Abstract
Varicella zoster virus (VZV) infection results in the establishment of latency in human sensory neurons. Reactivation of VZV leads to herpes zoster which can be followed by persistent neuropathic pain, termed post-herpetic neuralgia (PHN). Humans are the only natural host for VZV, and the strict species specificity of the virus has restricted the development of an animal model of infection which mimics all phases of disease. In order to elucidate the mechanisms which control the establishment of latency and reactivation as well as the effect of VZV replication on neuronal function, in vitro models of neuronal infection have been developed. Currently these models involve culturing and infecting dissociated human fetal neurons, with or without their supporting cells, an intact explant fetal dorsal root ganglia (DRG) model, neuroblastoma cell lines and rodent neuronal cell models. Each of these models has distinct advantages as well as disadvantages, and all have contributed towards our understanding of VZV neuronal infection. However, as yet none have been able to recapitulate the full virus lifecycle from primary infection to latency through to reactivation. The development of such a model will be a crucial step towards advancing our understanding of the mechanisms involved in VZV replication in neuronal cells, and the design of new therapies to combat VZV-related disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
During primary varicella-zoster virus (VZV) infection (varicella), the virus establishes a latent infection in sensory ganglia, mainly dorsal root ganglia (DRG) and trigeminal ganglia (TG), from where it can reactivate years later, resulting in re-initiation of productive replication with a consequence of the development of herpes zoster (shingles). Neurons within sensory ganglia are the primary site of latent infection, although there have also been sporadic reports of latency in ganglionic satellite cells (Hyman et al. 1983; Gilden et al. 1987; Croen et al. 1988; Schmidbauer et al. 1992; Lungu et al. 1995, 1998; Kennedy et al. 1998; LaGuardia et al. 1999; Levin et al. 2003). Sensory ganglia are part of the peripheral nervous system and sensory neurons can respond to mechanical, thermal, or chemical stimuli (Lawson 2005). Despite the critical importance of sensory ganglia in the lifecycle of VZV infection, not much is understood about the interactions between VZV, neurons, and their supporting cells. Specifically, factors controlling the establishment, maintenance, and reactivation from latency, as well as the impact of VZV replication on normal neuronal function remain poorly defined. This is largely due to the high species specificity of VZV, meaning the virus does not progress to a full cycle of lytic infection in most non-human cells (Weller and Stoddard 1952). This chapter will overview current experimental models that have been developed to study the interaction of VZV with ganglionic cells, with a particular focus on cell culture-based models of VZV infection as animal models of infection are detailed elsewhere in this volume.
2 Experimental VZV Infection of Primary Human Neurons from Dissociated Neural Tissue
The first in vitro study to examine VZV replication in human cells derived from neural tissue used cultures of human brain and ganglia cells. These cultures contained a mixed population of uncharacterised cells of mesenchymal and neuroglial origin, although due to multiple passaging neurons were not present (Gilden et al. 1978). VZV was successfully able to infect these cells and the infection occurred in a similar fashion to human lung fibroblasts, with the exception that cells of neural origin exhibited the formation of large intracytoplasmic vacuoles (Gilden et al. 1978).
Because of the inherent difficulty in obtaining fresh adult human sensory ganglia to derive primary neurons, several studies have utilised fetal sensory ganglia-derived neurons to study VZV neuropathogenesis. Although the capacity to obtain fresh fetal sensory ganglia remains a limiting factor, this approach is advantageous as humans are the only natural host for VZV and the virus generally does not replicate efficiently in cells of non-human origin (Weller and Stoddard 1952). Aborted fetal samples between 8 and 20 weeks of gestation are generally obtained and DRG harvested. Dissociation of the neurons within DRG is achieved usually after physically removing some of the surrounding epineurium before treatment with trypsin or collagenase to disrupt cell–cell junctions. To further dissociate the cells, they have been triturated through fire-polished pipettes or a fine gauge needle to obtain a uniform single cell suspension. Mitotic inhibitors have then often been employed to eliminate dividing non-neuronal cells such as fibroblasts, resulting in a relatively pure population of neurons, although some satellite cells often remain. After plating onto an extracellular matrix such as collagen, these cells can then be cultured in the presence of nerve growth factor (NGF) to ensure their survival (Wigdahl et al. 1986; Assouline et al. 1990; Somekh et al. 1992; Somekh and Levin 1993; Hood et al. 2003, 2006).
The first studies to utilise human fetal neurons were performed by Wigdahl et al. (1986) and Assouline et al. (1990). Using cell-associated and cell-free methods of infections, respectively, both studies showed that fetal neurons could be infected in vitro as demonstrated by the detection of viral proteins using immunofluorescence and electron microscopy to show the presence of viral particles within infected neurons (Wigdahl et al. 1986; Assouline et al. 1990). A cytopathic effect characterised by cellular degeneration and detachment of the cells was also observed within these infected neuronal cultures, consistent with productive replication. However, both studies also noted that infection of neurons progressed more slowly than infection of fibroblasts, a cell type highly permissive to productive VZV infection. Assouline et al. (1990) went on to characterise the temporal cascade of VZV protein expression and showed that the early protein encoded by open reading frame (ORF) 36, deoxypyrimidine kinase, accumulated to detectable levels prior to the immediate early gene IE62 and remained predominant in the cytoplasm, which was in contrast to the infection of fibroblasts where ORF36 was detected predominantly in the nucleus (Assouline et al. 1990). These studies showed that there are likely to be important differences between the nature of VZV replication in neurons compared to fibroblasts and the authors suggested that neurons may be able to control some aspects of viral replication to limit virus-induced damage (Wigdahl et al. 1986). Somekh and Levin (1993) also utilised dissociated fetal neurons to demonstrate infection with the attenuated vaccine strain vOKA; however, infection with the wild-type strain resulted in a much greater percentage of infected neurons, implying that the vaccine strain has attenuated neurotropism (Somekh and Levin 1993).
