flow in experimental peritumoral edema. Stroke 12:211–217
Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC (2003) Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol 23:9361–9374
Huncharek M, Muscat J, Geschwind JF (1999) K-ras oncogene mutation as a prognostic marker in non-small cell lung cancer: a combined analysis of 881 cases. Carcinogenesis 20:1507–1510
Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL (1998) Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12:149–162
Jensen RL (2006) Hypoxia in the tumorigenesis of gliomas and as a potential target for therapeutic measures. Neurosurg Focus 20:E24
Jögi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, Axelson H, Påhlman S (2002) Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci USA 99:7021–7026
Kaelin WG Jr (2002) Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer 2:673–682
Kaelin WG Jr, Ratcliffe PJ (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30:393–402
Keith B, Simon MC (2007) Hypoxia-inducible factors, stem cells, and cancer. Cell 129:465–472
Kim SJ, Rabbani ZN, Dewhirst MW, Vujaskovic Z, Vollmer RT, Schreiber EG, Oosterwijk E, Kelley MJ (2005) Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically resected non-small cell lung cancer. Lung Cancer 49:325–335
Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massague J (2009a) Tumor self-seeding by circulating cancer cells. Cell 139:1315–1326
Kim WY, Perera S, Zhou B, Carretero J, Yeh JJ, Heathcote SA, Jackson AL, Nikolinakos P, Ospina B, Naumov G, Brandstetter KA, Weigman VJ, Zaghlul S, Hayes DN, Padera RF, Heymach JV, Kung AL, Sharpless NE, Kaelin WG Jr, Wong KK (2009b) HIF2alpha cooperates with RAS to promote lung tumorigenesis in mice. J Clin Invest 119:2160–2170
Kondo K, Kim WY, Lechpammer M, Kaelin WG Jr (2003) Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol 1:E83
Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH (2000) Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene 19:5435–5443
Kronblad A, Jirstrom K, Ryden L, Nordenskjold B, Landberg G (2006) Hypoxia inducible factor-1alpha is a prognostic marker in premenopausal patients with intermediate to highly differentiated breast cancer but not a predictive marker for tamoxifen response. Int J Cancer 118:2609–2616
Le QT, Chen E, Salim A, Cao H, Kong CS, Whyte R, Donington J, Cannon W, Wakelee H, Tibshirani R, Mitchell JD, Richardson D, O’Byrne KJ, Koong AC, Giaccia AJ (2006) An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res 12:1507–1514
Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, Bicknell R, Taylor M, Gatter KC, Harris AL (2002) Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in Human breast cancer. Cancer Res 62:1326–1329
Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, Hjelmeland AB, Rich JN (2009) Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15:501–513
Lidgren A, Hedberg Y, Grankvist K, Rasmuson T, Vasko J, Ljungberg B (2005) The expression of hypoxia-inducible factor 1alpha is a favorable independent prognostic factor in renal cell carcinoma. Clin Cancer Res 11:1129–1135
Löfstedt T, Fredlund E, Holmquist-Mengelbier L, Pietras A, Ovenberger M, Poellinger L, Påhlman S (2007) Hypoxia inducible factor-2alpha in cancer. Cell Cycle 6:919–926
Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, Tanaka H, Cao Y, Berkenstam A, Poellinger L (2001) Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 414:550–554
Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM, Wykoff CC, Maher ER, Harris AL, Ratcliffe PJ, Maxwell PH (2002) HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 1:459–468
Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP (1999) Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med 341:1165–1173
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275
Maynard MA, Evans AJ, Shi W, Kim WY, Liu FF, Ohh M (2007) Dominant-negative HIF-3 alpha 4 suppresses VHL-null renal cell carcinoma progression. Cell Cycle 6:2810–2816
McCord AM, Jamal M, Shankavaram UT, Lang FF, Camphausen K, Tofilon PJ (2009) Physiologic oxygen concentration enhances the stem-like properties of CD133+ human glioblastoma cells in vitro. Mol Cancer Res 7:489–497
Nilsson H, Jögi A, Beckman S, Harris AL, Poellinger L, Påhlman S (2005) HIF-2alpha expression in human fetal paraganglia and neuroblastoma: relation to sympathetic differentiation, glucose deficiency, and hypoxia. Exp Cell Res 303:447–456
Noguera R, Fredlund E, Piqueras M, Pietras A, Beckman S, Navarro S, Påhlman S (2009) HIF-1alpha and HIF-2alpha are differentially regulated in vivo in neuroblastoma: high HIF-1alpha correlates negatively to advanced clinical stage and tumor vascularization. Clin Cancer Res 15:7130–7136
Ogden AT, Waziri AE, Lochhead RA, Fusco D, Lopez K, Ellis JA, Kang J, Assanah M, McKhann GM, Sisti MB, McCormick PC, Canoll P, Bruce JN (2008) Identification of A2B5+CD133- tumor-initiating cells in adult human gliomas. Neurosurgery 62:505–514; discussion 514–515
Park SK, Dadak AM, Haase VH, Fontana L, Giaccia AJ, Johnson RS (2003) Hypoxia-induced gene expression occurs solely through the action of hypoxia-inducible factor 1alpha (HIF-1alpha): role of cytoplasmic trapping of HIF-2alpha. Mol Cell Biol 23:4959–4971