2.1 Modulation of Neuronal Apoptosis by VZV
Our group used primary human fetal DRG-derived neurons to examine the interaction of VZV with this cell type (Hood et al. 2003) (Fig. 1). It was shown that during productive infection with VZV, apoptosis in fibroblasts increased proportionally to the number of infected cells. In contrast, VZV infected neurons appeared to be protected from apoptosis, highlighting a functional difference between VZV replication in neurons and fibroblasts (Hood et al. 2003). In a follow-up study we demonstrated a role for the VZV protein IE63 in the protection of neurons from apoptosis during productive infection (Hood et al. 2006). IE63 is encoded by ORF63 and is duplicated in the VZV genome, with this additional copy known as ORF70. In comparison to wild-type parental virus infection, infection of primary human neurons with either an ORF63 or an ORF70 deletion virus resulted in an increase in the percentage of neurons undergoing apoptosis (Hood et al. 2006). This protection from apoptosis by IE63 was further supported by experiments showing that the induction of apoptosis by withdrawal of NGF from cultured rat neurons was suppressed when these cells were first transfected with a plasmid encoding IE63 (Hood et al. 2006). At present the mechanism by which IE63 inhibits neuronal apoptosis has not been elucidated. In addition to IE63, other VZV genes may also play a function to prevent apoptosis. For example, the ORF66 protein product, which is expressed during productive infection as well as during latent infection of neurons (Cohrs et al. 2003), has been shown to play a role in preventing apoptosis in VZV-infected T cells (Schaap et al. 2005), although additional studies will be required to determine whether this gene product functions in an anti-apoptotic manner in neurons.
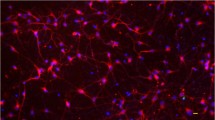
Light microscopy of dissociated human fetal dorsal root ganglia (DRG) neuronal cell culture. After culture of dissociated ganglionic cells in media containing nerve growth factor for 3 days, neurons (arrows) develop extensive axonal networks (arrowheads). Copyright © American Society for Microbiology, Hood et al. (2003), DOI: 10.1128/JVI.77.23.12852-12864.2003
Protecting neurons from apoptosis during the critical first stages of virus reactivation would likely allow for greater production of new virions for axonal transport to the skin and herpes zoster lesion formation (Hood et al. 2003). It may also be of benefit to the virus to actively resist the induction of apoptosis during the latent phase of infection. It can also be argued that resistance of neuronal apoptosis may also benefit the host, given that neurons are post-mitotic and therefore are not replaced following cell death. Interestingly, however, observations in human ganglia obtained post-mortem from patients suffering from herpes zoster and post-herpetic neuralgia (PHN) have revealed that these ganglia display regions of altered morphology, where damage to neuronal tissue and cell loss has occurred as a result of VZV reactivation (Head and Campbell 1900; Denny-Brown et al. 1944; Smith 1978; Watson et al. 1988, 1991; Steain et al. 2009). Thus, mechanisms responsible for this damage in vivo may be a consequence of the inflammatory process rather than VZV-induced apoptosis (Hood et al. 2003). Understanding the mechanisms that underlie VZV-induced cellular damage and PHN will ultimately require extension of in vitro-based findings to additional analysis of naturally infected human ganglia. In this respect, our group has gained access to adult human ganglia removed post-mortem from people suffering from herpes zoster at or near the time of death. Experiments to define the nature of the ganglionic cellular infiltrate and neuronal damage that accompanies VZV reactivation are currently underway, and it is hoped that these analyses will provide a better understanding of the factors that influence reactivation and the development of PHN.
3 Models of Latent Infection of Human Neurons
Another important aspect of the life cycle of VZV is the establishment of latency. In an attempt to create an in vitro model of latency, Somekh et al. (1992) treated neuronal cultures as well as satellite cell and mixed (neurons and satellite cells) cultures with the anti-herpes arabinosyl nucleoside analogue bromovinyl arabinosyl uracil (BVaraU) before infecting with cell-free VZV. They found that BVaraU was able to prevent a full productive infection in these cultures, and that no viral replication occurred even after BVaraU was removed 7 days post-infection. However, infectious VZV was able to be recovered from some of the mixed (neuronal and satellite cell) cultures a week after infection and BVaraU withdrawal, when the cells were trypsinized and co-cultured with human embryonic lung fibroblasts (HELFs) (Somekh et al. 1992). It was concluded from these experiments that satellite cells may play an important role in the establishment and/or maintenance of latency. However, important differences exist between this model and latency in vivo due to the requirement of BVaraU to establish latency in vitro and the absence of IE62, which has been detected in neurons during latency in vivo (Lungu et al. 1998). Since this initial report, there has been no new published work utilising cell-culture based models of latent VZV infection using cultures of dissociated human fetal DRG. Rather, the published studies of VZV latency in human neurons have relied predominantly on examination of naturally infected adult ganglia obtained post mortem (Gilden et al. 1983, 2001; Croen et al. 1988; Vafai et al. 1988; Mahalingam et al. 1992, 1993, 1996; Schmidbauer et al. 1992; Lungu et al. 1998; LaGuardia et al. 1999; Theil et al. 2003; Cohrs et al. 2003, 2005; Hufner et al. 2006; Cohrs and Gilden 2007; Verjans et al. 2007).
4 Experimental VZV Infection of Intact Ganglia
Dissociation of ganglia results in separation of neurons from satellite cells, which may affect their function and slow VZV spread in vitro, given that in vivo neurons are tightly enclosed by multiple satellite cells (Hanani 2005). Hanani et al. noted that “Isolated intact sensory ganglia should be the first choice for studying SGC (satellite glial cell)–neuron interactions as there is minimal tissue disruption” (Hanani 2005). Thus, to study fetal neurons in the context of a more intact anatomical architecture, two different approaches have been developed. The Arvin group reported the development of an in vivo model whereby severe combined immunodeficiency (SCID) mice were used following implantation of intact human fetal DRG under the kidney capsule. This model has provided a very useful means to study different routes of infection of DRG, and has been used to provide evidence for a role of VZV-infected T cells in the transfer of virus to the DRG (Zerboni et al. 2005). VZV also establishes a persistent infection within these DRG, which can last for up to 8 weeks post-infection in the absence of detectable infectious virus production (Zerboni et al. 2005). Additional studies using this model are detailed in (Zerboni et al. 2010). Disadvantages of the SCID-hu mouse model, however, are the extended period of time required to allow for the establishment of the DRG xenograph and costs associated with housing animals.
Our laboratory has established an intact explant fetal DRG culture model (Gowrishankar et al. 2007). By extracting fetal DRG from aborted fetuses between the ages of 14–20 weeks gestation and removing the surrounding epineurium, DRG can be cultured directly on glass coverslips in the presence of NGF. After 2–3 days post-explant extensive axonal growth can be seen protruding from the entire DRG (Gowrishankar et al. 2007). Characterization of the ganglia post-explanting has shown that the architecture of the ganglia is preserved, with neurons being surrounded by supporting satellite cells. Immunohistochemical staining has also demonstrated the presence of ganglionic cell markers, with neurons staining positive for the neural cell adhesion molecule (NCAM) and satellite cells positive for S100B (Gowrishankar et al. 2007), similar to normal human adult ganglia. This model allows VZV infection of neurons and satellite cells to be studied in the context of an intact ganglion in vitro. Following 48 h of cell-associated infection with VZV strain Schenke, discrete VZV glycoprotein-positive neurons were detected throughout cultured ganglia, suggesting productive infection and axonal transport of the virus from the periphery (Fig. 2). The number of infected neurons and supporting cells increased over time, indicating that cell-to-cell spread of the virus likely occurred within the infected DRG (Gowrishankar et al. 2007). The presence of virus particles in neurons, as well as in the extracelluar space, was also demonstrated by transmission electron microscopy (TEM) (Gowrishankar et al. 2007). Importantly, cell-free virus was shown to be released from these cultures, which is a unique feature of this model, as VZV typically remains highly cell-associated in culture (Weller 1953). The explant DRG model provides a number of opportunities to study various aspects of VZV replication within DRG, including innate immune responses to infection and effects of viral replication on neuronal function. This model could also be used to screen potential new vaccine candidates for neurotropism, and determine the basis of VZV neuropathogenesis using viral gene deletion viruses. This model may also have other uses such as studying neuronal signaling during VZV infection and anterograde and retrograde transport of VZV proteins in axons, which could be done in combination with explanted skin sections. This approach has previously been used in pioneering work by the Cunningham group to study axonal transport of herpes simplex virus (HSV) from human DRG (Penfold et al. 1994, 1996; Holland et al. 1998, 1999; Mikloska et al. 1999; Mikloska and Cunningham 2001), and a similar approach could be used to study VZV virion transport and assembly. Such studies may lead to the identification of new drug targets to limit axonal transport of the virus, which may prevent the establishment of latency or aid in the treatment of herpes zoster.
VZV antigen expression in infected intact explant human dorsal root ganglia (DRG). (a) Immunofluorescent staining of a DRG 48 h post infection with human VZV hyper immune serum and secondary antibody consisting of fluorescently conjugated anti-human AlexaFluor 594 (red staining). VZV antigen expression on the surfaces of distinct, scattered neurons (white arrows) and around the DRG body (green arrows). Boxed inset shows magnified image of VZV antigen positive neurons. (b) Immunofluorescent staining of a DRG at 48 h post infection with mouse anti-VZV gE monoclonal antibody and secondary antibody consisting of fluorescently conjugated anti-mouse AlexaFluor 594 (red staining). Nuclear blue DAPI staining is indicated. (c–e) Immunohistochemical detection of VZV infected DRG stained with mouse anti-VZV gB monoclonal antibody at 48 h post infection (c) and 72 h post infection (d) (brown staining) or mock infected DRG (e). Black arrows indicate infected neurons. Sections were lightly counterstained with hematoxylin (blue staining). Copyright © American Society for Microbiology, Gowrishankar et al. (2007), DOI: 10.1128/JVI.02793-06
5 VZV Infection of Neuronal Cell Lines
An alternative approach for studying VZV infection of primary neurons has been to examine infection of neuroblastoma cell lines. Such a surrogate model of neuronal infection affords distinct advantages over working with primary neurons in terms of cost, availability, cell number, ease of manipulation, a lack of donor variation, and a reduced risk of contamination with other infectious agents. Further, by using neuroblastomas, ethical issues that arise when using fetal tissues are avoided. Cell lines in general are also easier to genetically manipulate, and thus neuroblastomas have the potential to be used to evaluate the role of cellular genes in VZV infection. Although a caveat of using neuroblastoma cell lines is whether they adequately mimic primary neurons, it remains rather surprising that there have been so few reports exploring the nature and properties of VZV infection in neuroblastomas.
VZV infection of the human derived IMR-32 and the murine neuro-2A neuroblastoma cell lines have been studied (Bourdon-Wouters et al. 1990). Infection of IMR-32 cells, in both a cell-free and a cell-associated manner using infected MRC-5 cells, resulted in a cytopathic effect, cell death, and the release of cell-free virus. In contrast, infection of neuro-2A cells using a similar approach was non-productive, with no viral antigens detected within cells, despite persistence of VZV DNA, as detected by in situ hybridization (ISH) (Bourdon-Wouters et al. 1990). These differences may be due to the non-human origin of the neuro-2A cells (Bourdon-Wouters et al. 1990).
The SK-N-SH neuroblastoma cell line, which is also of human origin, as well as one of its derivatives SH-SY5Y (Biedler et al. 1973; Ross et al. 1983), has been used for infection with VZV (Cohen and Nguyen 1998; Cohen et al. 2001; Sato et al. 2002); however, the infection of these neuroblastomas has not yet been fully characterised. SH-SY5Y cells can be readily differentiated using retinoic acid and brain-derived neurotrophic factor (BDNF) to produce growth-arrested cells which have extended neuritic processes and express neuronal markers, rendering them similar to primary neurons (Encinas et al. 2000). SH-SY5Y cells have been shown to be more permissive to HSV-1 infection in their differentiated form due to an upregulation of Nectin-1 and -2 and HVEM (Gimenez-Cassina et al. 2006). Unpublished data from our group indicates that infection of both undifferentiated and differentiated SH-SY5Y cells with VZV results in a full productive infection, which shares many characteristics of infection with dissociated primary human fetal neurons. Mechanisms involved in the development of neuropathic pain during post-herpetic neuralgia are poorly understood. SY-SY5Y cells can be stably transfected to express transient receptor potential (TRP) vanilliod receptors (Lam et al. 2007) and the tetrodotoxin-resistant voltage-gated sodium channel alpha-subunit Nav1.8 (Dekker et al. 2005), giving properties of nociceptor (pain sensing) neurons. Therefore, these cells could be used to study the effects of VZV infection on neurotransmitter release and neuronal function, which may have implications for PHN. Thus the use of neuroblastomas may serve as a more practical alternative to using fetal neurons, at least for initial studies of various aspects of VZV infection, which could then be confirmed and extended using primary neuronal cultures or naturally infected tissue samples.