Peng J, Zhang L, Drysdale L, Fong GH (2000) The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc Natl Acad Sci USA 97:8386–8391
Percy MJ, Beer PA, Campbell G, Dekker AW, Green AR, Oscier D, Rainey MG, van Wijk R, Wood M, Lappin TR, McMullin MF, Lee FS (2008a) Novel exon 12 mutations in the HIF2A gene associated with erythrocytosis. Blood 111:5400–5402
Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, McMullin MF, Lee FS (2008b) A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med 358:162–168
Pietras A, Gisselsson D, Ora I, Noguera R, Beckman S, Navarro S, Påhlman S (2008) High levels of HIF-2alpha highlight an immature neural crest-like neuroblastoma cell cohort located in a perivascular niche. J Pathol 214:482–488
Pietras A, Hansford LM, Johnsson AS, Bridges E, Sjolund J, Gisselsson D, Rehn M, Beckman S, Noguera R, Navarro S, Cammenga J, Fredlund E, Kaplan DR, Påhlman S (2009) HIF-2alpha maintains an undifferentiated state in neural crest-like human neuroblastoma tumor-initiating cells. Proc Natl Acad Sci USA 106:16805–16810
Rankin EB, Tomaszewski JE, Haase VH (2006) Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66:2576–2583
Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH (2007) Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 117:1068–1077
Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, Simon MC, Keith B, Haase VH (2008) Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene 27:5354–5358
Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ (2005) Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol 25:5675–5686
Raza SM, Lang FF, Aggarwal BB, Fuller GN, Wildrick DM, Sawaya R (2002) Necrosis and glioblastoma: a friend or a foe? A review and a hypothesis. Neurosurgery 51:2–12; discussion 12–13
Rosenberger C, Griethe W, Gruber G, Wiesener M, Frei U, Bachmann S, Eckardt KU (2003) Cellular responses to hypoxia after renal segmental infarction. Kidney Int 64:874–886
Ryan HE, Lo J, Johnson RS (1998) HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 17:3005–3015
Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, Jakesz R, Kubista E, Birner P, Oberhuber G (2002) Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res 8:1831–1837
Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA (2003) The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood 102:1634–1640
Scortegagna M, Ding K, Zhang Q, Oktay Y, Bennett MJ, Bennett M, Shelton JM, Richardson JA, Moe O, Garcia JA (2005) HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood 105:3133–3140
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3:721–732
Shweiki D, Itin A, Soffer D, Keshet E (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63:5821–5828
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL (2003) Predominant role of hypoxia-inducible transcription factor (Hif)-1alpha versus Hif-2alpha in regulation of the transcriptional response to hypoxia. Cancer Res 63:6130–6134
Swinson DE, Jones JL, Richardson D, Wykoff C, Turley H, Pastorek J, Taub N, Harris AL, O’Byrne KJ (2003) Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non-small-cell lung cancer. J Clin Oncol 21:473–482
Tan EY, Campo L, Han C, Turley H, Pezzella F, Gatter KC, Harris AL, Fox SB (2007) BNIP3 as a progression marker in primary human breast cancer; opposing functions in in situ versus invasive cancer. Clin Cancer Res 13:467–474
Tian H, McKnight SL, Russell DW (1997) Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 11:72–82
Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL (1998) The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev 12:3320–3324
Volm M, Koomagi R (2000) Hypoxia-inducible factor (HIF-1) and its relationship to apoptosis and proliferation in lung cancer. Anticancer Res 20:1527–1533
Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, Ratcliffe PJ, Maxwell PH (1998) Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood 92:2260–2268
Yohena T, Yoshino I, Takenaka T, Kameyama T, Ohba T, Kuniyoshi Y, Maehara Y (2009) Upregulation of hypoxia-inducible factor-1alpha mRNA and its clinical significance in non-small cell lung cancer. J Thorac Oncol 4:284–290
Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL (2000) Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer 88:2606–2618
Zimmer M, Doucette D, Siddiqui N, Iliopoulos O (2004) Inhibition of hypoxia-inducible factor is sufficient for growth suppression of VHL-/- tumors. Mol Cancer Res 2:89–95
Acknowledgments
This work was supported by the Swedish Cancer Society, the Children’s Cancer Foundation of Sweden, the Swedish Research Council, the SSF Strategic Center for Translational Cancer Research – CREATE Health, Gunnar Nilsson’s Cancer Foundation and the research funds of Malmö University Hospital.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Pietras, A., Johnsson, A.S., Påhlman, S. (2010). The HIF-2α-Driven Pseudo-Hypoxic Phenotype in Tumor Aggressiveness, Differentiation, and Vascularization. In: Simon, M. (eds) Diverse Effects of Hypoxia on Tumor Progression. Current Topics in Microbiology and Immunology, vol 345. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2010_72
Download citation
DOI: https://doi.org/10.1007/82_2010_72
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-13328-2
Online ISBN: 978-3-642-13329-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)