6 VZV Infection of Rodent Neurons
Neurons of rodent origin have also been used to study VZV infection. However, as VZV is highly species specific and humans are the only natural host for the virus, infection of cells of non-human origin may not accurately reflect the virus lifecycle in vivo. Ganglionic neurons of rodent origin, however, are much easier to obtain than human fetal ganglia, and this has been the predominant driving force in pursuing this approach.
The first study of VZV infection using animal neurons in vitro was performed by Merville-Louis et al. (1989). A mixed population of dissociated neurons and supporting cells was established, with neurons consisting of approximately 10% of the culture. Cells were infected in either a cell-free or cell-associated manner, and in both cases no cytopathic effect was observed up to 10 days post-infection. In addition, no cell-free virus was released and virus was not able to be transferred to permissive MRC-5 cells. Viral antigens were detected in a small percentage of neurons, using human serum which was known to contain anti-VZV antibodies; however, antigen was detected only up until day 5 post-infection. Despite the lack of de novo virus production, VZV nucleic acids were detected within neurons by ISH, and this increased from 20% of neurons being positive at day 1 to 50% at day 6 post-infection. No ISH signal was detected in any of the non-neuronal cells. ISH specific for RNA transcripts of immediate early, early, and late genes showed that genes from all the temporal classes were expressed in neurons, although the IE63 transcript was found to give a stronger hybridization signal. From this study the authors concluded that the infection of rat neurons resulted in a non-productive, persistent infection, and that the IE63 gene may play a role in repressing a productive infection (Merville-Louis et al. 1989).
Subsequent studies using rat DRG neurons in vitro were able to achieve a productive infection using a “microculture” system (Kress and Fickenscher 2001; Schmidt et al. 2003). Neurons and infected fibroblasts were concentrated into very small volumes of media and incubated together for 4 h before additional culture media was added. Presumably this increased cell-to-cell contact between infected fibroblasts and neurons facilitated the spread of VZV. IE62, IE63, and glycoprotein E could be detected within neurons by immunofluorescence. Within 3–4 days post-infection, the majority of productively infected rat neurons died. This was characterised by a loss of viable cells within the culture, which was in contrast to control uninfected cultures, which appeared healthy. These studies went on to further examine the effects of VZV infection on rat neurons with respect to neuronal function and showed that VZV infection results in a gain-of-function conferring sensitivity to adrenergic agonists, including norepinephrine which is associated with pain (Kress and Fickenscher 2001; Schmidt et al. 2003). It was also shown that this effect was greatly reduced when rat neurons were infected with the vaccine OKA strain, despite the vaccine strain being able to infect an equal percentage of neurons as the wild-type strain (Schmidt et al. 2003). This contrasted with a prior study using human fetal neuron cultures, which showed that the vaccine strain did not infect neurons to the same degree as did the wild-type VZV strains (Somekh and Levin 1993). It was proposed that the induction of sensitivity to adrenergic stimulation following VZV infection could be responsible for heat hyperalgesia, which is commonly linked with PHN (Schmidt et al. 2003). In a separate study using rat neurons, a VZV IE63 expression construct was transfected into neurons derived from rat embryos to show that this caused an increase in calcitonin gene-related peptide (CGRP) release, which in vivo can increase the sensation of pain (Hamza et al. 2007). Thus expression of IE63 in neurons may play a role in the development of pain that is experienced during herpes zoster and PHN.
Isolated guinea pig and mouse enteric neurons have also been used to establish a model of lytic, latent, and reactivating VZV infection in vitro (Chen et al. 2003; Gershon et al. 2008). Enteric ganglia differ from dorsal root ganglia in that they not only contain afferent (sensory) neurons, but also efferent (motor) neurons and interneurons. To validate the use of enteric neurons for VZV infection, it has been shown in vivo that enteric neurons can harbor latent VZV (Gershon et al. 2009). Using this guinea pig-derived model it was demonstrated that infection of guinea pig enteric neuronal cultures with cell-free VZV resulted in an apparent latent infection. No cytopathic effect was observed, and transcripts for the latency-associated ORFs 4, 21, 29, 40, 62 and 63 could be detected (Chen et al. 2003). In addition, several proteins arising from these transcripts were detected in neurons by immunofluorescence where they were found in the cytoplasm of cells, which is indicative of latency (Lungu et al. 1998; Chen et al. 2003). Immunofluorescence staining also revealed that sensory neurons present in the culture, as well as other types of neurons, were able to be infected in a nonproductive manner, and these infected neurons could survive in culture for weeks, similar to uninfected controls (Chen et al. 2003). Interestingly, when these neurons were infected in a cell-associated manner, or when other non-neuronal cells from the bowel wall were present, a productive infection resulted (Chen et al. 2003). In this scenario, glycoproteins, which have not been reported to be produced during latency, were detected and the neurons died rapidly (Chen et al. 2003). It was proposed that the absence of the non-structural ORF61 protein may enable the virus to establish a latent infection when cell-free virus was used as the inoculum. It was subsequently shown that superinfection of latently infected enteric neurons with a viral vector expressing the ORF61 protein resulted in an apparent reactivation of the virus, with concomitant expression of viral glycoprotein and neuronal death (Gershon et al. 2008). This model has also been used to demonstrate that the cellular localization of IE63 and ORF29p is cell-type dependant, with both proteins accumulating in the cytoplasm of guinea pig enteric neurons and the nucleus of epithelial cells, and that the expression of ORF61p in neurons can drive their nuclear import in a protease-dependent fashion (Stallings et al. 2006; Walters et al. 2008). Infected guinea pig enteric neurons were also used to show a link between IE63 and the human antisilencing function 1 protein, which may influence transcription of genes during VZV infection (Ambagala et al. 2009).
In addition to the use of dissociated rodent neurons to study VZV infection, a number of studies have sought to utilise whole-animal models of infection. In this respect, in vivo VZV infection of weanling guinea pigs via various routes of inoculation, including intranasally, subcutaneously, intramuscularly, and via corneal infection has been reported to result in viremia and nasopharyngeal shedding of virus, which can lead to transmission of VZV to other animals (Myers et al. 1980, 1985; Matsunaga et al. 1982). In addition, the presence of VZV DNA in DRG of infected guinea pigs was also demonstrated by PCR, indicating that virus can access sensory ganglia in these animals (Lowry et al. 1993). Likewise, there has also been a report of VZV infection of mice via a corneal inoculation, resulting in viremia and detection of VZV DNA in various tissues including the trigeminal ganglia, the brain stem, and the spleen, up to 33 days post-infection, with the mice not suffering from any apparent VZV-related illness (Wroblewska et al. 1993). There has also been interest in creating an in vivo model of VZV infection using rats, and these have been used to study VZV latency and as a behavioral model of VZV-induced pain (Sadzot-Delvaux et al. 1990, 1995, 1998; Annunziato et al. 1998; Fleetwood-Walker et al. 1999; Kennedy et al. 2001; Sato et al. 2003; Grinfeld et al. 2004; Garry et al. 2005). The development and uses of rat models of latency is discussed further in (Cohen 2010).
7 VZV Infection of Ganglionic Satellite Cells
Neurons within the DRG and TG are surrounded by satellite cells, and in addition to providing physical support to neurons, satellite cells appear to be capable of affecting the microenvironment of the ganglia and may even play a role in neuronal signaling (review in Hanani 2005). Recently, satellite cells of the TG have also been shown to share some properties of antigen presenting cells, and therefore may also influence the immune response within ganglia (van Velzen et al. 2009). It is not surprising that satellite cells are required to perform immune surveillance, and antigen presentation within the ganglia, given neurons within sensory ganglia, like neurons of the central nervous system, fail to express major histocompatibility complex (MHC) molecules (Turnley et al. 2002). This may allow VZV to persist latently within neurons without recognition by the immune system. This would be beneficial for both the virus and the host as neurons are post-mitotic cells and therefore will not be replaced if killed as a result of infection (Joly et al. 1991). VZV DNA and RNA have been detected within satellite cells during latency (Croen et al. 1988; Schmidbauer et al. 1992; Kennedy et al. 1998; Lungu et al. 1998; LaGuardia et al. 1999). VZV antigens within satellite cells have also been reported in studies of explanted VZV-infected fetal ganglia (Gowrishankar et al. 2007) and in infected fetal ganglia xenographed into SCID mice (Zerboni et al. 2007; Reichelt et al. 2008). However, further studies examining the impact of VZV infection on satellite cell function are needed due to the important role these cells play in supporting neurons within the ganglia.
8 Conclusions and Perspectives
Productive, latent, and reactivated VZV infection of sensory neurons and their supporting satellite cells may result in alterations to normal cellular processes, which in combination with an immune-mediated response to viral replication may contribute towards the pain experienced during herpes zoster and subsequently during PHN. Thus, a greater understanding of how VZV interacts with cells of the sensory ganglia is required to develop new therapeutic strategies to prevent or manage these conditions.
The lack of an adaptive immune response is the greatest disadvantage to in vitro models of VZV neuronal infection, as well as the in vivo SCID-hu mouse model, and without an animal model that can mimic all phases of disease progression in the context of an immune response, studies of post-mortem ganglia affected by varicella, herpes zoster, and PHN will remain critical.
From the in vitro studies that have been conducted thus far, it is becoming clear that distinct differences exist between VZV replication in neuronal cells compared to other diploid cells. These differences could play a key role in enabling VZV to successfully replicate in neurons within sensory ganglia, for example, during the initial stages of virus reactivation, leading to herpes zoster. The precise viral and cellular mechanisms that underpin virus replication within this cell type, as well as those that facilitate latency, remain to be established.
Differences in VZV replication in vitro are also evident between studies and models, and which model is the most accurate representation of VZV infection in vivo remains unknown. Like during natural VZV replication in human skin, in both the explant fetal DRG model and in IMR32 neuroblastoma cells, cell-free virus release has been demonstrated (Bourdon-Wouters et al. 1990; Gowrishankar et al. 2007). This is in stark contrast to infection of other cell types in vitro (Weller 1953), including other neuroblastoma-based culture systems, where virus remains cell-associated (Abendroth, unpublished data). This may reflect differences in the virus lifecycle in these cells and perhaps a difference in the expression of mannose-6-phosphate receptor, which has been shown to affect cell-free virus release (Chen et al. 2004). Studies comparing neuronal infection in vitro to naturally infected human ganglia derived post-mortem or surgically are therefore needed to establish the robustness of these models, as well as to establish the role of the adaptive immune response.
Another difference observed between models of VZV neuronal infection in vitro is that VZV-infected rat and guinea pig enteric neurons do not survive infection (Merville-Louis et al. 1989; Chen et al. 2003; Gershon et al. 2008), which is in contrast to studies of primary human neuronal cells, in which VZV infection has been shown not to induce apoptosis (Hood et al. 2003). The IE63 protein has been shown to protect human neurons from apoptosis; however, the precise mechanism behind this protection is unknown (Hood et al. 2006). Further studies of the different models, animal vs. human neurons, may help to elucidate how IE63 modulates the apoptotic response of neurons.
Pain as a result of herpes zoster and especially PHN can be disabling and have a major negative impact on patient’s quality of life (Dworkin et al. 2001). In coming years the number of individuals suffering from herpes zoster and PHN is expected to rise, concomitant with the increasing number of patients who are elderly or immunosuppressed due to infection or therapies for cancer or transplantations. Therefore, VZV and associated diseases are likely to place a large burden on health care systems, and adequate treatment and prevention strategies are needed. To achieve this, further studies into the mechanisms involved in VZV replication in neuronal cells are necessary. In addition, given that VZV is able to readily infect neurons, like HSV, it may be a suitable vector for gene delivery into neuronal cells for the treatment of various nervous system disorders. This could be achieved only by further studies into the interaction between VZV and neuronal cells, and suitable attenuation of the virus to limit replication in other cell types.
References
Ambagala AP, Bosma T, Ali MA, Poustovoitov M, Chen JJ, Gershon MD, Adams PD, Cohen JI (2009) Varicella-zoster virus immediate-early 63 protein interacts with human antisilencing function 1 protein and alters its ability to bind histones h3.1 and h3.3. J Virol 83(1):200–209
Annunziato P, LaRussa P, Lee P, Steinberg S, Lungu O, Gershon AA, Silverstein S (1998) Evidence of latent varicella-zoster virus in rat dorsal root ganglia. J Infect Dis 178(Suppl 1):S48–S51
Assouline JG, Levin MJ, Major EO, Forghani B, Straus SE, Ostrove JM (1990) Varicella-zoster virus infection of human astrocytes, Schwann cells, and neurons. Virology 179(2):834–844
Biedler JL, Helson L, Spengler BA (1973) Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res 33(11):2643–2652
Bourdon-Wouters C, Merville-Louis MP, Sadzot-Delvaux C, Marc P, Piette J, Delree P, Moonen G, Rentier B (1990) Acute and persistent varicella-zoster virus infection of human and murine neuroblastoma cell lines. J Neurosci Res 26(1):90–97
Chen JJ, Gershon AA, Li ZS, Lungu O, Gershon MD (2003) Latent and lytic infection of isolated guinea pig enteric ganglia by varicella zoster virus. J Med Virol 70(Suppl 1):S71–S78
Chen JJ, Zhu Z, Gershon AA, Gershon MD (2004) Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell 119(7):915–926
Cohen JI (2010) Rodent models of varicella-zoster virus neurotropism. Curr Top Microbiol Immunol doi 10.1007/82_2010_11
Cohen JI, Nguyen H (1998) Varicella-zoster virus ORF61 deletion mutants replicate in cell culture, but a mutant with stop codons in ORF61 reverts to wild-type virus. Virology 246(2):306–316
Cohen JI, Sato H, Srinivas S, Lekstrom K (2001) Varicella-zoster virus (VZV) ORF65 virion protein is dispensable for replication in cell culture and is phosphorylated by casein kinase II, but not by the VZV protein kinases. Virology 280(1):62–71
Cohrs RJ, Gilden DH (2007) Prevalence and abundance of latently transcribed varicella-zoster virus genes in human ganglia. J Virol 81(6):2950–2956
Cohrs RJ, Gilden DH, Kinchington PR, Grinfeld E, Kennedy PG (2003) Varicella-zoster virus gene 66 transcription and translation in latently infected human Ganglia. J Virol 77(12):6660–6665
Cohrs RJ, Laguardia JJ, Gilden D (2005) Distribution of latent herpes simplex virus type-1 and varicella zoster virus DNA in human trigeminal Ganglia. Virus Genes 31(2):223–227
Croen KD, Ostrove JM, Dragovic LJ, Straus SE (1988) Patterns of gene expression and sites of latency in human nerve ganglia are different for varicella-zoster and herpes simplex viruses. Proc Natl Acad Sci USA 85(24):9773–9777
Dekker LV, Daniels Z, Hick C, Elsegood K, Bowden S, Szestak T, Burley JR, Southan A, Cronk D, James IF (2005) Analysis of human Nav1.8 expressed in SH-SY5Y neuroblastoma cells. Eur J Pharmacol 528(1–3):52–58
Denny-Brown D, Adam R, Fitzgerald P (1944) Pathological features of herpes zoster. Am Med Assoc Arch Dermatol 75:193–196
Dworkin RH, Nagasako EM, Johnson RW, Griffin DR (2001) Acute pain in herpes zoster: the famciclovir database project. Pain 94(1):113–119
Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Cena V, Gallego C, Comella JX (2000) Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem 75(3):991–1003
Fleetwood-Walker SM, Quinn JP, Wallace C, Blackburn-Munro G, Kelly BG, Fiskerstrand CE, Nash AA, Dalziel RG (1999) Behavioural changes in the rat following infection with varicella-zoster virus. J Gen Virol 80(Pt 9):2433–2436
Garry EM, Delaney A, Anderson HA, Sirinathsinghji EC, Clapp RH, Martin WJ, Kinchington PR, Krah DL, Abbadie C, Fleetwood-Walker SM (2005) Varicella zoster virus induces neuropathic changes in rat dorsal root ganglia and behavioral reflex sensitisation that is attenuated by gabapentin or sodium channel blocking drugs. Pain 118(1–2):97–111
Gershon AA, Chen J, Davis L, Krinsky C, Cowles R, Gershon MD (2009) Distribution of latent varicella zoster virus (VZV) in sensory ganglia and gut after vaccination and wild-type infection: evidence for viremic spread. The 34th Annual International Herpesvirus Workshop. Ithaca, New York, USA
Gershon AA, Chen J, Gershon MD (2008) A model of lytic, latent, and reactivating varicella-zoster virus infections in isolated enteric neurons. J Infect Dis 197(Suppl 2):S61–S65
Gilden DH, Gesser R, Smith J, Wellish M, Laguardia JJ, Cohrs RJ, Mahalingam R (2001) Presence of VZV and HSV-1 DNA in human nodose and celiac ganglia. Virus Genes 23(2):145–147
Gilden DH, Rozenman Y, Murray R, Devlin M, Vafai A (1987) Detection of varicella-zoster virus nucleic acid in neurons of normal human thoracic ganglia. Ann Neurol 22(3):377–380
Gilden DH, Vafai A, Shtram Y, Becker Y, Devlin M, Wellish M (1983) Varicella-zoster virus DNA in human sensory ganglia. Nature 306(5942):478–480
Gilden DH, Wroblewska Z, Kindt V, Warren KG, Wolinsky JS (1978) Varicella-zoster virus infection of human brain cells and ganglion cells in tissue culture. Arch Virol 56(1–2):105–117
Gimenez-Cassina A, Lim F, Diaz-Nido J (2006) Differentiation of a human neuroblastoma into neuron-like cells increases their susceptibility to transduction by herpesviral vectors. J Neurosci Res 84(4):755–767
Gowrishankar K, Slobedman B, Cunningham AL, Miranda-Saksena M, Boadle RA, Abendroth A (2007) Productive varicella-zoster virus infection of cultured intact human ganglia. J Virol 81(12):6752–6756
Grinfeld E, Sadzot-Delvaux C, Kennedy PG (2004) Varicella-Zoster virus proteins encoded by open reading frames 14 and 67 are both dispensable for the establishment of latency in a rat model. Virology 323(1):85–90
Hamza MA, Higgins DM, Ruyechan WT (2007) Two alphaherpesvirus latency-associated gene products influence calcitonin gene-related peptide levels in rat trigeminal neurons. Neurobiol Dis 25(3):553–560
Hanani M (2005) Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev 48(3):457–476
Head H, Campbell W (1900) The pathology of herpes zoster and its bearing on sensory localization. Brain 23:353–523
Holland DJ, Cunningham AL, Boadle RA (1998) The axonal transmission of herpes simplex virus to epidermal cells: a novel use of the freeze substitution technique applied to explant cultures retained on cover slips. J Microsc 192(Pt 1):69–72
Holland DJ, Miranda-Saksena M, Boadle RA, Armati P, Cunningham AL (1999) Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J Virol 73(10):8503–8511
Hood C, Cunningham AL, Slobedman B, Arvin AM, Sommer MH, Kinchington PR, Abendroth A (2006) Varicella-zoster virus ORF63 inhibits apoptosis of primary human neurons. J Virol 80(2):1025–1031
Hood C, Cunningham AL, Slobedman B, Boadle RA, Abendroth A (2003) Varicella-zoster virus-infected human sensory neurons are resistant to apoptosis, yet human foreskin fibroblasts are susceptible: evidence for a cell-type-specific apoptotic response. J Virol 77(23):12852–12864
Hufner K, Derfuss T, Herberger S, Sunami K, Russell S, Sinicina I, Arbusow V, Strupp M, Brandt T, Theil D (2006) Latency of alpha-herpes viruses is accompanied by a chronic inflammation in human trigeminal ganglia but not in dorsal root ganglia. J Neuropathol Exp Neurol 65(10):1022–1030
Hyman RW, Ecker JR, Tenser RB (1983) Varicella-zoster virus RNA in human trigeminal ganglia. Lancet 2(8354):814–816
Joly E, Mucke L, Oldstone MB (1991) Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science 253(5025):1283–1285
Kennedy PG, Grinfeld E, Bontems S, Sadzot-Delvaux C (2001) Varicella-Zoster virus gene expression in latently infected rat dorsal root ganglia. Virology 289(2):218–223
Kennedy PG, Grinfeld E, Gow JW (1998) Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc Natl Acad Sci USA 95(8):4658–4662
Kress M, Fickenscher H (2001) Infection by human varicella-zoster virus confers norepinephrine sensitivity to sensory neurons from rat dorsal root ganglia. FASEB J 15(6):1037–1043
LaGuardia JJ, Cohrs RJ, Gilden DH (1999) Prevalence of varicella-zoster virus DNA in dissociated human trigeminal ganglion neurons and nonneuronal cells. J Virol 73(10):8571–8577
Lam PM, Hainsworth AH, Smith GD, Owen DE, Davies J, Lambert DG (2007) Activation of recombinant human TRPV1 receptors expressed in SH-SY5Y human neuroblastoma cells increases [Ca(2+)](i), initiates neurotransmitter release and promotes delayed cell death. J Neurochem 102(3):801–811
Lawson SN (2005) The peripheral sensory nervous system: dorsal root ganglion neurons. In: Dyck PJ, Thomas PK (eds) Peripheral Neuropathy. Elsevier Saunders, Philadelphia, PA, pp 163–202
Levin MJ, Cai GY, Manchak MD, Pizer LI (2003) Varicella-zoster virus DNA in cells isolated from human trigeminal ganglia. J Virol 77(12):6979–6987
Lowry PW, Sabella C, Koropchak CM, Watson BN, Thackray HM, Abbruzzi GM, Arvin AM (1993) Investigation of the pathogenesis of varicella-zoster virus infection in guinea pigs by using polymerase chain reaction. J Infect Dis 167(1):78–83
Lungu O, Annunziato PW, Gershon A, Staugaitis SM, Josefson D, LaRussa P, Silverstein SJ (1995) Reactivated and latent varicella-zoster virus in human dorsal root ganglia. Proc Natl Acad Sci USA 92(24):10980–10984
Lungu O, Panagiotidis CA, Annunziato PW, Gershon AA, Silverstein SJ (1998) Aberrant intracellular localization of Varicella-Zoster virus regulatory proteins during latency. Proc Natl Acad Sci USA 95(12):7080–7085
Mahalingam R, Wellish M, Cohrs R, Debrus S, Piette J, Rentier B, Gilden DH (1996) Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc Natl Acad Sci USA 93(5):2122–2124
Mahalingam R, Wellish M, Lederer D, Forghani B, Cohrs R, Gilden D (1993) Quantitation of latent varicella-zoster virus DNA in human trigeminal ganglia by polymerase chain reaction. J Virol 67(4):2381–2384
Mahalingam R, Wellish MC, Dueland AN, Cohrs RJ, Gilden DH (1992) Localization of herpes simplex virus and varicella zoster virus DNA in human ganglia. Ann Neurol 31(4):444–448
Matsunaga Y, Yamanishi K, Takahashi M (1982) Experimental infection and immune response of guinea pigs with varicella-zoster virus. Infect Immun 37(2):407–412
Merville-Louis MP, Sadzot-Delvaux C, Delree P, Piette J, Moonen G, Rentier B (1989) Varicella-zoster virus infection of adult rat sensory neurons in vitro. J Virol 63(7):3155–3160
Mikloska Z, Cunningham AL (2001) Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J Virol 75(23):11821–11826
Mikloska Z, Sanna PP, Cunningham AL (1999) Neutralizing antibodies inhibit axonal spread of herpes simplex virus type 1 to epidermal cells in vitro. J Virol 73(7):5934–5944
Myers MG, Duer HL, Hausler CK (1980) Experimental infection of guinea pigs with varicella-zoster virus. J Infect Dis 142(3):414–420
Myers MG, Stanberry LR, Edmond BJ (1985) Varicella-zoster virus infection of strain 2 guinea pigs. J Infect Dis 151(1):106–113
Penfold ME, Armati P, Cunningham AL (1994) Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc Natl Acad Sci USA 91(14):6529–6533
Penfold ME, Armati PJ, Mikloska Z, Cunningham AL (1996) The interaction of human fetal neurons and epidermal cells in vitro. Cell Dev Biol Anim 32(7):420–426
Reichelt M, Zerboni L, Arvin AM (2008) Mechanisms of varicella-zoster virus neuropathogenesis in human dorsal root ganglia. J Virol 82(8):3971–3983
Ross RA, Spengler BA, Biedler JL (1983) Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst 71(4):741–747
Sadzot-Delvaux C, Arvin AM, Rentier B (1998) Varicella-zoster virus IE63, a virion component expressed during latency and acute infection, elicits humoral and cellular immunity. J Infect Dis 178(Suppl 1):S43–S47
Sadzot-Delvaux C, Debrus S, Nikkels A, Piette J, Rentier B (1995) Varicella-zoster virus latency in the adult rat is a useful model for human latent infection. Neurology 45(12 Suppl 8):S18–S20
Sadzot-Delvaux C, Merville-Louis MP, Delree P, Marc P, Piette J, Moonen G, Rentier B (1990) An in vivo model of varicella-zoster virus latent infection of dorsal root ganglia. J Neurosci Res 26(1):83–89
Sato H, Callanan LD, Pesnicak L, Krogmann T, Cohen JI (2002) Varicella-zoster virus (VZV) ORF17 protein induces RNA cleavage and is critical for replication of VZV at 37 degrees C but not 33 degrees C. J Virol 76(21):11012–11023
Sato H, Pesnicak L, Cohen JI (2003) Use of a rodent model to show that varicella-zoster virus ORF61 is dispensable for establishment of latency. J Med Virol 70(Suppl 1):S79–S81
Schaap A, Fortin JF, Sommer M, Zerboni L, Stamatis S, Ku CC, Nolan GP, Arvin AM (2005) T-cell tropism and the role of ORF66 protein in pathogenesis of varicella-zoster virus infection. J Virol 79(20):12921–12933
Schmidbauer M, Budka H, Pilz P, Kurata T, Hondo R (1992) Presence, distribution and spread of productive varicella zoster virus infection in nervous tissues. Brain 115(Pt 2):383–398
Schmidt M, Kress M, Heinemann S, Fickenscher H (2003) Varicella-zoster virus isolates, but not the vaccine strain OKA, induce sensitivity to alpha-1 and beta-1 adrenergic stimulation of sensory neurones in culture. J Med Virol 70(Suppl 1):S82–S89
Smith FP (1978) Pathological studies of spinal nerve ganglia in relation to intractable intercostal pain. Surg Neurol 10(1):50–53
Somekh E, Levin MJ (1993) Infection of human fetal dorsal root neurons with wild type varicella virus and the Oka strain varicella vaccine. J Med Virol 40(3):241–243
Somekh E, Tedder DG, Vafai A, Assouline JG, Straus SE, Wilcox CL, Levin MJ (1992) Latency in vitro of varicella-zoster virus in cells derived from human fetal dorsal root ganglia. Pediatr Res 32(6):699–703
Stallings CL, Duigou GJ, Gershon AA, Gershon MD, Silverstein SJ (2006) The cellular localization pattern of Varicella-Zoster virus ORF29p is influenced by proteasome-mediated degradation. J Virol 80(3):1497–1512
Steain MC, Sutherland JP, Rodriguez M, Buckland M, Cunningham AL, Slobedman B, Abendroth A (2009) Comparison of naturally infected ganglia during and after herpes zoster. The 34th Annual International Herpesvirus Workshop. Ithaca, New York, USA
Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T (2003) Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol 163(6):2179–2184
Turnley AM, Starr R, Bartlett PF (2002) Failure of sensory neurons to express class I MHC is due to differential SOCS1 expression. J Neuroimmunol 123(1–2):35–40
Vafai A, Murray RS, Wellish M, Devlin M, Gilden DH (1988) Expression of varicella-zoster virus and herpes simplex virus in normal human trigeminal ganglia. Proc Natl Acad Sci USA 85(7):2362–2366
van Velzen M, Laman JD, Kleinjan A, Poot A, Osterhaus AD, Verjans GM (2009) Neuron-interacting satellite glial cells in human trigeminal ganglia have an APC phenotype. J Immunol 183(4):2456–2461
Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD (2007) Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci USA 104(9):3496–3501
Walters MS, Kyratsous CA, Wan S, Silverstein S (2008) Nuclear import of the varicella-zoster virus latency-associated protein ORF63 in primary neurons requires expression of the lytic protein ORF61 and occurs in a proteasome-dependent manner. J Virol 82(17):8673–8686
Watson CP, Deck JH, Morshead C, Van der Kooy D, Evans RJ (1991) Post-herpetic neuralgia: further post-mortem studies of cases with and without pain. Pain 44(2):105–117
Watson CP, Morshead C, Van der Kooy D, Deck J, Evans RJ (1988) Post-herpetic neuralgia: post-mortem analysis of a case. Pain 34(2):129–138
Weller TH (1953) Serial propagation in vitro of agents producing inclusion bodies derived from varicella and herpes zoster. Proc Soc Exp Biol Med 83(2):340–346
Weller TH, Stoddard MB (1952) Intranuclear inclusion bodies in cultures of human tissue inoculated with varicella vesicle fluid. J Immunol 68(3):311–319
Wigdahl B, Rong BL, Kinney-Thomas E (1986) Varicella-zoster virus infection of human sensory neurons. Virology 152(2):384–399
Wroblewska Z, Valyi-Nagy T, Otte J, Dillner A, Jackson A, Sole DP, Fraser NW (1993) A mouse model for varicella-zoster virus latency. Microb Pathog 15(2):141–151
Zerboni L, Ku CC, Jones CD, Zehnder JL, Arvin AM (2005) Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc Natl Acad Sci USA 102(18):6490–6495
Zerboni L, Reichelt M, Arvin AM (2010) Varicella-zoster virus neurotropism in SCID mouse–human dorsal root ganglia xenografts. Curr Top Microbiol Immunol doi 10.1007/82_2009_8
Zerboni L, Reichelt M, Jones CD, Zehnder JL, Ito H, Arvin AM (2007) Aberrant infection and persistence of varicella-zoster virus in human dorsal root ganglia in vivo in the absence of glycoprotein I. Proc Natl Acad Sci USA 104(35):14086–14091
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Steain, M., Slobedman, B., Abendroth, A. (2010). Experimental Models to Study Varicella-Zoster Virus Infection of Neurons. In: Abendroth, A., Arvin, A., Moffat, J. (eds) Varicella-zoster Virus. Current Topics in Microbiology and Immunology, vol 342. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2010_15
Download citation
DOI: https://doi.org/10.1007/82_2010_15
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-12727-4
Online ISBN: 978-3-642-12728-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)